Abstract
Viral infections cause morbidity and mortality in allogeneic hematopoietic stem cell transplant (HSCT) recipients. To prevent and treat these, we have produced and infused cytotoxic T lymphocytes (CTLs) with specificity for Epstein–Barr virus (EBV), cytomegalovirus (CMV), and adenovirus (Adv), and shown that small numbers of infused cells proliferate in vivo and protect against all three viruses. Despite these encouraging results, broader implementation of this approach is limited by the need for infectious virus material (EBV), expensive production of clinical grade adenoviral vectors, and a prolonged (8–12 weeks) period of manufacture. There is also competition between virus-derived antigens within antigen-presenting cells (APCs), limiting extension to additional agents. We now describe an approach that uses DNA nucleofection of dendritic cells (DCs) with DNA plasmids that encode a range of immunodominant and subdominant viral antigens from CMV, EBV, BK, and Adv. Within 10 days, this methodology provides multivirus-reactive CTLs that lack alloreactivity. We further demonstrate that nucleofected DC stimulation can be combined with interferon-γ (IFN-γ) capture technology to produce even more rapid multivirus-CTL products for treatment of acute infection. These CTL generation procedures should increase the feasibility and applicability of T-cell therapy.
Introduction
Viral infections account for substantial morbidity and mortality in the immunocompromised host, in particular patients who have received allogeneic stem cell transplantation (SCT). Infections caused by persistent herpesviruses such as Epstein–Barr virus (EBV), cytomegalovirus (CMV), and herpes simplex virus, as well as by respiratory viruses such as respiratory syncytial virus, parainfluenza, and influenza are well known, whereas the importance of infections caused by adenovirus (Adv), BK virus, and human herpesvirus-6 have more recently been appreciated.1,2,3,4,5 Although pharmacological agents are standard therapy for some infections, they have substantial toxicities, generate resistant variants, and are frequently ineffective.
Immunotherapeutic strategies to restore virus-specific immunity offer an attractive treatment alternative. We and others have prepared donor-derived EBV-specific cytotoxic T lymphocytes (CTLs) using EBV-transformed lymphoblastoid cell lines (EBV-LCLs) as a source of antigen, and shown that infusion of these lines prevented and treated EBV-driven B cell lymphoproliferative diseases (EBV-LPDs) after allogeneic hematopoietic SCT (HSCT).6,7 Similarly, adoptively-transferred donor-derived CMV-specific CTL generated using CMV peptides, lysate, or vector-transduced antigen-presenting cells (APCs), were able to reconstitute immune responses against this virus and protect patients against the development of CMV disease or late recurrences.8,9,10,11,12,13,14 More recently, our group has produced trivirus-reactive CTL targeting EBV, CMV, and Adv by genetically modifying monocytes and EBV-LCL with a chimeric adenoviral vector expressing CMV-pp65 as a transgene. As few as 2 × 105/kg trivirus-specific CTL proliferated by several logs after infusion into HSCT recipients and appeared to protect the recipients against disease produced by all three viruses.15
Despite these encouraging clinical results, the broader implementation of T-cell immunotherapy is limited by (i) the infectious virus material (EBV/CMV/Adv) generally required for CTL generation, (ii) the expense of manufacture of clinical grade vectors for antigen presentation, and (iii) the prolonged period of culture (10–12-week manufacturing process) that is required to eliminate alloreactive T cells, with its attendant demands on technical skill and time. To address this latter problem, some groups have evaluated more rapid approaches for antigen-specific T-cell selection. Cobbold and colleagues selected CMV-specific CD8+ T cells from the blood of stem cell transplant donors using human leukocyte antigen (HLA)–peptide tetramers followed by selection with magnetic beads, and saw impressive clinical responses with eight of nine treated patients clearing their infection following infusion of tiny numbers of selected cells (median 8.6 × 103/kg).16 Selection of T cells that secrete interferon-γ (IFN-γ) after exposure to antigen (the IFN-γ capture assay) has also been used clinically by Feuchtinger and colleagues, who specifically selected Adv-specific T cells directly from peripheral blood after in vitro stimulation with viral peptides and showed that small numbers of cells (1.2–50 × 103/kg) were safe, protective, and effective in vivo.17,18 However, both these approaches are expensive and require a large starting blood volume, which is not always available, particularly in the matched unrelated donor setting. Thus, their use has been limited to restricted cases of urgent medical need.
We now describe an alternative good manufacturing practice-compliant method that overcomes all three limitations to allow the rapid generation of multivirus-specific CTL for immunotherapeutic purposes. We describe how DCs can be nucleofected with DNA plasmids encoding a range of immunodominant and subdominant viral antigens and used as in vitro T-cell stimulators to generate multivirus-reactive CTLs that target six different antigens from four common viruses within 10 days. Further, we demonstrate that T cell stimulation with nucleofected DCs can be combined with IFN-γ capture technology to produce a customized single- or multivirus-CTL product for rapid treatment of acute infection when there is an urgent need.
Results
Evaluation of nucleofected DCs as APCs
To determine whether DCs nucleofected with a viral antigen-encoding DNA plasmid could be used as APCs in T-cell stimulation protocols, we nucleofected the cells with the pShuttle plasmid encoding the immunodominant CMV-pp65 antigen fused with green fluorescent protein (GFP) (pShuttleGFPpp65), using the AMAXA/LONZA device (Amaxa–Lonza, Cologne, Germany). We first evaluated DC toxicity and transgene expression over a 60-hour period. The viability, defined as live cells/total initial cell numbers × 100, of nucleofected DCs was similar to non-nucleofected DCs between 6 and 24 hours postnucleofection (median 66% viable cells; range 58–74% versus median 69% viable cells; range 67–79%, respectively), but decreased in the nucleofected group after 24 hours (Figure 1a) (n = 3). Using flow cytometric analysis, GFP expression was detected in the nucleofected DCs as early as 6-hour postnucleofection (mean 38 ± 11% GFP+ cells) and remained stable over the 60-hour time course (Figure 1b). Nucleofection did not adversely affect the DC maturation status, as measured by expression of the costimulatory and activation markers CD80, CD83, CD86, and HLA-DR 24 hours postnucleofection, indicating that their antigen presenting capacity should be unimpaired (Figure 1c).
Figure 1.
Viability, transgene expression, and maturation state of nucleofected DCs. (a) DC viability, evaluated by Trypan blue exclusion, of nucleofected and unmodified cells in a time course from 0- to 60-hours postnucleofection. Viability was comparable in both DC populations until 24 hours postnucleofection (median 66%; range 58–74%) but thereafter progressively declined in the nucleofected population to median 29%; range 23–38% at the 60-hour time point. (b) Transgene expression (GFP) in nucleofected DCs, which was detectable as early as 6-hour postnucleofection with mean 38 ± 11% SD GFP+ cells, and remained stable until 60-hours postnucleofection in the live DC fraction. (c) The DC maturation state, evaluated by cell surface expression of CD83, CD80, CD86, and HLA-DR on untreated and nucleofected DCs 24 hours postnucleofection. DC, dendritic cell; GFP, green fluorescent protein.
Activation of CMV-specific CTL using nucleofected DCs as APCs
To determine whether pShuttleGFPpp65-nucleofected DCs could activate and expand pp65-specific T cells in vitro, we incubated T cells from CMV-seropositive individuals with nucleofected DCs at a 20:1 ratio and compared the specificity and function of the resultant CTLs with those produced by our conventional protocol with Ad5f35GFPpp65-transduced DCs as stimulators.19 Although gene transfer was significantly more efficient using the adenoviral vector (median 58% GFP positive cells, range 40–85%; n = 7) than the plasmid (median 43%, range 20–73%; n = 15) (P = 0.021) (Figure 2a), by day 9 of culture, the nucleofected DCs were more efficient in recruiting and expanding antigen-reactive pp65-specific T cells as assessed by pentamer and IFN-γ enzyme-linked immunospot (ELIspot) assays. Using the HLA-A2 pp65-NLV and the HLA-B7 pp65-TPR pentamers, the frequency of reactive cells increased from median 0.04% CD3+CD8+ T cells (range 0.01–1.75%) in peripheral blood to median 7.7% (range 1.7–22%) using nucleofected DCs, which was significantly higher than using transduced DCs, median 2.4% (range 0.79–16.3%; n = 7 donors, P = 0.01). The results for CTL lines from two representative donors are shown in Figure 2b. These results were confirmed by IFN-γ ELIspot assays using the pp65 pepmix to stimulate bulk CTLs, where there were 851.1 ± 130.8 SM spot-forming cells (SFC)/2 × 105 CTL in plasmid-reactivated CTL versus 559.9 ± 80.8 SM SFC/2 × 105 CTL for adenovector-reactivated CTL (n = 7 donors; P = 0.004). Overall, the expansion rate of the adenovector-reactivated cells was marginally higher than the plasmid-activated cells (1.9 ± 0.2-fold expansion versus 1.3 ± 0.4-fold expansion, respectively); however, the adenovector-reactivated CTL also contained an Adv-specific T-cell component that was absent in the plasmid-activated lines (Figure 2c).
Figure 2.
Transfection efficiency and stimulatory capacity of DNA plasmids versus adenoviral vectors. (a) GFPpp65 transgene expression (based on %GFP+ cells) in nucleofected (n = 15) or adenovector-transduced DCs (n = 7), 24–48-hours after modification. (b) Representative multimer staining from two HLA-A2 donors using the pp65-NLV pentamer performed 9 days after stimulation. The plasmid-activated CTL are shown in the left panels whereas the adenovector-activated CTL shown on the right-hand side. (c) The frequency of CMV-pp65 reactive T cells in plasmid- (black bars) or adenovector-activated CTL (gray bars) in response to the CMV-pp65 pepmix or a combination of the Hexon and Penton (Adv) pepmixes using IFN-γ ELIspot as a readout (n = 7 donors). Results represent the mean ± SE spot forming cells (SFCs) per 2 × 105 input cells. Control was IFN-γ release in response to stimulation with irrelevant pepmix. CTL, cytotoxic T-lymphocyte; CMV, cytomegalovirus; DC, dendritic cells; ELIspot, enzyme-linked immunospot; GFP, green fluorescent protein; HLA, human leukocyte antigen, IFN-γ, interferon-γ.
Expanded CTL lines retain their antigen specificity and functional ability
To assess whether pShuttleGFPpp65 plasmid-reactivated T cells could be further expanded while maintaining their antigen specificity, CTLs were restimulated weekly with Ad5f35GFPpp65-transduced EBV-LCL in the presence of interleukin-2 (IL-2). After 3 weeks of culture, we observed that the total number of T lymphocytes in cultures stimulated by the Adv vector was significantly higher than in cultures stimulated by plasmid-nucleofected DCs (43.5 ± 19.4 fold increase versus 20.06 ± 7.1 fold, respectively, and P = 0.027) (Figure 3a). However, there was no significant difference in the rate of expansion of antigen-specific CTLs as evaluated by the frequency of cells reactive with CMV-derived pentamers (Figure 3b,c).
Figure 3.
Specificity and function of CMVpp65-specific CTL after in vitro expansion. Plasmid- and adenovector-activated CTL were expanded by weekly restimulation using Ad5f35GFPpp65-transduced LCL in the presence of IL-2. On day 23 of culture T-cell expansion, specificity and function was assessed. (a) Overall T-cell expansion, based on cell counting using Trypan blue exclusion. Although the adenovector-activated cells expanded significantly more than the plasmid-activated cells, there was no difference in expansion of antigen-specific T cells based on pentamer analysis. (b) Representative NLV-pentamer staining of CTL lines from two donors on day 23, whereas (c) the expansion of the antigen-specific population of cells, based on HLA-A2 NLV and HLA-B7 TPR pentamer staining. (d) The frequency of CMV-pp65 reactive T cells in plasmid (black bars)- or adenovector (gray bars)-activated CTL in response to pp65 pepmix using IFN-γ ELIspot as readout (n = 7 donors). Results represent the mean ± SE SFC/2 × 105 input cells. Control was IFN-γ release in response to stimulation with irrelevant pepmix. (e) The CTL were functional in a standard 4-hour Cr51 assay and were able to lyse autologous pp65 pepmix-pulsed and antigen-expressing targets, with no recognition of mock or allogeneic targets. Data represent the mean ± SE% of target lysis from seven donors. CTL, cytotoxic T-lymphocyte; CMV, cytomegalovirus; ELIspot, enzyme-linked immunospot; IFN-γ, interferon-γ; LCL, lymphoblastoid cell line.
Phenotypic analysis of the bulk cultures demonstrated that the plasmid-reactivated CTLs were predominantly CD3+CD8+ (91 ± 2%). In contrast, the adenovector-reactivated CTL lines generated from the same donors were predominantly CD4+ with a minor CD8+ T-cell component (37.5 ± 18%) (data not shown). We next evaluated the antigen specificity and function of the expanded CMV-specific CTLs using pentamer staining, IFN-γ ELIspot, and cytotoxicity assays. Pentamer binding analysis on day 23 of culture using HLA-A2 NLV, representative examples from two donors are shown in Figure 3b) and HLA-B7 TPR (data not shown) showed that plasmid-reactivated CTL retained their antigen specificity. IFN-γ ELIspot confirmed the antigen specificity using the pp65 pepmix to stimulate CTL (2,434.4 ± 526.9 SM SFC/2 × 105 plasmid-activated CTL versus 1,726 ± 525.7 SM SFC/2 × 105 adeno-activated CTL, n = 7) (Figure 3d). Although plasmid stimulation favors expansion of the CD8+ CMV T cell population of CTLs, the overall population retains pp65-specific reactivity within both the CD8+ and CD4+ compartments (Supplementary Figure S1 online). The expanded CTL lines specifically killed target cells that expressed either whole pp65 antigen or pp65 pepmix-pulsed target cells, and the level of killing was equivalent, with no recognition of mock transfected or allogeneic target cells (Figure 3e). 3H proliferation assays showed CTL proliferation in response to pp65-transduced LCLs, without significant proliferation when CTLs were incubated with HLA-mismatched peripheral blood mononuclear cells (PBMCs) (data not shown).
Recruitment of viral-specific CTLs derived from low-frequency precursors
To test whether nucleofected DCs could be used to generate CTL against viruses irrespective of their precursor frequency in the peripheral circulation, we generated pShuttle plasmids encoding antigens from three viruses, EBV, BK, and Adv, for which the precursor frequencies are considered high, intermediate, and low respectively.15,20,21,22,23. The plasmids incorporated sequences encoding EBV latent membrane protein 2 (LMP2) and BamHI Z leftward reading frame 1 (BZLF1), BK-Large T, and Adv-Hexon and Penton (Figure 4). To optimize nucleofection, we titrated the DNA concentration for each plasmid and established that a concentration of between 5 and 10 µg of DNA was optimal, confirmed in three or more seropositive donors for each plasmid. A representative example is shown in Figure 4.
Figure 4.
Generation of CTL against less immunogenic viral antigens. DCs were nucleofected with pShuttle plasmids encoding Adv-Penton Adv-Hexon, BK-Large T, and EBV-LMP2-I-BZLF1. DNA for nucleofection was titrated from 2 to 20 µg DNA and the specificity of the resultant lines was compared to a positive control using DCs pulsed with a pepmix spanning the relevant antigen(s), with IFN-γ ELIspot as readout. Results are expressed as SFC/2 × 105 input cells. Control was IFN-γ release in response to stimulation with irrelevant pepmix. Representative examples from one donor are shown; results were confirmed in at least three donors. DCs, dendritic cells; ELIspot, enzyme-linked immunospot; IFN-γ, interferon-γ; SFC, spot-forming cells.
Generation of multivirus-specific CTL lines
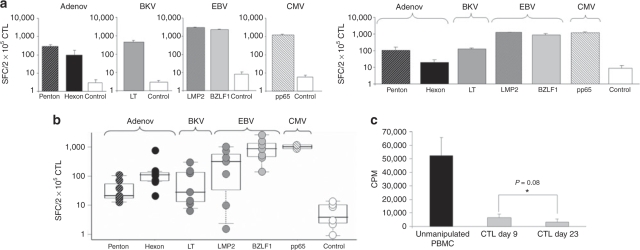
After successfully generating CTL with single virus specificity (Figures 2–4), we prepared a single culture that would contain multivirus-specific CTLs recognizing all four viruses (CMV, EBV, BK, and Adv). We nucleofected DCs separately with the five different plasmids (pShuttleGFPpp65, pShuttle-Hexon, pShuttle-Penton, pShuttle-Large T, and pShuttle LMP2-IRES-BZLF1), and then pooled the DCs and stimulated a single culture of autologous PBMC from seropositive donors at a responder:stimulator ratio of either 10:1 or 20:1 (PBMC:DC). Single virus-specific CTL generated from the same donors served as a positive control and specificity was assessed by IFN-γ ELIspot 9 days after stimulation using overlapping pepmixes. Figure 5a shows reactivity against all six stimulating antigens from all four viruses (pp65, Hexon, Penton, Large T, LMP2, and BZLF1). Importantly, the magnitude of the response generated in the single virus setting was not significantly different in the multivirus setting (n = 3; data not shown). Figure 5b shows that we can consistently make multivirus-specific CTLs from seropositive individuals (n = 7). The multivirus CTLs were 52.5 ± 14% CD3+ CD4+ and 41 ± 8% CD3+ CD8+ with a minor CD56+ population of 1.2 ± 0.2% (n = 4).
Figure 5.
Generation of multivirus-specific CTL. (a) IFN-γ ELIspot results from a representative donor comparing the specificity of single (left panel) versus multivirus (right panel) CTL lines reactive against Penton, Hexon, Large T, LMP2, BZLF1, and pp65 generated by T cell reactivation using nucleofected DCs as APCs. Results are expressed as SFC/2 × 105 input cells. Control was IFN-γ release in response to stimulation with irrelevant pepmix. (b) The specificity of multivirus CTL generated from seven donors, who were Adv, BK, and EBV seropositive. Two of seven were CMV seropositive. (c) These CTL should be safe for infusion because in a standard mixed lymphocyte reaction where alloreactive potential is measured by 3H incorporation following incubation with autologous or third party PBMC, the day 9 multivirus CTL proliferated minimally, similar to that of day 23 CTL. The presented results are the mean ± SD of five donors expressed as counts per minute (cpm) after thymidine uptake. Adv, adenovirus; CTL, cytotoxic T-lymphocyte; CMV, cytomegalovirus; ELIspot, enzyme-linked immunospot; EBV, Epstein–Barr virus; IFN-γ, interferon-γ; PBMC, peripheral blood mononuclear cell; SFC, spot-forming cells.
Safety of multivirus-specific CTL after a single stimulation
A single in vitro stimulation could produce 1.3-fold expansion of total cell numbers, and because tiny CTL numbers have proved sufficient for the reconstitution of antiviral immunity and to control active disease,15,18 it might be possible to infuse T cells after a single stimulation provided no alloreactive T cells with the potential to cause graft-versus-host disease remain in the cultures. Therefore we tested the alloreactive potential by incubating the CTLs with irradiated autologous or allogeneic PBMCs at a 1:1 ratio for 5 days. PBMCs incubated with irradiated allogeneic PBMCs served as a positive control, and CTLs from the same donor produced using three in vitro stimulations (day 23 CTL) served as the negative control. As shown in Figure 5c, proliferation of multivirus CTL obtained at day 9 of culture was not significantly different from that of the CTLs obtained from the more prolonged and restimulated cultures on day 23 (P = 0.08), whereas both were significantly less than the positive control (day 9 P = 0.00002, day 23 P = 0.00001). These results indicate that multivirus-specific CTL, generated after a single in vitro stimulation, should be safe for infusion from day 9 of culture.
Rapid protocol for generation of multivirus-specific CTLs
To determine whether our plasmid nucleofection strategy would also be effective for the most rapid antigen-specific T-cell production assay, we used the IFN-γ capture system to select T cells immediately after stimulation. We stimulated PBMCs from CMV, EBV, BK, and Adv seropositive donors with DCs nucleofected with all plasmids; 18–24 hours poststimulation, we assessed whether antigen-specific activated T cells could be rapidly selected using the IFN-γ capture system. Because of the small number of cells obtained by this ultrarapid process (3 × 104 CTL from a starting blood volume of 60 ml) we subsequently expanded the cells in vitro to allow detailed phenotypic and functional assessment and validation of the selected product.24 In IFN-γ ELIspot assays we detected CTLs reactive against all the stimulating antigens (Figure 6a). These cells were cytolytic, and at an E:T ratio of 40:1 we observed specific lysis of autologous phytohemagglutinin (PHA) blasts pulsed with pepmixes spanning pp65 pepmix (74%), LMP2 and BZLF1 (40%), and Large T (8%) with no recognition of allogeneic targets (Figure 6b). Although we were unable to detect specific lysis of Adv penton-pulsed PHA blasts, we detected specific reactivity by IFN-γ ELIspot as shown in Figure 6a, indicating that the reactive cells were either too low in frequency to lyse target cells or not cytolytic in our 4-hour assays.24 Importantly, these CTLs also specifically killed whole EBV- and pp65 antigen-expressing targets in a 4-hour chromium release assay (data not shown).
Figure 6.
Selection of multivirus CTL using the IFN-γ capture assay isolates both central and effector memory T cells. (a) Representative example of the specificity of multivirus CTLs that were selected by IFN-γ capture, and subsequently expanded in vitro to allow functional characterization. Specificity was measured by IFN-γ release in response to relevant and irrelevant pepmixes and results are expressed as SFC/2 × 105 input cells. (b) The selected and expanded cells are functional as measured by Cr51 release assay. Autologous PHA blasts, either alone or pulsed with the relevant pepmixes, were used as targets. Alloreactivity was assessed using allogeneic PHA blasts as a target. (c) shows, by flow cytometric analysis, that both central (CD62L+, CD45RO+) and effector memory (CD62L−, CD45RO+) T cells produce IFN-γ after stimulation with nucleofected DCs. Prior to stimulation PBMCs were selected or depleted for 62L using MACS bead separation. The purity of the selected/depleted fraction was >95% based on flow cytometric analysis. PBMCs were gated on CD3+ and CD45RO+. Selected/depleted CD62L fractions were stimulated with pShuttleGFPpp65 nucleofected DCs and IFN-γ production was evaluated by intracellular IFN-γ staining 18 hours after stimulation. Cells were gated on CD3+ and CD45RO+. Both CD8+ and CD8-(CD4+) T cells derived from central and effector memory T-cell subsets produced IFN-γ. CTL, cytotoxic T-lymphocyte; CMV, cytomegalovirus; IFN-γ, interferon-γ; PHA, phytohemagglutinin.
Rapid selection of multivirus-specific CTL with central memory phenotype
Proliferation and long-term T-cell persistence of infused T cells has been associated with infusion of cells with a central memory (CD62L+) rather than an effector memory phenotype (CD62L−) and some reports have suggested that only effector cells produce IFN-γ.25 Therefore, to determine whether the IFN-γ capture assay selected activated cells derived from both of these T-cell subsets, we selected CD62L+ and CD62L− T cells from peripheral blood using magnetic-activated cell sorter (MACS) beads. Figure 6c shows that the purity of CD3/CD45RO+ gated CD62L+ or CD62L− fractions (central and effector memory T cells, respectively) was >95%. We subsequently stimulated both fractions with DCs nucleofected with either pShuttleGFPpp65 (Figure 6c) or the combined plasmids (data not shown) and assessed IFN-γ secretion from the CD45RO+ T-cell fraction 24 hours poststimulation. Both CD8+ and CD8− (CD4+) central memory and effector memory populations were capable of specific cytokine secretion (Figure 6c), demonstrating that the IFN-γ capture system selects T cells with immediate effector function as well as those that should persist long-term in vivo.
Discussion
We have shown that DNA plasmid nucleofection can be used in a good manufacturing practice compliant manner to modify APCs and allow generation of CTL specific for four viruses that commonly cause disease in the immunocompromised host. We found that dendritic cells (DCs) nucleofected with a plasmid encoding the immunodominant pp65 antigen of CMV consistently reactivated pp65-specific T cells from CMV-seropositive donors to produce CTL lines with higher antigen-specific T-cell frequencies than lines generated using a standard protocol that employed an adenoviral vector expressing the same transgene.19 These CTL lines were functional as evaluated by IFN-γ ELIspot, and cytolytic when incubated with pp65 pepmix-pulsed and pp65 antigen-expressing target cells. Further, this system was robust and could be extended to other immunodominant and subdominant viral antigens derived from EBV, Adv, and BK virus to rapidly and reproducibly generate individual CTL lines with single or multiple viral specificities.
Viral reactivation is frequently seen in the immunocompromised host. After HSCT, for example, viral infections and reactivations are most common in the first 30 days but occur at a higher rate for ≥6 months.1,2,26,27,28,29,30 We have successfully used adoptive transfer of single or multivirus-specific CTL lines to prevent or treat infections caused by CMV, EBV, and Adv after HSCT, using whole antigen, either in the form of EBV-LCL or viral antigen-encoding adenoviral vectors, to activate and expand specific T cells.15,31 The use of full-length antigen in this process is essential for a number of reasons. First, the introduction of whole antigen that is physiologically processed in vivo by the APC antigen-processing machinery produces high-affinity CTL that should be effective in vivo. Second, the resulting T cells should recognize multiple epitopes, thus minimizing the likelihood of virus escape due to emergence of epitope loss variants. Third, the combination of both CD4 and CD8 epitopes usually contained in viral proteins can activate both CD4+ and CD8+ T cells, increasing the probability that both types of effector cells will be generated, and favoring the subsequent sustained expansion of transferred cells.15,21 Although this CTL generation system is well established and effective, the process is time consuming and labor intensive, requiring 8–12 weeks; 4–6 weeks for EBV-LCL generation; and 4–6 weeks for CTL expansion to produce sufficient cells for infusion and to reduce alloreactive T cells, which have the capacity to induce graft-versus-host disease in vivo. This limits the applicability and scalability of the approach and increases its costs.
In this study, we investigated whether nucleofection, using full-length antigen-encoding DNA plasmids, could be a nonviral alternative to APC modification, allowing the rapid generation of multivirus-specific CTLs with equivalent activity to “traditional” manufacturing approaches. Nucleofection delivers DNA directly into the nucleus of APCs resulting in high levels of transgene expression with minimal target cell toxicity.32,33 Further, plasmids have a proven track record of safety in vivo and have been used in both viral and tumor vaccination trials.34,35,36,37 On a practical level, clinical grade DNA can be rapidly and cost-effectively produced in large quantities and is stable for long-term storage.38
Our plasmids encoded viral antigens derived from CMV (pp65), Adv (Hexon and Penton), BK (Large T), and EBV (LMP2 and BZLF1), and we based this choice on the encouraging clinical results of our own and other groups showing that T cells directed against Adv-Hexon and Penton, and CMV-pp65 are effective and protective in vivo.14,15,18,39 In addition, BK-Large T-specific T cells have shown efficacy in vivo in the solid organ transplant setting.40 The antigens of choice for reactivation of EBV-specific T cells were less obvious because it is unclear which of the latent and/or lytic antigens are responsible for activating protective immunity. EBV-LCL express all latent and lytic EBV antigens and can simultaneously activate effector cells with wide varying precursor frequencies and affinities for different HLA molecules.7,41,42,43 In an effort to recapitulate this with the plasmid-based system, we used a construct encoding the EBV latent protein, LMP2, linked to the immunodominant lytic antigen BZLF1 via an internal ribosomal entry segment (IRES) sequence in order to stimulate T cells reactive against both antigens. We chose this antigen combination because both are broadly immunogenic across multiple HLA types and stimulate both CD4+ and CD8+ T cells in vitro.20,22,44,45 Further LMP2 is expressed in a majority of EBV+ tumors and also in healthy peripheral blood B cells.46,47 We were able to prevent antigenic competition for HLA molecules in APCs and thus ensure adequate stimulation of all six antigens by dividing the DCs and nucleofecting them separately with the five plasmids.21 We then recombined the APCs prior to coculture with T cells, ensuring that CTLs with multiple viral specificities were present in a single infusion.
By utilizing nucleofected DCs instead of LCLs as APC, we were able to decrease the APC generation time from 4–6 weeks to 5–7 days and reduce technician time accordingly. After PBMC stimulation, we assessed the specificity of the generated CTL lines by pentamer and IFN-γ ELIspot and found that the CTLs were significantly enriched for specific T cells after a single in vitro stimulation. However, although subsequent stimulations expanded the total number of antigen-specific T cells, it did not significantly increase the frequency of specific cells. Thus, maximum antigen-specific enrichment occurred within the first 9 days of culture (>200-fold enrichment), and corresponded to a reduction in alloreactive T cells to a level comparable to lines that had received at least three in vitro stimulations. Therefore, the CTLs generated in this way are ready for administration in 9–12 days rather than 4–6 weeks. To further shorten the process in case of urgent medical need, we evaluated whether antigen-specific multivirus CTL could be specifically selected after activation using an IFN-γ capture assay.17,48 We were able to select antigen-specific cells that were derived from both effector and central memory T-cell subsets. As a consequence, these cells should provide immediate effector function while retaining the capacity to persist long-term in vivo.25
Although we can prepare multivirus-specific CTLs rapidly by nucleofection, the direct isolation of tetramer-binding T cells or the selection of IFN-γ expressing T cells following stimulation with either recombinant protein or peptide stimulation is even more rapid. Both methods have been used clinically and have confirmed that small numbers of antigen-activated T cells can expand substantially in vivo in the presence of antigen, and protect against the targeted pathogen.16,18,48 However, there are associated complications; multimer selection is restricted to CD8+ T cells with known epitope peptide specificities and to viruses, such as CMV, with a high frequency of circulating reactive T cells.16 In contrast, the IFN-γ-capture assay selects specific T cells in an HLA unrestricted manner, but the frequency of circulating antigen-reactive cells and the stimulatory capacity of the immunogen limits the effectiveness of this approach.17,18,48 Thus, peptides, which are suboptimal stimulators, do not efficiently activate all specific cells, whereas recombinant protein is preferentially processed by the endosomal/lysosomal pathway, due to the route of antigen uptake, and thus predominantly activates CD4+ T cells. Instead, our more prolonged methodology has the advantage of optimally activating both CD4+ and CD8+ T cells using whole antigen as a stimulus. The activated T cells expand poststimulation with a consequent reduction in the alloreactive T-cell population. We estimate that from a starting blood volume of 100 ml of blood, we will generate between 5 and 15 × 106 nucleofected DCs that will be sufficient to stimulate 5–15 × 107 PBMC. Because, on average, we see a 1.3-fold increase in total cell numbers in a 9-day culture period, we produce sufficient cells to (i) perform safety and sterility testing, (ii) analyze the phenotype, function, and specificity, and (iii) freeze multiple doses of clinical grade CTL for repeat administration, should the need arise.
In summary, we have successfully produced CTL with simultaneous specificity for four viruses that are clinically relevant for the immunocompromised host. The approach we describe can be made good manufacturing practice compliant and should be extendable to additional pathogens. Because it is cheaper and faster than previously described methodology for such multispecific CTL, we hope it will contribute to a broadening of the application of these cells to viral disease.
Materials and Methods
Donors and cell lines. PBMCs from healthy EBV, CMV, Adv, and BK-seropositive volunteers were obtained with informed consent on a protocol approved by the Baylor College of Medicine institutional review board. PBMCs were used to generate DCs, CTL lines, and EBV-LCL and as allogeneic feeder cells. EBV-LCLs were generated from PBMC (5 × 106) using concentrated supernatants of the EBV B95-8 cell line in the presence of cyclosporin A (Sandoz, Holzkirchen, Germany). EBV-LCLs were maintained in RPMI 1640 (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS)(Hyclone) and 2-mmol/l L-glutamine (GlutaMAX-I; Invitrogen, Carlsbad, CA).
Monocyte isolation and DC generation. DCs were generated as previously described.15 Briefly, monocytes were isolated from PBMC by CD14 selection using MACS Beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in DC media (CellGenix plus 2 mmol/l L-glutamine) (CellGenix USA, Antioch, IL; GlutaMAX; Invitrogen) with 800 U/ml granulocyte macrophage-colony stimulating factor (Sargramostim Leukine; Immunex, Seattle, WA) and 1,000 U/ml IL-4 (R&D Systems, Minneapolis, MN) for 5 days. IL-4 and granulocyte macrophage-colony stimulating factor were replenished on day 3. On day 5, DCs were matured in DC media using a cytokine cocktail containing 10 ng/ml IL-1β, 10 ng/ml tumor necrosis factor-α (R&D Systems), 1 µg/ml prostaglandin E2 (Sigma, St Louis, MO), 800 U/ml granulocyte macrophage-colony stimulating factor and 1,000 U/ml IL-4.
Vectors. Ad5f35GFPpp65, as previously described,19 was purchased from the Vector Development Laboratory of the Center for Cell and Gene Therapy at Baylor College of Medicine, Houston, TX. The titer of the Ad5f35GFPpp65 vector was 5 × 1012 virus particles/ml or 5 × 1010 plaque-forming units/ml. All multiplicities of infection are based on virus particles.
Plasmids. The pShuttle DNA plasmid was used as a backbone for insertion of DNA sequences of the different viral antigens: Penton, Hexon, and Large T. The pShuttleGFPpp65 and the pShuttleLMP2-I-BZLF1 plasmids were generated as previously described.19,24 The sequences of Hexon and Penton were amplified by PCR from MSCV-Hexon-IRES-GFP and MSCV-Penton-IRES-GFP plasmids49 using primer sequences (5′-3′) Penton-Fw: TTGCATGCCAACCATGCGGCGCGCGG; Penton-Rv: AA TGAATTCGCTTGCTCAAAAAGTGCGGCTCG; Hexon-Fw: ATTGCA TGCACCATGGCTACCCCTTCGATGATGCC; Hexon Rv: AATGAATT CTTGCTTCTTTATGTTGTGGCGTTGCC, and after digestion with the restriction sites Sph1 and EcoR1 amplified PCR products were cloned into the multiple cloning sites of the pShuttle DNA plasmid backbone. The pShuttle-BKV-LargeT plasmid was a gift by Dr C. Ramos, Center for Cell and Gene Therapy at Baylor College of Medicine, Houston, TX. All plasmids were amplified in SCS110 competent cells (Stratagene, La Jolla, CA) and purified with the Endo Free Plasmid Maxi Kit (Qiagene, Hilden, Germany).
Genetic modification of DCs
Transduction: On day 5 immature DCs were harvested, pelleted, and transduced with Ad5f35GFPpp65 vector (multiplicity of infection of 500) in a volume of 100 µl of DC media at 37 °C. After 90–120 minutes of incubation, the DCs were resuspended in DC media containing the maturation cytokine cocktail described above and plated at a concentration of 1 × 106/ml in a 24-well plate. Transduced DCs were matured for 2 days.
Nucleofection: DCs were nucleofected 24 hours after maturation using the Amaxa DC nucleofection kit (Amaxa, Koeln, Germany). 0.5–1 × 106 DCs were prepared as per manufacturers' instructions and nucleofected with 2–20 µg plasmid DNA using the Amaxa program U2. After nucleofection DCs were resuspended in complete RPMI Media (10% FBS, 2 mmol/l GlutaMAX, and 1 mmol/l sodium pyruvate (Sigma)) supplemented with the cytokine maturation cocktail, and were matured for a further 12–18 hours before using in CTL stimulation protocols.
CTL generation. CD14 negative PBMCs were used as responder cells and stimulated with nucleofected or adenovector-transduced DCs at a S:R ratio of 1:20. Cells were cultured in CTL Media (RPMI 1640 supplemented with 45% Click's medium (Irvine Scientific, Santa Ana, CA), 2 mmol/l GlutaMAX TM-I, and 5% human AB serum) supplemented with 1,000 U/ml IL-4 at a concentration of 1 × 106/ml that has been shown to prevent activation-induced cell death in vitro.50 On day 9 or 10 CTLs were harvested, counted to assess viability using Trypan blue exclusion, and used for phenotypic and functional studies. To further expand the cells, CTLs were restimulated weekly at a S:R ratio of 1:4 with irradiated (40 Gy) Ad5f35GFPpp65-transduced autologous LCL, and cultures were supplemented with IL-2 (Proleukin; Chiron, Emeryville, CA) 50 U/ml twice weekly from day 12.
IFN-γ selection and expansion of stimulated T cells. After overnight stimulation with nucleofected DCs, IFN-γ secreting cells were selected using the IFN-γ secretion assay, cell enrichment, and detection kit (Miltenyi Biotec) as described previously.24 In brief, stimulated cells were harvested, resuspended in 106 cells/ml CTL media, and incubated with an anti-IFN-γ monoclonal antibody conjugated to CD45 antibody (Miltenyi Biotec) at 37 °C for 45 minutes to enable IFN-γ capture. Next, cells were labeled with anti-IFN-γ magnetic microbeads (Miltenyi Biotec), incubated for 15 minutes at 4 °C, and IFN-γ-positive secreting cells were selected using Miltenyi Mini-MACS column. To characterize the function and specificity of the IFN-γ selected T cells, we expanded them in culture for 2 weeks. Selected cells (2 × 104 per well) were placed in 24-well plates in the presence of 30 Gy-irradiated autologous PBMCs as feeders using a ratio of 1:100. Cells were cultured in CTL medium containing 10 ng/ml of IL-15 (R&D Systems). We supplemented the cultures with fresh medium and 10 ng/ml IL-15 on days 4, 7, and 10. On day 7, we added 30 Gy-irradiated autologous PBMCs at a ratio of 1:10.
Statistical analysis. All in vitro data were summarized by mean ± SD or SE, or median with range, and graphically presented by the box-plots. Analysis of variance and Student's t-test were used to analyze the ELISpot data after appropriate log transformation. Statistical significance was declared when P value <0.05.
Flow cytometry
Immunophenotyping: We surface-stained T cells with monoclonal antibodies to: CD3, CD4, CD8, CD14, CD16, CD45RO, CD56, CD62L and CD19 (Becton Dickinson, Franklin Lakes, NJ) and the generated DCs with monoclonal antibodies to CD80, CD83, HLA-DR, and CD86 (Becton Dickinson). Cells were washed once with phosphate-buffered saline (Sigma) containing 2% FBS, pelleted, and antibodies added in saturating amounts (5 µl). After 15 minutes of incubation at 4 °C in the dark, the cells were washed twice and analyzed. Approximately 10,000 live cells from each population were analyzed. Samples were acquired on a FACSCalibur flow cytometer and the data analyzed using Cell Quest software (Becton Dickinson).
Pentamer staining: We used soluble HLA-A*0201-NLVPMVATV (NLV) and HLA-B*0702 TPRVTGGGAM (TPR) unlabeled pentamers to detect T cells recognizing epitopes from CMV-pp65 antigen. Pentamers were prepared by Proimmune (Oxford, UK). For pentamer staining, 106 CTLs were stained with unlabeled pentamer, followed by Pro5 Flurotag (APC conjugated) (Proimmune) according to the manufacturer's instructions. These were costained with anti-CD8 fluorescein isothiocyanate, anti-CD3 PerCP, and CD4 PE antibodies for 30 minutes at 4 °C in the dark. The cells were washed twice and analyzed immediately with a FACSCalibur equipped with Cell Quest software (Becton Dickinson).
Intracellular IFN-γ staining: Effector and central memory T cells were isolated from PBMC by selection using CD62L microbeads and the MACS selection system (Miltenyi Biotec). Twenty-four hours after stimulation with nucleofected DCs T cells were harvested, washed once with phosphate-buffered saline (Sigma) containing 2% FBS, pelleted, and surface stained with CD8, CD62L, and CD45RO antibodies (Becton Dickinson) (5 µl/antibody/tube). After 15 minutes of incubation at 4 °C in the dark, the cells were washed twice, pelleted, fixed, and permeabilized with Cytofix/Cytoperm solution (BD Biosciences, Franklin Lakes, NJ) for 20 minutes at 4 °C in the dark. After washing twice with phosphate-buffered saline/2% FBS containing 0.1% saponin cells were incubated with 20 µl Pe–anti-IFN-γ antibody (BD, Biosciences) for 30 minutes at 4 °C in the dark. Cells were then washed twice with cold phosphate-buffered saline/2% FBS containing 0.1% saponin and at least 200,000 live cells from each population were analyzed with a FACSCalibur equipped with Cell Quest software (Becton Dickinson).
ELIspot assay: We used ELISpot analysis to quantify mono- and multivirus-specific T cells. The populations were serially diluted from 2 × 105 to 2.5 × 104 cells/well, and we measured the viral-specific activity of responder cells after direct stimulation with pepmixes spanning pp65 (CMV), LMP2 and BZLF1 (EBV), Hexon and Penton (Adv), and Large T (BK). All pepmixes, which are overlapping peptide libraries (15 mers overlapping by 11 amino acids) were purchased from JPT Technologies (Berlin, Germany). Each culture condition was run in triplicate. After 20 hours of incubation, plates were developed as previously described,24 dried overnight at room temperature in the dark, then sent to ZellNet Consulting, New York, NY for quantification. SFC and input cell numbers were plotted, and a linear regression calculated after excluding plateau data points. The frequency of T cells specific to each antigen was expressed as specific SFC per input cell numbers.
Proliferation assay
Alloreactivity: As previously described, we used CTLs or autologous PBMCs as responder cells and cultured them with autologous or allogeneic 30 Gy-irradiated PBMCs at a stimulator:responder (S:R) ratio of 1:1 for 5 days in 200-µl CTL medium in triplicate in a U-bottom 96-well plate (Nunc, Rochester, NY) at 37 °C and 5% CO2.24
Specificity: To test the specificity of CTL, we used mono- and multivirus-specific CTL as responder cells and cultured them with autologous and allogeneic LCL, which were pulsed with overlapping peptide libraries of the cognate viral antigens or transduced with the Ad5f35GFPpp65 vector. Cell proliferation was analyzed by 3H-thymidine incorporation. Cultures were pulsed with 0.037 MBq (1 mCi) 3H-thymidine/well (Amersham Biosciences, Piscataway, NJ) and 16-hours later, harvested onto glass fiber strips using a Brandel PHD cell harvester (Brandel, Gathersburg, MD). 3H-thymidine uptake was measured using a Matrix B liquid scintillation counter (Canberra Packard, Meriden, CT).
Cytotoxicity assay. We measured the cytotoxic specificity of each T-cell population in a standard 4-hour chromium (Cr51) release assay, using E:T ratios of 40:1, 20:1, 10:1, and 5:1. Generated CTLs were used as effectors. The targets were autologous LCLs or PHA blasts. LCLs were either nontransduced, transduced with Ad5f35GFPpp65 at an multiplicity of infection of 500 virus particles/cell, or pulsed with pp65 pepmix or an irrelevant pepmix as control. PHA blasts were pulsed with pepmixes spanning pp65, LMP2, BZLF1, Penton or Large T Ag. Allogeneic LCL or PHA blasts alone were used as controls. The target cells were labeled simultaneously for 1 hour with Cr51. The percentage of specific lysis was calculated as specific lysis [(experimental release-spontaneous release)/(maximum release-spontaneous release)] × 100.
SUPPLEMENTARY MATERIALFigure S1. CMV-pp65 T-cell reactivity. CMV pp65-specific CD4- (CD8+) and CD4+ T cell reactivity detected in 1 representative CTL line. Specificity was measured by IFN-γ release in response to pp65 pepmix and results are expressed as SFC/2x105 input cells. Control was IFN-γ release in response to stimulation with irrelevant pepmix.
Supplementary Material
CMV-pp65 T-cell reactivity. CMV pp65-specific CD4- (CD8+) and CD4+ T cell reactivity detected in 1 representative CTL line. Specificity was measured by IFN-γ release in response to pp65 pepmix and results are expressed as SFC/2x105 input cells. Control was IFN-γ release in response to stimulation with irrelevant pepmix.
Acknowledgments
This work was supported by NIH grants U54 HL081007, P01 CA094237, and a Baylor College of Medicine Center for Infection and Immunity Research award. A.M.L. was supported by the National Marrow Donor Program through funding from The Marrow Foundation. U.G. was funded by the Heinrich-Hertz Foundation, NRW, Germany.
REFERENCES
- Kim YJ, Boeckh M., and , Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28:222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- Myers GD, Bollard CM, Wu MF, Weiss H, Rooney CM, Heslop HE, et al. Reconstitution of adenovirus-specific cell-mediated immunity in pediatric patients after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:677–686. doi: 10.1038/sj.bmt.1705645. [DOI] [PubMed] [Google Scholar]
- Kadakia MP, Rybka WB, Stewart JA, Patton JL, Stamey FR, Elsawy M, et al. Human herpesvirus 6: infection and disease following autologous and allogeneic bone marrow transplantation. Blood. 1996;87:5341–5354. [PubMed] [Google Scholar]
- Comoli P, Binggeli S, Ginevri F., and , Hirsch HH. Polyomavirus-associated nephropathy: update on BK virus-specific immunity. Transpl Infect Dis. 2006;8:86–94. doi: 10.1111/j.1399-3062.2006.00167.x. [DOI] [PubMed] [Google Scholar]
- de Pagter PJ, Schuurman R, Meijer E, van Baarle D, Sanders EA., and , Boelens JJ. Human herpesvirus type 6 reactivation after haematopoietic stem cell transplantation. J Clin Virol. 2008;43:361–366. doi: 10.1016/j.jcv.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, et al. Long-term restoration of immunity against Epstein–Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- Comoli P, Basso S, Zecca M, Pagliara D, Baldanti F, Bernardo ME, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant. 2007;7:1648–1655. doi: 10.1111/j.1600-6143.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME., and , Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- Einsele H, Rauser G, Grigoleit U, Hebart H, Sinzger C, Riegler S, et al. Induction of CMV-specific T-cell lines using Ag-presenting cells pulsed with CMV protein or peptide. Cytotherapy. 2002;4:49–54. doi: 10.1080/146532402317251527. [DOI] [PubMed] [Google Scholar]
- Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Löffler J, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112:3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- Micklethwaite K, Hansen A, Foster A, Snape E, Antonenas V, Sartor M, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:707–714. doi: 10.1016/j.bbmt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuchtinger T, Lang P, Hamprecht K, Schumm M, Greil J, Jahn G, et al. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp Hematol. 2004;32:282–289. doi: 10.1016/j.exphem.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- Sili U, Huls MH, Davis AR, Gottschalk S, Brenner MK, Heslop HE, et al. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic T-lymphocyte lines using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003;26:241–256. doi: 10.1097/00002371-200305000-00008. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Leen AM, Buza EL, Taylor G, Huls MH, Heslop HE, et al. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. J Immunol. 2005;175:4137–4147. doi: 10.4049/jimmunol.175.6.4137. [DOI] [PubMed] [Google Scholar]
- Leen AM, Christin A, Khalil M, Weiss H, Gee AP, Brenner MK, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82:546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop AD, Taylor GS, Sauce D., and , Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein–Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- Comoli P, Hirsch HH., and , Ginevri F. Cellular immune responses to BK virus. Curr Opin Organ Transplant. 2008;13:569–574. doi: 10.1097/MOT.0b013e3283186b93. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Leen AM, Sun J, Nakazawa Y, Yvon E, Heslop HE, et al. Exploiting cytokine secretion to rapidly produce multivirus-specific T cells for adoptive immunotherapy. J Immunother. 2008;31:665–674. doi: 10.1097/CJI.0b013e318181b4bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C., and , Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y, et al. Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen. Biol Blood Marrow Transplant. 2008;14:1245–1252. doi: 10.1016/j.bbmt.2008.08.010. [DOI] [PubMed] [Google Scholar]
- de Pagter PJ, Schuurman R, Visscher H, de Vos M, Bierings M, van Loon AM, et al. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biol Blood Marrow Transplant. 2008;14:831–839. doi: 10.1016/j.bbmt.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Boeckh M, Erard V, Zerr D., and , Englund J. Emerging viral infections after hematopoietic cell transplantation. Pediatr Transplant. 2005;9 Suppl 7:48–54. doi: 10.1111/j.1399-3046.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- Peck AJ, Englund JA, Kuypers J, Guthrie KA, Corey L, Morrow R, et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110:1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison MG.Respiratory viral infections in transplant recipients Antivir Ther (Lond) 200712627–638.4 Pt B [PubMed] [Google Scholar]
- Leen A, Ratnayake M, Foster A, Heym K, Ahmed N, Rooney CM, et al. Contact-activated monocytes: efficient antigen presenting cells for the stimulation of antigen-specific T cells. J Immunother. 2007;30:96–107. doi: 10.1097/01.cji.0000211325.30525.84. [DOI] [PubMed] [Google Scholar]
- Landi A, Babiuk LA., and , van Drunen Littel-van den Hurk S. High transfection efficiency, gene expression, and viability of monocyte-derived human dendritic cells after nonviral gene transfer. J Leukoc Biol. 2007;82:849–860. doi: 10.1189/jlb.0906561. [DOI] [PubMed] [Google Scholar]
- Lenz P, Bacot SM, Frazier-Jessen MR., and , Feldman GM. Nucleoporation of dendritic cells: efficient gene transfer by electroporation into human monocyte-derived dendritic cells. FEBS Lett. 2003;538:149–154. doi: 10.1016/s0014-5793(03)00169-8. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Boyer JD, Ugen KE, Tebas P, Higgins TJ, Baine Y, et al. Plasmid vaccination of stable HIV-positive subjects on antiviral treatment results in enhanced CD8 T-cell immunity and increased control of viral “blips”. Vaccine. 2005;23:2066–2073. doi: 10.1016/j.vaccine.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Cattamanchi A, Posavad CM, Wald A, Baine Y, Moses J, Higgins TJ, et al. Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clin Vaccine Immunol. 2008;15:1638–1643. doi: 10.1128/CVI.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J, Boswell W, Smith J, Hersh E, Snively J, Diaz M, et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- Kutzler MA., and , Weiner DB. DNA vaccines: ready for prime time. Nat Rev Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuchtinger T, Richard C, Joachim S, Scheible MH, Schumm M, Hamprecht K, et al. Clinical grade generation of hexon-specific T cells for adoptive T-cell transfer as a treatment of adenovirus infection after allogeneic stem cell transplantation. J Immunother. 2008;31:199–206. doi: 10.1097/CJI.0b013e31815ef862. [DOI] [PubMed] [Google Scholar]
- Binggeli S, Egli A, Schaub S, Binet I, Mayr M., and , Steiger J. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;5:1131–1139. doi: 10.1111/j.1600-6143.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, et al. Long-term restoration of immunity against Epstein–Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein–Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG., and , Rickinson AB. Immediate early and early lytic cycle proteins are frequent targets of the Epstein–Barr virus-induced cytotoxic T cell response. J Exp Med. 1997;185:1605–1617. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh TA, Lin X, Jia H, Hui EP, Chan AT, Rickinson AB, et al. EBV latent membrane proteins (LMPs) 1 and 2 as immunotherapeutic targets: LMP-specific CD4+ cytotoxic T cell recognition of EBV-transformed B cell lines. J Immunol. 2008;180:1643–1654. doi: 10.4049/jimmunol.180.3.1643. [DOI] [PubMed] [Google Scholar]
- Qu L, Green M, Webber S, Reyes J, Ellis D., and , Rowe D. Epstein–Barr virus gene expression in the peripheral blood of transplant recipients with persistent circulating virus loads. J Infect Dis. 2000;182:1013–1021. doi: 10.1086/315828. [DOI] [PubMed] [Google Scholar]
- Carbone A, Gloghini A., and , Dotti G. EBV-associated lymphoproliferative disorders: classification and treatment. Oncologist. 2008;13:577–585. doi: 10.1634/theoncologist.2008-0036. [DOI] [PubMed] [Google Scholar]
- Mackinnon S, Thomson K, Verfuerth S, Peggs K., and , Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis. 2008;40:63–67. doi: 10.1016/j.bcmd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Leen AM, Sili U, Vanin EF, Jewell AM, Xie W, Vignali D, et al. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104:2432–2440. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- Vella AT, Dow S, Potter TA, Kappler J., and , Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CMV-pp65 T-cell reactivity. CMV pp65-specific CD4- (CD8+) and CD4+ T cell reactivity detected in 1 representative CTL line. Specificity was measured by IFN-γ release in response to pp65 pepmix and results are expressed as SFC/2x105 input cells. Control was IFN-γ release in response to stimulation with irrelevant pepmix.