Abstract
Metastatic ovarian cancer is the leading cause of death among women with gynecologic malignancies in the United States. The lack of effective treatment for patients with advanced ovarian cancer warrants development of innovative therapies. Cancer therapy using oncolytic viruses represents a promising new approach for controlling tumors. Vaccinia virus has been shown to preferentially infect tumor cells but not normal tissue. However, oncolytic therapy using recombinant viruses faces the limitation of viral clearance due to generation of neutralizing antibodies. In the current study, we found that cyclooxygenase-2 (Cox-2) inhibitors circumvented this limitation, enabling repeated administration of vaccinia virus without losing infectivity. We quantified the antivaccinia antibody response using enzyme-linked immunosorbent assay (ELISA) and neutralization assays to show that treatment of Cox-2 inhibitors inhibited the generation of neutralizing antibodies. Furthermore, we showed that combination treatment of Cox-2 inhibitors with vaccinia virus was more effective that either treatment alone in treating MOSEC/luc tumor-bearing mice. Thus, the combination of Cox-2 inhibitors and vaccinia virus represents a potential innovative approach to controlling ovarian tumors.
Introduction
Metastatic ovarian cancer is one of the most lethal gynecological malignancies. It is extremely difficult to cure and is responsible for the highest mortality rate among patients.1 An estimated 20,180 women will be diagnosed with ovarian cancer, and 15,310 will die from it in 2008.1 Although significant advancement has occurred in both surgical and chemotherapeutic techniques, the overall 5-year survival rate for all stages remains <50%.1,2 Whereas early detection improves the chances that ovarian cancer can be treated successfully, early stages of ovarian cancer are often asymptomatic. As a result, women with ovarian cancer are often not diagnosed until the disease is advanced in stage, making ovarian cancer one of the most deadly cancers of the female reproductive system. Existing therapies for advanced disease, such as chemotherapy and radiation therapy, rarely result in long-term benefits in patients with locally advanced and metastatic disease and often yield a high relapse rate as well as toxic side effects. The lack of effective treatment for patients with advanced ovarian cancer warrants development of innovative therapies. Hence, there is a critical need to develop new therapeutic approaches to control advanced stage ovarian carcinoma.
Cancer therapy using oncolytic viruses represents a promising new approach for controlling tumors. Oncolytic viruses such as measles, vaccinia and Sindbis viruses have been shown to target and lyse tumor cells directly. The viruses are also capable of spreading to adjacent tumor cells, thereby eradicating the target cells without seriously harming the normal tissues. In addition, as the cellular pathways by which these viruses lyse the cells are highly complex, the emergence of virus-resistant tumor cells is unlikely. Therefore, the selective targeting and replication of these viruses offer a potentially safe and effective alternative for combating cancers, such as ovarian cancer.
Recently, it has been demonstrated that the vaccinia virus can infect and kill both human and murine ovarian cancer cells (MOSEC) in vitro and, when injected intraperitoneally (i.p.) into mice, the virus preferentially infects tumor cells but not normal tissue.3 This work was done with the same strain used in this study.3 Other groups have confirmed the cancer-selectivity of vaccinia with the wild-type strain of vaccinia.4 The use of the oncolytic vaccinia is a potentially effective strategy for controlling ovarian cancer. The ability of vaccinia to preferentially infect ovarian cancer cells in vivo creates the opportunity to incorporate genes that are capable of generating potent tumor-specific immunity to further enhance the therapeutic effects of vaccinia.
Oncolytic therapy using recombinant vaccinia viruses most likely requires repeated treatment as the virus may infect only a portion of tumor cells in vivo. However, the presence of neutralizing antibodies specific to the vaccinia viral vector would prohibit the booster effect of the recombinant vaccinia viral vector and prevent successful vaccination with vaccinia.5 Neutralizing antibodies have also been shown to be critically inhibitory with other viral vectors such as herpes simplex virus, which is much more sensitive to innate immune-mediated clearance, and antibody clearance, than vaccinia.6 As the vaccinia virus has been used for the eradication of smallpox, a significant population has previously been immunized with vaccinia. These individuals may have a preexisting immunity against the vaccinia virus and may not be suitable candidates for the treatment with the same kind of vaccinia vector. Therefore, it is useful to find conditions for repeated vaccination and crucial to identify strategies that are able to circumvent this limitation.
The employment of cyclooxygenase (Cox) inhibitors may be able to circumvent the limitation of repeated treatment with vaccinia virus. Cox-2 inhibitors represent a new class of nonsteroidal anti-inflammatory drugs that reduce inflammation. Cox-2 has multiple procancerous effects such as stimulating angiogenesis by promoting prostaglandin E2, thromboxane A2, and prostacylin production and increasing expression of vascular endothelial growth factor within tumors.7,8 It has been shown that Cox inhibitors can attenuate antibody production by inhibiting antibody induction9,10 and as Cox-2 is highly expressed by B lymphocytes, specifically inhibiting Cox-2 is efficient in attenuating antibody responses to human papillomavirus virus–like particles.10
In the current study, we hypothesized that inhibition of Cox-2 would prolong the activity of vaccinia and limit the production of antivaccinia antibodies in mice previously vaccinated with vaccinia. We found that Cox-2 inhibitors prevent the generation of antivaccinia neutralizing antibodies, enabling repeated administration of vaccinia virus without losing infectivity in both mice preimmunized with vaccinia and in MOSEC tumor-bearing mice. Furthermore, we found that treatment of vaccinia in combination with Cox-2 inhibitors can be used to treat MOSEC/luc tumors in C57BL/6 mice as well as enhance the survival of tumor-bearing mice. Additionally, we demonstrate that the use of Cox-2 inhibitors and vaccinia in combination is more effective in treating tumors than either administration of vaccinia or Cox-2 inhibitors alone. The clinical implications of this study are discussed.
Results
Treatment with Cox-2 inhibitor enables repeated infection with vaccinia virus in treated mice
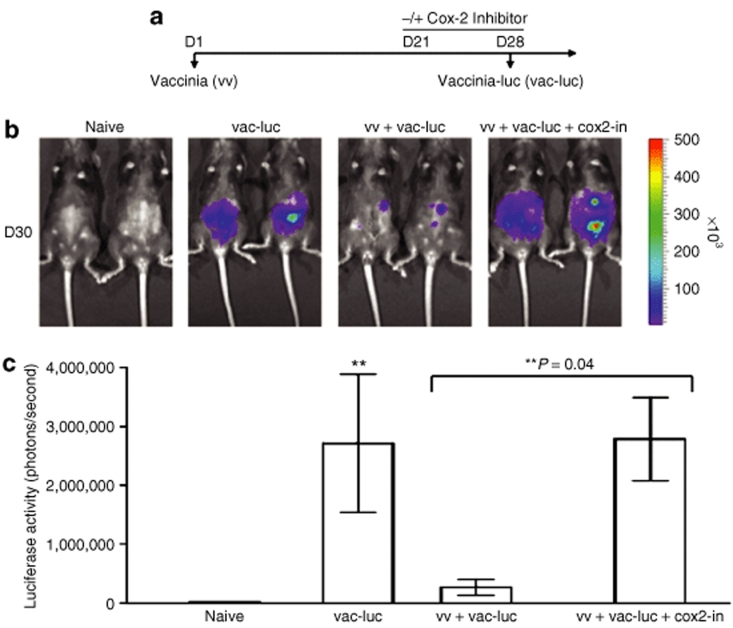
To determine if administration of Cox-2 inhibitors can allow us to repeatedly treat mice with recombinant vaccinia virus without losing the infectivity of vaccinia, we performed in vivo luminescence imaging assays in infected mice. C57BL/6 mice (five per group) were infected with wild-type vaccinia (vv) on day 1 (D1). Mice were treated with or without the Cox-2 inhibitor, Celecoxib orally from D21 to D28 at a dose of 100 mg/kg based on previous studies.11 We also tested lower doses of the Cox-2 inhibitor (10 mg/kg, 30 mg/kg) and found that administration of lower doses of Cox-2 inhibitor also enables repeated infection with vaccinia virus in treated mice (Supplementary Figure S1). We then infected the naive mice with luciferase-expressing vaccinia (vac-luc) on D28. The schematic regimen of treatment is depicted in Figure 1a. Naive mice injected only with luciferin served as a negative control. As shown in Figure 1b, preimmunized mice injected with vac-luc demonstrate the powerful effect of neutralizing antibodies; very little luciferase activity was seen as compared to the naive mice injected only with vac-luc. Preimmunized mice infected with vac-luc and treated with Cox-2 inhibitor showed increased luminescence expression on D30 compared to preimmunized mice reinfected with vac-luc in the absence of Cox-2 inhibitor, at levels comparable to those of the naive mice injected with vac-luc. A graphical representation of the luciferase activity is shown in Figure 1c (**P = 0.04). We have previously demonstrated the preferential infection by vaccinia virus in tumor cells compared to normal tissue.3 However, vaccinia can also infect normal cells in nontumor bearing mice as shown in Figure 1. Thus, our data indicate that treatment with Cox-2 inhibitor enables efficient reinfection with vaccinia virus in treated mice. We also tested another Cox-2 inhibitor, rofecoxib and observed that treatment with rofecoxib also enabled repeated infection with vaccinia virus in treated mice and inhibited the generation of neutralizing antibodies against vaccinia (data not shown).
Figure 1.
Luminescence imaging of mice infected with luciferase-expressing vaccinia (vac-luc) virus with or without treatment of Cox-2 inhibitor. (a) Schematic diagram demonstrating the regimen of vac-luc and Cox-2 inhibitor treatment in naive mice or mice previously infected with wild-type vaccinia. Naive mice injected with luciferin served as a negative control. C57BL/6 mice (five per group) were infected with wild-type vaccinia (vv) at a dose of 1 × 107 pfu/mouse on day 1 (D1). Mice were treated with Cox-2 inhibitor (100 mg/kg per day) orally from D21 to D28. Mice were then infected with vac-luc at a dose of 1 × 107 pfu/mouse on D28. Mice were imaged using the IVIS Imaging System Series 200. (b) Representative bioluminescence images of mice with or without previous wild-type vaccinia infection on D30. (c) Bar graph illustrating the luciferase activity (photons/seconds) of infected mice in each group on D30 (P = 0.04). Data shown are representative of two experiments performed (mean ± s.d.).
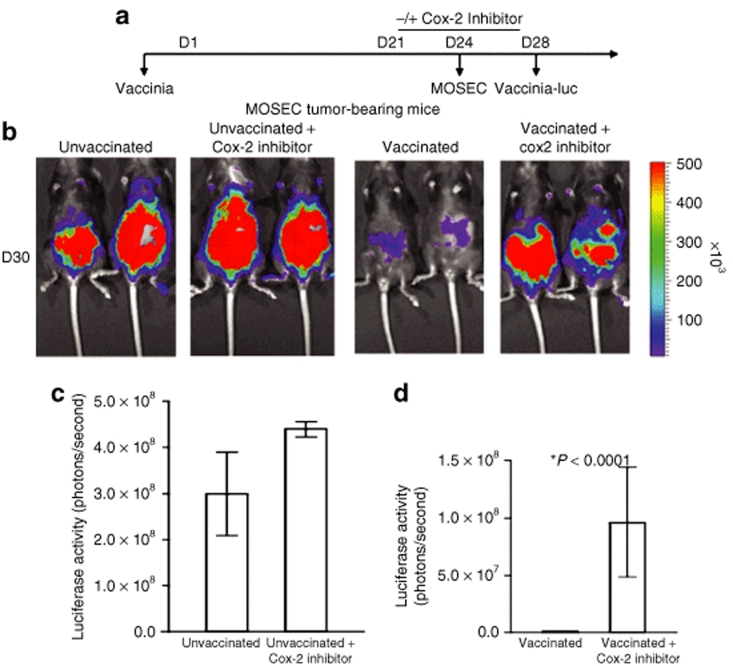
In order to determine whether Cox-2 inhibitor enabled reinfection with vaccinia-luciferase in tumor-bearing mice, we performed luminescence imaging in MOSEC tumor-bearing mice. C57BL/6 mice (five per group) were infected with wild-type vaccinia on D1. Mice were treated with or without Cox-2 inhibitor (100 mg/kg per day) orally from D21 to D28. Mice without vaccination treated with or without Cox-2 inhibitor served as controls. All groups of mice were then challenged with MOSEC cells on D24 and infected with vac-luc on D28. The schematic regimen of treatment is depicted in Figure 2a. Luciferase activities were determined by IVIS bioluminescent image system by injection of luciferin on D30. As shown in Figure 2b, MOSEC tumor-bearing mice reinfected with vac-luc and treated with Cox-2 inhibitor showed significantly higher intensity of luminescence, indicating more vaccinia infection compared to tumor-bearing mice without Cox-2 inhibitor treatment. A graphical representation of the luciferase activity is shown in Figure 2c (*P < 0.0001). In order to confirm these results, we also characterized the difference in the vaccinia virus titer in mice reinfected with vaccinia with or without administration of the Cox-2 inhibitor. Our data demonstrate that treatment with Cox-2 inhibitor led to an increase in vaccinia virus titer, thus enabling the reinfection of vaccinia virus (Supplementary Figure S2). Taken together, this data suggests that previous vaccinia infection can inhibit subsequent infections with the same type of vaccinia in vaccinated mice and treatment with Cox-2 inhibitors can circumvent this limitation, allowing the repeated vaccinia infection of mice without losing the infectivity.
Figure 2.
Luminescence imaging of MOSEC tumor-bearing mice infected with luciferase-expressing vaccinia virus with or without treatment of Cox-2 inhibitor. (a) Schematic diagram demonstrating the regimen of vac-luc and Cox-2 inhibitor treatment in MOSEC tumor-bearing mice previously infected with wild-type vaccinia. C57BL/6 mice (five per group) were infected with wild-type vaccinia at a dose of 1 × 107 pfu/mouse on day 1 (D1). Mice were treated with or without Cox-2 inhibitor (100 mg/kg per day) orally from D21 to D28. Mice without the first vaccination treated with or without Cox-2 inhibitor served as controls. All groups of mice were then challenged with 2 × 106 MOSEC cells/mouse on D24 and infected with vac-luc at a dose of 1 × 107 pfu/mouse on D28. Mice were imaged using the IVIS Imaging System Series 200. (b) Representative bioluminescence images of MOSEC tumor-bearing mice treated with vac-luc ± Cox-2 inhibitor. Images were acquired on D30. (c) Bar graph illustrating the luciferase activity (photons/second) in MOSEC tumor-bearing mice treated with or without Cox-2 inhibitor. (d) Bar graph illustrating the luciferase activity (photons/second) in MOSEC tumor-bearing mice treated with vac-luc ± Cox-2 inhibitor (*P < 0.0001). Data shown are representative of two experiments performed (mean ± s.d.).
Cox-2 inhibitor inhibits the generation of neutralizing antibodies against vaccinia virus infection in mice
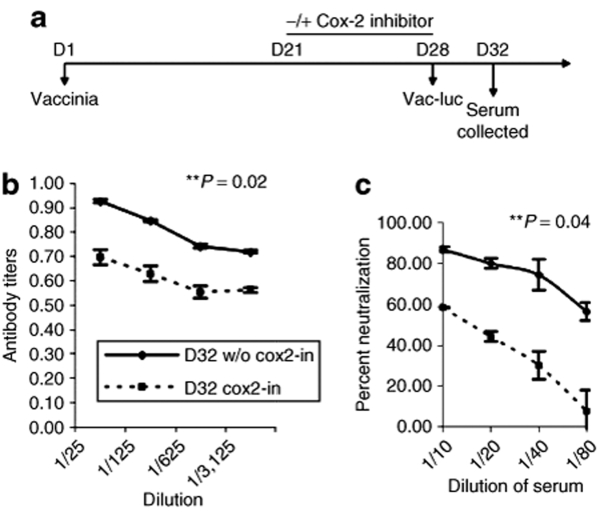
To further demonstrate the ability of Cox-2 inhibitor treatment to inhibit the generation of neutralizing antibodies against vaccinia virus, thereby enabling reinfection, we performed an enzyme-linked immunosorbent assay (ELISA) analysis to quantify antivaccinia antibody titers at different time points after reinfection with vac-luc in preimmunized mice. C57BL/6 mice (five per group) were infected with wild-type vaccinia on D1 and treated with or without Cox-2 inhibitor orally from D21 to D28 and then reinfected with vac-luc on D28 as outlined in the schematic regimen shown in Figure 3a. Serum was collected on D32 from each group of mice for characterization of the antivaccinia antibody response as described in Materials and Methods. As shown in Figure 3b, serum from preimmunized mice reinfected with vac-luc in the presence of Cox-2 inhibitors demonstrated significantly lower antivaccinia antibody titers compared to those of preimmunized mice reinfected with vac-luc alone on D32 (**P = 0.02). Our data indicate that treatment with Cox-2 inhibitors reduces the level of antivaccinia antibody response generated after vaccinia reinfection.
Figure 3.
Characterization of the antivaccinia antibody response to a 2nd vaccinia administration using ELISA and neutralization assay. (a) Schematic diagram demonstrating the regimen of vac-luc and Cox-2 inhibitor treatment in vaccinated mice. C57BL/6 mice (five per group) were infected with wild-type vaccinia (vv) at a dose of 1 × 107 pfu/mouse on day 1 (D1). Mice were then infected with vac-luc at a dose of 1 × 107 pfu/mouse on D28. Mice were treated with Cox-2 inhibitor (100 mg/kg per day) orally from D21 to D28. Serum was collected on D32 from each group of mice for characterization of the antivaccinia antibody response. (b) Line graph illustrating antivaccinia antibody titers in serum of infected mice treated with or without Cox-2 inhibitor on D32 (**P = 0.02). (c) Line graph depicting percentage of antibody-mediated neutralization of vaccinia virus infection in mice treated with or without Cox-2 inhibitor on D32 (*P = 0.0004). Data shown are representative of two experiments performed (mean ± s.d.).
For further characterization of the antivaccinia antibody response, serum collected from the preimmunized mice was used in a subsequent neutralization assay. Luciferase activity was determined using IVIS bioluminescent imaging system. As shown in Figure 3c, treatment with the Cox-2 inhibitor significantly diminished the percentage of neutralization in preimmunized mice on D32 (*P = 0.0004), which is consistent with our findings from the ELISA analysis. Taken together, our data suggest that Cox-2 inhibitors eliminate the generation of neutralizing antibodies against vaccinia virus in preimmunized mice.
Cox-2 inhibitor treatment enhances the long-term protective antitumor effects generated by treatment with oncolytic vaccinia virus
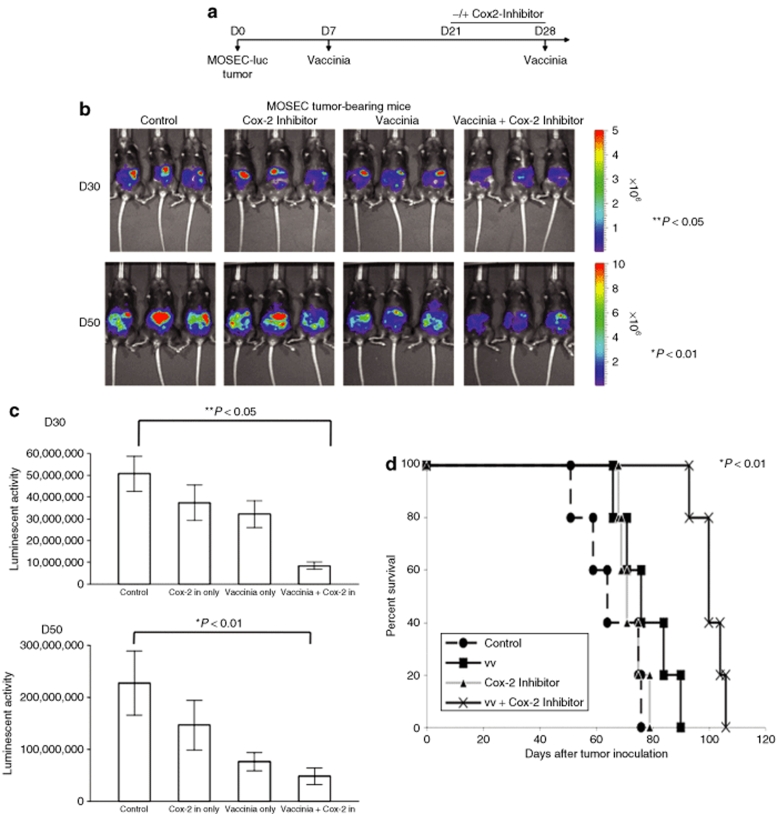
To determine if the observed reduction of antivaccinia neutralizing antibodies mediated by treatment with Cox-2 inhibitors would translate into enhanced antitumor treatment with vaccinia, we performed in vivo tumor protection experiments using C57BL/6 mice. C57BL/6 mice (five per group) were inoculated via intraperitoneal injection with luciferase-expressing MOSEC cells on D0 and immunized with vaccinia on D7. The mice were either treated with vaccinia virus alone on D28 (vaccinia), administered Cox-2 inhibitor alone daily from D21 to D28 (Cox-2 inhibitor) or treated with the vaccinia in combination with the Cox-2 inhibitor (vaccinia + Cox-2 inhibitor) as outlined in the schematic regimen shown in Figure 4a. Another group of MOSEC tumor-bearing preimmunized mice was used as a negative control. The tumor load was represented by luciferase activity determined using IVIS Bioluminescent Imaging System. As shown in Figure 4b,c, mice treated with vaccinia virus with the Cox-2 inhibitor exhibited significantly decreased tumor growth on both D30 (**P < 0.05) and D50 (*P < 0.01), compared to mice treated with vaccinia virus alone or Cox-2 inhibitor alone. We further characterized the survival in the different mice groups using Kaplan-Meier survival analysis. As shown in Figure 4d, the mice treated with the combination of vaccinia with Cox-2 inhibitor exhibited significantly improved survival compared to mice treated with vaccinia virus alone or treated with Cox-2 inhibitor alone (*P < 0.01). We also characterized the frequency of infiltrating CD4+ and CD8+ T cells and natural killer cells within the tumor following vaccinia infection and administration of Cox-2 inhibitor. Our data indicate that treatment with Cox-2 inhibitor does not significantly affect the number of infiltrating CD8+ and natural killer cells within the tumor in mice infected with vaccinia. However, we do observe a slight reduction in the number of CD4+ T cells in the tumor with Cox-2 inhibitor treatment (Supplementary Figure S3). Thus, treatment with Cox-2 inhibitor in combination with oncolytic vaccinia virus infection can enhance the long-term protective antitumor effects generated by vaccinia infection and improve survival in tumor-challenged mice.
Figure 4.
Characterization of antitumor effects against luciferase-expressing, murine ovarian tumors in mice treated with Cox-2 inhibitor and two injections of vaccinia. (a) Schematic diagram demonstrating the regimen of vaccinia and Cox-2 inhibitor treatment in luciferase-expressing MOSEC tumor-bearing mice. C57BL/6 mice (five per group) were intraperitoneally inoculated with 5 × 105 MOSEC-luc cells/mouse on D0. Seven days after tumor inoculation, all the tumor-bearing mice were treated with 1 × 107 pfu/mouse of vaccinia virus. The mice were either administered Cox-2 inhibitor alone (100 mg/kg per day) daily from day 21 (D21) to D28 (Cox-2 inhibitor), treated with vaccinia virus alone at a dose of 1 × 107 pfu/mouse on D28 (vaccinia) or treated with the vaccinia in combination with the Cox-2 inhibitor (vaccinia + Cox-2 inhibitor) as outlined in the schematic regimen. Mice were imaged using the IVIS Imaging System Series 200. (b) Representative bioluminescence images of MOSEC tumor-bearing mice treated with vaccinia alone, Cox-2 inhibitor alone or combination of vaccinia with Cox-2 inhibitor. Images were acquired on D30 (**P < 0.05) and D50 (*P < 0.01) after tumor inoculation. (c) Bar graph illustrating the luciferase activity (photons/second) in MOSEC tumor-bearing mice treated with treated with vaccinia alone, Cox-2 inhibitor alone or combination of vaccinia with Cox-2 inhibitor on D30 and D50. Data shown are representative of two experiments performed (mean ± s.d.). (d) Kaplan-Meier survival analysis of MOSEC tumor-bearing mice treated with vaccinia alone, Cox-2 inhibitor alone or combination of vaccinia with Cox-2 inhibitor (*P < 0.01).
In order to exclude the possibility that the enhanced antitumor effects generated by treatment with oncolytic vaccinia virus is due to enhancement of vaccinia virus replication by Cox-2 inhibitor, we treated MOSEC tumor cells with a wide range of concentrations of Cox-2 inhibitor for 24 hours and infected them with wild-type vaccinia for 96 hours. Vaccinia virus titer was then determined using plaque-forming assay. We found that treatment with Cox-2 inhibitor does not enhance the vaccinia virus replication at any of the concentrations tested (See Supplementary Figure S4). Our data suggest that the observed antitumor effects generated by vaccinia treatment is not contributed by the enhancement of vaccinia replication by Cox-2 inihbitor, but it is likely due to the inhibition of neutralizing antibody response.
Discussion
In the current study, we demonstrated that treatment with Cox-2 inhibitors efficiently inhibited generation of neutralizing antibodies against vaccinia virus infection in both preimmunized and tumor-bearing mice. Neutralizing antibodies created by inflammatory antiviral responses induced by the immune system are perhaps the most significant problem for systemic viral delivery and persistence. Administration of the anti-inflammatory Cox-2 inhibitor, Celecoxib, was shown to enable efficient repeated infection with vaccinia virus in treated mice without losing the infectivity of vaccinia. Furthermore, vaccinia virus administered intraperitoneally to ovarian tumor-bearing mice translated to long-term protective antitumor responses and increased survival for treated tumor-bearing mice. Our current findings show that the employment of Cox-2 inhibitors improves the therapeutic efficacy of vaccinia as a treatment for ovarian cancer.
Cox-2 inhibitors have also demonstrated direct antitumor effects against a multitude of different cancer models including liver cancer,12 ovarian cancer,13 colorectal cancer,14 and breast cancer.15,16 Although inhibition of Cox-2 activity is one mechanism by which Cox-2 inhibitors dampen malignant cell proliferation, emerging evidence using Cox-2 deficient and Cox-2 knockdown cells suggests that Cox-2 independent effects are also involved. For example, Cox-2 inhibitor SC-58125 was shown to decrease the content of intracellular glutathione, an antioxidant that protects cells from toxins, in normal and malignant human B cells.7 Celecoxib has close structural similarity to SC-58125 and was shown to inhibit cellular growth and dampen malignant cell proliferation, inducing apoptosis via a mitochondrial-dependent pathway.17,18,19,20 Meloxicam, a Cox-2 inhibitor selective for ovarian cancer, has shown strong antitumor effects against ovarian cancer by inducing apoptosis in ovarian cancer cells and downregulating production of prostaglandin E2.21 In addition to its antitumor properties, recent work convincingly demonstrates that Celecoxib can also act as a potent antiangiogenic agent.22,23 These findings have led to the clinical evaluation of selective Cox-2 inhibitors as an adjuvant to radiation24,25,26,27,28 and chemotherapy29,30 for treating solid tumors. Hence, Cox-2 inhibitors not only enable repeated infection with vaccinia virus, they also serve to contribute to the direct oncolytic effect of vaccinia virus.
The combination of Cox-2 inhibitors with immunotherapies using viral vectors has been explored to control tumor in other systems. For example, an adenoviral vector expressing human papillomavirus virus E7 protein used in combination with Cox-2 inhibitor significantly slowed the growth of large tumors and prolonged survival of vaccinated mice than either treatment alone.31 Additionally, recombinant poxviruses expressing carcinoembryonic antigen administered with Cox-2 inhibitor has been shown to elicit potent antitumor immunity and enhance long-term survival in carcinoembryonic antigen transgenic mice that developed spontaneous carcinoembryonic antigen-expressing intestinal neoplasms.32 Although the antitumor effect may be attributed to the direct inhibition of the tumor by Cox-2 inhibitors as well as the viral vector-based therapeutic vaccine, the observed improved antitumor effects may also be attributed to the ability of Cox-2 inhibitors to reduce generation of neutralizing antibodies, allowing the boost of viral vector. In both studies, the viral vector-based vaccines have been used to treat tumors with boost of the same vaccine.31,32
Intratumoral treatment with vaccinia vaccine strains have resulted in significant and reproducible efficacy in numerous clinical trials. Park et al. evaluated intratumoral injection of a targeted oncolytic poxvirus, JX-594, in a phase I trial in patients with refractory primary or metastatic liver cancer.33 Ten patients were found to be radiographically evaluable for objective responses and according to Response Evaluation Criteria in Solid Tumors, three patients had partial response, six had stable disease, and one had progressive disease. Liu et al. also tested the same oncolytic poxvirus, JX-594, and found antitumoral, antivascular, and antiviral activities in patients with liver cancer. Both trials found that treatment with oncolytic vaccinia was well-tolerated and resulted in antitumoral efficacy in three patients, despite high levels of neutralizing antibodies.34 Phase II trials using this virus are currently ongoing.
The investigation of Cox-2 inhibitors to augment immunotherapies renders it important to consider the safety of Cox-2 inhibitors for clinical use. Recently, the reported adverse cardiovascular effects of rofecoxib, a selective Cox-2 inhibitor, resulted in its removal from the market due to concerns of cardiovascular toxicity. Though reports focused on rofecoxib, the concern spread to include other Cox-2 inhibitors as it was thought that the problem stemmed from a class effect related to the degree of Cox-2 selectivity, though there has been little evidence to support this finding.35 Published scientific literature suggests that both specific and nonspecific Cox inhibitors may increase the risk of cardiovascular events, but that the effect varies between individual drugs. A note of caution may come from the observation that long-term administration of Cox-2 inhibitor may also produce significantly higher levels of Cox-2 and reactivate prostaglandin E2-associated growth factor signaling pathways in tumor and normal tissues, which may contribute to treatment toxicity.36 Nevertheless, Celecoxib is associated with a lower cardiovascular risk than rofecoxib and has the greatest evidence for cardiovascular safety.35 It would be clinically useful to administer Cox-2 inhibitors after surgical debulking of the tumor to maximize benefits of Cox-2 inhibitor use.
In summary, our findings suggest a novel therapeutic approach for the control of lethal ovarian cancer. Although the application of Cox-2 inhibitors has been shown to enable reinfection of vaccinia administered intraperitoneally to control ovarian cancer, it would be of interest to explore whether the administration of Cox-2 inhibitors would enable reinfection of systemically delivered vaccinia virus to control other tumors.37 The data generated serve as an important foundation for future experimental and clinical efforts to combine cancer vaccines with Cox-2 inhibition. The combination of preferential killing of ovarian cancers by vaccinia virus and the use of Cox-2 inhibitors to inhibit neutralizing antibodies and accordingly enable repeated infection without loss of infectivity of vaccinia virus represents a promising strategy for the control of ovarian cancer.
Materials and Methods
Mice and cell lines. Female C57BL/6 mice were purchased from the National Cancer Institute. All animals were maintained under specific pathogen-free conditions, and all procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals. Immortalized murine ovarian surface epithelial cell line MOSEC was generated as previously shown.38 Thymidine kinase negative (tk−) cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). MOSEC-luciferase (MOSEC-luc) cells were generated by transducing MOSEC with the retrovirus containing luciferase pLuci-thy1.1 and flow cytometry sorting following the protocol described in ref. 3.
Vaccinia virus. The Vac-luciferase (Lister strain, rVV4) was generated using a previously described protocol. The rVV4 is a hyper-attenuated recombinant derivative of the vaccine strain Lister of vaccinia virus. It contains two reporter genes (luc and lacZ) inserted into the thymidine kinase region of VV (tk−) as described.39 The Vac-WT (WR strain) was prepared as described previously.40
Cox-2 inhibitor. For confirming the common pharmacological anti-Cox-2 effect of Cox-2 inhibitors, we used Celecoxib in vaccinia infection and other assays. Celecoxib (100 mg/kg/day) was ground from the tablet and dissolved in water. Mice were given Cox-2 inhibitor by daily gavages during treatment.
Immunization of mice with Lister stain of vaccinia virus and collection of serum. Naive mice or mice preimmunized with Lister stain of vaccinia virus (intraperitoneal injection with 1 × 107 pfu/mouse on D1) were treated with Cox-2 inhibitor (Celecoxib, 100 mg/kg/day, D21–D28, daily by gavages, as outlined in Figure 1a). The other two groups (naive and preimmunized) of mice without Cox-2 inhibitor treatment were used as control. Postimmunization serum was obtained from the mice on D32 from each group of mice (five per group) for subsequent ELISA and virus neutralization assay, as outlined in Figure 3a.
ELISA. Titers of antivaccinia antibodies within the serum were represented and determined by ELISA in a standard protocol. Briefly, ELISA plate was coated with 100 µl/well of 10 µg/ml vaccinia virus antigen and incubated overnight at 4 ºC. Serum obtained from the postimmunization mice of each group was diluted in serial five-time folds and added to the coating well. Positive and negative controls were also included in each test. Plates were incubated in a humid chamber at 37 °C for 1 hour, followed by 1-hour incubation with horseradish peroxidase–conjugated antimouse secondary antibody (1:5000) after washing. The ELISA result was developed by adding 1-StepTM Turbo TMB-ELISA substrate (Pierce, Rockford, IL) and quenched with sulfuric acid, then quantified at 450 nm using a microplate reader (Bio-Rad, Hercules, CA).
Virus neutralization assay in vitro. Serum obtained from the postimmunization mice of each group described above was preheated at 55 °C 1 hour for inactivation of complement and diluted in serial two-folds with culture medium. Forty microliters of diluted serum and 10 µl of vaccinia encoding luciferase (2.5 × 104 pfu/mouse) were added to each well in a 96-well round bottom plate and incubated at 37 °C for 1 hour. After incubation, 10 µl of MOSEC cells (2.5 × 106/ml) were added into the vaccinia-luciferase/serum mixture in each well and incubated at 37 °C for 2 hours. The plate was centrifuged to spin down the cells and washed five times with PBS. Those MOSEC cells within each well were then transferred into 96-well flat bottom plate and incubated at 37 °C and 5% CO2 overnight. Bioluminescent activities indicating vaccinia infection were determined using IVIS image system by adding with luciferin in each well after 18 hours later.
Characterization of infection by vaccinia in mice. C57BL/6 mice were injected with 1 × 106 MOSEC cells/mouse intraperitoneally (mice with tumor). Five days later after the tumor challenge, mice with tumor or without tumor were divided into four groups (five per group) regarding the preimmunization of vaccinia virus (1 ×107 pfu/mouse) or not, and Cox-2 inhibitor treatment (Celecoxib, 100 mg/kg/day by gavages) or not (−/−, −/+, +/+, +/−). To determine the infectivity and persistence of vaccinia virus in vivo, each group of mice (five per group) were injected with 1 × 107 plaque-forming unit (pfu/mouse) vaccinia virus encoding luciferase gene intraperitoneally (D1). Luminescence activity images were recorded on D30, after the vaccinia-luciferase injection. The mice were injected with 0.2 ml of 15-mg/ml beetle luciferin (potassium salt; Promega, Madison, WI). After 10 minutes incubation, the mice were imaged using the IVIS 200 system (Xenogen, Alameda, CA). An integration time of 60 seconds were used for image acquisition. The relative time points of vaccinia immunization, Cox-2 inhibitor administration, tumor challenge and vaccinia-luciferase administration are depicted in panel a of Figures 1 and 2.
Determination of antitumor effects by vaccinia and Cox-2 inhibitor in mice. To demonstrate the effects of Cox-2 inhibitor administration on vaccinia virus infection and thus on controlling murine ovarian tumors, C57BL/6 mice were intraperitoneally challenged with 1 × 105 MOSEC cells expressing luciferase (MOSEC-luc) (D0). Five days later, the mice were examined using IVIS image system for the bioluminescent activities indicating basal tumor loading. Mice with excessive or diminished luminescent signals were excluded out of the experiment. Those with the same level of signal were divided into four groups (five per group) subjected to different treatment, including control, vaccinia virus alone (1 × 107 pfu/mouse, D7, i.p.), Cox-2 inhibitor (Celecoxib, 100 mg/kg/day, gavages) alone or combination. Each group of mice was treated again with the same regimen 3 weeks later as outlined in Figure 4a. The tumor load was checked and represented by luciferase activity determined using IVIS Bioluminescent Imaging System on D30 and D50. The survival of mice was recorded.
Characterization of cells infiltrating the tumor in mice treated with Cox-2 inhibitor and vaccinia virus. C57BL/6 mice (five per group) were intraperitoneally inoculated with 5 × 105 MOSEC-luc cells/mouse on D0. Seven days after tumor inoculation, all the tumor-bearing mice were treated with 1 × 107 pfu/mouse of vaccinia virus. The mice were treated with or without Cox-2 inhibitor (100 mg/kg per day) daily from D21 to D28 followed by reinfection with vaccinia on D28 as outlined in the schematic regimen in Figure 4a. Peritoneal cells were stained with CD4 or CD8 and interferon-γ or natural killer 1.1 and analyzed by flow cytometry of D30.
Characterization of vaccinia virus titer in mice reinfected with vaccinia with or without Cox-2 inhibitor treatment. C57BL/6 mice (five per group) were infected with wild-type vaccinia (vv) at a dose of 1 × 107 pfu/mouse on D1. Mice were treated with Cox-2 inhibitor (100 mg/kg per day) orally from D21 to D28. Mice were then infected with vac-luc at a dose of 1 × 107 pfu/mouse on D28. Mice were sacrificed on D31 and the vaccinia virus titers within peritoneal lavage (10 ml) were determined by plaque-forming assay by serial dilutions.
Statistical analysis. All data expressed as means ± s.d. are representative of at least two different experiments. Comparisons between individual data points were made using a Student's t-test. Differences in survival between experimental groups were analyzed using the Kaplan-Meier approach. The statistical significance of group differences will be assessed using the log-rank test.
SUPPLEMENTARY MATERIALFigure S1. Luminescence imaging of mice infected with luciferase-expressing vaccinia virus with different doses of Cox-2 inhibitor treatment.Figure S2. Characterization of vaccinia virus titer in mice reinfected with vaccinia with or without Cox-2 inhibitor treatment.Figure S3. Characterization of total intraperitoneal lymphocyte sub-populations in mice treated with Cox-2 inhibitor and vaccinia virus.Figure S4. Characterization of the vaccinia titer in the vaccinia infected MOSEC cells treated with Cox-2 inhibitor.
Supplementary Material
Luminescence imaging of mice infected with luciferase-expressing vaccinia virus with different doses of Cox-2 inhibitor treatment.
Characterization of vaccinia virus titer in mice reinfected with vaccinia with or without Cox-2 inhibitor treatment.
Characterization of total intraperitoneal lymphocyte sub-populations in mice treated with Cox-2 inhibitor and vaccinia virus.
Characterization of the vaccinia titer in the vaccinia infected MOSEC cells treated with Cox-2 inhibitor.
Acknowledgments
We thank Archana Monie (Johns Hopkins Medical Institutions) for critical review of the manuscript. This work was supported by ovarian cancer grants from the Alliance for Cancer Gene Therapy (ACGT), the NCDGG (1U19 CA113341-01) and the American Cancer Society (ACS).
REFERENCES
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. doi: 10.1007/978-1-4757-3587-1_4. [DOI] [PubMed] [Google Scholar]
- Hung CF, Tsai YC, He L, Coukos G, Fodor I, Qin L, et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14:20–29. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Liang W, Contag CH., and , Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68:2071–2075. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- Ryan EP, Bushnell TP, Friedman AE, Rahman I., and , Phipps RP. Cyclooxygenase-2 independent effects of cyclooxygenase-2 inhibitors on oxidative stress and intracellular glutathione content in normal and malignant human B-cells. Cancer Immunol Immunother. 2008;57:347–358. doi: 10.1007/s00262-007-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi YH, Liu RS, Song MM, Tian Y, Long J, Tu W, et al. Cyclooxygenase-2 promotes angiogenesis by increasing vascular endothelial growth factor and predicts prognosis in gallbladder carcinoma. World J Gastroenterol. 2005;11:3724–3728. doi: 10.3748/wjg.v11.i24.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EP, Pollock SJ, Pollack SJ, Murant TI, Bernstein SH, Felgar RE, et al. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174:2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- Ryan EP, Malboeuf CM, Bernard M, Rose RC., and , Phipps RP. Cyclooxygenase-2 inhibition attenuates antibody responses against human papillomavirus-like particles. J Immunol. 2006;177:7811–7819. doi: 10.4049/jimmunol.177.11.7811. [DOI] [PubMed] [Google Scholar]
- Niki Y, Takaishi H, Takito J, Miyamoto T, Kosaki N, Matsumoto H, et al. Administration of cyclooxygenase-2 inhibitor reduces joint inflammation but exacerbates osteopenia in IL-1 alpha transgenic mice due to GM-CSF overproduction. J Immunol. 2007;179:639–646. doi: 10.4049/jimmunol.179.1.639. [DOI] [PubMed] [Google Scholar]
- Xie H, Gao L, Chai N, Song J, Wang J, Song Z, et al. Potent cell growth inhibitory effects in hepatitis B virus X protein positive hepatocellular carcinoma cells by the selective cyclooxygenase-2 inhibitor celecoxib. Mol carcinog. 2008;48:56–65. doi: 10.1002/mc.20455. [DOI] [PubMed] [Google Scholar]
- Vital-Reyes V, Rodríguez-Burford C, Chhieng DC, Oelschlager DK, Reyes-Fuentes A, Barnes M, et al. Celecoxib inhibits cellular growth, decreases Ki-67 expression and modifies apoptosis in ovarian cancer cell lines. Arch Med Res. 2006;37:689–695. doi: 10.1016/j.arcmed.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Liou JY, Ghelani D, Yeh S., and , Wu KK. Nonsteroidal anti-inflammatory drugs induce colorectal cancer cell apoptosis by suppressing 14-3-3epsilon. Cancer Res. 2007;67:3185–3191. doi: 10.1158/0008-5472.CAN-06-3431. [DOI] [PubMed] [Google Scholar]
- Howe LR, Subbaramaiah K, Patel J, Masferrer JL, Deora A, Hudis C, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62:5405–5407. [PubMed] [Google Scholar]
- Lanza-Jacoby S, Miller S, Flynn J, Gallatig K, Daskalakis C, Masferrer JL, et al. The cyclooxygenase-2 inhibitor, celecoxib, prevents the development of mammary tumors in Her-2/neu mice. Cancer Epidemiol Biomarkers Prev. 2003;12:1486–1491. [PubMed] [Google Scholar]
- Ding H, Han C, Zhu J, Chen CS., and , D'Ambrosio SM. Celecoxib derivatives induce apoptosis via the disruption of mitochondrial membrane potential and activation of caspase 9. Int J Cancer. 2005;113:803–810. doi: 10.1002/ijc.20639. [DOI] [PubMed] [Google Scholar]
- Jendrossek V, Handrick R., and , Belka C. Celecoxib activates a novel mitochondrial apoptosis signaling pathway. FASEB J. 2003;17:1547–1549. doi: 10.1096/fj.02-0947fje. [DOI] [PubMed] [Google Scholar]
- Johnson AJ, Smith LL, Zhu J, Heerema NA, Jefferson S, Mone A, et al. A novel celecoxib derivative, OSU03012, induces cytotoxicity in primary CLL cells and transformed B-cell lymphoma cell line via a caspase- and Bcl-2-independent mechanism. Blood. 2005;105:2504–2509. doi: 10.1182/blood-2004-05-1957. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Shimizu T, Tago K, Nakamura M, Itoh H, Sonoda Y, et al. Celecoxib potently inhibits TNFalpha-induced nuclear translocation and activation of NF-kappaB. Biochem pharmacol. 2008;76:662–671. doi: 10.1016/j.bcp.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Xin B, Yokoyama Y, Shigeto T, Futagami M., and , Mizunuma H. Inhibitory effect of meloxicam, a selective cyclooxygenase-2 inhibitor, and ciglitazone, a peroxisome proliferator-activated receptor gamma ligand, on the growth of human ovarian cancers. Cancer. 2007;110:791–800. doi: 10.1002/cncr.22854. [DOI] [PubMed] [Google Scholar]
- Farooqui M, Li Y, Rogers T, Poonawala T, Griffin RJ, Song CW, et al. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. Br J Cancer. 2007;97:1523–1531. doi: 10.1038/sj.bjc.6604057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- Kang KB, Wang TT, Woon CT, Cheah ES, Moore XL, Zhu C, et al. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys. 2007;67:888–896. doi: 10.1016/j.ijrobp.2006.09.055. [DOI] [PubMed] [Google Scholar]
- Shin YK, Park JS, Kim HS, Jun HJ, Kim GE, Suh CO, et al. Radiosensitivity enhancement by celecoxib, a cyclooxygenase (COX)-2 selective inhibitor, via COX-2-dependent cell cycle regulation on human cancer cells expressing differential COX-2 levels. Cancer Res. 2005;65:9501–9509. doi: 10.1158/0008-5472.CAN-05-0220. [DOI] [PubMed] [Google Scholar]
- Nakata E, Mason KA, Hunter N, Husain A, Raju U, Liao Z, et al. Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int J Radiat Oncol Biol Phys. 2004;58:369–375. doi: 10.1016/j.ijrobp.2003.09.061. [DOI] [PubMed] [Google Scholar]
- Raju U, Nakata E, Yang P, Newman RA, Ang KK., and , Milas L. In vitro enhancement of tumor cell radiosensitivity by a selective inhibitor of cyclooxygenase-2 enzyme: mechanistic considerations. Int J Radiat Oncol Biol Phys. 2002;54:886–894. doi: 10.1016/s0360-3016(02)03023-7. [DOI] [PubMed] [Google Scholar]
- Pyo H, Choy H, Amorino GP, Kim JS, Cao Q, Hercules SK, et al. A selective cyclooxygenase-2 inhibitor, NS-398, enhances the effect of radiation in vitro and in vivo preferentially on the cells that express cyclooxygenase-2. Clin Cancer Res. 2001;7:2998–3005. [PubMed] [Google Scholar]
- van Wijngaarden J, van Beek E, van Rossum G, van der Bent C, Hoekman K, van der Pluijm G, et al. Celecoxib enhances doxorubicin-induced cytotoxicity in MDA-MB231 cells by NF-kappaB-mediated increase of intracellular doxorubicin accumulation. Eur J Cancer. 2007;43:433–442. doi: 10.1016/j.ejca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Altorki NK, Keresztes RS, Port JL, Libby DM, Korst RJ, Flieder DB, et al. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol. 2003;21:2645–2650. doi: 10.1200/JCO.2003.07.127. [DOI] [PubMed] [Google Scholar]
- Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, et al. Cyclooxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12:214–222. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- Zeytin HE, Patel AC, Rogers CJ, Canter D, Hursting SD, Schlom J, et al. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA.Tg/MIN mice. Cancer Res. 2004;64:3668–3678. doi: 10.1158/0008-5472.CAN-03-3878. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- Liu TC, Hwang T, Park BH, Bell J., and , Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- Howes LG. Selective COX-2 inhibitors, NSAIDs and cardiovascular events - is celecoxib the safest choice? Ther Clin Risk Manag. 2007;3:831–845. [PMC free article] [PubMed] [Google Scholar]
- Carothers AM, Moran AE, Cho NL, Redston M., and , Bertagnolli MM. Changes in antitumor response in C57BL/6J-Min/+ mice during long-term administration of a selective cyclooxygenase-2 inhibitor. Cancer Res. 2006;66:6432–6438. doi: 10.1158/0008-5472.CAN-06-0992. [DOI] [PubMed] [Google Scholar]
- Yu YA, Galanis C, Woo Y, Chen N, Zhang Q, Fong Y, et al. Regression of human pancreatic tumor xenografts in mice after a single systemic injection of recombinant vaccinia virus GLV-1h68. Mol Cancer Ther. 2009;8:141–151. doi: 10.1158/1535-7163.MCT-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- Chen B, Timiryasova TM, Haghighat P, Andres ML, Kajioka EH, Dutta-Roy R, et al. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J Immunother. 2001;24:46–57. doi: 10.1097/00002371-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Wu TC, Guarnieri FG, Staveley-O'Carroll KF, Viscidi RP, Levitsky HI, Hedrick L, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Luminescence imaging of mice infected with luciferase-expressing vaccinia virus with different doses of Cox-2 inhibitor treatment.
Characterization of vaccinia virus titer in mice reinfected with vaccinia with or without Cox-2 inhibitor treatment.
Characterization of total intraperitoneal lymphocyte sub-populations in mice treated with Cox-2 inhibitor and vaccinia virus.
Characterization of the vaccinia titer in the vaccinia infected MOSEC cells treated with Cox-2 inhibitor.