Abstract
Transient genetic manipulation of human neurons without chromosomal integration of the transgene would be valuable but has been challenging due to the quiescent nature of these postmitotic cells. In this study, we developed a set of baculoviral vectors for transient transduction in nondividing neurons derived from human embryonic stem cells (hESCs). Using a baculoviral vector equipped with the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), we observed a quick onset of transgene expression as early as day 1 after baculoviral transduction and a high efficiency of up to 80%. Strong transgene expression in the cultured human neurons was observed for more than 1 month and the signal was easily detectable even after 3 months. Using two baculoviral vectors carrying different transgenes, we found that co-transduction at a single neuron level was possible. After transplantation into the brain of nude mice, the baculovirus-transduced human neurons were integrated into the mouse brain and maintained transgene expression for at least 4 weeks, portending the usefulness of this technique in assisting neural transplantation. Therefore, by mediating efficient transient gene expression, baculoviral vectors can provide useful tools for both basic gene function studies in human neurons and therapeutic applications of these cells.
Introduction
Self-renewable and pluripotent human embryonic stem cells (hESCs) are a reliable and accessible source to provide unlimited amounts of human neurons that would otherwise be very difficult to obtain for basic studies and clinical applications.1 Transplantation of these human neurons in the diseased or injured central nervous system provides the potential for treatment of a broad spectrum of neurological diseases. At present, hurdles that limit the use of these hESC-derived cells for clinical transplantation include the poor survival and inappropriate functional recovery of grafted hESC-derived neurons.2,3 Genetic manipulation of these human neurons through gene transfer in culture prior to transplantation would be a possible way to overcome the problems, holding out the prospect of enhancing the survival, maturation, integration, and many other cellular properties of transplanted neurons in the brain.
One of the possible genetic manipulation strategies for hESC-derived neurons is integrating a transgene into the hESC genome and then deriving human neurons from the stably modified hESCs, in which the transgene expression is retained.4 This is a highly valuable approach for the purpose of using hESC-derived cells to deliver therapeutic genes for the applications that require persistent expression. However, as an approach for improving transplantation efficiency, stable expression of certain genes, for example, anti-apoptosis genes, poses the risk of tumor formation after cell transplantation.5 In addition, stable expression is not a physiological feature for other genes, like those encoding transcriptional factors, and the stable expression of these genes could be harmful to cells.6,7 In the case of using antibiotic selection to generate stably modified hESCs, the potential immune responses to the products of integrated antibiotic-resistance gene may impede the clinical use of these hESC-derived cells.8 Therefore, transient gene transfer to hESC-derived neurons is a more preferable alternative to improve transplantation efficiency. The success of this strategy depends on the availability of suitable vectors for human neurons that provide a transgene expression profile including quick onset expression, expression at a functional level, and transient expression but long enough to support neuronal transplantation. It would also be beneficial to have concomitant expression of multiple genes in view of complex processes involved in functional recovery of grafted neurons.
To develop a vector suitable for transient gene transfer into hESC-derived neurons, we tested in the current study the insect baculovirus Autographa californica multiple nucleopolyhedrovirus–based vectors. These vectors have been introduced as an effective delivery vehicle for transgene expression in a wide variety of mammalian cells.9,10 Upon transduction, the viruses exist mainly as episomes in the host cells and are considered as a nonintegrating vector. Recently, baculoviral vectors have also been applied in stem cell research, such as in transduction of human mesenchymal stem cells11 and the progenitor cells derived from them.12 In our lab, we have used baculoviral vectors for transient transduction of hESCs.4 The high transduction efficiency of the baculoviral vectors in hESCs prompts us to investigate the feasibility of using baculoviral vectors for transgene expression in hESC-derived neurons. As the first proof-of-concept study, we focused on the gene encoding enhanced green fluorescent protein (eGFP), using it as a reporter gene to evaluate four different expression cassettes in the context of baculoviral vectors.
Results
Generation of human neurons from HES-1 and HES-3
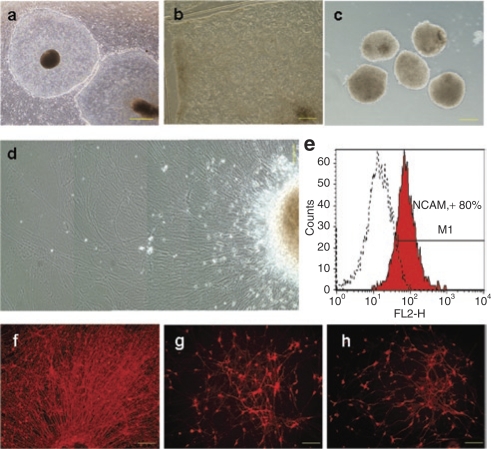
To test the baculoviral transduction of hESC-derived human neurons, two National Institutes of Health–recognized hESC lines, HES-1 (Figure 1a) and HES-3, were used to generate neurons using a well-established protocol.1 After neural differentiation was initiated with prolonged culturing of hESCs at high density, the areas containing neural progenitors were identified by their uniformly white-gray and opaque appearance under dark-field stereomicroscope. To obtain neural progenitors of high purity, these identified areas were further selected under phase-contrast microscope for their containment of rosettes-like structures (Figure 1b), before being cut into small cell clumps for expansion. The neural precursors were expanded for 6 weeks in the presence of basic fibroblast growth factor and epidermal growth factor in order to increase cell homogeneity. After that, the resulting neural spheres (Figure 1c) were dissected into small cell clumps and plated on culture dishes in medium without the growth factors for differentiation. These cell clumps attached to the coated plates rapidly and neurite outgrowth from the cell clumps was observed as early as day 1 after plating. After another 6-week culturing, differentiated cells showed typical neuronal morphology with very long extending neurites (Figure 1d). By using an antibody against NCAM, a surface marker for late stage of neuronal differentiation,13 flow cytometric analysis showed up to 80% of the cells derived from neural progenitor differentiation were NCAM positive (Figure 1e). These differentiated cells were stained positively by antibodies against neuronal markers such as βIII-tubulin (Figure 1f), MAP2ab (Figure 1g), and NF200 (Figure 1h), confirming further that large majority of the cells derived from our process were neurons. Similarly, homogenous human neurons derived from HES-3 (Figure 2f) were also generated using the same protocol.
Figure 1.
Production of homogenous human neurons from human embryonic stem cells. (a) HES-1 grown on feeder cells. (b) HES-1 overgrown on feeder cells for 4 weeks. The areas containing rosette-like structures are selected to produce neural spheres. (c) Six-week-old neural spheres. (d) Neurons derived by plating dissected neural spheres and allowing them to grow without growth factors for 6 weeks. (e) Flow cytometric analysis showing the NCAM expression in human neurons derived from HES-1. (f–h) Immunofluorescence staining showing the expression of neuronal marker (f) βIII tubulin in the periphery of a cluster of neurons and the expression of (g) MAP2ab and (h) NF200 in human neurons with complex multipolar morphology. Bar = 500 µm (a); 200 µm (b,c); 100 µm (d,f–h).
Figure 2.
Transgene expression in human neurons mediated by baculoviral vectors. (a) Phase contrast and fluorescence images of a group of live human neuron clusters transduced by baculoviral vectors carrying the expression cassette CMV.eGFP at a multiplicity of infection of 100 plaque-forming units per cell. The neurons were derived from HES-1. The pictures were taken 2 days after transduction. (b–e) Immunofluorescence staining showing the colocalization of neuronal markers (b) βIII tubulin, (c) MAP2ab, (d) NCAM, (e) NF200 with eGFP in HES-1–derived human neurons transduced by baculoviral vectors. (f) Immunofluorescence staining showing the colocalization of neuronal marker βIII tubulin and eGFP in human neurons derived from HES-3. The neurons were transduced by baculoviral vectors as described in (a). Bar = 500 µm (a); 200 µm (b,f); 100 µm (c–e). eGFP, enhanced green fluorescent protein; GFP, green fluorescent protein.
Baculoviral transduction of hESC-derived human neurons
We first tested baculoviral vector BV-CMV.eGFP carrying an eGFP gene driven by cytomegalovirus (CMV) promoter, a strong viral promoter that has been widely used for transgene expression in many cell types. In Figure 2a, live cell fluorescence image shows that most human neurons derived from HES-1 became eGFP positive 2 days after transduction at a multiplicity of infection (MOI) of 100 plaque-forming units (pfu) per cell. Intense eGFP fluorescence appeared not only in the neuronal cell bodies but also in the long extending neurites. Immunostaining shows that these baculovirus-transduced, eGFP-positive cells were positively stained by four different antibodies against neuronal markers, including βIII-tubulin (Figure 2b), MAP2ab (Figure 2c), NCAM (Figure 2d), and NF200 (Figure 2e). Baculoviral vectors were also able to efficiently transduce human neurons derived from another hESC line HES-3 (Figure 2f).
In our previous study, we found that transgene expression cassettes can have immense effect on baculoviral transduction efficiency in hESCs.4 Here, we investigated whether the use of different expression cassettes can also improve the transgene expression in hESC-derived human neurons. As shown in Figure 3, flow cytometric analysis indicated that the CMV promoter was more efficient in driving eGFP gene expression than the EF1α promoter, a commonly used cellular promoter, with 80% of cells transduced by BV-CMV.eGFP and 52% by BV-EF1α.eGFP (Figure 3). The woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) sequence originated from woodchuck hepatitis virus has been used to enhance the posttranscriptional processing of transgene mRNA and the following export of mRNA to cytoplasm from nucleus.14 WPRE might also stimulate translation per se.15 Here, we incorporated the WPRE sequence into the transgene expression cassettes of BV-CMV.eGFP and BV-EF1α.eGFP and observed an enhanced transgene expression from both vectors in human neurons. Using the WPRE sequence together with the EF1α promoter, the percentage of transduced neurons increased from 52% when using EF1α promoter only to 59% and the mean fluorescence intensity of eGFP increased from 1,082 to 1,331 (Figure 3). When compared with gene expression from the CMV promoter, the mean fluorescence intensity of eGFP from the CMV promoter plus the WPRE sequence increased by 1.6-fold from 1,475 to 2,348, although the percentage of transduced neurons remained at 80% (Figure 3). This result suggests that baculoviral vector–mediated transgene expression in hESC-derived human neurons can be greatly improved by the optimization of expression cassettes.
Figure 3.
Expression cassettes and transgene expression levels. Baculoviral vectors accommodating either the CMV or EF1α promoters, with or without the WPRE element, were tested at a multiplicity of infection of 100 plaque-forming units per cell in HES-1–derived human neurons. Flow cytometric analysis showing the percentage and mean fluorescence intensity (in parentheses) of eGFP 6 days after transduction. Representative live cell fluorescence and phase contrast images taken before flow cytometric analysis are shown below the histograms. BV, baculoviral vector; CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element. Bar = 100 µm.
The WPRE sequence prolonged transgene expression in hESC-derived human neurons
Previously, we showed that nonintegrating baculoviral vectors can mediate transient and short-term transgene expression in hESCs,4 in which the baculoviral vector–mediated transgene expression became silenced within 1 week. One possible factor that may contribute to the transgene silencing is the decrease of transgene copies in each cell with hESC proliferation. Therefore, it would be interesting to investigate the duration of transgene expression mediated by nonintegrating baculoviral vectors in differentiated, nondividing hESC-derived human neurons. Figure 4a,b shows changes of eGFP expression in hESC-derived human neurons over time. All four tested baculoviral vectors provided quick onset of transgene expression, starting as early as 1 day after transduction. The intense eGFP expression was maintained during the first week. Without the WPRE sequence, reduction in eGFP expression was obvious during the second week. With the help of the WPRE sequence, the transgene expressions from both CMV and EF1α promoters lasted for an extended period of time. Especially in the neurons transduced with BV-CMV.eGFP-WPRE, a significant number of the cells still remained eGFP positive even 3 months after transduction. Flow cytometric analysis demonstrated that 38 and 16% of hESC-derived human neurons transduced with BV-CMV.eGFP-WPRE maintained eGFP expression 6 and 12 weeks after transduction, respectively (Figure 4c). These results suggest that the incorporation of the WPRE sequence into an expression cassette is beneficial in prolonging transgene expression in hESC-derived human neurons.
Figure 4.
Expression cassettes and duration of transgene expression. Baculoviral vectors accommodating either the CMV or EF1α promoters, with or without the WPRE element, were tested at a multiplicity of infection of 100 plaque-forming units per cell in HES-1–derived human neurons. (a) Live cell fluorescence images were taken from day 3 to 91. (b) Quantitative representation of the change in eGFP intensity in live cells was measured from day 1 to 120. (c) Flow cytometric analysis showing the percentage and mean fluorescence intensity (in parentheses) of eGFP in HES-1–derived human neurons on day 42 and 84 after transduction with BV-CMV.eGFP-WPRE. AU, arbitrary units; BV, baculoviral vector; CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
Baculoviral vectors had little cytotoxicity to hESC-derived human neurons
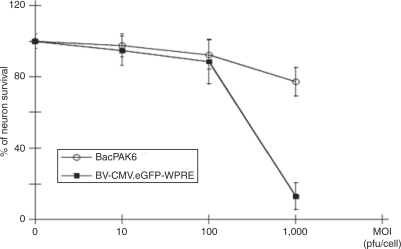
One of the advantages of using baculoviral vectors is their low cytotoxicity, which cause little microscopically observable cytopathic effects on the transduced cells. This property permits the use of baculoviral vector for gene delivery to sensitive cell type such as hESCs.4 In the above experiments, we observed the maintenance of neuron density during prolonged culture processes after transduction. To quantitatively evaluate the effects of baculoviral vector on the survival of hESC-derived human neurons, we used MTS assays. Upon transduction of the neurons with BV-CMV.eGFP-WPRE at an MOI of 10 pfu per cell, no obvious decrease in cell viability was detected 7 days after transduction and 95% of the human neurons survived. Although 89% of neurons were still alive at an MOI of 100 pfu per cell, the percentage plummeted to 13% at an MOI of 1,000 pfu per cell (Figure 5). It is known that some commonly used reporter gene products are toxic to neurons.16 To exclude the effect of overexpression of a reporter gene, baculoviral vectors carrying BacPAK6 viral DNA that do not express in mammalian cells were tested in hESC-derived human neurons. Figure 5 shows that 98 and 92% of human neurons survived at MOI of 10 and 100 pfu per cell, respectively. Even at the extremely high MOI of 1,000 pfu per cell, there were still 77% of live neurons. These results indicate that baculoviral vectors alone can be tolerated by hESC-derived human neurons at commonly used transduction doses.
Figure 5.
Effects of baculoviral vectors on the survival of HES-1–derived human neurons. Cells were prepared in a 96-well plate and transduced by BV-CMV.eGFP-WPRE or BacPAK6, a baculoviral vector without a mammalian gene expression cassette, at the indicated MOIs (plaque-forming units per cell). MTS assays were used to measure the neuronal survival 7 days after transduction. Cell viability is expressed as percentage of nontransduced control. Each point represents the mean ± SD of four wells. BV, baculoviral vector; CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; MOI, multiplicity of infection; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
Baculoviral vectors can be used for co-transduction of hESC-derived human neurons
To determine whether baculoviral vectors can be used to deliver and express two different exogenous genes in the same human neurons, a commercially available source of baculoviral vectors, Organelle Lights and our own baculoviral vectors were used to co-transduce hESC-derived human neurons. Organelle Lights ER-OFP or Mito-OFP, which express fluorescent protein OFP targeted to endoplasmic reticulum and mitochondria respectively, were first used for transduction on day 1, followed by transduction with BV-CMV.eGFP-WPRE on day 2. As shown in Figure 6, significant amount of human neurons were co-expressing ER-OFP and eGFP (Figure 6a) or Mito-OFP and eGFP (Figure 6b) on day 3. It was noticed that using the amount of OFP virus as indicated in the protocol from the manufacturer (138 µl diluted Organelle Lights transduction solution for 105 cells in our case) led to mistargeting of ER-OFP and Mito-OFP in the cells tested in the current study, resulting in strong OFP signals in the neurites of the human neurons (Figure 6a,b). To achieve proper targeting of ER-OFP or Mito-OFP, a low dose of OFP viruses (1/3 the amount recommended by manufacturer) was tested. As shown in Figure 6c, with a proper control over the transgene expression level, Mito-OFP clearly targeted the mitochondria of neurons, whereas in the same neuron, eGFP labeled the cell body and extending neurites.
Figure 6.
Co-transduction of human neurons using Organelle Lights and baculoviral vectors. HSE-1–derived human neurons were transduced by Organelle Lights ER-OFP or Mito-OFP on day 1 and BV-CMV.eGFP-WPRE on day 2. The expression of both fluorescence proteins was observed under a fluorescence microscope on day 3. (a,b) Live cell fluorescence and phase contrast images showing the expression of (a) ER-OFP and eGFP or (b) Mito-OFP and eGFP in human neurons. (c) The targeting of Mito-OFP in human neurons. Live cell fluorescence images of two single neurons showing that Organelle Light Mito-OFP signal was intense in the cytoplasm of the cell body, but not in the nucleus, whereas eGFP signal was detectable all over the neurons. The inset provides a greater magnification showing that the Organelle Light Mito-OFP–labeled mitochondria are located mainly in the neuronal cell body. Individual mitochondria can be seen in the axon hillock. Bar = 200 µm for left column images and 100 µm for right column images. BV, baculoviral vector; CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
Baculovirus-transduced hESC-derived human neurons survived and maintained transgene expression after transplantation into the brain of nude mice
To determine the feasibility of using baculovirus-modified human neurons for transplantation in the brain, hESC-derived human neurons were transduced with BV-CMV.eGFP-WPRE and then injected into the striatum of nude mice 3 days later. Immunostaining of brain sections collected 1 week after transplantation shows eGFP expression in the grafted area (Figure 7a–c). These eGFP-positive cells possessed outgrowing neurites, which could be up to 80 µm long (Figure 7c), indicating that grafted neurons started to reform neurite processes as early as 1 week after transplantation. Four weeks after transplantation, a significant number of eGFP-positive neurons were still detectable (Figure 7d). Many long eGFP-positive neurites were observed and these extending neurites were up to 250 µm in length (Figure 7e). There was no sign of neoplasm formation or other anatomical abnormalities in the grafted area (Figure 7a). These results indicate that human neurons derived in our study maintained their plasticity to integrate into a brain environment upon transplantation and that baculoviral transduction will not inhibit such neuronal function.
Figure 7.
Transplantation of baculovirus-transduced human neurons into the brain of nude mice. HES-1–derived human neurons were transduced by BV-CMV.eGFP-WPRE at a multiplicity of infection of 100 plaque-forming units per cell. These modified neurons were washed twice and transplanted into the striatum of nude mice 3 days after transduction. Brain sections were collected either (a–c) 1 or (d,e) 4 weeks after transplantation and stained with antibody against eGFP. Images (a,b,d,e) were taken under a fluorescence microscope and a confocal image was shown in c. (b) A high power image for the indicated area shown in a. The length of the two reformed neurite processes in the mouse brain was indicated in c and e. BV, baculoviral vector; CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
To study whether baculoviral transduction affected the immunogenicity of the hESC-derived neurons, which may lead to host defense responses to eliminate transplanted cells, the human neurons were transplanted into the striatum of immunocompetent mice 3 days after in vitro baculoviral transduction. Hematoxylin and eosin staining of brain sections showed heavy lymphocytic infiltration into the brain parenchyma of the grafts 1 week after transplantation (Figure 8). The severity of lymphocytic infiltration in the area transplanted with baculovirus-transduced neurons was similar to that in the area with untransduced neurons. Four weeks after transplantation, there was no obvious inflammatory cell infiltration in both transplant areas (Figure 8). These results suggest that baculoviral transduction may not obviously change the immunogenicity of hESC-derived neurons.
Figure 8.
Transplantation of baculovirus-transduced human neurons into the brain of immunocompetent mice. HES-1–derived human neurons were transduced by baculovirus at a multiplicity of infection of 100 plaque-forming units per cell. These modified neurons were then transplanted into the striatum of immunocompetent mice 3 days after transduction. Untransduced neurons were used as control. Brain sections were collected 1 or 4 weeks after transplantation. Images show the hematoxylin and eosin staining of brain sections. BV, baculoviral vector; hESC, human embryonic stem cell; PBS, phosphate-buffered saline. Note: Inflammatory infiltrates 1 week after human neuron transplantation in c,d. Bar = 1.0 mm (a,b); 50.0 µm (c–h).
Discussion
As postmitotic cells, neurons are difficult to be transfected by chemical- and physical-based gene transfer methods such as lipofection, electroporation, or nucleofection.17,18,19 Cytotoxicity and low survival rate that arise from the sensitiveness of neurons to these nonviral methods remain unsolved issues. Viral vectors derived from herpes simplex virus and adenovirus can transduce mammalian neurons with higher efficiency, but they are also toxic to the transduced neurons.17,18 Adeno-associated viruses (AAVs) have been widely tested in neurons,20,21 but these vectors provide a delayed transgene expression that has a peak level expression weeks after infection, which is because AAVs have a relatively slow nuclear import and require converting their single-stranded DNA genomes into double-stranded templates for transcription.18 AAV vectors have limited cloning capacity of ~5 kilobases, which precludes the use of a large expression cassette in an AAV system.18,22 Self-complementary AAV vectors can overcome the problem associated with single-stranded genome of AAV and provide much rapid transgene expression; however, the further reduction in gene cloning capacity of these vectors restrict their use to delivery of small genes and RNA-based therapy.23 Lentiviral and retroviral vectors can provide high transduction efficiency in neurons with low cell toxicity. Stable expression mediated by lentiviral vectors has made them the most commonly used vectors for the studies that require long-term functions of transgenes. Disappointingly, transduction with these retrovirus-based vectors is associated with random chromosomal integration and demands extra safety caution for both clinical and routine laboratory applications. Therefore, current gene transfer techniques in neurons have yet to keep up with requirements posed by both basic and clinical studies, especially those studies requiring efficient transient expression of transgenes.
Currently available nonviral and viral methods are usually tested in primary mammalian neuronal cultures from the mouse or rat, rarely in human neurons. Consequently, most of the gene function studies that lead to the development of potential therapeutic methods for human nervous system diseases are based on the mammalian neurons derived from rodents and further verification of the findings in human neurons is necessary. However, before the common use of hESCs, there were very limited sources for human neurons. One of the sources is the human tumor cell line–derived neurons, such as human neurons differentiated from the human teratocarcinoma cell line NTera 2 (ref. 24) or from human medulloblastoma-derived neural precursor cell lines.25 This type of human neurons may not closely mimic those human neurons in vivo and should be used with caution due to their tumor origins. Primary neural progenitor cells derived from fetal or adult human brain tissues provide another source for human neurons,26,27 but human neural progenitor cells are not accessible to general laboratories, variable in quality and not amenable for large-scale cell production due to their limited passaging capacity, which may prevent widespread experimental and therapeutic applications. When using these neurons in medical treatment, the method will meet ethical and logistical challenges in acquiring human tissues. Human neurons derived from hESCs are valuable in circumventing some of the above problems. The research and therapeutic potential of hESC-derived neurons can be further augmented by the development of effective gene transfer methods tailored for these cells.
Previous animal studies from others and our group suggest that baculoviral vectors are capable of transducing neurons.28,29,30,31,32 In this study, we successfully proved for the first time the gene transfer efficiency of ~80% in hESC-derived human neurons by baculoviral vectors. This high transduction efficiency would be useful in generating a relatively homogenous population of transduced neurons, largely eliminating the need for cell isolation before the use of these cells. Our results suggest that CMV promoter is more efficient than EF1α promoter for transgene expression in hESC-derived neurons. This is opposite to that in hES cells, where EF1α promoter is more efficient.4 This difference in promoter requirement for efficient transgene expression in the hES cells and their neuronal progenies is likely due to cellular differentiation status and related gene expression profiles, which has been evidently demonstrated in human mesenchymal stem cells and their derivatives.11,12
As one characteristic of baculoviral transduction, the transgenes are usually not integrated into the genome in host mammalian cells. Only with antibiotic selection could stable Chinese Hamster ovary cells expressing GFP be isolated following baculovirus transduction,33 suggesting that the frequency of spontaneous chromosomal integration of delivered DNA sequences without positive selection, if any, would be very low in transduced cells. Thus, the virus appears to be a vector without disturbing host genome and suitable for applications requiring transient gene expression. Although chromosomal integration is less likely, transgene expression in baculovirus-transduced human neurons remained for at least 3 months in vitro and 4 weeks in vivo. If a function gene is used to improve transplantation efficiency, this period might be sufficiently long for the gene products to assist transplanted neurons to integrate functionally into the host central nervous system. Nevertheless, progressive transcriptional gene silencing will inactivate the episomal expression cassettes of baculoviral vectors over time and shut down transgene expression in the end.
We have demonstrated in this study that the WPRE sequence is crucial in extending transgene expression in hESC-derived human neurons. WPRE, as a cis-acting RNA element, provides powerful effects on nuclear and cytoplasmic accumulation of RNA.34 WPRE is most effective when being inserted in 3′ UTR of a transgene and upstream of the polyadenylation signal. In integrating RNA vectors, this posttranscriptional regulatory element improves transcript termination, likely from enhanced polyadenylation, thus augmenting viral titers and transgene expression.35 WPRE can also increase transgene expression from DNA virus vectors based on AAV15,36 and adenovirus.37,38,39 In the context of baculoviral vectors, the advantage of using WPRE to improve transgene expression has been demonstrated in several differentiated cells by Yla-Herttuala's lab40 and in hESCs by our lab,4 although the exact underlying mechanism remains to be studied. It is of interest to notice that when our baculoviral vectors with the WPRE sequence were used for transduction of hESCs in vitro, transgene expression diminished toward background within 1 week (data not shown). Thus, besides benefiting from the posttranscriptional regulatory effects of WPRE, transgene expression for 3 months in hESC-derived neurons is also related to the postmitotic feature of these cells that prevents the dilution of transgenes with cell division.
One concern for the use of hESC-derived cells for brain repair is tumor formation after transplantation,2,41,42 due to the inability of current differentiation protocols to generate a pure population of specific cells. Purification of neurons using fluorescence-activated cell sorting (FACS) is one possible solution.13,43,44,45 Study using mouse embryonic stem cells with stable expression of a GFP gene under control of tyrosine hydroxylase promoter shows that the specificity of FACS selection method is affected by the specificity of the promoter and the chromosomal integration site of the transgene construct.44 To reduce the effect of transgene integration site on GFP expression, transient transduction of differentiated neurons will be more desirable than stable transduction of embryonic stem cells. To improve the specificity of FACS selection, it will be preferable to use a larger fragment of the promoter with tight control of cell type–specific gene expression or use multiple cell type–specific promoters to drive the expression of different reporter genes so as to increase the selectivity of FACS. The gene cloning capacity of baculoviral vector makes it possible to include a large promoter or multiple promoters to restrict transgene expression in a specific population of hESC-derived neurons. The ability of co-transduction of multiple genes with baculoviral vectors provides another possibility of using independent vectors that carry reporter genes under the control of different promoters to enhance the chance of using FACS to sort out subpopulation of neurons.
Besides the potentially important uses of baculoviral vectors for the enhancement and purification of human neurons, efficient and rapid genetic manipulation of hESC-derived neurons by baculoviral vectors could have general significance for genetic and genomic studies of genes related to neuronal functions.46,47 For example, co-transduction of different genes in human neurons would be useful to study the interaction of gene products in the same neurons. Moreover, the expression of disease-related genes in these human neurons from a baculoviral vector can be used to create disease models for human neuronal disorders that would be valuable for the selection of possible therapeutic targets and for drug screening.17,18,47,48,49 Looking ahead, the use of baculoviral vectors for transient genetic modification of hES cell–derived neurons will open up many new opportunities for both basic researches aiming to understand neuronal functions and medical treatments addressing neurological problems.
Materials and Methods
hESC culture and neuronal differentiation of hESCs. Two hESC lines HES-1 (National Institutes of Health code: ES01) and HES-3 (National Institutes of Health code: ES03), both of which are listed on National Institutes of Health Human Embryonic Stem Cell Registry, were obtained from ES Cell International (Singapore). HES-1 and HES-3 cells were amplified on mitotically inactivated mouse embryonic fibroblasts from CF-1 mouse strain obtained from American Type Culture Collection (Manassas, VA) seeded in gelatin (Sigma-Aldrich, St Louis, MO) -coated dishes in 80% knockout Dulbecco's modified Eagle's medium (KO-DMEM; Invitrogen, Carlsbad, CA) supplemented with 20% knockout serum replacement (Invitrogen), 2 mmol/l L-glutamine (Invitrogen), 0.1 mmol/l nonessential amino acids (Invitrogen), 0.1 mmol/l 2-mercaptoethanol (Invitrogen), 4 ng/ml basic fibroblast growth factor (Invitrogen), 50 U/ml penicillin, 50 µg/ml streptomycin as described before.4 The hESC colonies were subcultured every 7 days by mechanical slicing and replating into culture dishes with fresh mouse embryonic fibroblasts.
Neuronal differentiation of hESCs was achieved based on a protocol described by Reubinoff et al.1 First, spontaneous differentiation of hESCs was induced by prolonged culturing on feeders for 3–4 weeks. The areas containing neural progenitors were identified under dark-field stereomicroscope and phase-contrast microscope and selected for expansion. The selected areas were cut into small cell clumps and transferred to low cell binding six-well plates (Nalge Nunc International, Rochester, NY) containing Dulbecco's modified Eagle's medium/F12 (1:1) medium (Invitrogen) supplemented with B27 (1:50; Invitrogen), 2 mmol/l L-glutamine, 50 U/ml penicillin, 50 µg/ml streptomycin, 20 ng/ml human epidermal growth factor (Chemicon, Temecula, CA), and 20 ng/ml basic fibroblast growth factor. After expansion for 6 weeks, the round neural spheres were dissected into small cell clumps and then plated into dishes coated with poly-D-lysine (Sigma-Aldrich) and laminin (Sigma-Aldrich). Neuronal differentiation/maturation was induced by the withdrawal of the growth factors from the culture medium and culture for another 6 weeks.
Baculovirus preparation and cell transduction. The transfer plasmid pFastBac1 (Invitrogen) was used to generate recombinant baculoviruses with different expression cassettes. To construct a transfer vector containing the human CMV immediate early gene promoter and enhancer (CMV promoter) and the eGFP gene, a fragment with the CMV promoter and the eGFP gene was amplified from pEGFP-C1 (Clontech, Mountain View, CA) using PCR (forward primer: 5′-GGATCC TAGTTATTAATAGTAATCAAT-3′; reverse primer: 5′-GAATTC CTACTTGTACAGCT CGTC-3′) and inserted between BamHI and EcoRI of pFastBac1. The baculoviral vector generated with this transfer vector is named BV-CMV.eGFP. The generation of baculoviral vector BV-EF1α.eGFP that contains the human elongation factor-1α promoter (EF1α promoter) and the eGFP gene was described previously.4 To construct transfer vectors with WPRE, the eGFP-WPRE fragment from psubCMV-eGFP-WPRE (kindly provided by H. Büeler, University of Zurich, Zurich, Switzerland) was amplified using PCR (forward primer: 5′-GAATTCTACCGGTCGCCACCATG-3′; reverse primer: 5′-ACTAGTCAAAGGGAGATCC GACT-3′) and used to replace the eGFP gene in the above two transfer vectors. The viral vectors generated are named BV-CMV.eGFP-WPRE and BV-EF1α.eGFP-WPRE, respectively.
Baculoviral vectors carrying the above expression cassettes were produced and propagated in Sf9 insect cells according to the manual of Bac-to-Bac Baculovirus Expression System (Invitrogen). Budded viruses in the insect cell culture medium were filtered through 0.45 µm pore size filters (Millipore, Billerica, MA) to remove any cell debris and concentrated by centrifugation at 28,000 g for 60 minutes. Viral pellets were resuspended in 0.1 mol/l phosphate-buffered saline (PBS) and their infectious titers (pfu) were determined by plaque assay on Sf9 cells.
For baculoviral transduction, the hESC-derived neurons were prepared at a density of 105 cells per well in 24-well plate precoated poly-D-lysine and laminin by seeding 6-week-old neural spheres and growing in medium without epidermal growth factor and basic fibroblast growth factor as described above. Recombinant baculoviruses suspended in 100 µl PBS were mixed with 100 µl of neuron culture medium described above and added to human neuron cultures at a desired MOI. After incubation for 2 hours, 200 µl of fresh culture medium was added to each well. The transduction medium was replaced by fresh culture medium the next day. In some experiments, Organelle Lights ER-OFP and Organelle Lights Mito-OFP (kindly provided by Magnus Persmark from Invitrogen) were used according to the protocol from the manufacturer. Organelle Lights are ready-to-use baculovirus reagents for labeling cellular organelles and structures. Organelle Lights ER-OFP and Organelle Lights Mito-OFP express fluorescent protein OFP tagged by endoplasmic reticulum signal sequence of calreticulin/KDEL and leader sequence of E1 α-pyruvate dehydrogenase and can label the endoplasmic reticulum and mitochondria, respectively. In baculoviral co-transduction experiments, the human neurons were first transduced by Organelle Lights ER-GFP or Organelle Lights Mito-OFP. One day after, the neurons were transduced again using our own baculoviral vector BV-CMV.eGFP-WPRE.
Gene expression analysis, immunocytochemistry, and cell viability assay. The expression of fluorescent protein genes in human neurons transduced with baculoviral vectors was observed under a fluorescence microscope. To quantify the transduction efficiency, transduced human neurons were washed in PBS, trypsinized into single cells and analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). The use of eGFP as a reporter gene allows the evaluation of baculoviral transduction efficiency in terms of both the percentage of transduced cells and transgene expression levels as measured as the mean fluorescence intensity of eGFP. To investigate the duration of baculovirus-mediated transgene expression, fluorescence images of transduced human neurons were taken under the same imaging condition at various observation time points using digital camera DP70 from Olympus (Tokyo, Japan). These images were analyzed by Image-Pro Plus software (Media Cybernetics, Bethesda, MD) to quantify green fluorescence intensity based on a previously described protocol.50
Immunofluorescence staining was used to confirm the neuronal identity of transduced cells. First, the transduced neurons were washed with PBS and fixed with 4% paraformaldehyde for 15 minutes. This was followed by permeabilization with PBS containing 0.1% Triton X-100 for 30 seconds and blocking with 1% bovine serum albumin in PBS for 30 minutes. Primary monoclonal antibodies against human neuronal marker βIII tubulin (Promega, Madison, WI), MAP2ab (Santa Cruz Biotechnology, Santa Cruz, CA), NCAM (Santa Cruz Biotechnology), or NF200 (Santa Cruz Biotechnology) were used to incubate with the neuronal samples for 1 hour. After washing, secondary antibodies goat anti-mouse IgG or IgM-PE (Santa Cruz Biotechnology) were used to facilitate visualization. To detect the eGFP protein, Alexa Fluor 488–labeled rabbit anti-GFP antibody (Invitrogen) was included for double immunostaining. The samples were counterstained with Hoechst before observation under a fluorescence microscope.
To evaluate the effects of baculoviral vectors on cell viability, baculoviral vectors BV-CMV.eGFP-WPRE and BacPAK6 (Clontech), a control baculoviral vector without a mammalian gene expression cassette, were used to transduce HES-1–derived human neurons prepared in a 96-well plate at a density of 104 cells per well. Seven days after transduction, the viability of human neurons was assessed using CellTiter 96 AQueous One Solution Cell Proliferation Assay kit (Promega).
Cell transplantation and immunohistochemistry. For cell transplantation experiments, HES-1–derived human neurons were transduced with BV-CMV.eGFP-WPRE at an MOI of 100 pfu per cell. Three days after baculoviral transduction, these transduced neurons were harvested by trypsinization, washed twice with PBS and injected into the striatum of Balb/c nude mice or immunocompetent mice. In brief, adult female mice (weighing 20 g, 6–8 weeks old) were anesthetized using a mixture of ketamine and xylazine (150 mg/kg and 10 mg/kg, respectively) and positioned in a Kopf stereotaxic instrument after they demonstrated no footpad pinch reflex. A burr hole was drilled into the skull (anteroposterior: 0.0 mm, mediolateral: +2.0 mm, and dorsoventral: −3.0 mm from bregma and dura). A 10-µl Hamilton syringe connected with a 30G needle was aligned over the burr hole, and 1 × 105 baculovirus-transduced human neurons in 3 µl of PBS were injected into the left striatum of the mouse brain at a speed of 0.6 µl/min. The needle was withdrawn slowly over 3 minutes after injection. After surgery, the incisions were closed with sutures, and animals were allowed to recover under an infrared lamp before being returned to their cages.
One and four weeks after the xenotransplantation, mice were euthanized with an overdose of ketamine/xylazine. Transcardiac perfusion was performed using PBS followed by freshly prepared cold 4% paraformaldehyde. Brain specimens were left in cold 4% paraformaldehyde overnight, cryoprotected by incubation in 30% sucrose till the specimens sank and sectioned at −20 °C in 25 µm sections after being frozen in embedding medium (Jung tissue freezing medium; Leica Instruments, Nussloch, Germany). Sections were stained using a free-floating method by incubating in 0.3% Triton-X and 3% rabbit serum in PBS containing Alexa Fluor 488–labeled rabbit anti-GFP antibody at 4 °C overnight. Sections were rinsed three times in PBS before being mounted on slides and were observed under a fluorescence microscope or a confocal microscope. Hematoxylin and eosin staining was used for brain sections of immunocompetent mice.
All the handling and care of animals were carried out by following the Guidelines on the Care and Use of Animals for Scientific Purposes issued by the National Advisory Committee for Laboratory Animal Research, Singapore. The current study experimental protocols were approved by the Institutional Animal Care and Use Committee, Biological Resource Center, and the Agency for Science, Technology and Research of Singapore.
Acknowledgments
We thank other lab members for helpful discussion and support. The work was supported by Institute of Bioengineering and Nanotechnology, Biomedical Research Council, and Agency for Science, Technology and Research, Singapore.
REFERENCES
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Li JY, Christophersen NS, Hall V, Soulet D., and , Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Zeng J, Du J, Zhao Y, Palanisamy N., and , Wang S. Baculoviral vector-mediated transient and stable transgene expression in human embryonic stem cells. Stem Cells. 2007;25:1055–1061. doi: 10.1634/stemcells.2006-0616. [DOI] [PubMed] [Google Scholar]
- Shim JW, Koh HC, Chang MY, Roh E, Choi CY, Oh YJ, et al. Enhanced in vitro midbrain dopamine neuron differentiation, dopaminergic function, neurite outgrowth, and 1-methyl-4-phenylpyridium resistance in mouse embryonic stem cells overexpressing Bcl-XL. J Neurosci. 2004;24:843–852. doi: 10.1523/JNEUROSCI.3977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Morrow EM., and , Cepko CL. Misexpression of basic helix-loop-helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development. 2000;127:3021–3030. doi: 10.1242/dev.127.14.3021. [DOI] [PubMed] [Google Scholar]
- Geoffroy CG., and , Raineteau O. A Cre-lox approach for transient transgene expression in neural precursor cells and long-term tracking of their progeny in vitro and in vivo. BMC Dev Biol. 2007;7:45. doi: 10.1186/1471-213X-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates F., and , Daley GQ. Progress and prospects: gene transfer into embryonic stem cells. Gene Ther. 2006;13:1431–1439. doi: 10.1038/sj.gt.3302854. [DOI] [PubMed] [Google Scholar]
- Kost TA, Condreay JP., and , Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YC. Baculoviral vectors for gene delivery: a review. Curr Gene Ther. 2008;8:54–65. doi: 10.2174/156652308783688509. [DOI] [PubMed] [Google Scholar]
- Ho YC, Chung YC, Hwang SM, Wang KC., and , Hu YC. Transgene expression and differentiation of baculovirus-transduced human mesenchymal stem cells. J Gene Med. 2005;7:860–868. doi: 10.1002/jgm.729. [DOI] [PubMed] [Google Scholar]
- Ho YC, Lee HP, Hwang SM, Lo WH, Chen HC, Chung CK, et al. Baculovirus transduction of human mesenchymal stem cell-derived progenitor cells: variation of transgene expression with cellular differentiation states. Gene Ther. 2006;13:1471–1479. doi: 10.1038/sj.gt.3302796. [DOI] [PubMed] [Google Scholar]
- Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R., and , Isacson O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells. 2007;25:2257–2268. doi: 10.1634/stemcells.2006-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D., and , Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JE, Cordier WS, Harris ME, Weitzman MD., and , Hope TJ. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther. 1999;10:2295–2305. doi: 10.1089/10430349950016942. [DOI] [PubMed] [Google Scholar]
- Detrait ER, Bowers WJ, Halterman MW, Giuliano RE, Bennice L, Federoff HJ, et al. Reporter gene transfer induces apoptosis in primary cortical neurons. Mol Ther. 2002;5:723–730. doi: 10.1006/mthe.2002.0609. [DOI] [PubMed] [Google Scholar]
- Slack RS., and , Miller FD. Viral vectors for modulating gene expression in neurons. Curr Opin Neurobiol. 1996;6:576–583. doi: 10.1016/s0959-4388(96)80088-2. [DOI] [PubMed] [Google Scholar]
- Washbourne P., and , McAllister AK. Techniques for gene transfer into neurons. Curr Opin Neurobiol. 2002;12:566–573. doi: 10.1016/s0959-4388(02)00365-3. [DOI] [PubMed] [Google Scholar]
- Zeitelhofer M, Vessey JP, Xie Y, Tübing F, Thomas S, Kiebler M, et al. High-efficiency transfection of mammalian neurons via nucleofection. Nat Protoc. 2007;2:1692–1704. doi: 10.1038/nprot.2007.226. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J., and , Levivier M.Recombinant AAV-mediated gene delivery to the central nervous system J Gene Med 20046S212–S222.suppl. 1 [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Manfredsson FP, Foust KD, Rising A, Reimsnider S, Nash K, et al. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol Ther. 2006;13:463–483. doi: 10.1016/j.ymthe.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Davidson BL., and , Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- Wang S, Rosengren L, Hamberger A., and , Haglid K. Antisense inhibition of BCL-2 expression induces retinoic acid-mediated cell death during differentiation of human NT2N neurons. J Neurochem. 2001;76:1089–1098. doi: 10.1046/j.1471-4159.2001.00142.x. [DOI] [PubMed] [Google Scholar]
- Derrington EA, López-Lastra M, Chapel-Fernandez S, Cosset FL, Belin MF, Rudkin BB, et al. Retroviral vectors for the expression of two genes in human multipotent neural precursors and their differentiated neuronal and glial progeny. Hum Gene Ther. 1999;10:1129–1138. doi: 10.1089/10430349950018120. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- Keyoung HM, Roy NS, Benraiss A, Louissaint A, Jr, Suzuki A, Hashimoto M, et al. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nat Biotechnol. 2001;19:843–850. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- Sarkis C, Serguera C, Petres S, Buchet D, Ridet JL, Edelman L, et al. Efficient transduction of neural cells in vitro and in vivo by a baculovirus-derived vector. Proc Natl Acad Sci USA. 2000;97:14638–14643. doi: 10.1073/pnas.260472897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang X, Guo H., and , Wang S. Axonal transport of recombinant baculovirus vectors. Mol Ther. 2004;10:1121–1129. doi: 10.1016/j.ymthe.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang Y., and , Wang S. Neuronal gene transfer by baculovirus-derived vectors accommodating a neurone-specific promoter. Exp Physiol. 2005;90:39–44. doi: 10.1113/expphysiol.2004.028217. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang C, Zeng J, Xu X, Hwang PY, Yee WC, et al. Gene transfer to dorsal root ganglia by intrathecal injection: effects on regeneration of peripheral nerves. Mol Ther. 2005;12:314–320. doi: 10.1016/j.ymthe.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Wang J, Li B, Cai C, Zhang Y, Wang S, Hu S, et al. Efficient transduction of spiral ganglion neurons in vitro by baculovirus vectors. Neuroreport. 2007;18:1329–1333. doi: 10.1097/WNR.0b013e3282010b16. [DOI] [PubMed] [Google Scholar]
- Merrihew RV, Clay WC, Condreay JP, Witherspoon SM, Dallas WS., and , Kost TA. Chromosomal integration of transduced recombinant baculovirus DNA in mammalian cells. J Virol. 2001;75:903–909. doi: 10.1128/JVI.75.2.903-909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donello JE, Loeb JE., and , Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimoto T, Urbinati F, Perumbeti A, Jiang G, Zarzuela A, Chang LJ, et al. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14:1298–1304. doi: 10.1038/sj.gt.3302979. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Moccetti T, Mura A, Feldon J., and , Büeler H. Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther. 2000;7:1304–1311. doi: 10.1038/sj.gt.3301221. [DOI] [PubMed] [Google Scholar]
- Glover CP, Bienemann AS, Heywood DJ, Cosgrave AS., and , Uney JB.Adenoviral-mediated, high-level, cell-specific transgene expression: a SYN1-WPRE cassette mediates increased transgene expression with no loss of neuron specificity Mol Ther 20025509–516.5 Pt 1 [DOI] [PubMed] [Google Scholar]
- Xu ZL, Mizuguchi H, Mayumi T., and , Hayakawa T. Woodchuck hepatitis virus post-transcriptional regulation element enhances transgene expression from adenovirus vectors. Biochim Biophys Acta. 2003;1621:266–271. doi: 10.1016/s0304-4165(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Sims K, Ahmed Z, Gonzalez AM, Read ML, Cooper-Charles L, Berry M, et al. Targeting adenoviral transgene expression to neurons. Mol Cell Neurosci. 2008;39:411–417. doi: 10.1016/j.mcn.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Mähönen AJ, Airenne KJ, Purola S, Peltomaa E, Kaikkonen MU, Riekkinen MS, et al. Post-transcriptional regulatory element boosts baculovirus-mediated gene expression in vertebrate cells. J Biotechnol. 2007;131:1–8. doi: 10.1016/j.jbiotec.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Carson CT, Aigner S., and , Gage FH. Stem cells: the good, bad and barely in control. Nat Med. 2006;12:1237–1238. doi: 10.1038/nm1106-1237. [DOI] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF., and , Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Takahashi J, Watanabe K, Hayashi H, Morizane A, Koyanagi M, et al. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells. 2006;24:763–771. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Pruszak J, Ferree A, Viñuela A, Hong S, Isacson O, et al. Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells. 2007;25:1126–1135. doi: 10.1634/stemcells.2006-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund E, Pruszak J, Lardaro T, Ludwig W, Viñuela A, Kim KS, et al. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson's disease. Stem Cells. 2008;26:1526–1536. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends Neurosci. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Janson CG, McPhee SW, Leone P, Freese A., and , During MJ. Viral-based gene transfer to the mammalian CNS for functional genomic studies. Trends Neurosci. 2001;24:706–712. doi: 10.1016/s0166-2236(00)01954-8. [DOI] [PubMed] [Google Scholar]
- Cezar GG. Can human embryonic stem cells contribute to the discovery of safer and more effective drugs. Curr Opin Chem Biol. 2007;11:405–409. doi: 10.1016/j.cbpa.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Pouton CW., and , Haynes JM. Embryonic stem cells as a source of models for drug discovery. Nat Rev Drug Discov. 2007;6:605–616. doi: 10.1038/nrd2194. [DOI] [PubMed] [Google Scholar]
- Hoffman RM., and , Yang M. Whole-body imaging with fluorescent proteins. Nat Protoc. 2006;1:1429–1438. doi: 10.1038/nprot.2006.223. [DOI] [PubMed] [Google Scholar]