Abstract
Increased reactive oxygen species (ROS) production has been reported as a distinctive feature of different pathologies including cancer. Therefore, we assessed whether increased ROS production in the cancer microenvironment could be selectively exploited to develop a selective anticancer therapy. For this purpose, we constructed a novel chimeric promoter, based on a ROS-response motif located in the VEGF gene promoter placed, in turn, downstream of a second ROS-response motif obtained from the early growth response 1 (Egr-1) gene promoter. The activity of the chimeric promoter was largely dependent on variations in intracellular ROS levels and showed a high inducible response to exogenous H2O2. Transient expression of the thymidine kinase (TK) gene driven by the chimeric promoter, followed by gancyclovir (GCV) administration, inhibited human colorectal cancer and melanoma cell growth in vitro and in vivo. Moreover, electrotransfer of the TK gene followed by GCV administration exerted a potent therapeutic effect on established tumors. This response was improved when combined with chemotherapeutic drugs. Thus, we show for the first time that a distinctive pro-oxidant state can be used to develop new selective gene therapeutics for cancer.
Introduction
Increased reactive oxygen species (ROS) levels have been associated with numerous pathological conditions, including atherosclerosis, cardiovascular diseases, rheumatoid arthritis, neurodegenerative disorders, and cancer.1 ROS play a role in tumor development as DNA-damaging agents increasing mutation rates, leading to malignant transformation.2,3 Moreover, ROS act as mediators of signal transduction pathways related to cell proliferation,4,5,6,7 angiogenesis,8,9,10 and cell migration.11,12,13 Evidence from the literature indicates that a shift in cellular redox status may be a crucial event in the appearance of the malignant phenotype.3 Indeed, several studies have revealed higher levels of ROS in different types of human cancer tissues compared with their noncancerous counterparts.14,15,16,17,18 It is, therefore, plausible that the persistent oxidative stress of cancer cells is a differential feature of the tumor environment that may be exploited to develop a selective anticancer therapy.
Gene therapy is a relatively new strategy in clinical terms, and most clinical trials are still in early phases (www.wiley.co.uk/genetherapy/clinical/). More than 60% of clinical trials target cancers, and many obstacles remain to be overcome before cancer gene therapy becomes a routine procedure. Recently, it was shown that conditional targeting of a therapeutic gene using cancer-specific promoters to drive gene expression might become a useful strategy toward this end.19 The high levels of heterogeneity in gene expression from cell to cell, and from tumor to tumor, limit the potential use of a promoter obtained from a tumor-associated gene. Therefore, different groups aim to specifically target the tumor mass by taking advantage of defined microenvironmental differences between cancer tissues and normal cells. For instance, hypoxia can be used for the selective expression of therapeutic genes driven by hypoxia-response elements in cancer tissues.20,21 Thus, the conditional targeting of a cancer tissue by taking advantage of the particular characteristics of the tumor remains possible both for cancer gene therapy and to attack other diseases. DNA sequences responsive to ROS are present in the promoters of several redox-regulated genes.22 Particularly, oxidative stress was shown to regulate VEGF-A gene expression in gastric cancer cells.23 Additional examples of oxidative stress-responsive DNA sequences include a region present in the promoter of the early growth response 1 (Egr-1) gene.24 Interestingly, no sequence consensus has been found among the different response sequences defined to date.
We propose a new strategy that makes use of the increased levels of ROS in cancer cells to enhance therapeutic selectivity. Here, we demonstrate that the expression of the Herpes Simplex virus thymidine kinase (TK) gene, driven by a ROS-response chimeric promoter, was effective in inhibiting tumor cell growth in vitro and in vivo. Electrotransfer of this nonviral construct was able to strongly inhibit established human colorectal carcinoma and melanoma growth in a nude mouse model. The therapeutic effect of this construct was enhanced when combined with chemotherapeutic drugs.
Results
A ROS-response chimeric promoter is active in malignant cells
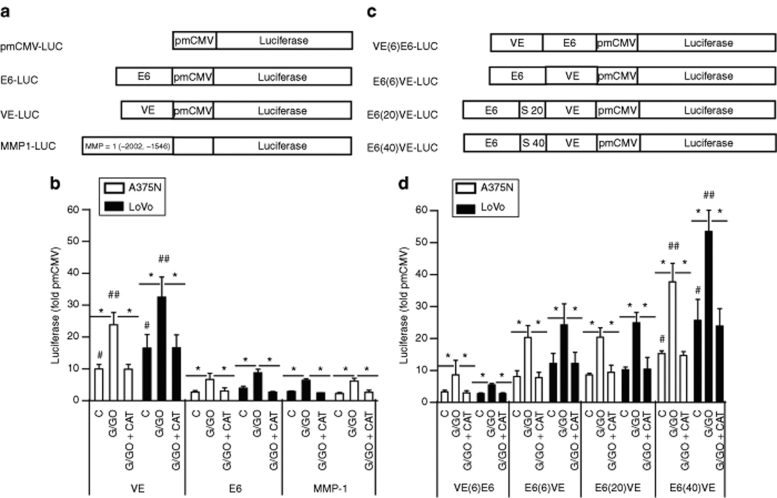
We initially explored the activities of ROS-response elements from different promoters, in an effort to develop novel selective gene therapeutics. We selected three DNA motifs that were previously described as redox-response DNA elements: a CG-rich motif located in the VEGF-A promoter (named VE),23 six-repeated CArG motives from the Egr-1 promoter (named E6),24 and a −2002, −1546 fragment corresponding to the matrix metalloproteinase-1 promoter (named MMP-1).13 The different fragments were cloned into the minimal cytomegalovirus (CMV) plasmid upstream of the luciferase reporter gene (Figure 1a).
Figure 1.
The reactive oxygen species-response chimeric promoter is active in malignant cells. (a) Schematic representation of different constructs containing a single motif or promoter sequence. (b) Oxidative stress responses of the different constructs described in a. LoVo and A375N cells transiently transfected with the different constructs were exposed to H2O2 generated by the G/GO system, in the presence or absence of exogenously added catalase. (*P < 0.001, the lines over the bars show the groups that were compared; #P < 0.0001, corresponds to the basal activity of VE, versus the basal activity of E6 or MMP-1; ##P < 0.0001, corresponds to VE activity in the presence of G/GO versus the activity of E6 and MMP-1 under the same conditions. (c) Schematic representations of the different chimeric constructs. The terms “S20” and “S40” mean that DNA spacers of 20 or 40 bp, respectively, were included. (d) Oxidative stress responses of the different chimeric constructs described in c. LoVo and A375N cells were treated as described in b. *P < 0.001 the lines over the bars show the groups that were compared; #P < 0.0001, corresponds to the basal activity of E6(40)VE versus VE; ##P < 0.0001, corresponds to the E6(40)VE activity in the presence of G/GO versus VE under the same conditions. Data are expressed as means ± SD values of three to seven independent experiments. C, control; G/GO, glucose/glucose oxidase; MMP-1, matrix metalloproteinase-1.
To evaluate the responsiveness of the different constructs to oxidative stress, A375N melanoma and LoVo colorectal cancer cells were transiently transfected with each plasmid and exposed to H2O2 generated by the glucose/glucose oxidase system.25 The VE element exhibited the highest basal activity, and inducible response, to H2O2, although all the elements tested were active to some extent (Figure 1b). These responses were redox-specific, as shown by their complete reversal upon addition of exogenous catalase (Figure 1b). As additional copies of the VE motif did not increase ROS response (data not shown), we decided to construct a chimeric promoter that will include the combination of different motives. In addition to the VE motif, we selected E6 because of it shorter length, and its slightly higher response to increased H2O2 levels, compared to MMP-1. Moreover, E6 can be additionally activated by ionizing radiation.24 The E6 and VE motifs were placed at different positions relative to the luciferase gene (Figure 1c). The 5′-E6(6)VE-3′ [hereafter termed E6(6)VE] chimera showed a basal and inducible activity higher than that of the 5′-VE(6)E6-3′ chimera (Figure 1d), but lower than that of VE alone, when the motifs were assessed in parallel (compare Figure 1d with 1b), suggesting that steric hindrance might hamper the activity of the chimeric promoter. In efforts to improve E6(6)VE activity, we moved the E6 and VE motifs either 20 or 40 bp apart, by introducing DNA spacers. The E6(40)VE chimera showed the best basal, and inducible, activities in the presence of exogenous H2O2 (Figure 1d). Notably, the E6(40)VE response to exogenous H2O2 was redox-specific, as shown by the complete reversal of inducible luciferase activity in the presence of catalase (Figure 1d). The basal luciferase activity driven by E6(40)VE was as strong as or higher than the activity of a SV40 promoter in various cell lines, including melanoma and colorectal cancer cells (Supplementary Figure S1).
Variation in endogenous ROS levels modulates E6(40)VE activity
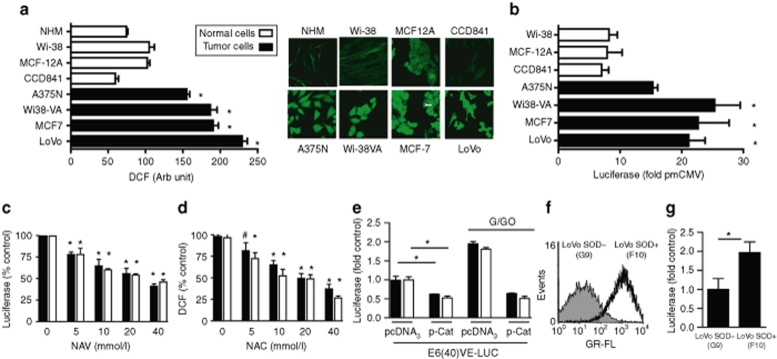
We next compared the basal activity of E6(40)VE in normal and malignant cells in response to ROS endogenous levels. First, we confirmed that malignant cells exhibited higher intracellular ROS levels than their normal counterparts. Indeed, quantification of dichlorofluorescein staining showed a two- to fivefold increase in intracellular ROS levels in malignant LoVo and A375N cells as well as in MCF-7 breast cancer cells, and in transformed WI-38VA fibroblasts compared to their normal counterparts (Figure 2a). Next, we transiently transfected normal and malignant cells lines with E6(40)VE-LUC, where luciferase activity was driven by the chimeric promoter. As expected, ROS dependent-luciferase activity was higher in malignant cells than in their respective normal counterparts (Figure 2b). We were unable to transfect primary cultures of melanocytes (data not shown).
Figure 2.
Variation in endogenous reactive oxygen species (ROS) levels modulates E6(40)VE activity. (a) Quantification of endogenous ROS in different cell types as assessed by DCF. Data represent the means ± SEMs of fluorescence levels of ~50 cells (*P < 0.01). Confocal microscopy photographs showing ROS levels in different cell types. Bar represent 20 µm. (b) Luciferase activity in cells transfected with E6(40)VE-LUC and the respective controls. Luciferase activity was normalized and quantified as described in Materials and Methods. (*P < 0.001). (c) Luciferase activity in LoVo (black) and A375N (white) cells transfected with E6(40)VE-LUC and treated with NAC (#P < 0.05, *P < 0.01) (d) ROS levels in NAC-treated cells measured by the DCF assay (*P < 0.01). (e) LoVo (black) and A375N (white) cells co-transfected with E6(40)VE-LUC and p-Cat, exposed or not to G/GO. Luciferase activity was expressed as -fold induction over control cells. (*P < 0.01). Data show the mean ± SD of three independent experiments. (f) Levels of ROS in LoVo SOD+(F10) and LoVo SOD−G9 cells as assessed by DCF followed by fluorescence-activated cell sorting analysis. Data show a representative result from three independent experiments. (g) Luciferase activity in stable LoVo SOD+(F10) and LoVo SOD−(G9) cell clones obtained from LoVo cells (see Results section for details). Cell clones were transfected with E6(40)VE-LUC. Luciferase activity was normalized as described and further quantified as a -fold induction over control G9 cells. Data are expressed as means ± SD values of three independent experiments (*P < 0.014). G/GO, glucose/glucose oxidase; SOD, superoxide dismutase.
Next, we evaluated whether E6(40)VE activity was dependent on intracellular redox status. First, we transfected LoVo and A375N cells with E6(40)VE-LUC and treated cells with the ROS scavenger N-acetylcysteine. Under these conditions N-acetylcysteine reduced luciferase activity and intracellular ROS levels in a dose-dependent manner (Figure 2c,d). Further confirmation that E6(40)VE activity was driven by intracellular ROS levels was obtained when luciferase expression driven by the chimeric motif was strongly reduced when cells were co-transfected with a plasmid encoding for human catalase (Figure 2e). Finally, the increase in luciferase activity induced by cell treatment with glucose/glucose oxidase system that augments H2O2 levels was strongly inhibited when cells were co-transfected with the plasmid encoding for human catalase (Figure 2e). Furthermore, we stably transfected LoVo cells with a construct containing a cDNA coding for the human CuZn-superoxide dismutase (SOD), as previous data demonstrated increased ROS levels in cells constitutively expressing SOD.13 The stable blasticidin-resistant clone LoVo SOD+(F10) showed the highest intracellular ROS levels compared to LoVo SOD−(G9) cells that were stably transfected with an empty vector (Supplementary Figure S2a,b). We confirmed by dichlorofluorescein quantification that LoVo SOD+(F10) produced higher levels of H2O2 than LoVo SOD−(G9) cells (Figure 2f). LoVo SOD+(F10) cells exhibited 2.5-fold increased luciferase activity after transfection with E6(40)VE-LUC, compared to LoVo SOD−(G9) control cells, further demonstrating that E6(40)VE activity correlated with increased intracellular ROS levels (Figure 2g). The sum of the evidence clearly demonstrates that the activity of the chimeric promoter was ROS-dependent.
Expression of TK driven by the ROS-responsive promoter inhibited the in vitro and in vivo proliferation of malignant cells
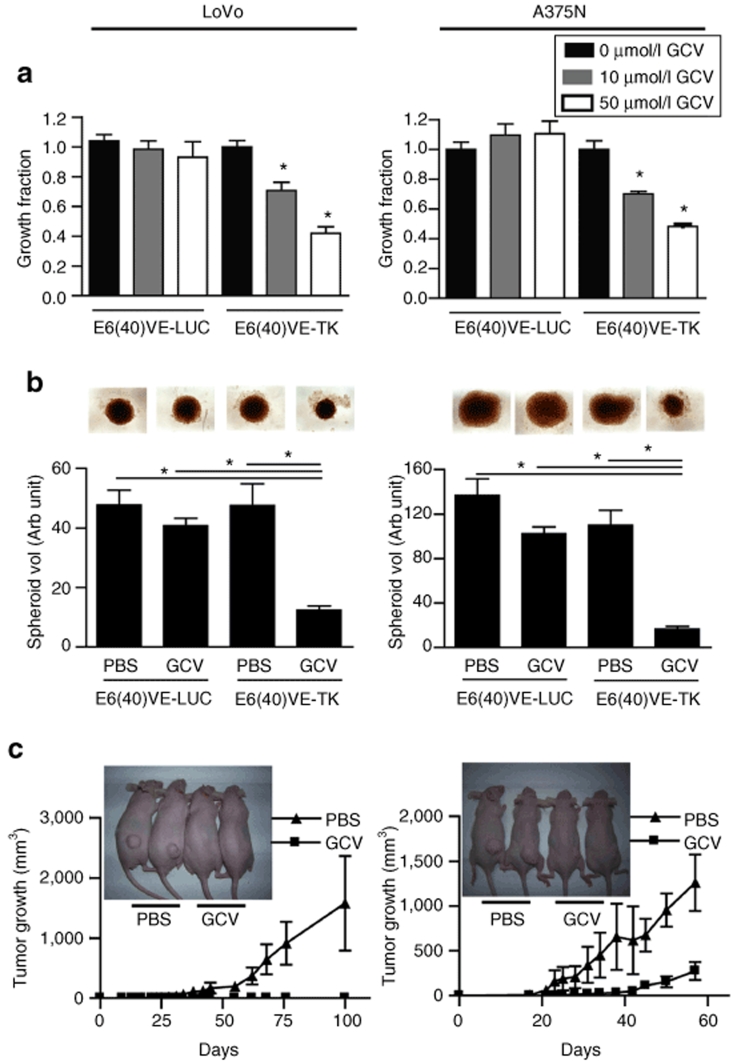
Based on the evidence mentioned earlier, we next determined if E6(40)VE might drive the expression of a therapeutic gene. As a proof of concept we cloned the TK gene downstream of E6(40)VE to generate E6(40)VE-TK. Transient expression of E6(40)VE-TK led to a significant inhibition of LoVo and A375N cell proliferation in the presence of gancyclovir (GCV) (Figure 3a). No significant inhibition was observed in control cells transfected with E6(40)VE-LUC, whether or not these cells were exposed to GCV, or when TK was expressed in the absence of GCV (Figure 3a). Moreover, normal CCD-481 human colonic cells were completely resistant to TK expression driven by E6(40)VE, as almost no death was observed in transfected cells in the presence of GCV, compared to a massive death of malignant cells under the same conditions (Supplementary Figure S3 and Supplementary Materials and Methods).
Figure 3.
TK expression driven by the reactive oxygen species-responsive chimeric promoter, followed by GCV, inhibits in vitro and in vivo cell growth. (a) In vitro growth inhibition of LoVo and A375N cells transiently transfected with E6(40)VE-TK plasmid, and exposed to GCV. “Growth fraction” means the percentage of surviving cells, compared to control cells. Transfection efficiencies were 40–60%. Data show the mean ± SD values of three independent experiments (*P < 0.0001). (b) Spheroids made of LoVo or A375N cells transiently transfected with E6(40)VE-LUC and E6(40)VE-TK plasmids. Spheroids were grown in the presence or absence of 50 µmol/l GCV. Data represent the mean ± SD of measurements from three to eight spheroids, corresponding to one of two independent experiments. Inset: photomicrographs (×25) of spheroids taken after 20 days of GCV treatment (*P < 0.01). (c) In vivo tumorigenicity of LoVo and A375N cells transiently transfected with E6(40)VE-TK. Mice were treated intraperitoneal with GCV (50 mg/kg), or vehicle, every day during the first 15 days after cell inoculation (n = 5–7 mice per group); (*P < 0.0002 for the melanoma model). Inset: photographs of mice taken at 60 days, showing the presence of tumors in control mice only. GCV, gancyclovir; PBS, phosphate buffered saline; TK, thymidine kinase.
E6(40)VE was also able to drive TK expression in multicellular spheroids. Indeed, we observed a strong reduction in the growth capacity of spheroids made of LoVo or A375N cells previously transfected with E6(40)VE-TK, when exposed to GCV, compared to the same spheroids without GCV, or compared to spheroids made of cells transfected with E6(40)VE-LUC followed by GCV (Figure 3b). This effect was observed immediately, after only 2 days of culture (Supplementary Figure S4).
To evaluate the capacity of E6(40)VE to drive TK expression in vivo, LoVo and A375N cells were transiently transfected with E6(40)VE-TK and injected subcutaneously into nude mice. No mouse injected with LoVo cells expressing TK, and treated with GCV, developed a tumor (Figure 3c). In addition, mice injected with A375N, and receiving the same treatment as animals receiving LoVo cells, showed significant tumor growth delay compared to control mice (Figure 3c). It can be concluded that transient expression of TK driven by E6(40)VE, followed by GCV, was sufficient to strongly inhibit the in vivo growth of colorectal cancer and melanoma cells.
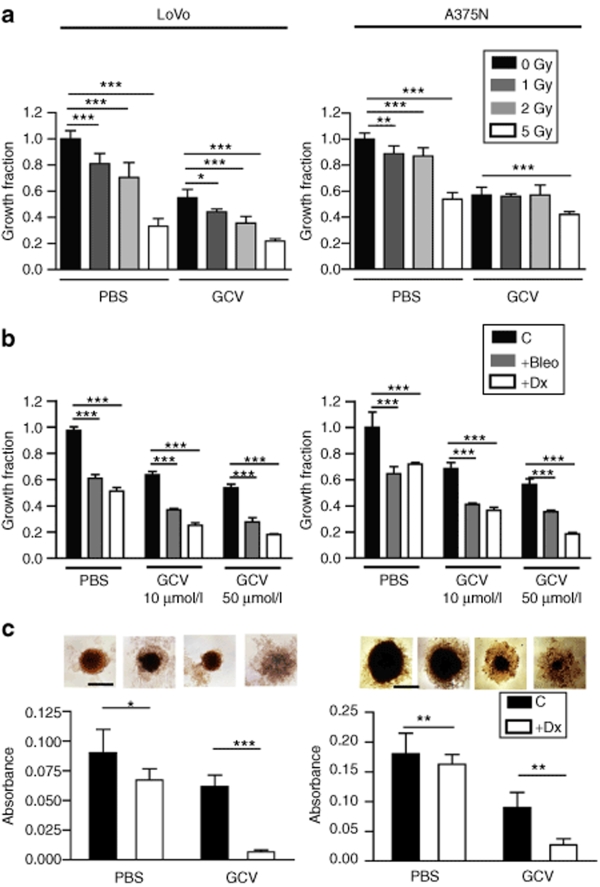
The combination of E6(40)VE-TK with γ irradiation, bleomycin, or doxorubicin, enhanced the inhibition of tumor cell growth in vitro, in cell monolayers and spheroids
Ionizing radiation, and some chemotherapeutic drugs such as bleomycin (Bleo) and doxorubicin (Dx), are associated with the formation of reactive oxygen intermediates, and direct damage to DNA.24,25,26 We hypothesized that the combination with chemotherapeutic drugs or ionizing radiation might enhance the therapeutic potential of the ROS-responsive plasmid in a setting that resembles a clinical situation. Using luciferase expression as reporter gene, we confirmed that the activity of E6(40)VE was stimulated by increasing doses of ionizing radiation (Supplementary Figure S5). Next, we performed in vitro studies in which LoVo and A375N cells were transiently transfected with E6(40)VE-TK, followed by GCV combined either with γ irradiation, Bleo, or Dx. The combination of E6(40)VE-TK/GCV with γ irradiation enhanced the inhibition of LoVo cell proliferation compared to the proliferation levels seen with any other treatment, and the optimum inhibition occurred at 2Gy γ irradiation (Figure 4a). In addition, the combination of γ irradiation and E6(40)VE-TK/GCV treatment was slightly more effective on A375N melanoma cells than any other treatment, when E6(40)VE-TK/GCV was combined with 5Gy γ irradiation (Figure 4a). Moreover, the combination of E6(40)VE-TK/GCV treatment with either Bleo or Dx enhanced cell growth inhibition, compared to the inhibitions noted when each treatment was applied separately (Figure 4b). Apoptosis was the main form of cell death induced by Dx or E6(40)VE-TK/GCV as single agent or in combination (Supplementary Figure S6 and Supplementary Materials and Methods). On the other hand, no enhancement of cell growth inhibition was observed when LoVo and A375N cells were transfected with E6(40)VE-LUC, followed by GCV in combination with either γ irradiation, Bleo, or Dx (Supplementary Figure S7).
Figure 4.
A combination of TK expression driven by the reactive oxygen species-responsive chimeric promoter, and GCV, with g radiation, Bleo, or Dx, enhances in vitro cell growth inhibition. (a) LoVo and A375N cells were transiently transfected with E6(40)VE-TK and exposed to g radiation in the presence or absence of 10 µmol/l GCV (***P < 0.001, **P < 0.01). (b) LoVo and A375N cells were transiently transfected with E6(40)VE-TK and exposed to Dx (0.5 µmol/l), or Bleo (20 µmol/l), in the presence or absence of 10 or 50 µmol/l GCV. Growth inhibition was measured by the MTT assay and “growth fraction” refers to the inhibition seen in comparison with untreated cells (***P < 0.001). (c) Spheroids made of LoVo or A375N cells, transiently transfected with E6(40)VE-TK, were exposed to GCV (50 µmol/l), Dx (0.5 µmol/l), or both. Spheroid growth was measured using the MTT assay. Data show the mean ± SD of three to five measurements within a representative experiment of two experiments. Each measurement includes one spheroid of A375N cells and a pool of three spheroids for LoVo cells. Inset: photomicrographs (×25) of spheroids taken at day 20 (*P < 0.05 and **P < 0.01). Data show the means ± SD values of three independent experiments and lines over the bars indicate the groups that were compared. Bleo, bleomycin; C, control; Dx, doxorubicin; GCV, gancyclovir; PBS, phosphate buffered saline; TK, thymidine kinase.
We further evaluated the effect of the combination of E6(40)VE-TK/GCV treatment and Dx on spheroid growth. Spheroids made of cells transiently transfected with E6(40)VE-TK were exposed to GCV and Dx treatments and the viabilities of the spheroids were assessed using the MTT assay. We observed enhanced inhibition of spheroid growth of both cell types after the combination treatment, compared to the inhibitions noted when each treatment was applied separately (Figure 4c). Overall, these series of experiments demonstrate that E6(40)VE-TK and GCV can be combined with γ irradiation, and chemotherapeutic drugs, to enhance antitumor effects.
Electrotransfer of E6(40)VE-TK, followed by GCV, with or without chemotherapy, inhibited the in vivo growth of established colorectal cancer and melanoma
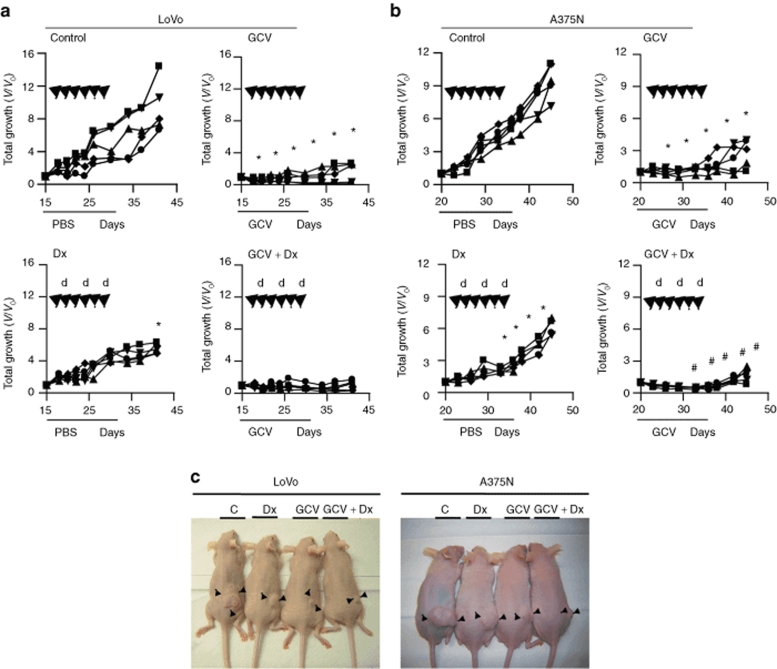
We finally decided to assess whether E6(40)VE was sufficiently potent to drive TK expression, and thus to exert a therapeutic effect, on established tumors. For this purpose, mice harboring LoVo or A375N tumors (150–200 mm3 average diameter) received six intratumor administrations of E6(40)VE-TK followed by local electroporation, once every 2 days. Starting on the day of the first electroporation, control mice received intraperitoneal (i.p.) injections of phosphate buffered saline for 15 consecutive days. A second group received GCV i.p. for 15 consecutive days. A third group received Dx i.p. on three occasions, once every 4 days, starting at the second round of electroporation. A fourth group received both GCV and Dx (Figure 5a,b).
Figure 5.
Combination of TK expression driven by the reactive oxygen species-responsive chimeric promoter, and GCV, with or without chemotherapy, inhibited the in vivo growth of established tumors. (a,b) Nude mice harboring established LoVo and A375N tumors were distributed into four groups (n = 5 per group): control (C), Dx, GCV, and GCV+Dx. All mice received six intratumoral injections of 50 µg of naked plasmid by electroporation, every 2 days. GCV (50 mg/kg) or PBS was administrated i.p from day 0 to 15, as indicated. Dx (5 mg/kg) was administrated i.p 1 hour prior to electroporation, when indicated. Growth curves represent the growths of individual tumors, relative to tumor size at the beginning of the treatment (V/V0). Black arrows indicate the times of E6(40)VE-TK plasmid electrogene transfer; “d” indicates Dx administration, the line in the x-axis is the duration of PBS or GCV administration. (c) Photographs of LoVo and A375N mice obtained at 20 days of treatment. (*P < 0.0001 control versus GCV; *P < 0.008 control versus Dx and #P < 0.0001 GCV versus GCV+Dx). Dx, doxorubicin; GCV, gancyclovir; i.p., intraperitoneal; PBS, phosphate buffered saline; TK, thymidine kinase.
Figure 5 shows the -fold increase in tumor volumes in the different individuals of each experimental group. To facilitate the visualization, the data are presented in separate figures although the experiments for each tumor type were performed simultaneously. Control mice showed a 7–14-fold increase in tumor volumes in both tumor models (Figure 5a,b). Electro-E6(40)VE-TK transfer followed by GCV administration resulted in strong growth inhibitions, compared to control groups, in mice carrying either LoVo or A375N tumors. In fact, tumor growth was completely inhibited up to day 25 after treatment initiation in all mice treated with GCV. Conversely, Dx treatment alone inhibited tumor growth to a minor extent, although all mice showed significant reductions in their tumor masses (Figure 5c). Interestingly, E6(40)VE-TK/GCV, in combination with Dx, significantly improved the inhibition of tumor growth, even when compared to E6(40)VE-TK/GCV administered alone. In addition, 2/5 of LoVo tumors in mice treated with the combination approach were completely eliminated, and did not resume growth until the end of the experiment. These data demonstrate that TK expression driven by ROS-responsive elements can be combined with chemotherapeutic agents to increase therapeutic efficacy.
Discussion
Our results demonstrate that a ROS-responsive chimeric promoter containing two elements, from the VEGF-A and the Egr-1 promoter regions, was effective to drive the expression of the herpes simplex TK suicide gene, in a tumor-selective gene-directed enzyme/prodrug therapy, both in vitro and in vivo. The activity of the ROS-responsive chimeric promoter was largely dependent on ROS levels.
The most effective response element combination was obtained when the VE element was placed downstream, and 40 bp away from the E6 element. The VE ROS-response element is located at position −88/−50 of the VEGF-A promoter, and a single copy of this element was sufficient to confer maximal basal and inducible activity when placed in a heterologous promoter system.23 The E6 motif contains six copies of the CArG elements of the Egr-1 gene.27 Egr-1 activity is regulated by ionizing radiation and the Egr-1 promoter region has been used to drive tumor necrosis factor-α expression in radiation gene therapy clinical trials.28 The CArG elements were shown to control the radiation response of Egr-1.29 However, CArG elements are essentially regulated by a mechanism involving ROS that are produced after cell exposure to ionizing radiation.24
Dual specificity chimeric promoters have been described; these contain hypoxia-response elements in combination with estrogen responsive promoters,30 cytokine inducible enhancers,31 and ionizing radiation-responsive elements.32 These hybrid promoters have been shown to be active as gene enhancers. Our novel chimeric promoter reacted not only to increased ROS levels but also to ionizing radiation, further enhancing its potential therapeutic capacity. Interestingly, recent evidence demonstrates that hypoxia, a hallmark of cancer tissues, enhanced ROS production. Oxygen deprivation stimulates mitochondria to produce ROS at complex III of the electron chain; these ROS in turn activate the hypoxia inducible factor 1, α subunit, a paradigmatic hypoxia-related protein.3,33 On these bases we can hypothesize that the present chimeric promoter will have the potential to react to a combination of factors (ROS, hypoxia, and ionizing radiation) that are highly distinctive of cancer tissue, making our promoter a promising tool for gene therapy.
Anthracyclines are one of the most effective classes of anticancer drugs ever developed, but, despite their extensive use in cancer treatment, their mechanism of action remains controversial.25 In addition to their capacity to intercalate with DNA, anthracyclines have been shown to generate ROS in cancer cells by increasing intracellular hydrogen peroxide levels.34 Moreover, the Bleo antiproliferative effect was shown to occur in part through the generation of free radicals.35 In line with this, both Dx and Bleo showed additive effects, when combined in vitro with the novel chimeric promoter-driven TK expression, in the presence of GCV. Moreover, Dx enhanced the antitumor effect induced by the combination of TK and GCV. Thus, the present data suggest that both Bleo and Dx can be combined with the ROS-responsive chimeric promoter to improve overall therapeutic efficacy.
The ROS-responsive chimeric construct was directly applied to tumors by electroporation. Electrically mediated delivery of plasmids encoding therapeutic genes has been efficiently used in animal models of cancer treatment.36 Complete tumor regression, and the development of an antitumor memory, has been achieved by intratumor electroporation of plasmids encoding for cytokines such as interleukin-2 (IL-2), IL-12, and IL-18, sometimes combined with TK, in immunocompetent models of cancer.37,38,39 Combined electrogene therapy using plasmids expressing herpes virus TK and IL-12, is highly efficient against murine carcinomas in vivo.39 More recently, electroporation has been applied for intratumoral delivery of Bleo in melanoma patients; this procedure improved the efficacy of the chemotherapeutic drug.40 Our immunodeficient model did not allow us to explore the capacity of the ROS-responsive chimeric construct to induce the development of a specific antitumor memory. Despite this fact, we were able to show that tumor growth remained completely suppressed for as long as 40 days, when gene therapy was combined with chemotherapy. In several mice injected with colorectal cancer cells, tumors regressed completely. In long-term follow-up, we observed that some of the tumors ceased growing, whereas others resumed growth. It is expected, therefore, that the therapeutic efficacy of our construct will be greatly improved in immunocompetent systems.
Aberrant redox signaling mechanisms are critical not only in cancer but in various diseases. Increased formation of free radicals was shown to be associated with atherosclerotic lesions and hypertension.41,42 Redox imbalance in synovial tissues led to increased mutation levels of the p53 gene in rheumatoid arthritis.43 Moreover, the inflammatory infiltrate that leads to cartilage erosion appears to be influenced by ROS-sensitive signaling pathways.44 The complications that hyperglycemia exerts on patients suffering from type 2 diabetes also appear to be related to abnormally high levels of ROS.45 Finally, oxidative stress was shown to contribute to the cascade of events leading to dopamine cell degeneration in Parkinson's disease, and to the marked accumulation of amyloid-B peptide in Alzheimer's patients.46 Based on these data, we anticipate that the present strategy can be applied, by using the appropriate therapeutic gene, to any of the diseases mentioned earlier.
In conclusion, we have shown for the first time that an augmented oxidative state encountered in tumor cells can be used as a new approach for cancer gene therapy. The new therapeutic vector as presently designed and administered was shown to be very potent for direct intratumor application alone or in combination with chemotherapeutic drugs currently in use. The first phase I clinical trial using a plasmid containing IL-12 administered by electroporation has been recently performed in melanoma patients suggesting that gene transfer with in vivo electroporation is a safe strategy for clinical application.47 The present studies and previous data7 indicate that tumor cells express higher ROS levels than normal cells indicating its potential usefulness for systemic treatment, provided specific targeting can be achieved, to avoid side effects. In addition, it will be necessary to establish the minimal ROS levels required to drive the chimeric promoter to rule out potential toxicity to normal organs. Although we were unable to observe signs of toxicity in autopsies of treated animals, it will be important to assess whether the combination of nonviral gene therapy and chemotherapy, as proposed here, might enhance toxicity in chronic treatment. Finally, the enhanced therapeutic efficacy of the combination of our ROS-responsive element with chemotherapy would be reduced when combined with some drugs such as glutathione whose mechanism of action relies on their capacity to reduce intracellular ROS levels.48 On the other hand, the anticancer activity of mainstay drugs such as paclitaxel49 in addition to Bleo and Dx are strongly dependent on ROS activity, suggesting a potential combination with the ROS-responsive element described here.
Materials and Methods
Cell lines and culture. Human colorectal carcinoma LoVo cells (CCL-229), WI38-VA transformed fibroblasts (CCL-75.1), MCF-7 breast cancer cells (HTB-22), CCD-841 normal colon cells (CRL-1790), MCF-12A normal breast cells (CRL-1790), and WI38 normal fibroblasts (CCL-75), were obtained from the ATCC. Normal melanocytes obtained from primary cultures of neonatal foreskins (NHM), and A375N melanoma cells, were kindly provided by Estela Medrano, Houston, TX. The CCD-841, WI38, and MCF-12 cells were used between passages 6 and 20. Cell lines were maintained in DMEM/F12 (Invitrogen-Life Sciences, Grand Island, NY) supplemented with 10% (vol/vol) FBS (NatoCor, Cordoba, Argentina), 2 mmol/l L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37 °C, and under 5% (vol/vol) CO2. All additional reagents were obtained from Sigma-Aldrich (St Louis, MO).
Vector constructions. The pmCMV plasmid was obtained by inserting a basal CMV promoter, containing the transcription start site and a TATA box, between the XhoI and HindIII sites of plasmid pGL-3-basic (Promega, Madison, WI). A synthetic VE motif containing the GC boxes of the VEGF-A promoter (-88/-55, GenBank accession no. AF095785.1), and a synthetic E6 motif containing six CarG copies of the enhancer element of the Egr-1 promoter gene (GenBank accession no. AJ245926), were independently cloned between the MluI and XhoI sites of pmCMV, to obtain the VE-LUC and E6-LUC plasmids. The sequences of oligonucleotides used for VE motif cloning were: 5′-CGGGGCGGGCCGGGGGCGGGGTCCGGCGGGGCGGAGA-3′ and 5′-CGC GTCTCCGCCCCGCCGGGACCCCGCCCCCGGCCGCCCCCGGTAC-3′. The sequences of oligonucleotides used for the cloning of the of the E6 motif were: 5′-CGCGTCCATATAAGGCCATATAAGGCCATATAAGGCCATATAAGGCCATATAAGGCCATATAAGGC-3′ and 5′-TCGAGCCTTATATGGCCTTATATGGCCTTATATGGCCTTATATGGCCATATGGCCTTATATGGA-3′. Briefly, double-stranded molecules derived from the complementary single-stranded oligonucleotides described earlier were obtained by mixing 0.05 nmol of the appropriate oligonucleotides in 10 ml, heating to 55 °C for 5 minutes, and cooling to room temperature. The synthetic oligonucleotides, but also with terminal KpnI and MluI restriction enzyme cloning sites, were treated as described earlier and cloned into E6-LUC and VE-LUC vectors to obtain E6(6)VE-LUC and VE(6)E6-LUC plasmids. The E6(40)VE vector was obtained by cloning a spacer sequence of 40 bp into the MluI site of E6(6)VE-LUC. This spacer was obtained from the complementary single-stranded oligonucleotides 5′-CGCGTACCTCTTAGTACATATGAATCGATGCTTAGTAGCAAA-3′ and 5′-CGCGTTTGCTAGCATCGATTCATATGTACTAGTA-3′. The MMP-1 promoter (−2002, −1546, GenBank accession no. AF023338.1) was cloned by PCR of genomic DNA obtained from T47-D breast cancer cells, using primers 5′-CGGGGTACCACAGTGTATGAGACTCTTCCAGGG-3′ and 5′-CCCCTCCCCTTATGGATTCCTG-3′. The MMP-1 promoter was cloned between the KpnI and MluI restriction sites of the pmCMV vector, yielding plasmid MMP-1-LUC.
Generation of an H2O2-overproduction cell model, by stable expression of SOD. The CuZnSOD complete cDNA (from a plasmid kindly provided by Fernando Larcher; CIEMAT, Madrid, Spain) was obtained by PCR, with appropriate primers, flanked by HindIII and XbaI restriction sites, sequenced, and cloned into pcDNA6 to obtain CuZnSOD-pcDNA6. Subconfluent cultures of LoVo cells were stably transfected with the CuZnSOD-pcDNA6 expression vector using Lipofectamine 2000 (Invitrogen). Control cells were transfected with empty pcDNA6. For selection of stable transfectants, blasticidin (10 µg/ml; Sigma-Aldrich) was added to the cells 24 hours after transfection and maintained for 3 weeks. Blasticidin-resistant clones were obtained by dilution cloning.
Assessment of ROS levels. To measure ROS levels produced by cells stably transfected with SOD cDNA, cells were incubated for 40 minutes with 10 µmol/l of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, Molecular Probes), at 37 °C, washed twice with phosphate buffered saline, harvested with trypsin, and evaluated by flow cytometry. For assessment of ROS levels produced by paired normal and malignant cells or in N-acetylcysteine treatment, cells were incubated 15 minutes with DCHF-DA, cells and were analyzed under a confocal (Pascal LSM510; Carl Zeiss, Okerkochen, Germany) or epifluorescence microscope (Olympus BX51) respectively.
Luciferase assays. Luciferase was assayed using the “Dual Luciferase Reporter Assay” (Promega), with the aid of a Genius Luminometer (Tecan, Männedorf, Switzerland). To correct for transfection efficiencies, all cells were co-transfected with the Renilla luciferase construct pRL-LUC. For experiments on H2O2 induction, 5 × 105 LoVo or A375N cells were seeded in wells of 24-well tissue culture plates, and 24 hours later, cells were co-transfected with 0.1 mg/well of pRL-LUC and 0.8 mg/well of either pmCMV, VE-LUC, E6-LUC, MMP1-LUC, E6(6)VE-LUC, VE(6)E6-LUC, E6(20)VE-LUC, or E6(40)VE-LUC, using Lipofectamine. Twenty-four hour after transfection, cells were exposed to H2O2 generated by glucose (10 mmol/l) and glucose oxidase (20 mU/ml), for 6 hours. For assessment of E6(40)VE-LUC activities in F10 and G9 cells, cells were treated as described earlier, but luciferase activities were measured 24 hours post-transfection. For experiments comparing E6(40)VE-LUC activities in normal and tumor cells, cells were seeded in dishes 35 mm in diameter and co-transfected with 3.2 mg of pmCMV or E6(40)VE-LUC and 0.4 mg of pRL-LUC. To evaluate the effect of ROS scavenging, 24 hours E6(40)VE-LUC-transfected cells were treated with different concentrations of N-acetylcysteine for 24 hours. For experiments involving co-transfection with catalase, human cDNA catalase (kindly provided by Fernando Larcher) was cloned into pcDNA3 to yield p-Cat. p-Cat was co-transfected with E6(40)VE-LUC (1mg of each plasmid, and 0.1 mg pRL-LUC, per well).
In vitro proliferation assays. LoVo cells or A375N cells (5 × 105) seeded in 24-well multiwell plates, were transiently transfected with 1.8 mg of E6(40)VE-LUC or E6(40)VE-TK, and 0.5 mg pEGFP, in each well. pEGFP transfection was used to evaluate transfection efficiency. Twenty-four hours after transfection, cells were reseeded at low density (1/8 dilution) in complete medium, left to settle for 3–5 hours, and exposed to GCV. GCV was added in fresh medium each 48 hours, and after 4 days, cell survival was evaluated by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich) assay. Briefly, the culture medium was aspirated and replaced with 500 ml of fresh medium with 5 mg/ml MTT, for 1 hour, washed with phosphate buffered saline, and dissolved using 500 ml of DMSO; the absorbances were obtained at 570 nm. For γ irradiation, cells were irradiated with 1, 2, or 5 Gy 24 hours after the first GCV administration. Similarly, when chemotherapeutic treatments were used, cells were exposed to 20 µmol/l Bleo or 0.5 µmol/l Dx 24 hours after the first GCV administration.
Spheroid assays. Cells (5 × 105) were seeded in 24-well multiwell plates and co-transfected with 1.8 mg of E6(40)VE-LUC, or E6(40)VE-TK, and 0.5 mg pEGFP, in each well. Twenty-four hours after transfection, cells were trypsinized and seeded into wells of a 96-well agarose-coated plate (10,000 cells per well). GCV (50 µmol/l) was added the day after seeding, and replaced every 72 hours. Spheroids were kept at 37 °C under 5% (vol/vol) CO2, sized twice a week, and grown over 15 days. When administered in combination with GCV, Dx (0.5 µmol/l) was added at day 4 after seeding. In this case, spheroids were grown for a further 15–20 days and survival evaluated using the MTT assay. Briefly, spheroids were incubated with 5 mg/ml MTT for 2 hours at 37 °C, aspirated, centrifuged, dissolved in 50 ml of DMSO, and scanned at 570 nm using a spectrophotometer.
In vivo studies. For in vivo studies, LoVo and A375N cells seeded in dishes 100 mm in diameter were transiently transfected with 24 mg E6(40)VE-TK per plate. Twenty-four hour later, 4 × 106 LoVo or 2.5 × 106 A375N cells were injected subcutaneously into the flanks of a 20-day-old male or female athymic nu/nu mice (obtained from the animal facility of the National Atomic Energy Commission; Buenos Aires, Argentina). Mice were given GCV (50 mg/kg) i.p, or vehicle, daily, for 10 days.
To assess the effect of treatments on established tumors, 4 × 106 LoVo or 2.5 × 106 A375N cells were injected subcutaneously into the flanks of nude mice. When average tumor sizes reached ~150–200 mm3, mice were anesthetized with ketamine/xylazine (78 and 11.5 mg/kg, respectively) before treatment commenced. All mice were injected intratumorally with 50 mg of E6(40)VE-TK in 100 µl of phosphate buffered saline, and immediately electroporated using a two-needle electrode (5 mm between electrodes; Genetronics, San Diego, CA). Six pulses (200 V/20 ms) were applied every 2 days with an Electro Square Porator (ECM 830; BTX, San Diego, CA). GCV (50 mg/kg), or vehicle, was administered i.p. daily between day 0 and 15. In some experiments, Dx (5 mg/kg) was administered i.p. Tumor sizes were measured in two dimensions, using a caliper, twice a week. Tumor volumes were calculated from the equation: tumor volume (mm3) = (Dm × Dm × DM)/2, where Dm and DM are the minor and major tumor diameters. Animal care and experimental procedures followed institutional guidelines approved by the National Institutes of Health.
Statistical analysis. We used a two-tailed Student's t-test to compare two sets of data. One-way analysis of variance was used to compare three or more sets of data. Tukey's, Sheffe or Dunnett's tests were used as post hoc tests. Welch test and Games-Howells as post hoc tests were used when variances were heterogeneous. When parametric tests were not possible to apply, we used Kruskal–Wallis test with Mann–Whitney as post hoc test.
SUPPLEMENTARY MATERIALFigure S1. E6(40)VE chimeric promoter activity was comparable to that of an SV40 promoter. Different cell lines, including LoVo (colorectal cancer), A375N (melanoma), T-84 (normal colon), Paju (neuroblastoma), and U2OS (osteosarcoma), were transfected either with pmCMV, E6(40)VE-LUC, or SV40-Luc (a plasmid wherein an SV40 promoter drives luciferase activity). Luciferase activity was normalized, for transfection efficiencies, using the pRL-Luc plasmid, and the activities are expressed as -fold inductions, compared to cells transfected with pmCMV.Figure S2. ROS levels of different clones stably transfected with a plasmid containing the human CuZn-superoxide dismutase gene. (a) ROS activities of the different clones obtained after transfection of LoVo cells with a plasmid encoding for CuZn-superoxide dismutase, and selection in the presence of blasticidin. Clone F10 of LoVo cells showed the highest intracellular ROS levels. ROS production was evaluated using the DCF assay and fluorescence was acquired on a minimum of 10,000 cells, by flow cytometry. (b) Levels of ROS assessed by DCF, followed by FACS analysis, in F10 cells and F10 cells treated with exogenous catalase.Figure S3. E6(40)VE-TK activity in normal and malignant cells. LoVo, A375N and CCD-841 cells were transiently co-transfected with E6(40)VE-TK and renilla plasmid and exposed to 10mM GCV (black columns) or 50mM GCV (white columns). Data was expressed as percentage of dead cells divided by the amount of transfected cells using renilla expression as a readout. (*P < 0.001).Figure S4. Time course of spheroid growths made of LoVo or A375N cells, transfected with E6(40)VE-TK, followed by GCV. Photomicrographs of spheroids treated, or not treated, with GCV, were taken at the indicated times.Figure S5. Gamma irradiation response of the E6(40)VE promoter. LoVo and A375N cells were transiently transfected with E6(40)VE-LUC and exposed to 1, 2, 5 and 10 Gy of gamma radiation. Luciferase activity was used as a readout and data are expressed as increased activity over non-irradiated control cells (*P < 0.01).Figure S6. Apoptosis was the main form of cell death induced by E6(40)VE-TK/GCV as single agent or in combination with Dx. Apoptosis and necrosis of LoVo and A375N cells were evaluated after transiently transfected with E6(40)VE-TK exposed to GCV alone or in combination with 0.5 mM Dx.Figure S7. Transfection of tumor cells with E6(40)VE-LUC, followed by GCV treatment, did not enhance the growth inhibition induced by γ irradiation, Bleo or Dx. LoVo and A375N cells were transiently transfected with E6(40)VE-LUC and exposed to different levels of g radiation (a,b), Dx, or Bleo (c,d), in the presence or absence of administration of 10 or 50 mM GCV. “Growth fraction” indicates the percentage of surviving cells, compared to control cells.Materials and Methods.
Supplementary Material
E6(40)VE chimeric promoter activity was comparable to that of an SV40 promoter. Different cell lines, including LoVo (colorectal cancer), A375N (melanoma), T-84 (normal colon), Paju (neuroblastoma), and U2OS (osteosarcoma), were transfected either with pmCMV, E6(40)VE-LUC, or SV40-Luc (a plasmid wherein an SV40 promoter drives luciferase activity). Luciferase activity was normalized, for transfection efficiencies, using the pRL-Luc plasmid, and the activities are expressed as -fold inductions, compared to cells transfected with pmCMV.
ROS levels of different clones stably transfected with a plasmid containing the human CuZn-superoxide dismutase gene. (a) ROS activities of the different clones obtained after transfection of LoVo cells with a plasmid encoding for CuZn-superoxide dismutase, and selection in the presence of blasticidin. Clone F10 of LoVo cells showed the highest intracellular ROS levels. ROS production was evaluated using the DCF assay and fluorescence was acquired on a minimum of 10,000 cells, by flow cytometry. (b) Levels of ROS assessed by DCF, followed by FACS analysis, in F10 cells and F10 cells treated with exogenous catalase.
E6(40)VE-TK activity in normal and malignant cells. LoVo, A375N and CCD-841 cells were transiently co-transfected with E6(40)VE-TK and renilla plasmid and exposed to 10mM GCV (black columns) or 50mM GCV (white columns). Data was expressed as percentage of dead cells divided by the amount of transfected cells using renilla expression as a readout. (*P < 0.001).
Time course of spheroid growths made of LoVo or A375N cells, transfected with E6(40)VE-TK, followed by GCV. Photomicrographs of spheroids treated, or not treated, with GCV, were taken at the indicated times.
Gamma irradiation response of the E6(40)VE promoter. LoVo and A375N cells were transiently transfected with E6(40)VE-LUC and exposed to 1, 2, 5 and 10 Gy of gamma radiation. Luciferase activity was used as a readout and data are expressed as increased activity over non-irradiated control cells (*P < 0.01).
Apoptosis was the main form of cell death induced by E6(40)VE-TK/GCV as single agent or in combination with Dx. Apoptosis and necrosis of LoVo and A375N cells were evaluated after transiently transfected with E6(40)VE-TK exposed to GCV alone or in combination with 0.5 mM Dx.
Transfection of tumor cells with E6(40)VE-LUC, followed by GCV treatment, did not enhance the growth inhibition induced by γ irradiation, Bleo or Dx. LoVo and A375N cells were transiently transfected with E6(40)VE-LUC and exposed to different levels of g radiation (a,b), Dx, or Bleo (c,d), in the presence or absence of administration of 10 or 50 mM GCV. “Growth fraction” indicates the percentage of surviving cells, compared to control cells.
Acknowledgments
We specially thank Veronica Lopez, Eduardo Cafferata, and Diego Viale for their technical support, to Federico Prada and Pablo Salgado for their valuable assistance with statistical analysis and to Cristina Cambiaggio for her continuous support. This work was supported by grants from the National Agency for Promotion of Science and Technology, Argentina. We acknowledge the continuous support of Fundacion Rene Baron and Friends of Instituto Leloir for the War against Cancer (AFULIC), Argentina.
REFERENCES
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M., and , Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Jackson AL., and , Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- Halliwel B. Oxidative stress and cancer: have we moved forward. Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, et al. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci USA. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal EA, Day AM., and , Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Laurent A, Nicco C, Chéreau C, Goulvestre C, Alexandre J, Alves A, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2007;65:948–956. [PubMed] [Google Scholar]
- Shono T, Ono M, Izumi H, Jimi SI, Matsushima K, Okamoto T, et al. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol Cell Biol. 1996;16:4231–4239. doi: 10.1128/mcb.16.8.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M, Voest E, Amano S, Beerepoot L, Takashima S, Tolentino M, et al. Reactive oxygen intermediates increased vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98:1466–1471. doi: 10.1172/JCI118962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KK, Ranganathan AC, Mansouri J, Rodriguez AM, Providence KM, Rutter JL, et al. Elevated sod2 activity augments matrix metalloproteinase expression: evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin Cancer Res. 2003;9:424–432. [PubMed] [Google Scholar]
- Szatrowski TP., and , Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Policastro L, Molinari B, Larcher F, Blanco P, Podhajcer OL, Costa CS, et al. Imbalance of antioxidant enzymes in tumor cells and inhibition of proliferation and malignant features by scavenging hydrogen peroxide. Mol Carcinog. 2004;39:103–113. doi: 10.1002/mc.20001. [DOI] [PubMed] [Google Scholar]
- Toyokuni S, Okamoto K, Yodoi J., and , Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M., and , Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Al-Gayyar MM, Eissa LA, Rabie AM., and , El-Gayar AM. Measurements of oxidative stress status and antioxidant activity in chronic leukaemia patients. J Pharm Pharmacol. 2007;59:409–417. doi: 10.1211/jpp.59.3.0011. [DOI] [PubMed] [Google Scholar]
- Ko D, Hawkins L., and , Yu DC. Development of transcriptionally regulated oncolytic adenoviruses. Oncogene. 2005;24:7763–7774. doi: 10.1038/sj.onc.1209048. [DOI] [PubMed] [Google Scholar]
- Ido A, Uto H, Moriuchi A, Nagata K, Onaga Y, Onaga M, et al. Gene therapy targeting for hepatocellular carcinoma: selective and enhanced suicide gene expression regulated by a hypoxia-inducible enhancer linked to a human alpha-fetoprotein promoter. Cancer Res. 2001;61:3016–3021. [PubMed] [Google Scholar]
- Ballinger JR. Imaging hypoxia in tumor. Semin Nucl Med. 2001;31:321–329. doi: 10.1053/snuc.2001.26191. [DOI] [PubMed] [Google Scholar]
- Liu H, Colavitti R, Rovira II., and , Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- Schäfer G, Cramer T, Suske G, Kemmner W, Wiedenmann B., and , Höcker M. Oxidative stress regulates vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J Biol Chem. 2003;278:8190–8198. doi: 10.1074/jbc.M211999200. [DOI] [PubMed] [Google Scholar]
- Datta R, Taneja N, Sukhatme VP, Qureshi SA, Weichselbaum R., and , Kufe DW. Reactive oxygen intermediates target CC(A/T)6GG sequences to mediate activation of the early growth response 1 transcription factor gene by ionizing radiation. Proc Natl Acad Sci USA. 1993;90:2419–2422. doi: 10.1073/pnas.90.6.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G., and , Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Chen J., and , Stubbe J. Bleomycins: towards better therapeutics. Nat Rev Cancer. 2005;5:102–112. doi: 10.1038/nrc1547. [DOI] [PubMed] [Google Scholar]
- Scott SD, Joiner MC., and , Marples B. Optimizing radiation-responsive gene promoters for radiogenetic cancer therapy. Gene Ther. 2002;9:1396–1402. doi: 10.1038/sj.gt.3301822. [DOI] [PubMed] [Google Scholar]
- Senzer N, Mani S, Rosemurgy A, Nemunaitis J, Cunningham C, Guha C, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol. 2004;22:592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- Marples B, Scott SD, Hendry JH, Embleton MJ, Lashford LS., and , Margison GP. Development of synthetic promoters for radiation-mediated gene therapy. Gene Ther. 2000;7:511–517. doi: 10.1038/sj.gt.3301116. [DOI] [PubMed] [Google Scholar]
- Hernandez-Alcoceba R, Pihalja M, Nunez G., and , Clarke MF. Evaluation of a new dual-specificity promoter for selective induction of apoptosis in breast cancer cells. Cancer Gene Ther. 2001;8:298–307. doi: 10.1038/sj.cgt.7700304. [DOI] [PubMed] [Google Scholar]
- Modlich U, Pugh CW., and , Bicknell R. Increasing endothelial cell specific expression by the use of heterologous hypoxic and cytokine-inducible enhancers. Gene Ther. 2000;7:896–902. doi: 10.1038/sj.gt.3301177. [DOI] [PubMed] [Google Scholar]
- Greco O, Marples B, Dachs GU, Williams KJ, Patterson AV., and , Scott SD. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Ther. 2002;9:1403–1411. doi: 10.1038/sj.gt.3301823. [DOI] [PubMed] [Google Scholar]
- Guzy RD., and , Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- Wagner BA, Evig CB, Reszka KJ, Buettner GR., and , Burns CP. Doxorubicin increases intracellular hydrogen peroxide in PC3 prostate cancer cells. Arch Biochem Biophys. 2005;440:181–190. doi: 10.1016/j.abb.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PM., and , Tisdale MJ. Role of lipid-mobilising factor (LMF) in protecting tumour cells from oxidative damage. Br J Cancer. 2000;90:1274–1278. doi: 10.1038/sj.bjc.6601669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller LC, Ugen K., and , Heller R. Electroporation for targeted gene transfer. Expert Opin Drug Deliv. 2005;2:255–268. doi: 10.1517/17425247.2.2.255. [DOI] [PubMed] [Google Scholar]
- Lucas ML., and , Heller R. IL-12 gene therapy using an electrically mediated nonviral approach reduces metastatic growth of melanoma. DNA Cell Biol. 2003;22:755–763. doi: 10.1089/104454903322624966. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang X., and , Xia X. Regression of tumor growth and induction of long-term antitumor memory by interleukin 12 electro-gene therapy. J Natl Cancer Inst. 2002;94:762–768. doi: 10.1093/jnci/94.10.762. [DOI] [PubMed] [Google Scholar]
- Goto T, Nishi T, Kobayashi O, Tamura T, Dev SB, Takeshima H, et al. Combination electro-gene therapy using herpes virus thymidine kinase and interleukin-12 expression plasmids is highly efficient against murine carcinomas in vivo. Mol Ther. 2004;10:929–937. doi: 10.1016/j.ymthe.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Byrne CM, Thompson JF, Johnston H, Hersey P, Quinn MJ, Michael Hughes T, et al. Treatment of metastatic melanoma using electroporation therapy with bleomycin (electrochemotherapy) Melanoma Res. 2005;15:45–51. doi: 10.1097/00008390-200502000-00008. [DOI] [PubMed] [Google Scholar]
- Yuan XM., and , Li W. The iron hypothesis of atherosclerosis and its clinical impact. Ann Med. 2003;35:578–591. doi: 10.1080/07853890310016342. [DOI] [PubMed] [Google Scholar]
- Romero JC., and , Reckelhoff JF. State-of-the-Art lecture. Role of angiotensin and oxidative stress in essential hypertension. Hypertension. 1999;34:943–949. doi: 10.1161/01.hyp.34.4.943. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Echeverri F, Yeo M, Zvaifler NJ., and , Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 1997;94:10895–10900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane G, Fitzgerald O, Beeton C, Cawston TE., and , Bresnihan B. Early joint erosions and serum levels of matrix metalloproteinase 1, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 in rheumatoid arthritis. Arthritis Rheum. 2001;44:2263–2274. doi: 10.1002/1529-0131(200110)44:10<2263::aid-art389>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Eriksson JW. Metabolic stress in insulin's target cells leads to ROS accumulation. A hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581:3734–3742. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M., and , Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block KI, Koch AC, Mead MN, Tothy PK, Newman RA., and , Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007;33:407–418. doi: 10.1016/j.ctrv.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Alexandre J, Batteux F, Nicco C, Chéreau C, Laurent A, Guillevin L, et al. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119:41–48. doi: 10.1002/ijc.21685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
E6(40)VE chimeric promoter activity was comparable to that of an SV40 promoter. Different cell lines, including LoVo (colorectal cancer), A375N (melanoma), T-84 (normal colon), Paju (neuroblastoma), and U2OS (osteosarcoma), were transfected either with pmCMV, E6(40)VE-LUC, or SV40-Luc (a plasmid wherein an SV40 promoter drives luciferase activity). Luciferase activity was normalized, for transfection efficiencies, using the pRL-Luc plasmid, and the activities are expressed as -fold inductions, compared to cells transfected with pmCMV.
ROS levels of different clones stably transfected with a plasmid containing the human CuZn-superoxide dismutase gene. (a) ROS activities of the different clones obtained after transfection of LoVo cells with a plasmid encoding for CuZn-superoxide dismutase, and selection in the presence of blasticidin. Clone F10 of LoVo cells showed the highest intracellular ROS levels. ROS production was evaluated using the DCF assay and fluorescence was acquired on a minimum of 10,000 cells, by flow cytometry. (b) Levels of ROS assessed by DCF, followed by FACS analysis, in F10 cells and F10 cells treated with exogenous catalase.
E6(40)VE-TK activity in normal and malignant cells. LoVo, A375N and CCD-841 cells were transiently co-transfected with E6(40)VE-TK and renilla plasmid and exposed to 10mM GCV (black columns) or 50mM GCV (white columns). Data was expressed as percentage of dead cells divided by the amount of transfected cells using renilla expression as a readout. (*P < 0.001).
Time course of spheroid growths made of LoVo or A375N cells, transfected with E6(40)VE-TK, followed by GCV. Photomicrographs of spheroids treated, or not treated, with GCV, were taken at the indicated times.
Gamma irradiation response of the E6(40)VE promoter. LoVo and A375N cells were transiently transfected with E6(40)VE-LUC and exposed to 1, 2, 5 and 10 Gy of gamma radiation. Luciferase activity was used as a readout and data are expressed as increased activity over non-irradiated control cells (*P < 0.01).
Apoptosis was the main form of cell death induced by E6(40)VE-TK/GCV as single agent or in combination with Dx. Apoptosis and necrosis of LoVo and A375N cells were evaluated after transiently transfected with E6(40)VE-TK exposed to GCV alone or in combination with 0.5 mM Dx.
Transfection of tumor cells with E6(40)VE-LUC, followed by GCV treatment, did not enhance the growth inhibition induced by γ irradiation, Bleo or Dx. LoVo and A375N cells were transiently transfected with E6(40)VE-LUC and exposed to different levels of g radiation (a,b), Dx, or Bleo (c,d), in the presence or absence of administration of 10 or 50 mM GCV. “Growth fraction” indicates the percentage of surviving cells, compared to control cells.