Abstract
Dendritic cell (DC)–based vaccines are a promising strategy for tumor immunotherapy due to their ability to activate both antigen-specific T-cell immunity and innate immune effector components, including natural killer (NK) cells. However, the optimal mode of antigen delivery and DC activation remains to be determined. Using M protein mutant vesicular stomatitis virus (ΔM51-VSV) as a gene-delivery vector, we demonstrate that a high level of transgene expression could be achieved in ~70% of DCs without affecting cell viability. Furthermore, ΔM51-VSV infection activated DCs to produce proinflammatory cytokines (interleukin-12, tumor necrosis factor-α, and interferon (IFN)α/β), and to display a mature phenotype (CD40highCD86high major histocompatibility complex (MHC II)high). When delivered to mice bearing 10-day-old lung metastatic tumors, DCs infected with ΔM51-VSV encoding a tumor-associated antigen mediated significant control of tumor growth by engaging both NK and CD8+ T cells. Importantly, depletion of NK cells completely abrogated tumor destruction, indicating that NK cells play a critical role for this DC vaccine-induced therapeutic outcome. Our findings identify ΔM51-VSV as both an efficient gene-delivery vector and a maturation agent allowing DC vaccines to overcome immunosuppression in the tumor-bearing host.
Introduction
Many cancer vaccines are primarily designed to target adaptive T-cell immunity but increasing evidence has demonstrated that concomitant activation of natural killer (NK) cells is crucial for the generation of optimal antitumor activity. In this regard, NK cells not only provide an additional effector mechanism to directly control tumor growth1 but also secrete cytokines, such as interferon (IFN)γ, which facilitate the development of effective CD8+ cytotoxic T-lymphocyte (CTL) responses.2 In some cases, NK cells are the sole effector population against tumors after vaccination.3 Thus, it is important to select those vaccine approaches that are capable of both activating and linking innate and adaptive immunity.
Dendritic cells (DCs) are an ideal cancer vaccine platform as the most potent antigen-presenting cells for priming both CD4+ and CD8+ T cells. Moreover, cross talk between DCs and NK cells results in DC maturation and NK activation, providing favorable conditions for the induction of antitumor CTL and Th immune responses.2 Indeed, we, and others, have demonstrated that ex vivo differentiated DCs can engage T-lymphocytes and NK cells following adoptive transfer.1,4,5 However, the outcome of immune responses is dependent on DC maturational status and the availability of specific antigen components. For instance, immature DCs are susceptible to NK killing and are likely to induce T-cell tolerance.6,7 Similarly, DCs fail to prime optimal CTL responses in the absence of cognate interactions with CD4+ T cells.8 Finally, factors produced following DC activation, including type-I IFN (IFNα/β), are known to play an important role in the activation of NK and T cells.9,10 Thus, developing optimal strategies for DC maturation and antigen loading is critical to the success of DC-based cancer immunotherapy.
Genetic modification with a recombinant virus has been demonstrated as an effective approach to deliver tumor antigens into DCs.11 Viral transduction offers the advantage of a continuous supply of antigen and a broad spectrum of major histocompatibility complex (MHC) epitopes presented by DCs.11 Of these viral vectors, adenovirus (Ad), lentivirus, and vaccinia virus are the most intensively studied due to their ability to infect a wide variety of cells including DCs.11,12,13 More importantly, these recombinant viral vectors appear to be able to induce DC maturation, though controversial results have also been reported.13,14 Considerable effort is currently being devoted to further manipulate these vectors for the improvement of their specificity and effect on DC function.15,16,17
Vesicular stomatitis virus (VSV) is a negative-strand RNA virus in the family Rhabdoviridae that has been used successfully as an oncolytic agent or vaccine vector in animal models.18 VSV can infect multiple cell types and its replication is naturally attenuated in normal tissues by the innate IFN (IFNα/β) response. Most wild-type strains of VSV inhibit gene expression and suppress IFN production via the matrix (M) protein; however, deletion of methionine 51 (M51) in M prevents this blockade.19 Thus, infection with VSV carrying this mutation (ΔM51-VSV) produces a marked IFN response in normal cells, facilitating its neutralization in healthy tissues.19 These properties may make ΔM51-VSV an ideal vector to transduce DCs for the development of DC-based cancer vaccines.
In this article, we have examined the utility of ΔM51-VSV as a vector for antigen loading and activation of DC. We demonstrate that VSV infection is an effective and nontoxic method to create a DC vaccine that potently engages both NK and T-cell immunity in a tumor-bearing host.
Results
ΔM51-VSV can efficiently infect DCs in vitro
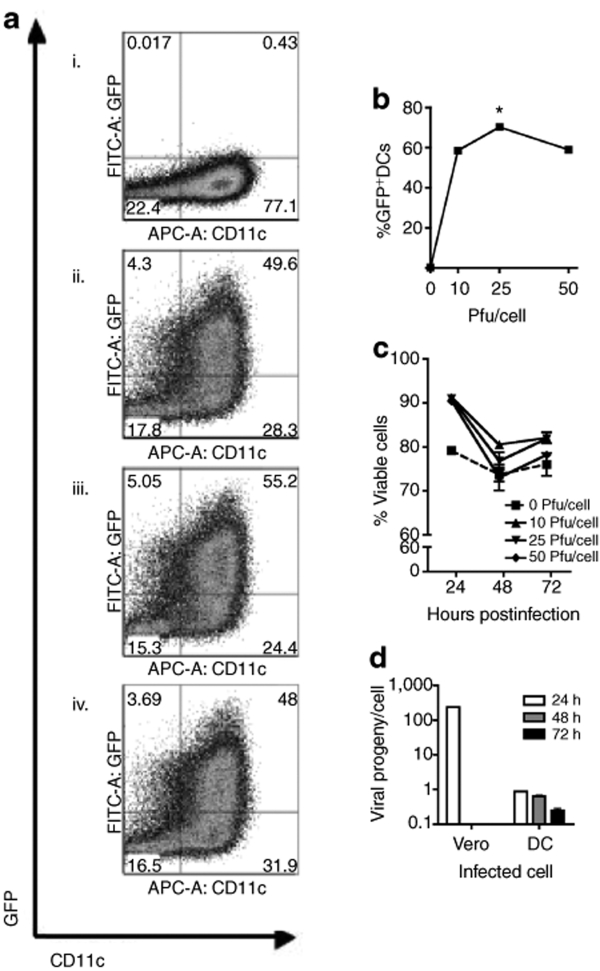
A recent report indicated that bone marrow–derived myeloid DCs could tolerate infection by an M protein mutant of VSV and these cells remained viable for at least 24 hours (ref. 20). With the goal of using ΔM51-VSV-infected DCs as a vaccine platform, we extended the previous study to determine the multiplicity of infection (MOI) that would give maximal transgene expression without causing significant DC mortality. To this end, bone marrow–derived DCs were infected at 10, 25, or 50 plaque-forming units (pfu)/cell with ΔM51-VSV carrying green fluorescent protein (GFP) (VSV/GFP) and analyzed 24, 48, and 72 hours later for transgene (GFP) expression and viability. As shown in Figure 1a,b, 10 pfu/cell of VSV/GFP resulted in substantial GFP expression (58.6 ± 0.7%) in cultured CD11c+ DC at 24 hours postexposure, suggesting that DCs can be readily loaded with an antigen via ΔM51-VSV infection. Although an MOI of 25 increased GFP expression to 70.3 ± 1.2%, no further increase was obtained by doubling the viral dose to 50 pfu/cell (58.9 ± 0.9%). GFP was not measured in DCs infected with ΔM51-VSV containing no transgene (VSV/MT) excluding the possibility that the signal was due to infection-induced autofluorescence (not shown). Importantly, infection by all three MOIs did not compromise DC viability, which was equivalent to or higher than mock-infected cells (Figure 1c) for at least 3 days in culture, suggesting that myeloid DCs can tolerate relatively high MOIs of this mutant virus and remain viable to interact with host immune cells upon in vivo inoculation. As 25 pfu ΔM51-VSV/cell gave maximal transgene expression without toxicity, we decided to use this MOI for all subsequent experiments. To confirm the clinical feasibility of this strategy, we tested the ability of ΔM51-VSV to infect DCs derived from human CD34+ peripheral blood mononuclear cells. Similar to murine bone marrow–derived DCs, the highest transduction efficiency occurred when human DCs were infected with 25 pfu ΔM51-VSV/cell. At 24 hours after infection, 64.6 ± 0.5 % of human CD11c+ cells were GFP+ (Supplementary Figure S1a,b; P < 0.001). In addition, these cells remained 96.6 ± 0.9% viable at this time point (Supplementary Figure S1c). Taken together, these findings imply that DCs can be readily transduced with antigen using ΔM51-VSV and administered to patients for cancer immunotherapy.
Figure 1.
DCs are efficiently transduced using VSV and remain viable for at least 72 hours after infection. (a) Triplicate wells of DCs were infected with 0 (i) (mock treatment), 10 (ii), 25 (iii), or 50 (iv) pfu/cell VSV/GFP for 4 hours in minimal media, washed thrice in PBS, and returned to culture in DC media. Representative dot pots depicting GFP expression versus CD11c are shown. (b) GFP expression by DCs (gated on CD11c+ cells) was quantified 24 hours postinfection by flow cytometry (*P < 0.0001 compared to all other groups). (c) Cell viability was determined by 7-amino actinomycin-D staining 24, 48, or 72 hours postinfection or mock treatment. (d) Samples of infected cell supernatants were collected 24, 48, and 72 hours after infection and washing and viral progeny was quantified by plaque assay. Vero cells were infected using identical conditions as a positive control for productive infection. Data are representative of three independent trials. APC, allophycocyanin; DC, dendritic cell; FITC, fluorescein isothiocyanate; GFP, green fluorescent protein; PBS, phosphate-buffered saline; pfu, plaque-forming units; VSV, vesicular stomatitis virus.
To determine whether infected DCs produce viral progeny, supernatants were collected every 24 hours for 3 days following infection with 25 pfu/cell VSV. Infected DCs released minimal amounts of virus (0.8 pfu/cell) in comparison to highly permissive Vero cells, where the virus production was 300 times higher in the first 24 hours (Figure 1d). After 24 hours, the production of VSV by infected DCs declined further, demonstrating that only minimal viral replication occurs in DCs. No comparisons could be made to Vero at these time points because these cells were killed by the VSV infection within the first 24 hours. Thus, DCs are susceptible to infection with ΔM51-VSV viruses but do not permit productive replication.
VSV infection elicits maturation of DC
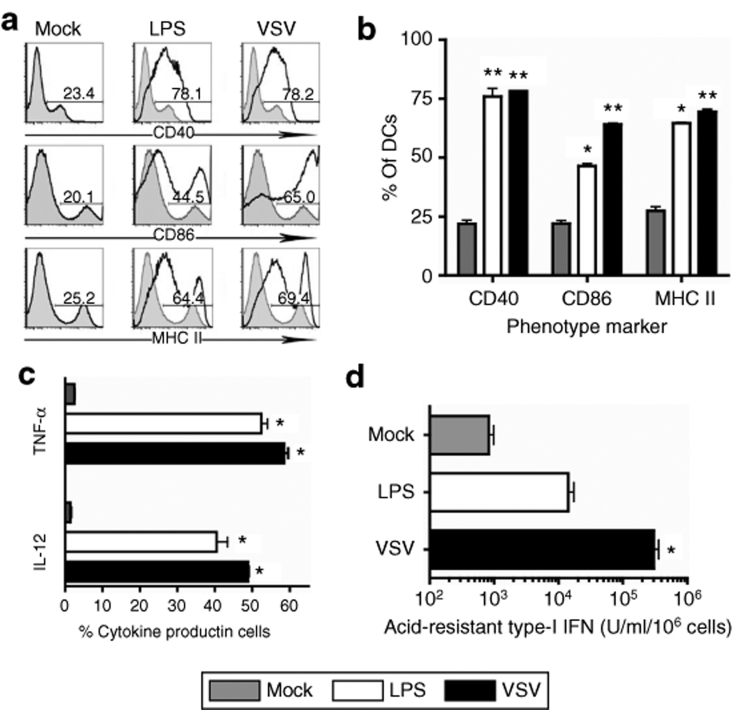
Another important aspect determining the overall efficiency of DC vaccination is the maturation status of DCs following ex vivo manipulation. To determine whether ΔM51-VSV infection at an MOI of 25 affected DC maturation, we analyzed both co-stimulatory molecule expression and cytokine production by DCs using flow cytometry. Approximately 80% of the cells generated in our mock-infected culture conditions were positive for CD11c (Figure 1a) but only 20–30% of them expressed high levels of CD40, CD86, and MHC II, suggesting a heterogeneous population of mostly immature DCs (Figure 2a,b; Mock). Consistent with surface marker expression, only minimal production of the proinflammatory cytokines tumor necrosis factor-α and interleukin-12 was measured in unmanipulated DCs (<5%, Figure 2c). However, 65–80% of CD11c+ DCs expressed high levels of CD40, CD86, and MHC II 24 hours after infection with VSV/MT, indicating a strong induction of maturation (Figure 2a,b, P < 0.001 compared to mock-treated cells). This level of maturation was similar to (CD40) or greater than (CD86 and MHC II, P < 0.05) that induced by lipopolysaccharide (LPS) treatment (Figure 2b). Similarly, significantly more DCs were activated to produce interleukin-12 and tumor necrosis factor-α following treatment with LPS or VSV/MT compared to their mock-treated counterparts (P < 0.001, Figure 2c). As the ΔM51-VSV vector used in this study is an IFN-inducing mutant, we also assayed for type-I IFN concentration in supernatants before and after VSV/MT infection of DCs using a bioassay based on inhibition of wild-type VSV growth in L929 cells. Indeed, levels of IFNα/β were much higher in the supernatants of infected DCs (Figure 2d, P < 0.05 compared to mock- and LPS-treated cells), confirming the IFN-inducing capacity of the mutant VSV vector. Taken together, the upregulation of maturational markers and cytokine production by DCs following infection indicates a mature phenotype, suggesting that in addition to antigen loading, transduction with ΔM51-VSV allows simultaneous activation of DCs.
Figure 2.
VSV infection induces maturation and cytokine production by DCs. DCs were infected with 25 pfu/cell VSV/MT, treated with LPS, or mock-treated for 4 hours in minimal media, washed thrice in PBS, and returned to culture. (a) Representative histograms depicting CD40, CD86, and MHC II expression from mock (shaded) and stimulated (black line) samples are shown. (b) Summarized data for CD40, CD86, and MHC II expression by DCs (**P < 0.001 compared to mock-treated cells; *P < 0.05, significantly different from mock- and VSV-treated cells). (c) 12 hours following infection, intracellular staining for IL-12 and TNF-α was performed (**P < 0.001, significant increase compared to mock-treated cells). (d) Supernatants were collected 12 hours after infection and analyzed for acid-resistant type-I IFN based on VSV plaque reduction assay as described in materials and methods (*P < 0.05 compared with mock- and LPS-treated cells). Bars represent means ± SEM and data from three independent infections. DC, dendritic cell; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; MHC, major histocompatibility complex; pfu, plaque-forming units; TNF, tumor necrosis factor; VSV, vesicular stomatitis virus.
ΔM51-VSV-infected DCs confer therapeutic effect against lung metastatic melanoma
Having demonstrated the ability of the VSV vector to introduce antigen into and activate DCs, we next assessed the therapeutic capacity of VSV expressing an ovalbumin (OVA)-derived CD8+ T-cell epitope, SIINFEKL (VSV/SIIN), as a model tumor antigen to transduce DCs for immunization of mice with established tumors. C57BL/6 mice were injected intravenously with 1 × 106 B16F10-OVA cells, which consistently results in the formation of visible tumor nodules on the lung surface in 10 days and becomes fatal within 21–25 days in untreated mice (J. Boudreau and Y. Wan, unpublished results). Thus, we chose to treat animals with VSV/SIIN-transduced DCs (DC-VSV/SIIN) at day 10 after tumor inoculation and kill them 11 days later for the enumeration of lung metastases. We included DCs infected with VSV/MT (DC-VSV/MT) and phosphate-buffered saline (PBS) as controls for virus infection and mock treatment, respectively. Interestingly, although the lowest tumor burden was measured in mice vaccinated with DC-VSV/SIIN (157.2 ± 34.3 lung nodules, P < 0.01 compared to PBS; P < 0.05 compared to DC-VSV/MT), mice immunized with DC-VSV/MT were also significantly protected (285.8 ± 41.9 lung nodules), compared to those treated with PBS (657.0 ± 95.0 lung nodules, P < 0.05) (Figure 3a), suggesting that in addition to SIINFEKL-specific CD8+ T cells, nonantigen-specific effects are involved in tumor destruction. To compare with other maturation approaches, we included DCs activated by LPS and pulsed with or without SIINFEKL peptide (DC/LPS-SIIN and DC/LPS, respectively), or transduced with recombinant Ad encoding no transgene (DC/Ad-BHG) or SIINFEKL (DC/Ad-SIIN). As shown in Figure 3b, LPS-treated DCs failed to control tumor growth even when pulsed with SIINFEKL peptide. Conversely, similar to VSV infection, DC/Ad-BHG elicited a significant decrease in lung tumor burden (325.0 ± 67.6 lung nodules, P < 0.001), which was enhanced when the Ad used to transduce DCs encoded SIINFEKL (DC/Ad-SIIN, 213.7 ± 16.8, P < 0.05). These results suggest that maturation per se is insufficient and that viral vectors may provide additional signals that enable DCs to activate therapeutic antitumor immunity in tumor-bearing mice.
Figure 3.
VSV-infected DCs mediate therapeutic tumor destruction. (a) Mice were challenged with 1 × 106 B16-OVA cells intravenously and immunized 10 days later with PBS, DC-VSV/MT, or DC-VSV/SIIN. Mice were killed 11 days after immunization (21 days after challenge), lungs were collected and tumour nodules were counted using a dissecting microscope (significantly greater tumor burden in PBS compared to DC-VSV/MT (**P < 0.001) and DC-VSV/SIIN (**P < 0.001) *P < 0.05, significant difference between DC-VSV/MT and DC-VSV/SIIN). (b) Mice were immunized with DCs treated LPS with or without SIINFEKL peptide pulsing, or infected with recombinant Ad carrying SIINFEKL (DC/Ad-SIIN) or no transgene (DC/Ad-BHG). Significant reduction compared to PBS, **P < 0.001. Bars represent means ± SEM from two independent experiments with 5 mice/group. DC, dendritic cell; LPS, lipopolysaccharide; OVA, ovalbumin; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
Because a small amount of VSV could be produced by infected DCs (Figure 1d), it is possible that tumor destruction by DC/VSV might be partially attributable to VSV-mediated oncolysis.19 To examine this possibility, mice bearing 10-day-old lung metastatic tumors were treated with 1 × 106 DC-VSV/MT or 25 × 106 pfu VSV/MT (equivalent to the dose used for in vitro infection of 1 × 106 DCs) by footpad injection. No VSV could be detected by plaque assay from lung homogenates of mice 3 days after ΔM51-VSV alone or DC/VSV inoculation (data not shown). Furthermore, a single injection with 107 to 109 pfu ΔM51-VSV had no effect on the growth of B16 lung metastasis (B. Bridle and J. Boudreau, unpublished results), indicating that oncolysis does not play a significant role in the tumor destruction mediated by DC/VSV.
Activation of both CD8+ T cells and NK cells by ΔM51-VSV-transduced DCs
We and others have previously shown that the prophylactic, nonantigen-specific antitumor activity generated by DCs is mediated by NK cells and activation of both NK cells and CD8+ T cells is often associated with maximum efficacy.1,4,5 To determine whether these populations could also be activated in tumor-bearing hosts, mice bearing 10-day-old lung metastatic tumors were immunized with PBS or DCs infected with either VSV/MT or VSV/SIIN. Eleven days after immunization, mice were killed and activation of splenic CD8+ and NK cells was assessed.
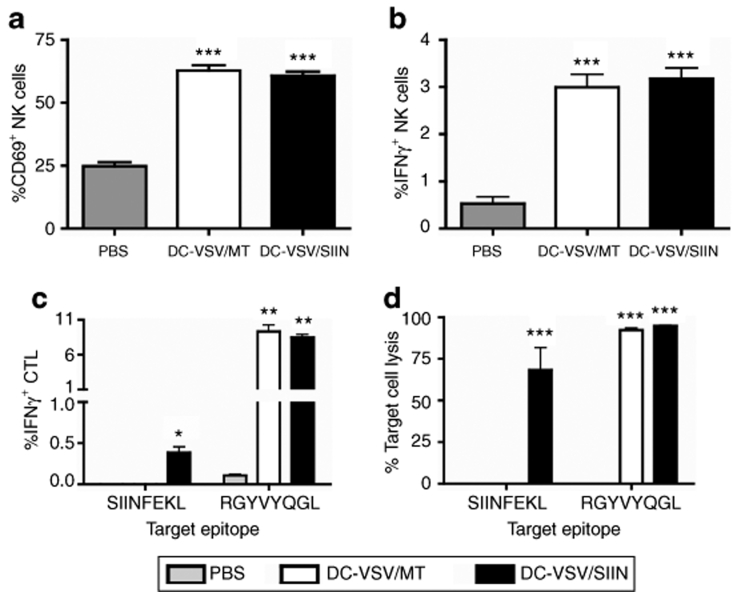
Following a brief culture in vitro, basal levels of CD69 expression (24.87 ± 1.52%, Figure 4a) and IFNγ secretion (0.53 ± 0.14%, Figure 4b) were detected in NK cells from PBS-treated animals. However, the frequency of NK cells, which stained positive for CD69 and IFNγ, was increased 2.5- and 5.8-fold, respectively (P < 0.0001), in mice injected with DCs infected with either recombinant virus, demonstrating that VSV-infected DCs mediate sustained activation of NK cells in the presence of established tumors.
Figure 4.
Immunization with VSV-infected DCs elicits NK and CTL activation in tumor-bearing hosts. Mice bearing 10-day-old B16-OVA lung metastases were immunized with DC-VSV/SIIN or DC-VSV/MT, or unimmunized (PBS). Eleven days following immunization, splenic NK cells were analyzed for activation by flow cytometry based on (a) CD69 expression and (b) IFNγ production (***P < 0.0001, compared with PBS-immunized). (c) To analyze CTL activation, IFNγ production following in vitro peptide restimulation was measured by flow cytometry (*P < 0.05 significantly greater IFNγ production following restimulation with SIINFEKL peptide compared to PBS and DC-VSV/MT-immunized mice; **P < 0.001, significantly greater production of IFNγ following restimulation with RGYVYQGL peptide compared to PBS-immunized. (d) In vivo T-cell cytotoxic activity was measured as described in materials and methods (***P < 0.0001, significantly greater target cell killing compared to unpulsed targets. Bars illustrate means ± SEM. Data are representative of two independent experiments with 5 mice/group. CTL, cytotoxic T-lymphocyte; DC, dendritic cell; IFN, interferon; NK, natural killer; OVA, ovalbumin; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
To characterize the antigen-specific CD8+ T-cell responses elicited by the DC/VSV inoculum, we measured immunity against epitopes derived from the transgene (SIINFEKL) or the nucleoprotein of VSV (RGYVYQGL). As shown in Figure 4c, IFNγ production in response to SIINFEKL was measured in splenic CD8+ T cells that were harvested from mice immunized with DC-VSV/SIIN, but not with DC-VSV/MT or PBS (P < 0.05). Similar frequencies of CD8+ T cells specific for the viral peptide, RGYVYQGL, were detected in mice treated with either DC-VSV/SIIN or DC-VSV/MT, confirming antigen specificity and similar loading between the two recombinant viruses.
To determine the cytolytic ability of activated CD8+ T cells, we performed in vivo cytotoxicity assays. Consistent with the observations for cytokine production, mice immunized with DC-VSV/SIIN could clear target cells pulsed with either SIINFEKL or RGYVYQGL, whereas DC-VSV/MT-immunized animals only eliminated lymphocytes pulsed with the viral peptide (Figure 4d, P < 0.0001). Taken together, these results demonstrate that ΔM51-VSV-infected DCs are an effective vaccine platform, capable of activating antigen-specific CD8+ T cells and NK cells in tumor-bearing hosts.
NK cells play a predominant role in tumor suppression
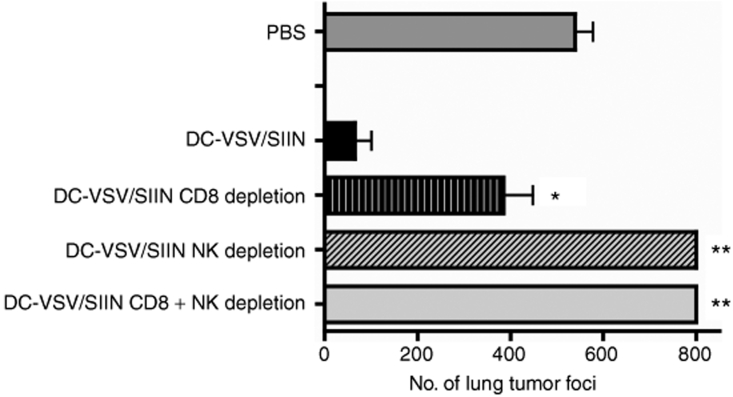
To determine the requirement of CD8+ T cells and NK cells for the tumor destruction induced by DC-VSV/SIIN, in vivo depletion of these subsets was performed by repeated intraperitoneal injections of depleting antibodies. As shown in Figure 5, depletion of CD8+ T cells reduced tumor destruction (P < 0.01), compared to immunized mice without depletion, but tumor burdens were still significantly lower than in PBS-immunized mice (P < 0.01). A more profound effect was observed when either NK cells or both NK cells and CD8+ cells were concurrently depleted: tumor clearance was completely abrogated and tumor burdens were even greater than in PBS-treated animals (P < 0.001). These findings demonstrate that both CD8+ T cells and NK cells are involved in tumor growth control following immunization with DC-VSV/SIIN and that NK cells appear to play a predominant and critical role for tumor destruction.
Figure 5.
Tumor destruction mediated by VSV-infected DCs involves both CD8+ and NK cells. Mice were challenged and immunized as described in Figure 3. CD8 and NK-depleting antibodies were administered throughout the immunization period the number of lung tumor metastases was enumerated 21 days after challenge (*P < 0.01 and **P < 0.001 compared to DC-VSV/SIIN-immunized). Bars represent means ± SEM and two independent experiments with 5 mice/group. DC, dendritic cell; NK, natural killer; VSV, vesicular stomatitis virus.
ΔM51-VSV-infected DCs can elicit tumor destruction using a native tumor-associated antigen
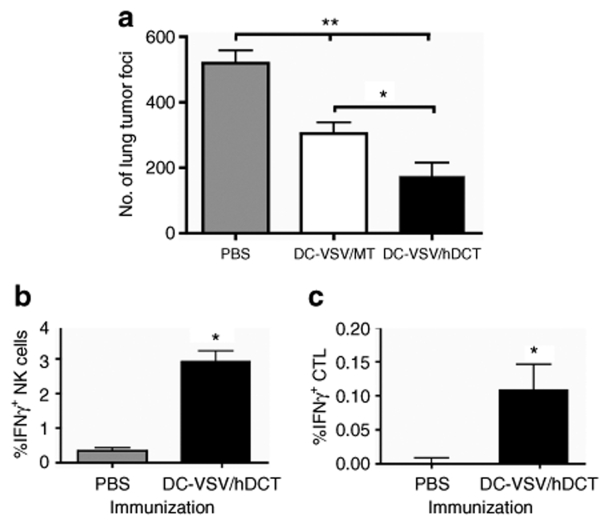
Clinical cancer immunotherapy will require immune targeting of native tumor-associated antigens. With this in mind, we constructed a recombinant ΔM51-VSV vector that expresses the human melanoma–associated antigen, dopachrome tautomerase (hDCT), and used this vector to transduce DCs for immunization of tumor-bearing mice. Human DCT is highly homologous (84%) to its murine counterpart,21 which is endogenously expressed by B16-OVA cells. CD8+ T-cell immunity against a dominant epitope SVYDFFVWL, which is 100% conserved between mouse and human, has been associated with B16 tumor rejection.8 Similar to DC-VSV/SIIN, immunization with DC-VSV/hDCT in mice carrying 10-day-old lung metastases could significantly reduce tumor burdens compared to PBS and DC-VSV/MT treated mice (Figure 6a, P < 0.01 and P < 0.001, respectively). Moreover, activation of NK cells and SVYDFFVWL-specific CD8+ T cells was evident by intracellular staining of IFNγ (Figure 6b,c).
Figure 6.
DCs infected with VSV carrying a native tumour antigen mediate tumor protection. Mice were challenged with 106 B16-OVA cells i.v., and treated with PBS or immunized with DC-VSV/hDCT. Eleven days after immunization, mice were killed and spleens and lungs were collected. (a) Tumor nodules on lung surfaces were counted using a dissecting microscope (significantly greater tumor burden in PBS-treated compared to DC-VSV/MT (**P < 0.01) and DC-VSV/hDCT (**P < 0.001); *P < 0.05, significantly different tumor burdens between DC-VSV/MT and DC-VSV/hDCT). (b) IFNγ production by splenic NK cells was measured after in vitro culture without stimulation (*P < 0.05 compared to PBS-treated). (c) CD8+ T cells were restimulated with SVYVYQGL peptide in vitro and IFNγ production was measured (*P < 0.05 compared to PBS-treated). DC, dendritic cell; IFN, interferon; OVA, ovalbumin; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
Discussion
DCs are the most potent antigen-presenting cells and have proven highly effective as cellular vehicles for eliciting antigen-specific immune responses. However, although DC vaccines are often successful for immunization of animals to resist subsequent tumor challenges, only rarely can they eradicate pre-existing well-established tumors.22,23 Thus, further refinement of this strategy is required to elicit therapeutic immunity. In this report, we demonstrate that recombinant ΔM51-VSV infection of DCs simultaneously mediates high-level transgene expression and DC maturation. Administration of ΔM51-VSV-infected DCs to tumor-bearing mice caused a significant reduction in tumor burden, an effect mediated by CD8+ T cells and NK cells. Furthermore, we demonstrate that ΔM51-VSV can be used to efficiently transduce human DCs, suggesting that ΔM51-VSV infection of DCs may represent a promising protocol for the development of clinical DC-based therapeutic cancer vaccines.
As demonstrated in this study and in previous reports, using a virus to deliver a tumor-associated antigen-encoding gene to DCs offers several benefits, including: (i) high-efficiency of gene transfer ensuring sufficient and continuous supply of antigen, (ii) endogenous protein production facilitating the loading of MHC class-I, and (iii) exposure to the full-length protein permitting epitope selection that covers the entire molecule.6,12 Furthermore, viral infection may provide inflammatory signals that mediate DC maturation and cytokine production.16 In this study, we report that up to 70% of cultured DCs can be transduced with ΔM51-VSV vectors without compromising cell viability. Additionally, infection with ΔM51-VSV induces DC maturation and proinflammatory cytokine production conducive to subsequent immune priming. In particular, recombinant ΔM51-VSV induces high levels of type-I IFN secretion by DCs that may not only attenuate viral replication and spread but also further improve DC function. Studies from others have indeed demonstrated that IFNα can exert important effects on the differentiation and function of DCs and that when pretreated with IFNα, DCs are potent activators of CTL response and proliferation.24,25 Moreover, IFNs can directly provide important T-cell survival signals in the tumor microenvironment,26 suggesting that ΔM51-VSV-infected DC vaccines may offer an additional advantage to overcome tumor-associated immune suppression and enhance T-cell function.
We noticed that the CD8+ T-cell response against the viral nucleoprotein was always higher than that against the transgene. Coincidently, all three epitopes, SIINFEKL (OVA), SVYDFFVWL (DCT), and RGYVYQGL (VSV) share MHC I H-2kb and thus it is likely that competition for antigen presentation accounts for the magnitude of the T-cell response. As the gene for the VSV nucleoprotein is upstream of that for the transgene, a greater amount of VSV nucleoprotein will favor presentation of the VSV epitope over transgene products.27 This possibility requires further investigation for the improvement of VSV-based vaccines.
Although the focus of DC-based immunization strategies has typically been activation of CD4+ and CD8+ T cells,6 DC:NK cross talk is increasingly appreciated as an important component of the immune response following DC-based vaccination. DCs have been shown to activate NK cells for both cytokine production and cytotoxicity through contact-dependent mechanisms and/or soluble factors.28,29 As a result, activated NK cells can provide cytokines such as IFNγ to facilitate Th1/CTL responses30 or function as effectors to mediate rapid tumor destruction.4 However, the optimal method for manipulation of DCs to engage NK activation, especially in tumor-bearing hosts, remains to be determined. In fact, previous reports have demonstrated that a growing tumor could impair NK function by tumor-derived factors or tumor-associated suppressor cells31,32 and that NK-cell development in the bone marrow was interrupted in cancer-bearing hosts.33 Interestingly, however, despite the multiple mechanisms accountable for the tumor-mediated inhibitory effect on NK-cell function, our results indicate that ΔM51-VSV-infected DCs are able to overcome the immunosuppressive environment and trigger NK-mediated antitumor activity. More importantly, activation of NK cells appears to be critical to achieve maximum tumor destruction, as depletion of NK cells completely abrogated the benefit of DC-ΔM51-VSV therapy. Furthermore, the rapid control of tumor growth and cytokine secretion by activated NK may allow for the optimal development or effector function of antigen-specific CTL.
The mechanisms by which ΔM51-VSV-infected DC could induce NK activation remain to be determined. Our previous studies showed that CpG/LPS-matured DCs were equally potent to DC/ΔM51-VSV to elicit NK-mediated protection in a prophylactic setting, but they had no impact on mice with established tumors.1 A possible explanation for this discrepancy is that compared to transient treatment with CpG/LPS, viral infection may provide persistent TLR/maturation signals to DCs required for bypassing regulatory inhibitory mechanisms such as regulatory T cells and myeloid suppressor cells.34 This notion is supported by the similar results obtained from Ad-infected DCs. Although a small amount of VSV could be produced by infected DCs, a single inoculation of VSV via footpad injection had no impact on tumor growth in our study. However, using a skin B16-OVA model, Diaz et al. have recently reported that repeated intratumoral injection with higher doses of VSV could induce both NK cell and CD8+ T-cell responses leading to tumor regression.35 Other studies have also confirmed that VSV functions as both an oncolytic virus and an immune modulator but the appropriate delivery (e.g., route, dose, and frequency) is essential.18,19 Nevertheless, these results point to the possibility that combining DC vaccination and oncolytic therapy may represent a novel and effective anticancer approach.
Our studies identify recombinant ΔM51-VSV as a novel gene-delivery vector that can effectively transduce and activate DCs. The potency of DC-ΔM51-VSV vectors for activation of both NK and CD8+ T cells in tumor-bearing hosts offers a new platform for the development of therapeutic vaccines that can overcome tumor-associated immunosuppression.
Materials and Methods
Mice and cell cultures. Female 6- to 8-week-old C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in our specific pathogen-free facility. All animal experimentation was approved by the McMaster Animal Research Ethics Board and concurred with the guidelines established by the Canadian Council on Animal Care. B16-OVA cells, a murine melanoma line stably transfected with chicken egg OVA, were cultured in MEM-F11 media supplemented with 10% fetal bovine serum, 2 mmol/l L-glutamine, 1× β-mercaptoethanol, 1× vitamin solution, and antibiotics. G418 (800 µg/ml) was used to maintain OVA expression in the B16-OVA line. Vero and L929 cells were propagated in α-MEM supplemented with 10% fetal bovine serum, 2mmol/l L-glutamine, and antibiotics (all cell culture reagents from Invitrogen, Grand Island, NY).
Recombinant VSV. All VSV vectors used were constructed using an M protein mutant of the Indiana serotype (ΔM51-VSV) and were created by subcloning PCR fragments between the XhoI and NheI sites of the plasmid pΔM51 (ref. 19). VSV/SIINFEKL-Luc (VSV/SIIN) contains a modified version of luciferase bearing the immunodominant class-I epitope from OVA (SIINFEKL) tagged to the N-terminus.36 VSV/hDCT carries a human melanoma–associated antigen, DCT. VSV/GFP harbors the GFP and the control virus, VSV/MT, contains no transgene. ΔM51-VSV vectors were propagated in 293T cell cultures and purified by centrifugation on a sucrose gradient.37
Recombinant Ad. Generation and purification of recombinant E1- and E3-deleted Ads encoding no transgene (Ad-BHG) or expressing SIINFEKL-luciferase (Ad-SIIN) have been previously described.37,38
DC culture. Murine bone marrow–derived DCs were generated in the presence of 40 ng/ml recombinant murine GM-CSF (PeproTech, Rocky Hill, NJ), as described.1,39
Human DCs were derived from CD34+ progenitors isolated from leukapheresis products obtained from patients undergoing peripheral blood stem cell transplants under approval of the McMaster Research Ethics Board. Briefly, mononuclear cells were isolated by centrifugation on Ficoll-Paque (GE Healthcare, Uppsala, Sweden) and CD34+ cells were purified by positive magnetic selection (Miltenyi Biotech, Bergisch Gladbach, Germany) according to manufacturer's instructions. CD34+ cells were seeded into polystyrene culture plates in RPMI (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% human AB serum, antibiotics (Sigma-Aldrich, Oakville, Ontario, Canada), recombinant human granulocyte-macrophage colony-stimulating factor (100 ng/ml), stem cell factor (25 ng/ml), FMS-like tyrosine kinase-3 ligand (Flt-3L, 25 ng/ml), and tumor necrosis factor-α (2.5 ng/ml from days 0 to 5 and 5 ng/ml from days 6 to 7) (all cytokines were obtained from CellGenix, Freiburg, Germany) for 7 days before infection with ΔM51-VSV viruses.
Infection of DCs. After a 7-day culture period, DCs were infected with recombinant ΔM51-VSV or Ad at various pfu/cell or treated with 2 µg/ml LPS with or without 1 µg/ml SIINFEKL peptide for 4 hours in minimal media and washed thrice with PBS before in vivo immunization or continued culture for in vitro analysis. Assessment of DC maturation following infection was carried out by analyzing surface marker expression and intracellular cytokine production by flow cytometry (FACSCanto; BD Biosciences, Mississauga, Ontario, Canada). Flow cytometric data were analyzed using FlowJo software (Tree Star, Ashland, OR), and gates were set based on isotype control antibodies. To exclude the possibility of changes in DC autofluorescence based on VSV infection, unstained control cells infected with VSV-MT were used to confirm similar background fluorescence.
Viral titering in culture supernatants. Supernatants were collected from VSV/MT-infected DCs 24, 48, and 72 hours after infection. As a control for productive infection, Vero cells were infected in parallel for 24 hours. Viral titers were quantified by plaque assay on Vero monolayers and are expressed as viral progeny per infected cell.
Peptides and flow cytometry reagents. Kb-restricted peptides from OVA (SIINFEKL), DCT (SVYDFFVWL), or the VSV nucleoprotein (RGYVYQGL) were purchased from PepScan Systems (Lelystad, The Netherlands), Dalton Chemicals (Toronto, Ontario, Canada), and Biomer Technology (San Francisco, CA), respectively. All flow cytometry antibodies and fluorescent reagents were purchased from BD Biosciences.
IFNα/β bioassay. DCs were washed thrice with PBS 4 hours post-VSV/MT infection and replated in DC media to culture for an additional 12 hours. Supernatants were collected and acid neutralized to eliminate residual ΔM51-VSV and neutralize nontype-I IFN cytokines before bioassay.40,41 Neutralized supernatants were serially diluted and used to treat L929 cells for 24 hours. As control, dilutions of an IFNβ stock of known concentration (Sigma-Aldrich) were assayed in parallel. L929 cells were then infected with wild-type VSV expressing GFP overnight. GFP expression was scanned using the Typhoon Trio Variable Mode Imager (Amersham Biosciences, Piscataway, NJ) and fluorescence was compared between standard and experimental wells to give a quantitative measure of type-I IFN in the supernatants of infected cells.
Tumor treatment. C57BL/6 mice were given 1 × 106 B16-OVA cells by intravenous tail-vein injection to establish lung metastatic tumors. Ten days later, DC-based vaccines (1 × 106 cells/mouse) were administered via subcutaneous footpad injection. In some experiments, mice were depleted of NK cells or CD8+ T cells by injection of corresponding antibodies. Purified antibody (250 µg, clone 53-6.72 for CD8 depletion) and/or 100 µl ascites (clone PK136 for NK depletion) were injected intraperitoneally on days 9, 11, and 18 with respect to tumor challenge. Twenty-one days following tumor inoculation, mice were killed and lungs were collected. The number of surface tumor nodules was counted using a dissecting microscope. In the event that there were too many tumor nodules to differentiate to obtain a reliable count, a value of 800 was assigned based on an attempted count of these lungs.
In vivo CTL assay. We modified an existing protocol1,42 to simultaneously measure antigen-specific killing against RGYVYQGL and SIINFEKL targets in vivo. Splenocytes from naïve Ptprc mice (Taconic Farms, Hudson, NY), which express the CD45.1 alloantigen, were collected and pulsed with RGYVYQGL, SIINFEKL, or no peptide and labeled with discreet concentrations of carboxyfluorescein succinimidyl ester dye. Target cells were mixed at a 1:1:1 ratio and injected intravenously into tumor-bearing C57BL/6 mice (which express CD45.2) 11 days after treatment with DC vaccines. Splenic CD45.1+ cells were analyzed after overnight in vivo exposure based on their level of carboxyfluorescein succinimidyl ester fluorescence. Ratios of the total number of remaining RGYVYQGL or SIINFEKL-pulsed targets were compared to unpulsed targets to quantify the extent of antigen-specific killing as described.42
Intracellular cytokine staining. To quantify the frequency of IFNγ-producing CD8+ T cells, splenocytes from treated animals were restimulated with specific peptides (1 µg/ml) for 5 hours in the presence of Brefeldin A and anti-CD28 antibody (BD Biosciences). For NK-cell analysis, splenocytes were incubated overnight without additional restimulation. Brefeldin A was added in the last 6 hours. Cells were first treated with FcBlock, stained to detect surface markers and subsequently permeabilized using the Cytofix/Cytoperm solution from BD Biosciences and stained with antibody against IFNγ according to the manufacturer's instructions. Lymphocytes were examined by flow cytometry (BD FACSCanto) and analyzed using FlowJo software.
Statistical analysis. Data were analyzed using Student's t-tests or one- and two-way analysis of variance with Tukey's or Bonferonni's post hoc test, as appropriate, using GraphPad Prism 4.0b software (Graph Pad Software, La Jolla, CA). In all cases, P < 0.05 was taken as significantly different from chance and data are presented as means ± SE.
Supplementary MaterialFigure S1. DCs derived from human CD34+ PBMCs are efficiently transduced with VSV.
Supplementary Material
DCs derived from human CD34+ PBMCs are efficiently transduced with VSV.
Acknowledgments
We gratefully acknowledge the technical assistance of Natasha Kazdhan for production of the recombinant VSV vectors and Stephen Hanson for the IFN bioassay. This work was supported by the Ontario Cancer Research Network and grants to Y.W. from the Canadian Institutes of Health Research (MOP-67066) and B.D.L. from the Canadian Cancer Society (017103). J.E.B. is supported by studentships from the Natural Sciences and Engineering Research Council of Canada.
References
- Karimi K, Boudreau JE, Fraser K, Liu H, Delanghe J, Gauldie J, et al. Enhanced antitumor immunity elicited by dendritic cell vaccines is a result of their ability to engage both CTL and IFN gamma-producing NK cells. Mol Ther. 2008;16:411–418. doi: 10.1038/sj.mt.6300347. [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- Saudemont A, Jouy N, Hetuin D., and , Quesnel B. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7-H1 that stimulates T cells. Blood. 2005;105:2428–2435. doi: 10.1182/blood-2004-09-3458. [DOI] [PubMed] [Google Scholar]
- Kim A, Noh YW, Kim KD, Jang YS, Choe YK., and , Lim JS. Activated natural killer cell-mediated immunity is required for the inhibition of tumor metastasis by dendritic cell vaccination. Exp Mol Med. 2004;36:428–443. doi: 10.1038/emm.2004.55. [DOI] [PubMed] [Google Scholar]
- Adam C, King S, Allgeier T, Braumüller H, Lüking C, Mysliwietz J, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood. 2005;106:338–344. doi: 10.1182/blood-2004-09-3775. [DOI] [PubMed] [Google Scholar]
- Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Screpanti V, Yagita H, Grandien A, Ljunggren HG, Smyth MJ, et al. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–129. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- Steitz J, Tormo D, Schweichel D., and , Tüting T. Comparison of recombinant adenovirus and synthetic peptide for DC-based melanoma vaccination. Cancer Gene Ther. 2006;13:318–325. doi: 10.1038/sj.cgt.7700894. [DOI] [PubMed] [Google Scholar]
- Lucas M, Schachterle W, Oberle K, Aichele P., and , Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Hiroishi K, Eguchi J, Hiraide A., and , Imawari M. Dendritic cell therapy with interferon-alpha synergistically suppresses outgrowth of established tumors in a murine colorectal cancer model. Gene Ther. 2006;13:78–87. doi: 10.1038/sj.gt.3302608. [DOI] [PubMed] [Google Scholar]
- Sloan JM, Kershaw MH, Touloukian CE, Lapointe R, Robbins PF, Restifo NP, et al. MHC class I and class II presentation of tumor antigen in retrovirally and adenovirally transduced dendritic cells. Cancer Gene Ther. 2002;9:946–950. doi: 10.1038/sj.cgt.7700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckpot K, Aerts JL., and , Thielemans K. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene Ther. 2007;14:847–862. doi: 10.1038/sj.gt.3302947. [DOI] [PubMed] [Google Scholar]
- Gamvrellis A, Leong D, Hanley JC, Xiang SD, Mottram P., and , Plebanski M. Vaccines that facilitate antigen entry into dendritic cells. Immunol Cell Biol. 2004;82:506–516. doi: 10.1111/j.0818-9641.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- Yao Y, Li P, Singh P, Thiele AT, Wilkes DS, Renukaradhya GJ, et al. Vaccinia virus infection induces dendritic cell maturation but inhibits antigen presentation by MHC class II. Cell Immunol. 2007;246:92–102. doi: 10.1016/j.cellimm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26:326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa N, Koretomo R, Murakami S, Sakurai F, Mizuguchi H, Nakagawa S, et al. Factors involved in the maturation of murine dendritic cells transduced with adenoviral vector variants. Virology. 2008;374:411–420. doi: 10.1016/j.virol.2007.12.043. [DOI] [PubMed] [Google Scholar]
- Lopes L, Fletcher K, Ikeda Y., and , Collins M. Lentiviral vector expression of tumour antigens in dendritic cells as an immunotherapeutic strategy. Cancer Immunol Immunother. 2006;55:1011–1016. doi: 10.1007/s00262-005-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF., and , Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Brzoza KL., and , Hiltbold EM. Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells. J Virol. 2006;80:2194–2205. doi: 10.1128/JVI.80.5.2194-2205.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J, Brück J, Gambotto A, Knop J., and , Tüting T. Genetic immunization with a melanocytic self-antigen linked to foreign helper sequences breaks tolerance and induces autoimmunity and tumor immunity. Gene Ther. 2002;9:208–213. doi: 10.1038/sj.gt.3301634. [DOI] [PubMed] [Google Scholar]
- Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, et al. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176:1411–1420. doi: 10.4049/jimmunol.176.3.1411. [DOI] [PubMed] [Google Scholar]
- Tuettenberg A, Schmitt E, Knop J., and , Jonuleit H. Dendritic cell-based immunotherapy of malignant melanoma: success and limitations. J Dtsch Dermatol Ges. 2007;5:190–196. doi: 10.1111/j.1610-0387.2007.06179.x. [DOI] [PubMed] [Google Scholar]
- Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- Santodonato L, D'Agostino G, Nisini R, Mariotti S, Monque DM, Spada M, et al. Monocyte-derived dendritic cells generated after a short-term culture with IFN-alpha and granulocyte-macrophage colony-stimulating factor stimulate a potent Epstein-Barr virus-specific CD8+ T cell response. J Immunol. 2003;170:5195–5202. doi: 10.4049/jimmunol.170.10.5195. [DOI] [PubMed] [Google Scholar]
- Hiroishi K, Tüting T., and , Lotze MT. IFN-alpha-expressing tumor cells enhance generation and promote survival of tumor-specific CTLs. J Immunol. 2000;164:567–572. doi: 10.4049/jimmunol.164.2.567. [DOI] [PubMed] [Google Scholar]
- Kretzschmar E, Buonocore L, Schnell MJ., and , Rose JK. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J Virol. 1997;71:5982–5989. doi: 10.1128/jvi.71.8.5982-5989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli-Esposti MA., and , Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- Ferlazzo G. Natural killer and dendritic cell liaison: recent insights and open questions. Immunol Lett. 2005;101:12–17. doi: 10.1016/j.imlet.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A.Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming Nat Immunol 200451260–1265.et al [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI., and , Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- Richards JO, Chang X, Blaser BW, Caligiuri MA, Zheng P., and , Liu Y. Tumor growth impedes natural-killer-cell maturation in the bone marrow. Blood. 2006;108:246–252. doi: 10.1182/blood-2005-11-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Huang CT, Huang X., and , Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Miller M, Rekas G, Dayball K, Wan YH., and , Bramson J. The efficacy of electroporated plasmid vaccines correlates with long-term antigen production in vivo. Vaccine. 2004;22:2517–2523. doi: 10.1016/j.vaccine.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Mirakhur B., and , Peluso RW. In vitro assembly of a functional nucleocapsid from the negative-stranded genome RNA of a defective interfering particle of vesicular stomatitis virus. Proc Natl Acad Sci USA. 1988;85:7511–7515. doi: 10.1073/pnas.85.20.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TC, Dayball K, Wan YH., and , Bramson J. Detailed analysis of the CD8+ T-cell response following adenovirus vaccination. J Virol. 2003;77:13407–13411. doi: 10.1128/JVI.77.24.13407-13411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau J, Koshy S, Cummings D., and , Wan Y. Culture of myeloid dendritic cells from bone marrow precursors. J Vis Exp. 2008;17:769. doi: 10.3791/769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner JS., and , Wertz G. Interferon production in mice by vesicular stomatitis virus. J Virol. 1968;2:1360–1361. doi: 10.1128/jvi.2.11.1360-1361.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Bermayer HP, Weidinger G., and , Jungwirth C. Release of interferon-induced translation inhibition by tRNA in cell-free extracts from mouse erythroleukemia cells. Eur J Biochem. 1977;76:541–551. doi: 10.1111/j.1432-1033.1977.tb11624.x. [DOI] [PubMed] [Google Scholar]
- Coles RM, Mueller SN, Heath WR, Carbone FR., and , Brooks AG. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J Immunol. 2002;168:834–838. doi: 10.4049/jimmunol.168.2.834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DCs derived from human CD34+ PBMCs are efficiently transduced with VSV.