Abstract
Tumor cells harbor unique genetic mutations, which lead to the generation of immunologically foreign antigenic peptide repertoire with the potential to induce individual tumor-specific immune responses. Here, we developed an in situ tumor vaccine with the ability to elicit antitumor immunity. This vaccine comprised an E1B-deleted oncolytic adenovirus expressing β-defensin-2 (Ad-BD2-E1A) for releasing tumor antigens, recruiting and activating plasmacytoid dendritic cells (pDCs). Intratumoral injections of Ad-BD2-E1A vaccine inhibited primary breast tumor growth and blocked naturally occurring metastasis in mice. Ad-BD2-E1A vaccination induced potent tumor-specific T-cell responses. Splenic and intratumoral DCs isolated from Ad-BD2-E1A-immunized mice were able to stimulate or promote the differentiation of naive T cells into tumor-specific cytotoxic T cells. We further found that the increased numbers of mature CD45RA+CD8α+CD40+ pDCs infiltrated into Ad-BD2-E1A-treated tumors. The antitumor effect of Ad-BD2-E1A vaccination was abrogated in toll-like receptor 4 (TLR4) deficient mice, suggesting the critical role of TLR4 in the induction of antitumor immunity by Ad-BD2-E1A. The results of this study indicate that in situ vaccination with the oncolytic BD2-expressing adenovirus preferentially attracts pDCs and promotes their maturation, and thus elicits potent tumor-specific immunity. This vaccine represents an attractive therapeutic strategy for the induction of individualized antitumor immunity.
Introduction
Tumor cells contain antigenic peptide repertoire derived from largely random mutations, an estimated 11,000 genetic mutations per cell,1 in addition to the shared self-tumor-associated antigens. Furthermore, tumor-specific antigenic peptides can also be generated by peptide splicing.2,3 Different from the self-tumor-associated antigens, this antigenic peptide repertoire derived from largely random mutations, or peptide splicing would be immunologically foreign to the host and could induce potent specific antitumor immunity. Yet, tumors harboring these mutated antigens are usually unable to induce robust immune responses, probably due to the lack of danger signals and co-stimulation.4,5
It is generally believed that tumor-specific or tumor-associated antigens need to be presented by professional antigen-presenting cells (APCs). Dendritic cells (DCs), the most potent APCs, play a pivotal role in the induction of immune responses.6,7 They are located in peripheral tissues, where they can detect incoming pathogens and thus act as “sentinels” of the immune system.6,8 DCs must possess the ability to migrate, a function largely regulated by DC maturation stages9,10,11 and to interact with chemokines, which are released by a variety of cells.12,13,14 Numerous studies reveal that tumor-associated DCs generally have an immature phenotype, characterized by high CD1a expression and low expression of the co-stimulatory molecules CD80, CD86, and CD40 (refs. 15,16,17). This immature phenotype may reflect the lack of effective DC maturation signals in situ.
Recently, it has been reported that β-defensins, a group of antimicrobial peptides of the innate immunity, have selective chemotactic activities on DCs and promote DC maturation through toll-like receptor (TLR) signaling pathway.18,19 Here, we developed an in situ tumor vaccine with the ability to elicit individual tumor-specific immunity. We hypothesized that a recombinant oncolytic virus vaccine expressing β-defensin-2 (BD2) would have dual effects: (i) oncolytic adenoviral activity against local tumor, releasing a broad spectrum of tumor antigens, and (ii) BD2-mediated induction of systemic antitumor responses by attraction of endogenous DCs to the site of tumor, and stimulation of DC maturation in order to prime T cell–mediated antitumor responses. Consequently, maturing DCs will uptake and process tumor antigens, and migrate into lymph organs or tissues, where they prime tumor-specific naive T-cells, resulting in the activation of potent tumor-specific immune responses. Our data demonstrate that such a vaccine is effective in inducing tumor-specific cellular immunity by attracting plasmacytoid DCs (pDCs), and promoting their maturation.
Results
Construction of recombinant oncolytic adenovirus-encoding BD2 and assessment of chemotactic properties of BD2
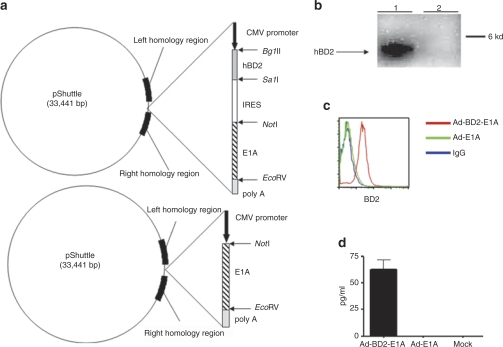
The replication-competent E1B-deleted adenoviruses were found to selectively destroy tumor cells with minimal toxicity to normal cells.20,21,22 In our study, we generated the recombinant replication-competent E1B-deleted adenovirus Ad-BD2-E1A by inserting an expression cassette consisting of human BD2 complementary (cDNA) transcriptionally linked to E1A via encephalomyocarditis virus internal ribosome entry site (IRES) into the E1 and E3-deleted adenovirus (Figure 1a). As a control for Ad-BD2-E1A, we used the previously generated recombinant replication-competent adenovirus-encoding E1A (Ad-E1A),23 which is E1B-deleted and resembles the E1B-deleted ONYX-015 (ref. 22). The recombinant adenoviruses were produced and titrated as described previously.23 The expression of the human BD2 by the recombinant Ad-BD2-E1A was verified by western blot assay (Figure 1b). In addition, we showed BD2 expression in infected mouse mammary adenocarcinoma JC cell line by intracellular staining (Figure 1c) and protein secretion from infected JC cells measured by enzyme-linked immunosorbent assay (Figure 1d).
Figure 1.
Construction and evaluation of the Ad-BD2-E1A. (a) Construction of bicistronic Ad-BD2-E1A and Ad-E1A. Schematic representation of pShuttle vectors is shown. (b) In vitro expression of BD2 (~4 kd). Western blot analysis of cell extracts from HEK-293 cells infected with Ad-BD2-E1A (lane 1) and Ad-E1A (lane 2). Western blots were performed with rabbit antihuman BD2 mAb as described in Materials and Methods. (c) Expression of BD2 in JC tumor cells. JC cells were transduced with Ad-BD2-E1A and Ad-E1A. After 48 hours, the cells were analyzed by intracellular hBD2 staining. (d) Secretion of human BD2 was assessed by ELISA in the supernatants of JC cells transduced with Ad-E1A or Ad-BD2-E1A. CMV, cytomegalovirus; ELISA, enzyme-linked immunosorbent assay; FITC, fluorescein isothiocyanate; hBD2, human β-defensin-2; IRES, internal ribosome entry site.
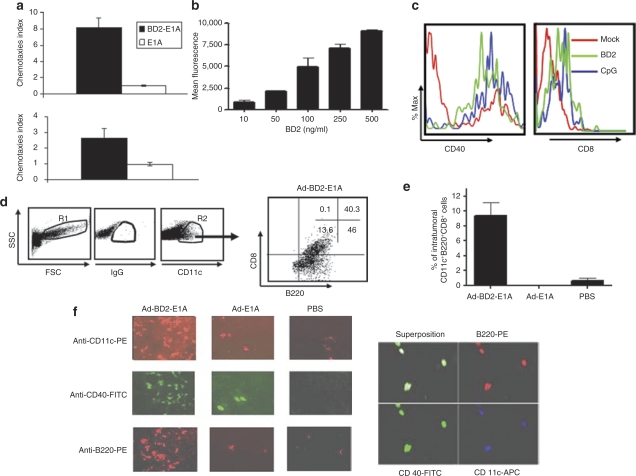
Toward characterization of the human BD2 expressed by Ad-BD2-E1A, we unexpectedly found that the expressed BD2 was able to attract both mouse myeloid and plasmacytoid subsets of DCs in a standard chemotaxis in vitro assay described previously. Figure 2a shows that supernatants from pShuttle-BD2-transfected cells induced chemotactic migration of BM-derived pDCs 8.1-fold higher than the supernatants from the mock-transfected cells (P < 0.01). BD2 induced migration of myeloid DCs 2.6-fold higher than spontaneous migration (P < 0.01). The ability of human BD2–attracting mouse pDCs was further confirmed by testing a purified recombinant human BD2 (from PeproTech, Rocky Hill, NJ) in the in vitro chemotaxis assay (Figure 2b). Thus, human BD2 appeared chemotactic for mouse DCs, and more effective in chemo-attracting mouse plasmacytoid than attracting mouse myeloid DCs. Moreover, recombinant human BD2 induced higher levels of CD40 and CD8 expression in pDCs (Figure 2c), but the expression levels of CD80 and CD86 were not affected by BD2 stimulation (data not shown).
Figure 2.
Chemotactic properties of BD2 on dendritic cells (DCs). (a) Chemotaxis of DCs was measured by migration through a polycarbonate filter with 5 µm pore size. Cos-1 cells were transfected with pShuttle-BD2-E1A (BD2-E1A), pShuttle-E1A (E1A), or pShuttle (mock) with Gene Porter. The cell supernatants collected 48 hours post-transfection were used as an assay media. Migrated cells were harvested and counted by automatic Z2 COULTER Cell Counter. Chemotaxis index is the fold-increase in the number of migrating cells in the presence of test factors over the spontaneous cell migration (mock-transfected cells). Each experiment was performed twice with triplicate wells. Top panel: plasmacytoid DCs; bottom panel: myeloid DCs. (b) In vitro migration of mouse plasmacytoid DCs to different concentrations of recombinant human BD2. Plasmacytoid DCs were labeled with Green-CMFDA cell tracker and added to the upper chamber. Fluorescence of cells migrated through the microporous membrane was measured in triplicates. Fluorescence intensity of spontaneously migrating cells into the media without BD2 was subtracted from each condition. (c) Plasmacytoid DCs were cultured overnight with 500 ng/ml of recombinant BD2, 5 µmol/l CpG ODN or left untreated (mock). The expression of CD40 and CD8a was evaluated by flow cytometry on CD11c+B220+ gated pDCs. (d) Tumor single-cell suspensions were stained with anti-mouse mAb CD11c, B220, CD8α. DCs were gated as large cell population (gate R1) and CD11c+ positive cells (gate R2). Dot plots shown are representative of results from three mice per group. Percentage of cells in each quadrant is shown. (e) Frequency of CD11c+B220+CD8+ plasmacytoid DCs in JC tumors assessed by flow cytometry in three mice per group. (f) Immunofluorescent staining of frozen sections of JC carcinoma tissue. Plasmacytoid DCs were identified in tumor sections from Ad-BD2-E1A, Ad-E1A, and PBS-injected mice by labeling with anti-mouse mAb to CD11c, B220 and CD40 (left panel). Superposition of CD11c, B220, and CD40 in Ad-BD2-E1A injected tumor (right panel). FSC, forward scatter; PBS, phosphate-buffered saline; SSC, side scatter.
To investigate whether in situ Ad-BD2-E1A vaccination may attract DCs in vivo, mammary carcinoma JC murine model that can support human adenovirus replication24 was selected for the study. We injected subcutaneously (s.c.) established JC tumors with 1010 infectious units (ifu) Ad-BD2-E1A or control Ad-E1A vector and surgically excised the tumors 3 days after Ad injection. We evaluated expression of CD11c, CD11b and B220, and CD205 markers representing chief subsets of CD11c+ DCs, i.e., CD11b+CD8α−/CD45RA− (myeloid DCs), CD8α+/−/CD45RA+ (pDCs), CD8α+/CD205+, and CD8α−/CD205+ (Langerhans cells). Interestingly, BD2 induced influx of predominant CD11c+B220+CD8α+ pDCs into the tumors (Figure 2d,e). In addition, immunohistochemical staining with anti-CD11c showed a marked increase of CD11c+B220+ DCs inside the tumors injected with Ad-BD2-E1A, compared with Ad-E1A tumors (Figure 2f). CD11c+ DCs were distributed throughout the Ad-BD2-E1A-injected tumors, while CD11c+ DCs were predominantly accumulated in the periphery of the tumor treated with Ad-E1A or phosphate-buffered saline (PBS), suggesting an active attraction of DCs deeply into the tumor mass after in situ Ad-BD2-E1A vaccination. To examine the maturation status of the tumor-infiltrating DCs, the tumor sections were co-stained with rat anti-mouse CD40. As shown in Figure 2f (right panel), a significant fraction of CD11c+B220+ DCs in Ad-BD2-E1A-injected tumors expressed CD40+, while only limited numbers of CD40+ DCs were detected in Ad-E1A-injected tumors, suggesting that in situ Ad-BD2-E1A vaccination not only attracts DCs, but also promotes their activation inside the tumor. In addition, pDCs from the Ad-BD2-E1A injected tumors showed increased levels of CD8α (40% CD8α+ pDCs), indicating a more activated phenotype compared to Ad-E1A (3% CD8α+ pDCs) and PBS (4% CD8α+ pDCs) injected tumors (Figure 2d and data not shown).
Induction of systemic antitumor immunity in Ad-BD2-E1A-vaccinated mice
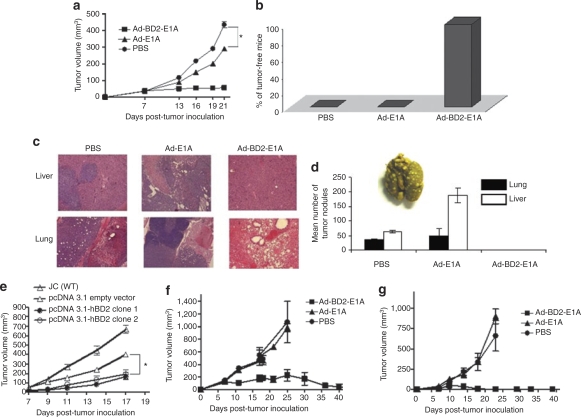
The capability of human BD2–attracting mouse DCs prompted us to use murine model to evaluate antitumor effect of the oncolytic Ad-BD2-E1A vaccine. As human BD2 is a foreign antigen to mouse, we first tested whether intratumoral injection of Ad-BD2-E1A induces immune responses to reject the expressed human BD2 in treated mice. Treatment with adenovirus started when s.c. established tumors reached ~5 mm in diameter. Balb/c mice of each group were intratumorally vaccinated three times (7, 8, and 14 days after tumor inoculation) with 1010 ifu of Ad-BD2-E1A or 50 µl PBS. One group of mice in the experiment was immunized with 7.5 µg human recombinant BD2 protein in complete Feuds' adjuvant. As shown in Supplementary Figure S1, vaccination with Ad-BD2-E1A or 7.5 µg human BD2 in complete Feuds' adjuvant only induced weak antibody response. Because positive mouse antisera to human BD2 were not available, we used a goat antihuman BD2 as a positive control. Furthermore, we failed to detect any significant T-cell response in a standard interferon (IFN)-γ enzyme-linked immunosorbent spot (ELIspot) assay in which 5 µg/ml human BD2 protein was used for in vitro stimulation (data not shown). The result suggested that human BD2 has low immunogenicity in Balb/c host possibly owing to that it is a small molecule, and exhibits high percentage of amino acid sequence identity to murine homologue.25 Thus, antitumor efficacy of Ad-BD2-E1A in situ vaccination was first tested in the low immunogenic and highly metastatic JC mammary carcinoma mouse model. As shown in Figure 3a, vaccination with Ad-BD2-E1A blocked JC tumor growth, while vaccination with Ad-E1A only partially inhibited the primary tumor, compared with PBS treatment, suggesting that intratumoral injection of Ad-BD2-E1A might induce antitumor immunity for controlling the primary tumor.
Figure 3.
Treatment with Ad-BD2-E1A inhibits the growth of established tumors and blocks the secondary tumors formation. (a) 5 × 105 JC cells were injected s.c. into the right flank of Balb/c mice. Groups of mice (N = 6) were injected intratumorally with 1 × 1010 ifu of Ad-BD2-E1A, Ad-E1A, or PBS on 7, 8, and 14 days after JC cell inoculation. Tumors were resected when they reached about 10 mm in diameter in PBS group (day 15–16 after the first adenoviral treatment), *P < 0.05. (b) Resistance to rechallenged JC tumor after in situ Ad-BD2-E1A vaccination. After treatment and resection of JC tumors as described above, mice were rechallenged with 105 JC tumor cells in the opposite flank. Percentages of tumor-free mice are shown 3 weeks after rechallenge. (c) Ad-BD2-E1A treatment blocks metastasis of JC breast cancer cells to the lungs and livers. Mice were killed on day 22 after the first adenoviral injection. Organs from three mice of each group were collected and fixed in Bouin's solution. Hematoxylin–eosin staining was performed on formalin-fixed paraffin-embedded lung and liver tissues from mice bearing JC breast tumors. Representative pictures were from nine sections (three sections, 5 µm-thick from three different mice of each group) at ×20 magnification. (d) Mean number of pulmonary and liver surface tumor nodules counted under dissecting microscope. Representative gross image of fixed lungs with visible JC metastatic nodules is shown. Representative results from two experiments (N = 3). (e) JC cells were transfected with pcDNA 3.1 (Invitrogen) expressing human BD2 or pcDNA empty vector. After 4–6 weeks of Zeocin selection (500 µg/ml), the drug-resistant clones were inoculated into the right flanks of six mice. Tumor growth was measured twice a week and mice inoculated with JC (wild type) cells were killed when tumors were >15 mm in diameter, *P < 0.05. (f) Parental E.G7-OVA tumors were established s.c. into the right flank of C57BL/6 mice (N = 7). Graph shows tumor growth in mice intratumorally injected with Ad-BD2-E1A, Ad-E1A, and PBS as described above. (g) Mice (N = 7) were inoculated with E.G7-OVA tumor cells (distant tumors) s.c. into the left flank before first treatment with adenovirus. Graph depicts the growth of these distant tumors, which were not treated with the adenoviruses. Error bars represent standard error of the mean. OVA, ovalbumin; PBS, phosphate-buffered saline; s.c., subcutaneously; WT, wild type.
To test whether in situ vaccination with Ad-BD2-E1A can induce systemic antitumor responses, we resected tumors in the mice of different groups 2 weeks after the first intratumoral injection, and then rechallenged the mice with JC cells. It was found that Ad-BD2-E1A-treated mice were completely resistant to JC tumor rechallenge, while Ad-E1A-treated mice still developed JC tumors (Figure 3b). We further tested whether intratumoral treatment with Ad-BD2-E1A blocks naturally occurring metastases, because subcutaneous JC tumor is known to be able to metastasize to lungs, liver, and other organs in mice. JC tumor–bearing mice were intratumorally vaccinated three times (7, 8, and 14 days after tumor inoculation) with 1010 ifu of Ad-BD2-E1A, or Ad-E1A, or PBS. Approximately 3 weeks after the first adenoviral injection mice were killed and their lungs and livers were collected for hematoxylin and eosin staining (Figure 3c and Supplementary Figure S2) and surface tumor nodules enumeration (Figure 3d). It was found that no or little metastatic nodules were found in Ad-BD2-E1A-treated mice, while significant numbers of metastatic nodules were found in Ad-E1A-treated mice. Taken together, these data indicate that in situ vaccination of highly metastatic and low immunogenic breast adenocarcinoma JC tumor model with Ad-BD2-E1A effectively blocks the primary tumor growth, as well as, controls tumor metastases, probably by inducing tumor-specific immune responses.
In order to investigate whether BD2 by itself is sufficient to control JC tumor growth, we generated JC cell lines stably expressing BD2 and inoculated the BD2-expressed or control JC cells into Balb/c mice. As shown in Figure 3e, BD2-expressed tumors grew with a lower efficiency compared with control JC tumors, but kept growing during the whole observed period. The overall size of BD2-expressed tumor was bigger than Ad-BD2-E1A-treated tumor (Figure 3a), suggesting that the expressed BD2 contributes to eradication of tumors, likely by attraction and activation of APCs, but the oncolytic activity of Ad-BD2-E1A in synergy with BD2 reaches the maximal control of JC tumor. Further studies are necessary to understand whether BD2 plays a role in direct suppression of tumor growth by using immune-deficient mice.
We also tested the antitumor efficacy of Ad-BD2-E1A intratumoral administration in E.G7 tumor model. We inoculated s.c. tumors on the right and the left flanks of mice. Viruses were injected only into the right tumor and the growth of both tumors was monitored. Interestingly, we found that intratumoral injection of Ad-BD2-E1A efficiently eradicated not only injected but also distant tumors (Figure 3f,g).
Potent cellular immune responses induced by in situ Ad-BD2-E1A vaccination
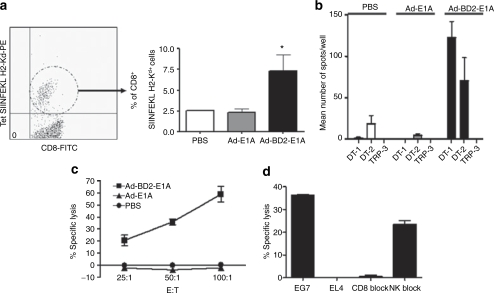
Because E.G7 tumor expresses the well-defined ovalbumin (OVA) antigen, we intensively evaluate antigen-specific immune responses in immunized EG7-bearing mice. To test the frequency of antigen-specific cytotoxic lymphocytes in peripheral blood of E.G7-bearing mice, peripheral blood mononuclear cells were purified and stained with anti-CD8 and SIIFNFEKL-H2-Kb tetramers (CD8-specific OVA epitope, designated as OT-1). As expected, the percentage of SIINFEKL-specific cytotoxic T lymphocytes (CTL) was significantly increased in Ad-BD2-E1A-vaccinated mice (Figure 4a). In addition, Ad-BD2-E1A-intratumoral vaccinations induced high frequency of OVA-specific CD4+ and CD8+ T cells. As shown in Figure 4b, large numbers of T cells from Ad-BD2-E1A-vaccinated EG7-mice secreted IFN-γ in response to stimulation of OT-1 peptide and OT-2 peptide (CD4-specific OVA epitope ISQAVHAAHAEINEAGR). In contrast, little IFN-γ-producing T-cells were detected in Ad-E1A or PBS vaccinated mice. These T-cell responses were OVA-specific, as T cells from Ad-BD2-E1A-treated E.G7-bearing mice did not respond to tyrosinase-related protein-2 H2-Kb-restricted peptide. Functional status of the activated T-cells was assessed by testing cytotoxic activity to 51Cr-labeled target E.G7 or EL-4 control cells after coculture of splenocytes with OVA protein over a week. As shown in Figure 4c, splenocytes from Ad-BD2-E1A-immunized animals had active specific cytotoxicity to E.G7 target cells, but not to irrelevant EL-4 cells (Figure 4d). Splenocytes from Ad-E1A- or mock-immunized mice had weak cytotoxicity (Figure 4c). This cytotoxicity was primarily mediated by CD8+ T, because it was blocked by anti-CD8 mAb, but not by excess of Yac-1 cells (Figure 4d).
Figure 4.
Enhanced E.G7-tumor-specific T-cell responses induced by intratumoral administration of Ad-BD2-E1A. (a) Peripheral blood was collected from the E.G7-bearing mice 10 days after the final adenoviral immunization. Left panel, CD8+ and SIINFEKL H2-Kb+ double positive cells were gated in a circular gate. Right panel, percentage of tetramer-positive CD8+ T cells in the peripheral blood of three mice in each group. *P < 0.05 between tetramer-positive CD8 T cells in the blood of Ad-BD2-E1A-treated group compared with Ad-E1A and PBS groups. (b) Splenocytes from E.G7-bearing mice treated with Ad-BD2-E1A, Ad-E1A, or PBS were analyzed in IFN-γ ELIspot assay with either 1 µg/ml of SIINFEKL peptide (OT-1), ISQAVHAAHAEINEAGR (OT-2), or TRP-2 (irrelevant H2-Kb-restricted) peptides. The number of IFN-γ-producing lymphocytes was evaluated in triplicate wells. Average ± SD for each mouse was calculated and the assays were repeated twice. (c) Splenocytes from E.G7-bearing mice were tested for cytolytic activity in a standard 6-hour 51Cr-release assay. Target cells labeled with 51Cr were placed in each well of 96-well plates, and 50 µl of effector T cells for each dilution was added. The supernatant from each well was harvested, and the amount of 51Cr radioactivity released was measured in a γ counter. (d) E.G7-specific CTL response in Ad-BD2-E1A treated mice. EL-4 cell line was used as irrelevant control. Two micrograms of monoclonal anti-mouse CD8 mAb was added for the blocking assay for splenocytes from Ad-BD2-E1A treated mice; E:T (effector:target) ratio is 50:1. Yac-1 cells were used for blocking the NK-activity at a Yac-1 to E.G7 cell ratio of 50:1. FITC, fluorescein isothiocyanate; IFN, interferon; NK, natural killer; PBS, phosphate-buffered saline.
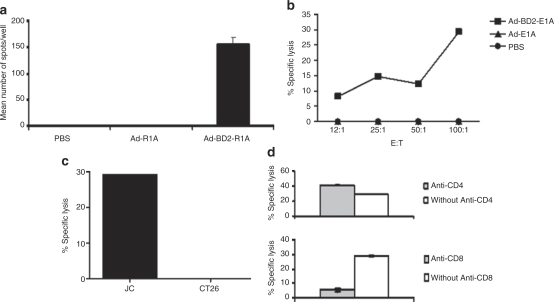
We also tested JC tumor–specific immune response in the adenovirus immunized JC-bearing mice. As shown in Figure 5a, high frequency of T cells from Ad-BD2-E1A immunized mice produced IFN-γ in response to stimulation of JC cell lysate-pulsed DCs, whereas, little IFN-γ-producing T-cells were detected in Ad-E1A or PBS vaccinated JC-mice. Furthermore, Ad-BD2-E1A intratumoral injection induced robust CTL response as evidenced by that splenocytes from Ad-BD2-E1A immunized mice effectively killed 51Cr-labeled JC cell (Figure 5b,c), but not irrelevant CT26 control (Figure 5c), in comparison with splenocytes from adenoviral control or PBS control. Anti-CD8 mAb blocked CTL activity detected in Ad-BD2-E1A immunized mice indicating CTL is mediated by CD8 T cells (Figure 5d).
Figure 5.
Potent JC-specific CTL responses induced by Ad-BD2-E1A in situ vaccinations. (a) Splenocytes from JC-bearing mice treated with Ad-BD2-E1A, Ad-E1A or PBS were analyzed with IFN-γ ELIspot assay. DCs from naive mice pulsed with JC or CT26 (control) tumor lysates were used as antigen-presenting cells at 1:100 ratio. (b) CTL response was induced by Ad-BD2-E1A intratumoral vaccination. Splenocytes were tested for cytolytic activity with a standard 6-hour 51Cr-release assay. CTLs (1.5 × 106 cells) were stimulated with JC tumor lysates at a T cell: JC tumor cell ratio of 2:1 for 5 days. Target cells labeled with 51Cr were placed in each well of 96-well plates, and 50 µl of effector T cells for each dilution was added. The supernatant from each well was harvested, and the amount of 51Cr radioactivity released was measured in a γ counter. (c) JC-specific CTL response in Ad-BD2-E1A treated mice. CT26 colon carcinoma used as irrelevant control. E:T (effector:target) ratio is 100:1. (d) 2 µg of monoclonal anti-mouse CD4 or CD8 Abs were added for blocking CTL activity of splenocytes from Ad-BD2-E1A treated mice; E:T ratio is 100:1. The assays were performed three times or more. Abs, antibodies; CTL, cytotoxic T lymphocytes; DCs, dendritic cells; IFN, interferon; PBS, phosphate-buffered saline.
DCs from Ad-BD2-E1A-treated mice prime potent tumor-specific CTLs
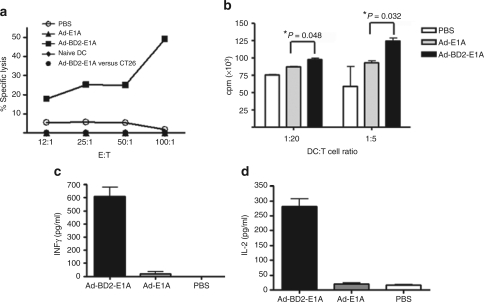
The mature phenotype of tumor-infiltrating pDCs and higher percentage of CD11c+CD8+ DCs in spleens of Ad-BD2-E1A-treated mice (data not shown) prompted us to test whether the mature tumor-infiltrated pDCs would migrate into lymphoid organs after uptaking tumor antigens to prime tumor-specific immune responses. We isolated CD11c+ DCs from the spleens of mice treated with different Ad vectors and cocultured them with splenocytes from naive mice in vitro over a week in order to prime T-cell responses. After in vitro priming, the splenocytes were used for testing CTL activity against target JC tumor cells. As shown in Figure 6a, DCs from the Ad-BD2-E1A, but not from Ad-E1A or mock-vaccinated mice were able to prime tumor-specific CTL responses. These CTLs were able to lyse the JC tumor cells (at 49 ± 5.3% specific lysis), but not unrelated CT26 cells. Cytotoxicity was mediated by CTLs, because it was not abrogated in the presence of an excess of Yac-1 cells (data not shown). Thus, although the study did not exclude the possibility that JC tumor cells migrated and primed splenic DCs in the lymphoid organ, in combination of the metastasis data form differently treated mice (Figure 3) and higher percentage of CD11c+CD8+ DCs present in spleens of Ad-BD2-E1A-treated mice, the result hints that in situ Ad-BD2-E1A vaccination not only enhances tumor infiltration of DCs but also likely facilitates their homing to lymphoid organs for T cell priming.
Figure 6.
DCs from Ad-BD2-E1A-treated mice stimulate antitumor T cell responses. (a) Splenic DCs from Ad-BD2-E1A immunized mice prime CTL response. Balb/c mice were inoculated with JC cells, and intratumorally injected with different adenoviral vector. Splenic DCs were isolated from Ad-BD2-E1A, Ad-E1A, or PBS-treated mice with anti-mouse CD11c microbeads. Naive splenocytes were cocultured with the DCs at the ratio 100:1 for 7 days as described in Materials and Methods. Splenocytes were tested for cytolytic activity against JC cells in a standard 6-hour 51Cr-release assay. CT26 colon carcinoma cells served as irrelevant control. The assay was repeated two times. (b) Tumoral DCs from Ad-BD2-E1A treated mice enhance CD8 T-cell responses. Tumors were established s.c. into both flanks of six mice of each treatment group. Forty-eight hours after the adenoviral injections, tumors were resected and tumoral DCs were purified as described in Materials and Methods. Graded numbers of CD11c+-enriched tumoral DCs cultured for 3 days in vitro were cocultured with 3 × 105 purified naive CD8+ T cells in the presence of anti-CD3 for 3 days. In the last 16 hours, 3H-thymidine (1 µCi/well) was added into the cell culture to monitor the T-cell proliferation, (c) IFN-γ and (d) IL-2 secretion were measured 16 hours later. cpm, counts per minute; DCs, dendritic cells; E:T, effector-to-target ratio; IL, interleukin; IFN, interferon; PBS, phosphate-buffered saline; s.c., subcutaneously.
In addition, we evaluated whether the purified CD11c+ DCs from Ad-BD2-E1A-injected tumors can promote proliferation and cytokine production of naive CD8+ T cells. CD8+ T cells exhibited higher proliferation (Figure 6b) and more robust IFN-γ and interleukin (IL)-2 secretion (Figure 6c,d) when cocultured with tumor-infiltrating DCs from Ad-BD2-E1A group compared with from Ad-E1A and PBS groups, in the presence of anti-CD3. These results support that Ad-BD2-E1A enables tumor-infiltrating DCs to acquire enhanced ability to prime CD8+ T-cell responses in immunized mice.
Antitumor effects of Ad-BD2-E1A were abrogated in TLR4 deficient mice
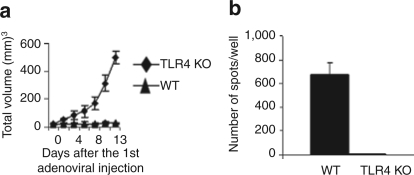
We further tested whether human BD2-expressing oncolytic adenoviral vaccine is functional in TLR4-deficient mice as in wild type mice, as it was reported that TLR4 is an endogenous ligand for mouse BD2.19 Six wild type and TLR4 deficient mice bearing JC tumors were three times (7, 8, and 14 days after tumor inoculation) intratumorally injected with 1010 ifu of Ad-BD2-E1A virus. We found that the antitumor properties of Ad-BD2-E1A were dependent on functional TLR4, as primary JC tumor growth was not inhibited by Ad-BD2-E1A intratumoral treatment in TLR4 deficient mice (Figure 7a). Consistently, INF-γ ELIspot data showed that T-cell response was completely abrogated in TLR4 deficient mice (Figure 7b). The result implies that the ability of in situ Ad-BD2-E1A vaccination to induce tumor-specific responses requires existence of functional TLR4 in immunized host.
Figure 7.
Ad-BD2-E1A-induced immune responses are abrogated in TLR4-deficient mice. JC tumor cells were subcutaneously inoculated into right flank of TLR4-deficient and wild type (WT) Balb/c mice (N = 6 in each group). Mice were intratumorally treated with 1010 ifu of Ad-BD2-E1A three times as described in Figure 3. (a) Tumor growth was monitored until tumors reached about 10 mm in diameter in TLR4-deficient group (15–16 days after first intratumoral injection). Error bars represent standard error of the mean. (b) IFN-γ ELIspot assay was used to determine the number of JC-specific T cells induced in TLR4-deficient and WT Balb/c mice by Ad-BD2-E1A vaccine. IFN, interferon; TLR, toll-like receptor.
Discussion
We have demonstrated that intratumoral immunization with the oncolytic BD2-expressing adenovirus vaccine elicits potent systemic tumor-specific immunity by attracting DCs and promoting their maturation. Importantly, we found that intratumoral vaccination with Ad-BD2-E1A preferentially attracts and activates pDCs which exhibit a mature CD11c+B220+CD8α+CD40+ phenotype. The results of this study imply the therapeutic potential of this oncolytic BD2-expressing adenovirus vaccine for inducing individual tumor-specific immune responses.
β-Defensins contribute to host defense by disrupting the cytoplasmic membrane of microorganisms. These natural peptide antibiotics also possess immunoadjuvant properties by chemo-attracting and activating immature APCs to induce systemic immunity.18,26 Several studies have provided evidence that β-defensin enhanced immunogenicity of the fused antigens, which are nonimmunogenic by facilitating delivery of these antigens to immature DCs and enhancing DCs' antigen presentation and maturation.18,19,26,27 Recently, it was reported that mature human BD2 was chemotactic for mouse cells and promoted antigen- and tumor-specific immune response in mouse model.18,28 Our study provides additional evidence to support the biological activity of the human BD2 in mice, in detail, intratumoral immunization with the oncolytic BD2-expressing adenovirus vaccine elicits potent tumor-immune responses in the mammary carcinoma JC murine model by chemo-attracting and upregulating immature pDC. Early studies suggested that β-defensins induced chemotaxis of APCs by binding to CCR6, which is preferentially expressed by immature DCs and memory T cells,27 while β-defensins activate APCs via TLRs, specifically, mouse BD2 activates immature DCs by acting on TLR419 and human BD3 activated APC by acting on TLR1/2 (ref. 29). Recent emerging evidence argued that CCR6 is not a functional receptor for β-defensins by demonstrating that β-defensins also induced migration of monocytes and mast cells that do not express CCR6 (ref. 30). Based on these studies, we tested Ad-BD2-E1A-mediated antitumor activity in TLR4 knockout mice. We found that Ad-BD2-E1A required the functional TLR4 to trigger tumor-specific immune response. Although mouse pDCs express dominantly TLR9 and TLR7,31 the low level expression of TLR4 was also detected in these cells.32 However, Funderburg et al. recently reported that human BD2 was unable to activate human monocytes that abundantly express TLR4 (ref. 29). The finding prevents us from drawing any conclusion on TLR4 as a receptor for hBD2 to regulate pDCs activation and the antitumor activity in Ad-BD2-E1A treated mice. If TLR4 does not involve human BD2 mediating pDC activation, what role does the TLR play in Ad-BD2-E1A inducing antitumor immunity? One straightforward speculation is that the oncolytic adenoviral vector not only mediates oncolytic activity against tumor cells, also induces nonspecific immune responses by activation of TLR4 signaling. However, adenovirus activation of TLR4 signaling has never been reported, and the speculation is unable to explain the intratumoral injection of Ad-E1A controlled tumor growth and metastasis with much lower efficiency than Ad-BD2-E1A in our study. Clearly, this study does not answer how TLR4 involves Ad-BD2-E1A-mediating antitumor immune responses and whether human BD2 triggering TLR4 signaling only occurs in the differentiated pDCs. Our study demonstrates that human BD2 does activate murine pDCs. The tumor-infiltrating pDCs after Ad-BD2-E1A vaccination exhibited a mature CD45RA+CD8α+CD40+ phenotype, as found by immunohistochemistry and flow cytometry, whereas, the pDCs in the tumors injected with the control Ad-E1A exhibited an immature CD11c+CD45RA+CD8α− phenotype with low expression of CD40. Furthermore, the purified recombinant hBD2 exhibited a high efficiency in induction of CD8 and CD40 expression. CD8 has been seen as a maturation or activation marker for pDC,33 because the surface expression of CD8 is upregulated on murine pDC34,35 upon stimulation with microbial products or CpG. Further study should identify how BD2 regualtes activation of pDCs and what signaling pathway BD2 does use to trigger pDC maturation.
An interesting finding of this study is the preferential attraction of CD11c+CD8α+CD45RA+ pDCs by intratumoral vaccination with the oncolytic BD2-expressing adenovirus vaccine. Human BD2–mediated preferential attraction of pDCs was further confirmed by testing purified recombinant human BD2 and human BD2–expressed supernatants to attract pDC in a standard chemotaxis in vitro assay. Although the in vitro studies also indicate the ability of human BD2 to attract myeloid DCs, we did not detect a significant number of tumor-infiltrating myeloid DCs in the tumor model, which supports the study from Dr Zwirner's group that human BD2 is not chemotactic to mouse myeloid DCs.30 Clearly, further studies are needed to elucidate the mechanism underlining the preferential attraction of pDCs. Plasmacytoid DCs are major cellular source of IFN-α upon viral infection.36,37,38 IFN-α is one of the most potent antitumor cytokines that is routinely used for the treatment of several malignant diseases39 as it can enhance natural killer cells' and CTL responses.40,41 Based upon the observed correlation of antitumor activity and tumor infiltration of mature pDCs, it is likely that the tumor-infiltrating pDCs undergo maturation by BD2 stimulation, secrete INF-α in situ, as well as, migrate into lymphoid organs or tissues where they prime tumor-specific CTL responses. DCs isolated from the spleens of Ad-BD2-E1A-treated mice promoted differentiation of naive T cells into antitumor effector T cells, which supports the speculation. The biological functions of pDCs are contradictive as they show a striking functional plasticity. For example, although type-I INF production commits pDCs to differentiate into mature DCs driving Th1- and Th2-type immune responses,32 treatment with IL-3 and CD40L converts these cells into promoters of immune suppression through the generation of IL-10-producing T regulatory cells.42 In addition, pDCs show the antiviral immunogenic properties to protect other cells from viral infections and promote survival of antigen-activated T cells by expression of INF-α.43 Thus, it is particularly challenging to predict what kind of role pDCs do play during in vivo immune responses and whether they work as an immune stimulus or an immune suppressor. Further studies are needed to fully understand and characterize pDC.
Currently oncolytic adenoviral vaccines show encouraging therapeutic results in clinical trials.44 However, local tumor killing by replication-competent adenovirus is not sufficient to elicit potent antitumor immunity. According to our results intratumoral vaccination with the oncolytic adenovirus Ad-E1A could not recruit and activate professional APCs at the tumor site to induce systemic antitumor immunity. Other in situ therapies are based on targeting intratumoral DCs by the tumoral expression of different chemokines such as macrophage inflammatory protein-3α,17 secondary lymphoid tissue chemokine,45 Epstein–Barr virus–induced molecule 1 ligand CC chemokine,46 macrophage-derived chemokine (CCL22) (ref. 47). However, the higher tumor infiltration by naive, immature DCs may not be sufficient to prime robust antitumor immune responses. For example, in the study reported by Furumoto et al., the facilitation of tumor infiltration of DCs in response to macrophage inflammatory protein-3α expression was not potent to eradicate tumor. An additional intratumoral injection of CG-rich motifs (“danger” signal) was required in order to induce DC maturation and induction of antitumor immunity.17 Here, we developed the in situ vaccine, which combines the oncolytic activity of the adenovirus to release unique tumor-associated antigens, and the BD2-chemokine-mediated attraction and activation of APCs. This vaccine capable of inducing potent individual tumor-specific immunity is a promising tumor vaccine candidate.
Materials and Methods
Cell lines and animals. JC murine mammary adenocarcinoma, E.G7, CT26 murine colon carcinoma, COS-1, human embryonic kidney cells (HEK-293), and Yac-1 cells were purchased from American Type Culture Collection (Manassas, VA). Balb/c female mice (4–6 week old) used in this study were purchased from Harlan Labs (Indianapolis, IN). TLR4-deficient mice (C.C3-Tlr4Lps-d/J) were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were housed in a specific pathogen-free animal facility at Baylor College of Medicine (Houston, TX).
Generation of the recombinant adenoviral vectors. Generation of the Ad-E1A pShuttle transfer vector was previously described.23 To generate recombinant adenovirus encoding mature human BD2, i.e., Ad-BD2-E1A, we constructed a pShuttle transfer vector comprised of BD2 and E1A expression cassette under the cytomegalovirus promoter; the E1A gene was transcriptionally linked to the BD2 gene via an encephalomyocarditis virus IRES (Figure 1a). Human mature BD2 cDNA was amplified by PCR using the primer sets, 5′-AAT TAA AGA TCT ATG GGT ATA GGC GAT CCT-3′ and 5′-TAT ATT GTC GAC TTA TGG CTT TTT GCA GCA TTT-3′ and human lymph node cDNA pool (BD Biosciences, Palo Alto, CA) as a template. The resulting cDNA was 141 bp and had sticky 5′ BglII/SalI ends. IRES of encephalomyocarditis virus was amplified with the primer set 5′-AAT TAA GTC GAC GCC CCT CTC CCT CCC CCC-3′ and 5′-TAT ATT GCG GCC GCT GTG GCC ATA TTA TCA TC-3′ on pIRES template (BD Biosciences, Palo Alto, CA). The resulting cDNA was 610 bp and had sticky 5′ SalI/NotI ends. Human BD2 and IRES PCR products were inserted into the Ad-E1A pShuttle vector by ligation of a 3-fragments with cohesive ends. The sequences of the inserts in the plasmids were confirmed by sequencing. Homologous recombination and subsequent amplification of the recombinant virus were carried out in HEK-293 cells according to the manufacturer's instructions (Qbiogene, Carlsbad, CA). Final stock of the amplified recombinant virus Ad-BD2-E1A was titrated using the Adeno-X Rapid Titer Kit (BD Biosciences, Palo Alto, CA), and stored at −80 °C.
Western blot. Human BD2 expression was detected by western blot. Briefly, HEK-293 cells were infected with Ad-BD2-E1A or Ad-E1A (negative control) for 48 hours. Cells were lysed in a buffer containing 1% Triton X-100, 150 mmol/l NaCl, 50 mmol/l Tris–HCl (pH 7.4) and 1× mammalian protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO). Samples were electrophoresed in a 4–20% gradient sodium dodecyl sulfate-polyacrylamide gel and onto 0.22-µm nitrocellulose membrane (Hybond, Amersham Biosciences, Sunnyvale, CA). Immunodetection was performed with rabbit anti-hBD2 antibodies (Alpha Diagnostics, San Antonio, TX) at 1 µg/ml in PBS with 0.1% Tween 20 (PBS-T) and 2.5% nonfat dried milk overnight at 4 °C. The membrane was then incubated with anti-rabbit IgG-horseradish peroxidase conjugated antibody (Amersham Biosciences) diluted 1:2,000 in PBS-T for 1 hours at room temperature (RT). Chemiluminescent signal was detected with ECL Plus western blotting detection kit (Amersham Biosciences).
Intracellular staining. JC cells transduced with Ad5-BD2-E1A or Ad5-E1A were fixed and permeabilized using BD Cytofix/Cytoperm kit (BD Biosciences, San Diego, CA). BD2 expression was assessed by intracellular staining using with 1 µg/ml of goat antihuman BD2 (PeroTech, Rocky Hill, NJ) for 30 minutes at RT. Washed cells were incubated with 1:1,000 of donkey anti-goat-FITC for 30 minutes at RT and expression was analyzed by flow cytometry.
BD2 enzyme-linked immunosorbent assay. JC cells were transduced with 40 multiplicity of infection of Ad-BD2-E1A overnight. As BD2 is a charged peptide that is tightly bound to the membranes of JC cells, cell layers were washed with 300 µl of sterile water.48 Human BD2 was detected in the washings using enzyme-linked immunosorbent assay kit (PeroTech).
DC isolation and generation. Spleen- or tumor-derived DCs were isolated following the protocol for mouse CD11c mouse micromagnetic beads (Miltenyi Biotec, Auburn, CA). Cells were cultured for 2–3 days before the assays in RPMI 1640 with 10% fetal bovine serum, 20 ng/ml murine granulocyte–macrophage colony-stimulating factor (Biosource International, Camarillo, CA), and 20 ng/ml mIL-4 (R&D Systems, Minneapolis, MN).
Bone marrow–derived myeloid DCs were generated from female Balb/c mice by culturing bone marrow cell suspensions as previously described28,49 in RPMI 1640 supplemented with 10% fetal calf serum, 20 ng/ml murine granulocyte–macrophage colony-stimulating factor, and 20 ng/ml of mIL-4. After 7 days of BM culture, >80% of the cells were CD11c+MHCII+ CD11b+CD8α− myeloid DCs. Plasmacytoid DCs were generated by culturing bone marrow cells in RPMI 1640 containing 100 ng/ml Flt3 ligand (R&D Systems) for 10 days (ref. 50).
DC chemotaxis assay. Chemotaxis assay was performed as described by Biragyn et al.28 with minor modifications. DCs (2 × 105 cells) were seeded into the upper compartment of microchemotaxis chamber (5-µm pore size; Costar, London, UK). Supernatants harvested from transiently transfected cos-1 cells with a pShuttle vector expressing mature human BD2 molecule (pShuttle-BD2), pShuttle-E1A, or pShuttle (mock) were added to the lower compartment to analyze migration toward gradient. Cells were incubated at 37 °C in humidified air with 5% CO2 for 1.5 hours. Migrated cells were harvested and counted by automatic Z2 COULTER Cell Counter (Beckman Coulter, Hialeah, FL). The results (mean ± SE of triplicate samples) are presented as the chemotactic index, defined as the fold increase in the number of migrating cells in the presence of test factors over the spontaneous cell migration into supernatant from nontransfected cos-1 cells. Each experiment was performed twice in triplicate wells. Human BD2 expression was detected in supernatants of pShuttle-BD2 by immunoprecipitation with antihuman BD2 Abs (data not shown). In experiments with recombinant BD2 protein (PeproTech) chemotaxis of pDCs was measured by migration through a polycarbonate filter with 5 µm pore size in 96-Multiwell HTS Fluoroblok plates (BD Biosciences, San Jose, CA). Mouse pDCs were enriched (95% pure) from Flt3L-stimulated bone marrow cell cultures by flow sorting with anti-CD11c and anti-mouse plasmacytoid dendritic cell antigen antibodies (Miltenyi Biotec). Cells were (10,000) labeled with Green-CMFDA cell tracker (Invitrogen, Carlsbad, CA) and added to the upper chamber in a total volume of 50 µl for 4 hours at 37 °C and 5% CO2. Lower chamber was filled with media alone (background control) or with different doses of recombinant human BD2. Fluorescence of cells, which had migrated through the microporous membrane, was measured using the FLUOstar OPTIMA reader (BMG Labtech, Durham, NC). The mean fluorescence of spontaneously migrated cells (to the media without BD2) was subtracted from the total number of migrated cells for each condition. The assay was performed in triplicates.
Vaccine efficacy study in mouse models. JC tumor cells (5 × 105) were inoculated s.c. in Balb/c mice. When tumors developed ~5 mm in diameter (7 days after tumor inoculation), mice were intratumorally injected with 1010 ifu of virus Ad-BD2-E1A or Ad-E1A or PBS. The injections were repeated on days 8 and 14 after tumor inoculation. Tumor volumes were measured every 2 days with a caliper. In rechallenge experiments 105 JC cells were inoculated 2 days after the tumor resections. E.G7-OVA cells were inoculated s.c. in the right flank of C57BL/6 mice. Primary tumors were treated with the viral vectors as described above. The day before the first treatment of primary tumors, mice were inoculated s.c. with E.G7-OVA tumor cells (distant tumors) into the left flank. Tumors were surgically resected when they grew >10 mm in diameter to avoid unnecessary animal suffering (day 21–25 postinoculation).
Immunohistochemistry. Freshly obtained tumor tissue was embedded in OCT (Sakura Finetek USA, Torrance, CA), snap-frozen on dry ice, and stored at −20 °C. Cryostat sections (5-µm thick) were air-dried at RT, fixed in cold acetone (10 minutes), rinsed in PBS (pH 7.4), and the nonspecific binding was blocked by incubation with 1% goat serum (Vector Laboratories, Burlingame, CA). Sections were then stained for 1 hour in the presence of anti-mouse monoclonal Abs conjugated with the fluorochromes: CD11c-PE, CD45RA (B220)-PE, CD40-FITC (BD Biosciences, Palo Alto, CA) diluted 1:50 in PBS.
Flow cytometric analysis of tumor suspension cells. Single-cell tumor suspensions were prepared by crushing tumors through a 70-µm pore nylon cell strainer (Falcon; BD Biosciences, Franklin Lakes, NJ) using a 3-ml syringe plunger and rinsing with cold PBS/5 mmol/l EDTA. Cells were pelleted by centrifugation at 4 °C at 400 g for 5 minutes, and then red blood cells were lysed in ammonium chloride cell lysis buffer for 5 minutes at RT. Cells were incubated with 0.5 µg of Fc block/106 cells (BD Biosciences, San Diego, CA). Anti-mouse CD11c-APC, CD45RA-PE, and CD8α-PerCP (all from BD Biosciences, San Diego, CA) were used for staining the tumor suspension cells.
IFN-γ ELIspot assay. To determine the frequency of T cells producing IFN-γ in response to a specific stimulus, an ELIspot assay was performed. DCs were isolated before the ELIspot assay using CD11c magnetic beads from the spleens of naive mice and pulsed with JC or CT26 (irrelevant control) tumor lysates (at a ratio of one tumor cell equivalent to one DC) for 48 hours. Millipore MultiScreen-HA plates were coated with 10 µg/ml of monoclonal anti-mouse IFN-γ AN18 antibody (Mabtech AB, Nacka, Sweden). Wells were blocked with RPMI medium with 10% fetal bovine serum. Splenocytes (105 cells/well) were added and cultured for 20 hours at 37 °C in a 5% CO2 incubator in complete medium (RPMI, 10% fetal bovine serum, 1% nonessential amino acids, penicillin, streptomycin, glutamine) in the presence of 5,000 DCs/well pulsed with JC or CT26 cell lysates. After washes, a second biotinylated monoclonal antibody to mouse IFN-γ (R4-6A2, Mabtech AB) was applied to the wells at a concentration of 1 µg/ml, followed by incubation with streptavidin–alkaline phosphatase complexes (Vector Laboratories). Plates were then developed with the alkaline phosphatase substrate, 3-amino-9 ethylcarbazole (Sigma-Aldrich). Numbers of spots in the wells were scored by ZellNet Consulting (New York, NY) with an automated ELIspot reader system (Carl Zeiss, Thornwood, NY). The number of IFN-γ-producing lymphocytes was evaluated in triplicate wells. Average ± SD for each mouse was calculated. The assays were repeated twice.
Cytotoxicity assay. Splenocytes were tested for cytolytic activity in a standard 6-hour 51Cr-release assay. Briefly, splenocytes were stimulated for 5 days with OT-1 peptide (20 µg/ml) or JC cell lysates at a T-cell tumor ratio of 2:1 in ELIspot medium with 20 U/ml of IL-2 (R&D Systems). Tumor cells were labeled at 37 °C with 51Cr (150 µCi) (Amersham Biosciences) in 500 µl of complete RMPI 1640 medium for 2 hours, washed twice with RPMI 1640, and resuspended in ELIspot medium. Different numbers of effector cells were incubated with a constant number of target cells (1 × 104/well) in 96-well U-bottom plates (200 µl/well) for 6 hours at 37 °C. The effector-to-target cell ratios in triplicate were 1:100, 1:50, 1:25, 1:12. The supernatant from each well was harvested and the amount of 51Cr radioactivity released was measured by LS 6500 Multi-Purpose Scintilation Counter (Beckman Coulter). Two micrograms of monoclonal anti-mouse CD8α (BD Biosciences, San Diego, CA) were added for blocking assays. Yac-1 cells were used for blocking the natural killer cell activity at a Yac-1: E.G7-OVA cell ratio of 50:1. The percent specific lysis was calculated using the formula % specific lysis = (E−S)/(M−S), where E is the average counts per minute released from target cells in the presence of effector cells, S is the spontaneous counts per minute released in the presence of medium only, and M is the maximum counts per minute released in the presence of 2% sodium dodecyl sulfate. The assays were repeated three times or more.
Priming of naive T cells in vitro. DCs from Ad-BD2-E1A, Ad-E1A, and PBS-treated mice were isolated using CD11c magnetic beads. Splenocytes from naive mice were incubated with the DCs (from each group) at the splenocytes–DC ratio of 100:1. Splenocytes were incubated in ELIspot medium for 5–7 days and DCs were added every 2 days with ½ of medium change. Cytotoxicity assay was performed as described above.
T-cell proliferation assay. DCs were purified by using mouse CD11c micromagnetic beads (Miltenyi Biotec) from E.G7 tumors, which were resected 48 hours after the intratumoral injections of adenoviruses. Graded numbers of CD11c+-enriched tumoral DCs were cocultured with 3 × 105 of purified naive CD8+ T cells in 96-well flat-bottom plates coated with anti-mouse CD3 (clone 17A2; BD Biosciences, San Diego, CA) for 3 days. In the last 16 hours, 3H-thymidine (1 µCi/well) was added into the cell culture to monitor the 3H-thymidine incorporation.
Statistical analysis. All data are reported as mean ± SE. Statistical comparison was made using the two-tailed Student's t-test, and a value of P < 0.05 was accepted as significant.
SUPPLEMENTARY MATERIALFigure S1. A low level of anti-BD2 antibody response induced by Ad-BD2-EIA in situ vaccination.Figure S2. Ad-BD2-EIA treatment blocks metastasis of JC breast cancer cells to the lung and livers indicated by hematoxylin–eosin staining of the tumor sections.
Supplementary Material
A low level of anti-BD2 antibody response induced by Ad-BD2-EIA in situ vaccination.
Ad-BD2-EIA treatment blocks metastasis of JC breast cancer cells to the lung and livers indicated by hematoxylin–eosin staining of the tumor sections.
Acknowledgments
We thank Bangxing Hong and Lifeng Wang in the lab for technical assistance. This work was supported by grants from the US National Institutes of Health (R01CA90427, R01CA116677, and R01AI68472 to S.-Y.C., and R01 CA100841 to X.F.H.) and US Army Prostate Cancer program (DOD W81XWH-04-1-0194 to X.F.H.).
REFERENCES
- Stoler DL, Chen N, Basik M, Kahlenberg MS, Rodriguez-Bigas MA, Petrelli NJ, et al. The onset and extent of genomic instability in sporadic colorectal tumor progression. Proc Natl Acad Sci USA. 1999;96:15121–15126. doi: 10.1073/pnas.96.26.15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Yewdell JW., and , Yang JC. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427:252–256. doi: 10.1038/nature02240. [DOI] [PubMed] [Google Scholar]
- Vigneron N, Stroobant V, Chapiro J, Ooms A, Degiovanni G, Morel S, et al. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304:587–590. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. Inducing autoimmune disease to treat cancer. Proc Natl Acad Sci USA. 1999;96:5340–5342. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne WB, Srinivasan R, Wolchok JD, Hawkins WG, Blachere NE, Dyall R, et al. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., and , Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol. 2003;51:59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- Behrens G, Li M, Smith CM, Belz GT, Mintern J, Carbone FR, et al. Helper T cells, dendritic cells and CTL Immunity. Immunol Cell Biol. 2004;82:84–90. doi: 10.1111/j.1440-1711.2004.01211.x. [DOI] [PubMed] [Google Scholar]
- De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D., and , Stockinger B. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–178. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D., and , Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Robbiani DF, Finch RA, Jäger D, Muller WA, Sartorelli AC., and , Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Allavena P, Vecchi A., and , Mantovani A. The role of chemokines in the regulation of dendritic cell trafficking. J Leukoc Biol. 1999;66:1–9. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Allavena P, D'Amico G, Luini W, Bianchi G, Kataura M, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S, et al. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. 2003;200:255–268. doi: 10.1002/path.1344. [DOI] [PubMed] [Google Scholar]
- Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto K, Soares L, Engleman EG., and , Merad M. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J Clin Invest. 2004;113:774–783. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA., and , Kwak LW. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002;100:1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- Freytag SO, Rogulski KR, Paielli DL, Gilbert JD., and , Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- Huang XF, Ren W, Rollins L, Pittman P, Shah M, Shen L, et al. A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. Cancer Res. 2003;63:7321–7329. [PubMed] [Google Scholar]
- Wang Y, Hallden G, Hill R, Anand A, Liu TC, Francis J, et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328–1335. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- Bals R, Wang X, Meegalla RL, Wattler S, Weiner DJ, Nehls MC, et al. Mouse β-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun. 1999;67:3542–3547. doi: 10.1128/iai.67.7.3542-3547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. β-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Huang GT, Zhang HB, Kim D, Liu L., and , Ganz T. A model for antimicrobial gene therapy: demonstration of human β-defensin 2 antimicrobial activities in vivo. Hum Gene Ther. 2002;13:2017–2025. doi: 10.1089/10430340260395875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV, et al. Human-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA. 2007;104:18631–18635. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soruri A, Grigat J, Forssmann U, Riggert J., and , Zwirner J. β-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol. 2007;37:2474–2486. doi: 10.1002/eji.200737292. [DOI] [PubMed] [Google Scholar]
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, et al. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochrein H, O'Keeffe M., and , Wagner H. Human and mouse plasmacytoid dendritic cells. Hum Immunol. 2002;63:1103–1110. doi: 10.1016/s0198-8859(02)00748-6. [DOI] [PubMed] [Google Scholar]
- Brasel K, De Smedt T, Smith JL., and , Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- O'Keeffe M, Hochrein H, Vremec D, Scott B, Hertzog P, Tatarczuch L, et al. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood. 2003;101:1453–1459. doi: 10.1182/blood-2002-03-0974. [DOI] [PubMed] [Google Scholar]
- Nakano H, Yanagita M., and , Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- Jonasch E., and , Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6:34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- Platsoucas CD, Fox FE, Oleszak E, Fong K, Nanno M, Ioannides CG, et al. Regulation of natural killer cytotoxicity by recombinant α interferons. Augmentation by IFN-α7, an interferon similar to IFN-αJ. Anticancer Res. 1989;9:849–858. [PubMed] [Google Scholar]
- Vertuani S, Bazzaro M, Gualandi G, Micheletti F, Marastoni M, Fortini C, et al. Effect of interferon-α therapy on epitope-specific cytotoxic T lymphocyte responses in hepatitis C virus-infected individuals. Eur J Immunol. 2002;32:144–154. doi: 10.1002/1521-4141(200201)32:1<144::AID-IMMU144>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Gilliet M., and , Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Brière F, et al. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- Kirk CJ, Hartigan-O'Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, et al. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–2070. [PubMed] [Google Scholar]
- Hillinger S, Yang SC, Zhu L, Huang M, Duckett R, Atianzar K, et al. EBV-induced molecule 1 ligand chemokine (ELC/CCL19) promotes IFN-γ-dependent antitumor responses in a lung cancer model. J Immunol. 2003;171:6457–6465. doi: 10.4049/jimmunol.171.12.6457. [DOI] [PubMed] [Google Scholar]
- Guo J, Wang B, Zhang M, Chen T, Yu Y, Regulier E, et al. Macrophage-derived chemokine gene transfer results in tumor regression in murine lung carcinoma model through efficient induction of antitumor immunity. Gene Ther. 2002;9:793–803. doi: 10.1038/sj.gt.3301688. [DOI] [PubMed] [Google Scholar]
- Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Conway BA, et al. Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A low level of anti-BD2 antibody response induced by Ad-BD2-EIA in situ vaccination.
Ad-BD2-EIA treatment blocks metastasis of JC breast cancer cells to the lung and livers indicated by hematoxylin–eosin staining of the tumor sections.