Abstract
The microtubule-associated protein tau (MAPT) and α-synuclein (SNCA) genes play central roles in neurodegenerative disorders. Mutations in each gene cause familial disease, whereas common genetic variation at both loci contributes to susceptibility to sporadic neurodegenerative disease. Here, we demonstrate exquisite gene regulation of the human MAPT and SNCA transgene loci and functional complementation in neuronal cell cultures and organotypic brain slices using the herpes simplex virus type 1 (HSV-1) amplicon-based infectious bacterial artificial chromosome (iBAC) vector to express complete loci >100 kb. Cell cultures transduced by iBAC vectors carrying a 143 kb MAPT or 135 kb SNCA locus expressed the human loci similar to the endogenous gene. We focused on analysis of the iBAC-MAPT vector carrying the complete MAPT locus. On transduction into neuronal cultures, multiple MAPT transcripts were expressed from iBAC-MAPT under strict developmental and cell type–specific control. In primary neurons from Mapt−/− mice, the iBAC-MAPT vector expressed the human tau protein, as detected by enzyme-linked immunosorbent assay and immunocytochemistry, and restored sensitivity of Mapt−/− neurons to Aβ peptide treatment in dissociated neuronal cultures and in organotypic slice cultures. The faithful retention of gene expression and phenotype complementation by the system provides a novel method to analyze neurological disease genes.
Introduction
The microtubule-associated protein tau (MAPT or tau) and α-synuclein (SNCA) genes and encoded proteins play central roles in neurodegenerative disorders.1,2 Both proteins are found aggregated in neurons in disease, point mutations in each gene cause rare dominantly inherited familial forms of disease, and common genetic variation at both genomic loci affects gene expression and splicing, contributing to susceptibility to sporadic neurodegenerative diseases.
The tau protein is the major component of the intracellular neurofibrillary tangles observed in many neurodegenerative disorders collectively known as tauopathies,3 which include Alzheimer's disease, frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD). The MAPT gene locus is located on chromosome 17 and consists of 16 exons. Six isoforms are expressed in the adult central nervous system produced by alternative splicing of exons 2, 3, and 10. Alternative splicing of exon 10 leads to a protein containing either three (3R; exon 10−) or four (4R; exon 10+) tandem repeats of a microtubule-binding motif.3 Coding point mutations, and splice site mutations increasing exon 10 inclusion, cause FTDP-17, a rare dominantly inherited condition,4,5 whereas common noncoding variation is found to be associated with the sporadic diseases PSP6,7,8,9,10 and CBD,11,12 and more recently with Alzheimer's disease.13 The SNCA locus is on chromosome 4 and contains 6 exons. Three separate point mutations in SNCA (A53T, A30P, and E46K),14,15,16 as well as duplications17 or triplications18 of the wild-type locus, are known to cause rare familial Parkinson's disease (PD). Moreover, polymorphisms present 10 kb upstream of the start of SNCA transcription, have been shown to increase reporter gene activity, and been associated with sporadic PD.19 It is, therefore, clear that genetic variation across both loci is implicated in mechanisms of neurodegenerative disease.
Biological models to study gene expression from the SNCA and MAPT loci are urgently required if we are to understand the molecular mechanisms of neurodegenerative disease. Infectious vectors are an efficient means of delivering genes to cells, but the size of most genomic loci generally precludes their use in the context of viral constructs. We have recently developed an efficient viral delivery and expression system for genomic DNA loci >100 kb in size, which we have termed the infectious bacterial artificial chromosome (iBAC).20,21,22,23,24,25,26 The iBAC is based on the herpes simplex virus type 1 (HSV-1) amplicon vector. HSV-1 amplicons are promising tools for infectious genomic DNA locus delivery because HSV-1 has a high transgene capacity of ~160 kb, high-titer amplicon stocks can be produced free from viral gene contamination by a helper virus-free packaging system,27 and the resulting virion particles have a broad cell tropism across a wide range of species. We have previously demonstrated the ability of the iBAC system: (i) to efficiently deliver and express by infecting a genomic DNA locus >100 kb in size to a range of cell types, including primary cells;20 (ii) to express genomic DNA loci at a physiologically relevant level;20,22,25 (iii) to deliver a locus to correctly retain complex features including promoter regulation22,25 and alternative splicing;24 and (iv) to correct the cellular deficiency in cells from patients with the neurodegenerative disorder Friedreich's ataxia.26
Here, we describe the design and construction by homologous recombination (HR) gap-end joining of iBAC vectors expressing the complete genomic loci of MAPT or SNCA. The vectors expressed genomic locus inserts in neuronal cultures, including primary mouse neuronal cultures, organotypic slice cultures, and dopaminergic neurons, differentiated from embryonic stem (ES) cells. As our primary model, we focused on expression from the iBAC-MAPT vector and demonstrated tau expression was under correct developmental and cell type–specific regulation. Functional tau expression was verified in biological assays demonstrating the restoration of sensitivity to Aβ peptide treatment in iBAC-MAPT transduced primary neurons and organotypic brain slices prepared from Mapt−/− mice.
We propose that the iBAC vector will allow the development of improved cell culture models to facilitate future detailed studies of the effects of genetic variation in disease. Such vectors have significant advantages over complementary DNA-based systems, which typically overexpress a single splice variant from a strong heterologous promoter and fail to capture the complexity of expression from the context of a genomic DNA locus.
Results
Construction of iBAC vectors
We have devised a general strategy suitable for the expression of genomic DNA loci in a wide range of neuronal culture systems by combining three technologies: (i) the high capacity of the bacterial artificial chromosome (BAC) cloning vector; (ii) the ability to precisely manipulate large BAC inserts with base-pair resolution using HR in bacteria; and (iii) the high capacity and neural tropism of the HSV-1 amplicon vector. Here, we demonstrate the utility of the system by first expressing two genes critically involved in neurodegenerative diseases in a variety of neuronal cultures, and then by focusing on the iBAC-MAPT vector to demonstrate correct developmental and cell-type control of RNA and protein expression.
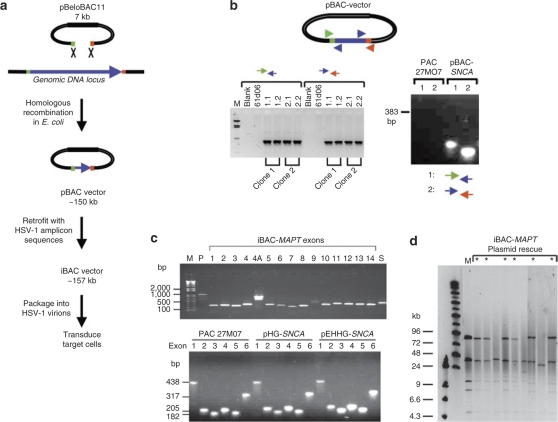
The broad cloning strategy we employed is shown in Figure 1a. The exact genomic DNA insert selected for excision is defined by 55 nucleotide (nt) homology arm sequences at the 5′ and 3′ ends of the genomic DNA locus. An additional 25 nt, complementary to the pBeloBAC11 vector, is added at the 3′ end of the recombination primer to allow the homology arms to be incorporated onto pBeloBAC11 by PCR. HR in Escherichia coli is used to excise the insert from the original high capacity library cloning vector [in this case, a P1 plasmid-based artificial chromosome (PAC)] and place it into pBeloBAC11 to create a pBAC vector. Cre-loxP recombination is then used to retrofit the pBAC vector with a plasmid carrying the origin of replication (oriS) and packaging signal (pac) from HSV-1 to create an iBAC vector suitable for packaging as an HSV-1 amplicon for subsequent delivery to neuronal cells by viral transduction.
Figure 1.
General strategy for infectious bacterial artificial chromosome (iBAC) vector construction by gap end-joining and Cre-loxP recombination. (a) Genomic DNA inserts carrying whole gene loci are transferred from the original library clone into pBeloBAC11 by homologous recombination in Escherichia coli to create a pBAC vector. Cre-loxP retrofitting then incorporates the replication and packaging elements from herpes simplex virus type 1 (HSV-1) to create an iBAC vector. The iBAC-MAPT and iBAC-SNCA vectors contain genomic inserts of 143 and 135 kb, respectively. (b) Correct transfer of the genomic DNA insert by gap end-joining is confirmed by PCR across the newly formed junctions. PAC61D06 and PAC27M07 refer to the original library clones. (c) The presence of all exons was confirmed by PCR for iBAC-MAPT and iBAC-SNCA vectors. (d) Plasmid rescue assay for the 157 kb iBAC-MAPT vector confirms intact packaging and delivery of the MAPT genomic locus insert. Total DNA extracted from transduced fibroblasts was transformed into E. coli and bacterial colonies selected on chloramphenicol/ampicillin. Plasmid DNA was prepared from resistant bacterial colonies, digested with Not1, and resolved by pulsed field gel electrophoresis. An asterisk indicates a bacterial colony confirming intact rescue; 6/9 colonies contained an intact plasmid. M, marker lane running positive control iBAC-MAPT plasmid.
Specifically, we first obtained PAC clones PAC61D06 and PAC27M07, covering the complete genomic locus of the human MAPT and SNCA genes, respectively. To construct iBAC-MAPT, HR was used to transfer a 143 kb genomic DNA region of PAC61D06 carrying the complete MAPT locus to pBeloBAC11, creating pBAC-MAPT. Briefly, the pBeloBAC11 plasmid was amplified by PCR using HR, primers HR1 and HR2. The 7.3 kb pBeloBAC11 PCR product incorporating 55 bp homology arms at each end was electroporated into E. coli bacteria carrying the pBAD-ETγ-Tet plasmid induced to express RecE and RecT and recombinant clones carrying the desired product were identified by pulsed field gel electrophoresis. The pBAC-MAPT insert comprises 9 kb of promoter sequence and 134 kb of genomic DNA, including two alternative polyadenylation sequences at the 3′ end of the MAPT locus. pBAC-SNCA was created in parallel, carrying a 135 kb genomic DNA insert carrying the 111 kb SNCA locus, 18 kb of 5′ sequence, and 6 kb of 3′ sequence using primers HR3 and HR4. The efficiency of recombination producing the correct product was 2/16 and 2/12 colonies screened for pBAC-MAPT and pBAC-SNCA, respectively. Construction of the new vector was confirmed by analysis of the junctions formed by recombination in independent clones (Figure 1b). Cre-loxP recombination was used to retrofit pBAC-MAPT and pBAC-SNCA with pHG or pEHHG, plasmids carrying either the HSV-1 amplicon packaging elements with (pEHHG) or without (pHG) episomal retention elements.20,22 We confirmed the presence of all MAPT or SNCA exons in our final vectors by exon PCR (Figure 1c).
Infectious iBAC-MAPT and iBAC-SNCA amplicons were prepared, as previously described.20,22,27 Finally, the packaging and delivery of intact genomic DNA inserts >100 kb by HSV-1 virions was confirmed by plasmid rescue of iBAC vector from transduced human fibroblasts back into bacteria, as previously described.20,22 Total DNA extracted from transduced human fibroblasts was transformed into E. coli and bacterial colonies selected on chloramphenicol/ampicillin. The 157 kb iBAC-MAPT plasmid was shown to be packaged and delivered intact with an efficiency of 67% (6/9 bacterial colonies carried intact iBAC-MAPT vector as analyzed by Not1 restriction enzyme digestion and pulsed field gel electrophoresis; Figure 1d), an efficiency in very close agreement with our previous data.20,22
iBAC-SNCA is expressed in differentiated mouse neuronal models
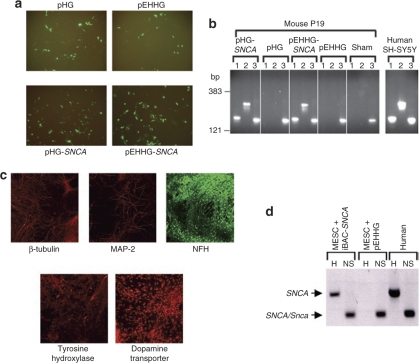
Expression from iBAC-SNCA was demonstrated by transduction into two murine neuronal-like cell culture models (Figure 2). The first model was the mouse P19 cell line, which was transduced with iBAC-SNCA or control vectors at a multiplicity of infection (MOI) of five (Figure 2a) and RNA prepared from the cultures 48-hour postinfection. Reverse transcription PCR (RT-PCR) using primers specific for human SNCA demonstrated expression of the human SNCA transgene in cells transduced by the iBAC-SNCA vectors but not the pHG and pEHHG control infections (Figure 2b).
Figure 2.
Delivery and expression of iBAC-SNCA in mouse neuronal models. (a) Expression of vector backbone-encoded green fluorescent protein visualized by fluorescence microscopy confirmed delivery of iBAC-SNCA and control vectors to mouse P19 cells transduced at an MOI of five. pHG and pEHHG are two variants on the iBAC vector backbone (see Materials and Methods for details). (b) Species-specific reverse-transcriptase PCR (RT-PCR) was performed on RNA extracted from transduced P19 cells. RT-PCR products corresponding to expression of the SNCA transgene were seen following iBAC-SNCA transduction but not in untransduced cells or cells transduced with pHG or pEHHG alone. The nonspecies specific SNCA/Snca primer pair (NS) confirms the cells express endogenous Snca. RNA extracted from human SH-SY5Y cells is a positive control for SNCA expression. RT-PCR primer pairs: 1, human specific SNCA 5′; 2, human specific SNCA 3′; 3, nonspecific SNCA/Snca. (c) Immunofluorescence analysis of mouse ET14tg2a ES cells after differentiation into dopaminergic neurons confirms expression of neuronal markers such as β-tubulin, microtubule associated protein 2 (MAP-2) and neurofilament heavy chain (NFH) a well as tyrosine hydroxylase (TH) and the dopamine transporter (DAT), markers specific for dopaminergic neurons. (d) Species-specific RT-PCR was performed on RNA extracted from differentiated ES cell-derived dopaminergic neurons transduced with iBAC-SNCA or the control pEHHG vector at an MOI of one. RT-PCR confirms expression of the iBAC-SNCA transgene and endogenous Snca expression. RNA extracted from differentiated MESC2.10 human mesencephalic cells is a positive control for SNCA expression. RT-PCR primer pairs: H, human-specific SNCA 3′; NS, nonspecific SNCA/Snca.
SNCA has shown to be intimately involved in the regulation of dopamine homeostasis, representing a likely mechanism underlying the pathology of PD. Dopaminergic neurons, therefore, represent an attractive target for iBAC-SNCA transduction. Mouse ET14tg2a ES cells were induced to differentiate into dopaminergic neurons, as previously described.28 The ES cells developed a characteristic neuronal morphology and expressed neuronal markers β-tubulin, microtubule associated protein 2 and neurofilament protein, heavy chain (NFH), as well as specific markers of dopaminergic neurons, dopamine transporter, and tyrosine hydroxylase (Figure 2c). Differentiated ES cells were efficiently transduced with iBAC-SNCA vectors (MOI = 1) and expressed the SNCA transgene (Figure 2d).
Expression of iBAC-MAPT in primary neurons is under developmental control and mirrors endogenous splicing regulation
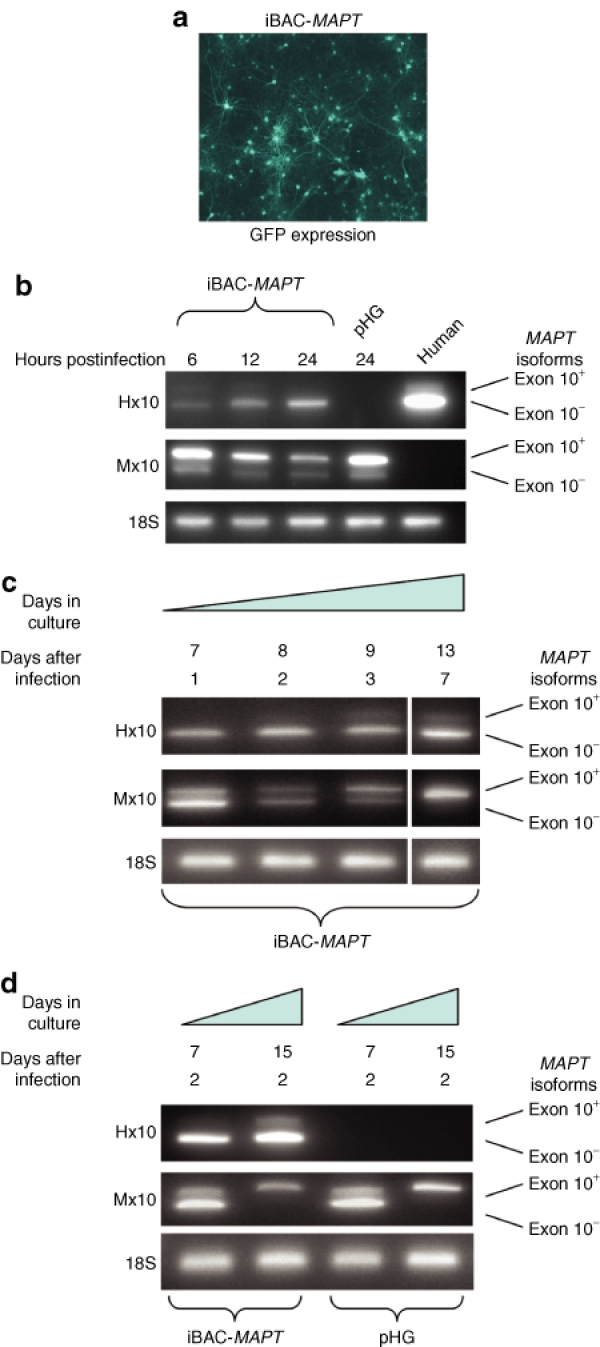
Primary neurons were prepared from new-born P0 wild-type C57BL6 mice and transduced with iBAC-MAPT at an MOI of one, after 6 days in culture. Expression from the vector backbone-encoded green fluorescent protein (GFP) reporter gene measured 48 hours post-transduction confirmed efficient transduction (Figure 3a). At 6, 12, and 24 hours postinfection RNA was prepared from the neuronal cultures and RT-PCR carried out to specifically detect human MAPT transgene expression using PCR primers specific for human MAPT flanking the alternatively spliced exon 10 (Hx10). PCR products of 390 and 297 bp demonstrate expression of exon 10+(4R) and exon 10−(3R) MAPT isoforms, respectively.29 MAPT transgene expression can be detected as early as 6 hours postinfection of primary cortical neurons (Figure 3b). Mouse Mapt exon 10 specific primers (Mx10) were able to detect mouse Mapt transcript in all neuronal cultures, as both exon 10+(4R) and exon 10−(3R) forms. We next compared the relative expression levels of transgene-derived exon 10+(4R) and exon 10−(3R) MAPT at later time points (Figure 3c). Neurons were transduced at an MOI of one after 6 days in culture and RNA was harvested 1, 2, 3, and 7 days post-transduction. Initially, a low amount of iBAC-MAPT derived exon 10+(4R) MAPT was seen after 7 days in culture, but levels of exon 10+(4R) MAPT rise after prolonged culture periods of up to 13 days. This broadly coincides with the developmental switch of Mapt expression in rodent neurons from the exon 10−(3R) to exon 10+(4R) form (Figure 3c), previously reported to occur at day 8, becoming complete by day 15 (ref. 30). We next determined that the important parameter was the length of time of in vitro culture rather than the time post-transduction. Mouse cortical neurons in culture for either 5 or 15 days were transduced with iBAC-MAPT at an MOI of one and gene expression analyzed 2 days postinfection. Expression from iBAC-MAPT in neurons in culture for 7 days showed no detectable exon 10+(4R) MAPT expression (Figure 3d). However, detection of the exon 10+(4R) MAPT form was readily detectable as a fraction of total MAPT expression after 15 days in culture. Semiquantitative analysis of band intensity performed by measuring pixel intensity on ethidium bromide–stained gels showed that the exon 10+(4R) MAPT form represented ~20% of MAPT expression after 15 days in culture. After this time, the switch to the exon 10+(4R) endogenous Mapt transcript is complete.
Figure 3.
iBAC-MAPT transgene expression in mouse primary neurons is regulated by endogenous developmental cues. (a) Vector backbone-encoded GFP expression 48 hours post-transduction confirms highly efficient delivery of iBAC-MAPT (MOI = 1) to primary mouse neuronal cultures prepared from P0 newborn pups. (b) Species-specific RT-PCR was performed on RNA extracted from transduced cells 6, 12, and 24 hours post-transduction. MAPT transgene expression is first seen 6 hours post-transduction. RNA extracted from human G16-9 glioblastoma cells is a positive control for MAPT expression. (c) P0 mouse primary cultures were transduced with iBAC-MAPT after 6 days in culture. RNA was extracted 1, 2, 3, or 7 days post-transduction and species-specific RT-PCR was performed. Relative expression of the exon 10+(4R) splice forms of both MAPT and Mapt increases with developmental maturation of neurons in culture. (d) P0 mouse primary cultures were maintained in vitro for either 5 or 13 days before transduction with iBAC-MAPT (MOI = 1). RNA was extracted 2 days post-transduction and species-specific RT-PCR was performed. The relative expression of both MAPT and Mapt exon 10+(4R) increased over time. Measuring relative band strength by quantifying pixel intensity showed the exon 10+(4R) form represents 100% of Mapt and ~20% of MAPT expression after 15 days in culture. Primer pairs: Hx10: human exon 10; Mx10: mouse exon 10.
Taken together, these data show that the temporal regulation of expression of the iBAC-MAPT human transgene is similar to that of endogenous mouse Mapt. Cells expressing iBAC-MAPT after only 7 days in culture expressed predominantly exon 10−(3R) endogenous mouse Mapt and human MAPT. After 15 days in culture endogenous expression is exclusively of mouse exon 10+(4R) Mapt. Simultaneously, expression of exon 10+(4R) from the iBAC-MAPT human transgene rises from <1% to ~20% of the level of exon 10−(3R) MAPT after 15 days growth in vitro, a level of expression of exon 10+(4R) MAPT similar to that reported in MAPT transgenic mice.31,32,33,34,35 Notably, the infection of iBAC-MAPT has no effect on splicing of endogenous Mapt.
iBAC-MAPT protein expression in Mapt−/− primary neuronal cultures
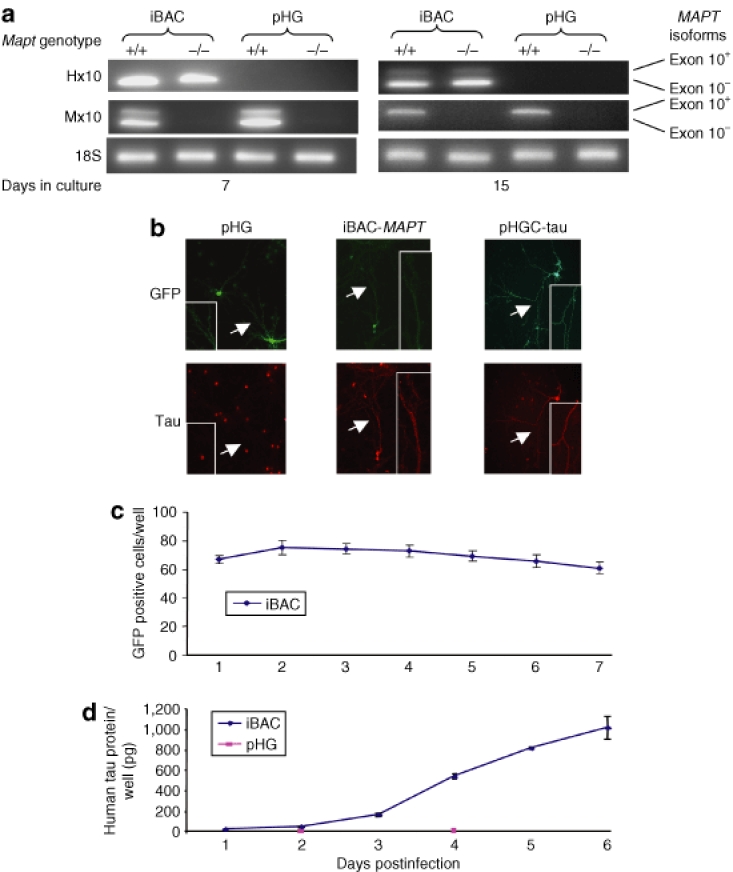
We next undertook expression studies in Mapt−/− neurons derived from the Mapttm1(GFP)Klt strain,36 which provides an advantageous Mapt null background to detect vector-encoded tau protein and allows functional studies to be undertaken. Mapt−/− P0 neurons were cultured for 5 or 13 days in vitro and transduced by iBAC-MAPT at an MOI of one. RNA was prepared from the cells 2 days post-transduction and species-specific RT-PCR was performed. The iBAC-MAPT transgene showed the same developmentally regulated increase in expression of exon 10+(4R) as seen in Mapt+/+ cultures (Figure 4a). Mapt−/− P0 neurons were then cultured for 5 days in vitro, transduced by iBAC-MAPT at an MOI of one and human tau protein expression was detected in transduced cells by immunocytochemistry 9 days post-transduction (Figure 4b). Axonal staining of tau protein can clearly be seen. A human tau specific enzyme-linked immunosorbent assay was then used to demonstrate a time-dependent increase of tau protein expression in Mapt−/− P0 primary neuronal cultures transduced by iBAC-MAPT. Mapt−/− P0 neurons were cultured for 5 days in vitro, transduced by iBAC-MAPT at an MOI of one and gene expression levels measured over time. Prolonged transduction of primary cultures was sustained over several days assayed by GFP reporter gene expression (Figure 4c), which correlated with a steady buildup of protein expression reaching 1,000 pg tau protein/well, measured by enzyme-linked immunosorbent assay over the 6-day period (Figure 4d).
Figure 4.
iBAC-MAPT transgene RNA and protein expression in mouse Mapt−/− primary neurons. (a) The iBAC-MAPT transgene RNA is expressed in primary neurons prepared from Mapt−/− P0 pups. P0 mouse primary cultures were transduced with iBAC-MAPT (MOI = 1) after either 5 or 13 days in culture. RNA was extracted 2 days post-transduction and species-specific RT-PCR was performed. The human exon 10+(4R) MAPT form is expressed in a developmentally regulated way, only present in mature neurons after 15 days in vitro. Primer pairs: Hx10, human exon 10; Mx10, mouse exon 10. (b) Immunocytochemistry confirms expression of tau protein in Mapt−/− neurons following transduction by iBAC-MAPT, but not pHG. P0 mouse Mapt−/− primary cultures were transduced with iBAC-MAPT or control vectors (MOI = 1) after 5 days in culture and immunocytochemistry was performed 9-days post-transduction. pHGC-tau is a positive control amplicon vector expressing human MAPT complementary DNA. Axonal staining of GFP and tau protein is indicated by an arrow and is seen in detail in the inset. The red cell-body staining is a nonspecific signal associated with the secondary antibody and seen in Mapt−/− neurons transduced with the pHG negative control vector. (c) Counts of GFP positive cells over time following iBAC-MAPT transduction. Prolonged iBAC vector backbone-encoded GFP expression confirms prolonged vector retention. (d) Tau protein levels per well measured by enzyme-linked immunosorbent assay over time following iBAC-MAPT or pHG transduction. iBAC-MAPT maintains prolonged tau protein expression leading to a steady increase in tau protein levels in tranduced cells. (c) and (d) P0 mouse Mapt−/− primary cultures were transduced after 5 days in culture and transduced at MOI = 1.
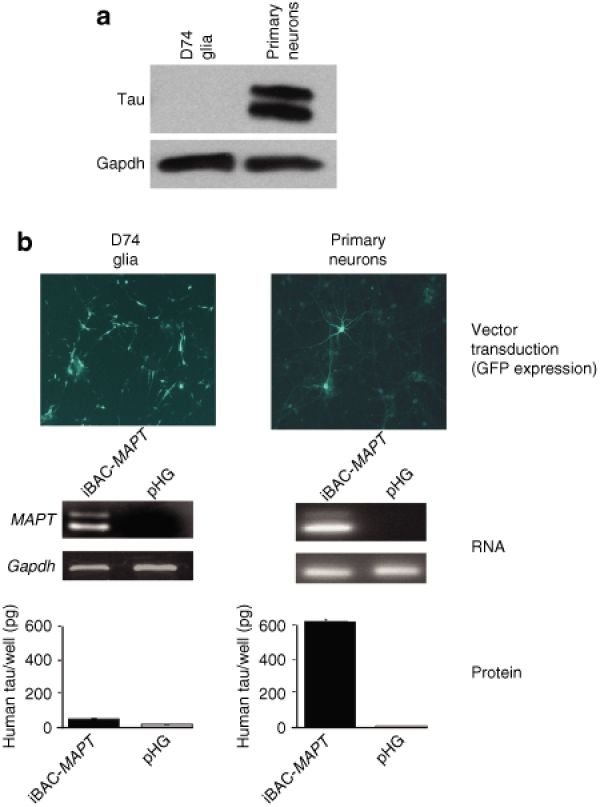
Correct cell-type regulation of tau protein expression from iBAC-MAPT
Having shown correct developmentally regulated splicing of MAPT exon 10, we next sought to demonstrate correct cell-specific expression from iBAC-MAPT to mirror endogenous expression. Western blot analysis shows that rat D74 glioma cells do not express endogenous tau protein, whereas primary mouse cortical cultures express high levels of tau (Figure 5a). Infection of D74 glioma cells with iBAC-MAPT was highly efficient (~60%) at an MOI of one. Following transduction, MAPT mRNA was easily detectable in both cell lines 48 hours post-transduction. However, tau protein was present at negligible amounts in D74 cells compared to primary cortical neurons 5 days post-transduction (Figure 5b). This accurately reflects the regulation of endogenous tau protein expression in the respective cell types. Overall, the iBAC-MAPT transgene mRNA is alternatively spliced and the resulting protein expression is subject to correct physiological regulation in a cell-type specific manner.
Figure 5.
iBAC-MAPT exhibits correct cell-type specific regulation of tau protein expression. (a) Primary neurons, but not D74 glial cells, strongly express endogenous tau protein by western blot. (b) D74 glia and primary neuronal cultures were transduced with iBAC-MAPT (MOI = 1) after 1 or 5 days in culture, respectively. Vector backbone-encoded GFP expression confirms efficient iBAC-MAPT delivery to both the D74 glial cell line and primary neuronal cultures 24-hours post-transduction. RT-PCR confirms iBAC-MAPT transgene RNA is expressed in both glial and neuronal cells 2-days post-transduction. However, transgenic tau protein measured by enzyme-linked immunosorbent assay is expressed only in transduced neuronal cells, but not in glial cells, an expression pattern matching that observed for endogenous expression.
Expression of iBAC-MAPT in tau knockout neurons restores Aβ sensitivity
We then devised two biological assays to detect restoration of tau function in primary neurons and organotypic slice cultures, derived from Mapt−/− mice. Primary neuronal cultures from Mapt−/− embryos have previously been shown to be resistant to Aβ peptide-induced toxicity,33 suggesting that the tau protein may mediate the neurotoxicity of Aβ fibrils. Therefore, to test the functionality of the iBAC-MAPT transgene we examined whether iBAC-MAPT could restore the responsiveness to Aβ peptide in the Mapt−/− neurons. Primary Mapt−/− P0 neurons were transduced with iBAC-MAPT or the pHG control vector at an MOI of one and fibrillar Aβ1-42 peptide (prepared at 100 µmol/l) was added to cultures 5 days post-transduction to a final concentration of 5 µmol/l and imaged for GFP expression 2 days later. Mapt−/− primary neurons transduced with pHG remained insensitive to treatment with Aβ1-42 peptide, whereas iBAC-MAPT transduced neurons showed signs of degeneration visible by microscopy indicating that tau protein expression was restoring Aβ1-42 toxicity in these cells (Figure 6a). Quantitative analysis of cell counts showed iBAC-MAPT transduction significantly restored sensitivity to Aβ1-42 peptide (Figure 6b).
Figure 6.
Expression of iBAC-MAPT in Mapt−/− primary neuronal cultures restores sensitivity to Aβ1-42 peptide toxicity. (a) P0 mouse Mapt−/− primary neurons cultured for 5 days were transduced with iBAC-MAPT or the control pHG vector at MOI = 1 and 5 days later were treated with Aβ1-42 peptide (5 µmol/l for 2 days) and then imaged for GFP expression. All transduced neurons express vector-encoded GFP reporter gene but neurons transduced with iBAC-MAPT show cell death and degeneration of axonal morphology compared to cells transduced with a control vector (pHG) after treatment with Aβ1-42 peptide. (b) Quantitative analysis of GFP-positive neurons in Mapt−/− primary neuronal cultures shows a highly significant increase in Aβ1-42 peptide mediated cell death after iBAC-MAPT transduction compared to the control vector pHG (**P < 0.01 by Student's t-test). The experiment was performed in triplicate three times and the highly significant result was obtained each time. Data from a representative experiment is shown; error bars represent SD.
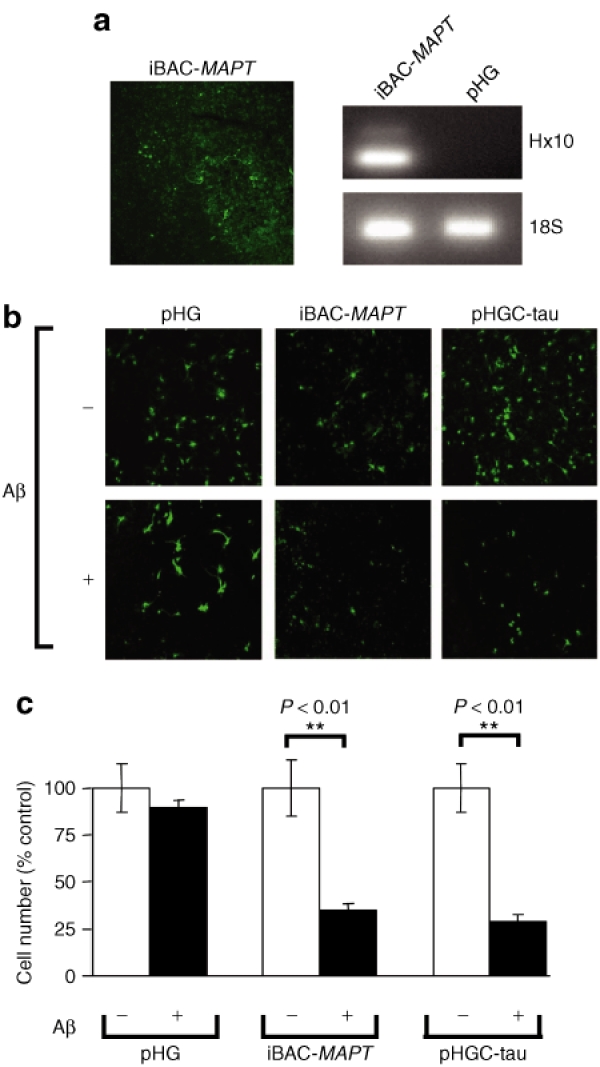
We next used organotypic brain slices as an improved neuronal model because they are viable for several weeks longer than dissociated cultures of primary neurons. Organotypic slice cultures were prepared from Mapt−/− pups, and transduced with iBAC-MAPT. We found that we could obtain efficient vector transduction and sustained expression of MAPT transgene in this model (Figure 7a). Similar to the Mapt−/− dissociated primary neuronal cell culture system, exposing brain slices to 20 µmol/l Aβ1-42 peptide for 48 hours, 7 days post-transduction, led to increased cell death in iBAC-MAPT transduced neurons compared with transduction with the pHG control vector (Figure 7b). Quantitative analysis of cell counts showed iBAC-MAPT transduction conferred sensitivity to Aβ1-42 peptide to Mapt−/− organotypic slice cultures (Figure 7c).
Figure 7.
Delivery and expression of iBAC-MAPT in Mapt−/− organotypic slice cultures. (a) Delivery of iBAC-MAPT to organotypic slice cultures of Mapt−/− mouse brain. Vector delivery is confirmed by vector-encoded GFP reporter gene and MAPT expression is assayed by RT-PCR detection of MAPT RNA. Slices were cultured for 25 days before vector transduction and imaged 2 days later. RNA was harvested for RT-PCR 4 days post-transduction. (b) Organotypic slice cultures transduced with iBAC-MAPT, or the positive control MAPT complementary DNA vector (pHGC-tau), and then treated with Aβ1-42 peptide, show increased cell death compared to slices transduced with a negative control vector (pHG). Slices cultured for 25 days were transduced using 1 × 105 transducing units/slice, and 7 days later treated with Aβ1-42 peptide (20 µmol/l for 2 days) and then imaged for GFP expression. (c) Quantitative analysis of GFP-positive cells in Mapt−/− organotypic slice cultures shows a highly significant increase in Aβ-peptide mediated cell death after transduction with iBAC-MAPT or the positive control pHGC-tau vector compared to the negative control vector pHG (**P < 0.01 by Student's t-test). The experiment was performed in triplicate three times and the highly significant result was obtained each time. Data from a representative experiment is shown; error bars represent SD.
Discussion
We describe here an advanced methodology for the delivery and expression of neurological disease loci to primary neuronal culture systems for functional analysis. The method makes use of the broad availability of BAC and PAC genomic DNA library clones, the ability for precise manipulation of vectors by HR, and the high transgene capacity and neuronal tropism of HSV-1 amplicon vectors. The resulting vectors are able to deliver and express functional genes of >100 kb in size under physiological regulation, including the generation of multiple splice variants. Such a system will likely find broad utility in the analysis of the genetic basis of neurological disease.
The MAPT and SNCA loci represent important neurodegenerative disease genes. Rare point mutations affecting coding regions and splice sites in MAPT cause FTDP-17 and point mutations in, or duplications or triplications of, SNCA cause familiar PD. Moreover, common genetic variation at the MAPT locus is associated with increased susceptibility to the movement disorders PSP, CBD, PD and, more recently, with Alzheimer's disease, the most common dementia. The association of the MAPT locus with PD,37,38,39,40,41 a synucleinopathy, as well as with tauopathies, suggests that tau plays a critical role in a range of neurodegenerative diseases, although the mechanisms are poorly understood. There is clearly an increasing need to understand the genetic basis of such diseases to better understand the biological pathways. This may in turn help generate preventative therapies to delay the onset of disease symptoms, or even lead to curative treatments.
The neurofibrillary tangles, which form in CBD and PSP, are predominantly formed of exon 10+(4R) and the diseases are referred to as 4R-tauopathies. Recent work by our group and others has started to uncover potential mechanisms underlying the association of the common MAPT H1 haplotype with PSP and CBD.42,43,44,45 We previously found that the MAPT H1 variant expressed up to 43% more exon 10+(4R) MAPT in human brain tissue than did the other common variant, H2, immediately suggesting a disease mechanism based on excess exon 10+(4R) expression.42 In a further study,43 we found that H2 expressed twofold more exon 2+3+ MAPT than did H1, possibly related to the involvement of the amino-terminal region as a projection domain regulating microtubule spacing. In agreement with the potential susceptibility risk conferred by H1, a recent large-scale genetic study found that the H2 haplotype is under positive selection in European populations.46 There is clearly mounting evidence for the role of noncoding DNA in neurodegenerative disease and new gene vector technologies, such as the one we describe here, are required for the manipulation, delivery, and expression of disease genes such as MAPT and SNCA. A BAC-based genomic DNA expression system capable of undergoing precise sequence-specific manipulation, followed by efficient delivery and expression in neuronal cells would greatly help to further dissect the genetic mechanisms underlying the association with disease.
Focusing on MAPT as a model, we demonstrate that a gene delivered in this manner is subject to developmental and cell-type specific regulation of gene splicing and protein expression. The human iBAC-MAPT transgene responds to the changing endogenous physiological splicing signals present in maturing neurons and increases the relative expression of exon 10+(4R) compared to exon 10−(3R). This relative increase in expression of exon 10+(4R) from the MAPT transgene in maturing mouse neurons occurs as endogenous Mapt expression switches from exon 10−(3R) to exon 10+(4R). Transgene expression is also regulated at the protein level, only being expressed in appropriate neuronal cell types and matching endogenous expression patterns. The tau protein expressed from the iBAC-MAPT transgene is functional, restoring sensitivity of Mapt−/− neurons to Aβ1-42 peptide. Finally, we show the broad utility of the system through the parallel construction of an iBAC-SNCA vector and expression of SNCA in differentiated dopaminergic neurons.
The use of the iBAC system combined with HR in bacteria is applicable for expression of almost any gene. Approximately 95% of all human gene loci are 150 kb in size or less (see www.ensembl.org), ensuring that the expression system has broad applicability. The strategy first requires identifying a BAC or PAC clone carrying the gene of interest, and then uses subcloning by gap end-joining to excise the required insert out of the starting library clone and into a second high-capacity vector. In both cases described here, a PAC insert is placed into a BAC vector, but we have also performed the reverse in unpublished studies. The new construct is then retrofitted using Cre-loxP recombination, packaged into HSV-1 transducing virion particles, and delivered to cells for gene expression.
Neurodegenerative disease is fast becoming become a huge burden on health care. Developing functional genomic analysis in the post-sequence era offers us new oppurtunities to better understand disease mechanisms. The new gene expression tools such as the one described will allow gene manipulation, delivery, and expression for functional analysis of noncoding sequence variation in differentiated neuronal cultures.
Materials and Methods
Vector construction and amplicon preparation. PAC clones PAC61D06 (Genome Systems, St Louis, MO) and PAC27M07 (Roswell Park Cancer Institute; RPCI-1 library, Buffalo, NY) were obtained carrying the complete genomic loci for MAPT and SNCA, respectively. Homologous recombination was performed using gap-end joining to subclone the required 143 kb and 135 kb inserts carrying MAPT and SNCA, respectively, using RecE/T technology.47 Briefly, 80 nt primers were designed incorporating 55 nt homology arms, defining either the 5′ or 3′ end of the genomic locus being excised with the addition of 25 nt at the 3′ end of the primer to amplify the large insert BAC cloning vector pBeloBAC11. All primer sequences for vector construction and expression analysis are given in Supplementary Table S1. The 80 nt primers were then used to amplify a 7.3 kb pBeloBAC11 vector fragment by PCR from a BamH1 linearized and gel-purified vector template using Pfu Turbo HotStart high-fidelity polymerase (Stratagene, La Jolla, CA). The PCR product was purified through a PCR purification column (Qiagen, Valencia, CA), eluted in 90 µl of water, digested with DpnII to remove template DNA, and column-purified again. Electrocompetent DH10B E. coli cells carrying the starting PAC construct and pBAD-ETγ-Tet, a modified variant of pBAD-ETγ (itself a kind gift of A.F. Stewart) kindly provided by Y. Saeki, were induced to express recombination genes by the addition of arabinose at a final concentration of 1%. The linear pBeloBAC11 PCR product incorporating 55 nt homology arms at each end was introduced into the cells by electroporation. After incubation for 75 minutes in SOC medium at 37 °C, bacteria were plated on chloramphenicol. Bacterial colonies carrying correctly recombined plasmids were identified. In many cases, chloramphenicol resistant bacteria carrying correctly recombined pBeloBAC11 clones carrying the genomic DNA insert were also resistant to kanamycin, indicating that the original PAC construct was retained. Retransformation of isolated plasmid DNA into E. coli and selection on chloramphenicol allowed bacterial clones resistant only to chloramphenicol to be isolated. Cre/loxP recombination was performed in vitro, as previously described.25 The pHGC-tau MAPT complementary DNA expression vector was constructed by subcloning the MAPT complementary DNA coding sequence from pcDNA3-tau (a gift from B. Hyman, Massachusetts General Hospital, Charlestown, MA) into the pHGCX amplicon vector.27 HSV-1 amplicon vector preparations were packaged using an improved helper virus–free packaging system, as previously described.27 HSV-1 amplicon preparations were concentrated through a 25% sucrose solution and titered on the G16-9 glioma cell line. Titers of around ~2–3 × 107 transducing units/ml were routinely obtained.
Primary neuronal cultures. Cultures of primary cortical neurons were performed, with modifications, as previously described.48 The Mapttm1(GFP)Klt Mapt−/− mouse strain36 in which an enhanced GFP expression cassette has been inserted into exon 1 of the Mapt gene was obtained from Jackson Laboratories (Bar Harbor, ME). C57BL6 mice were obtained from Charles River (Boston, MA). Briefly, P0 pups were euthanized in CO2 and the cerebral cortices were isolated and incubated in Hanks' buffered saline solution containing 0.25% trypsin (Invitrogen, Carlsbad, CA) and 0.2% DNAase (Sigma, St Louis, MO) for 15 minutes at 37 °C. Horse serum (Invitrogen) was added and the tissue was rinsed twice in phosphate buffered saline. Cells were dissociated in Neurobasal medium (Invitrogen) supplemented with B27 supplement (Invitrogen), 50 mmol/l β-mercaptoethanol and 200 mmol/l L-glutamine by repeated trituration through a fire-polished Pasteur pipette. The cells were then pelleted at 150 g for 3 minutes and resuspended in 10 ml of neurobasal medium plus supplements. Cells were filtered through a 40 µm mesh and plated. For biological assays, cells were plated at high density (1,500 cells/mm2) in polyethyleneimine precoated dishes, replacing half the medium every 3–4 days. Neurons were kept in culture for at least 5 days before being infected with viral vectors.
D74 (HveC) rat glial cells were previously generated in our laboratory and grown as described.49 Mouse ET14tg2a ES cells (a kind gift of A. Smith, Edinburgh, UK) were induced to differentiate into dopaminergic neurons using the Mouse Dopaminergic Neuron Differentiation Kit (R&D Systems, Abingdon, UK) following the manufacturer's protocol with reference to published methods.28
Gene expression assays. RNA was prepared from cells using Trizol Reagent (Invitrogen). RT was carried out on 1 mg total RNA using iSCRIPT (Bio-Rad, Hercules, CA). Semiquantitative RT-PCR was carried out using Hotstart Taq polymerase (Roche, Basel, Switzerland). Human and mouse exon 10 primers used were as described.29 All RT-PCR primer sequences are given in the Supplementary Table S1. Semiquantitative analysis of band intensity was performed by measuring pixel intensity on ethidium bromide–stained gels using NIH-distributed Image J software (NIH, Bethesda, MD).
Protein detection. For immunocytochemistry antibodies used were Tau-5 (BD Biosciences, Franklin Lakes, NJ) and Anti Human Tau (Pierce, Rockford, IL). For protein detection, a Human Tau (total) ELISA Kit (Biosource, Camarillo, CA) was used, according to the manufacturer's recommendations. Each sample was run in triplicate. Immunostaining was performed as follows: 8 days postinfection, cells were fixed in 1% paraformaldehyde, 20 mmol/l L-lysine (Sigma), and 400 mmol/l sodium periodate (Sigma) in 0.1 mol/l phosphate buffered saline for 1 hour. This was followed by treatment with 100 mmol/l β-mercaptoethanol in 0.4% Triton X-100/0.1 mol/l phosphate buffered saline for 1 hour at RT and successively exposed to Avidin-Biotin solutions (Avidin/Biotin blocking kit, Vector Laboratories, Burlingame, CA), before being neutralized with 10% donkey normal serum for 1 hour at 37 °C. Anti-tau primary antibodies were added as follows: Mouse Tau-5 at 1:100 and Pierce antihuman tau at 1:50, and left overnight at 4 °C. Control plates were left in normal serum overnight. The following day a biotin-SP-conjugated donkey antimouse IgG (ImmunoResearch Laboratories, West Grove, PA) was applied to all plates at a 1:100 concentration for 2 hours, and finally a 1:100 solution in 0.1 mol/l phosphate buffered saline of Streptavidin Alexa Fluor 568 conjugate (Molecular Probes, Eugene, OR) was added for 1 hour. Cells were then rinsed, coverslipped and analyzed using a Zeiss LSM confocal microscope (Carl Zeiss, Jena, Germany).
Brain slice preparation and infection. Organotypic brain tissue slices were aseptically prepared from postnatal day 5 to 7 (P5–P7) mice. The pups were killed, their brains rapidly excised and 350 micron sections made using a Leica Vibratome VT1000S. Slices were cultured on tissue culture plate inserts (Millicell-CM, 0.4 mm pore size, Millipore, Bedford, MA), fed with filtered minimum essential medium (50%), 25% fetal bovine serum, and 25% Hanks' buffered saline solution, L-glutamine (1 mmol/l) and D-glucose (36 mmol/l) supplemented with antibiotics and antimycotics at a final concentration of 10 U/ml penicillin, 10 µg/ml streptomycin, and 25 ng/ml amphotericin B. The slices were maintained in an incubator at 36 °C. After 3 weeks in culture the organotypic brain slices were treated with clodronate liposomes for 24 hours (supplied by N. Van Rooijen, VUMC, the Netherlands). The slices were then intensely rinsed in the medium, and 4 days later were infected with amplicons. HSV-1 amplicon transduction was carried out using 40 µl of vector of amplicon in Hanks' buffered saline solution applied onto each slice. The titer of amplicons: iBAC-MAPT: 2 × 107 transducing units/ml; pHG and pHGC-tau vectors: 1 × 108 transducing units/ml. Three hours later the amplicon solution was rinsed with medium, and the slices were cultured for 7 days before treatment with Aβ1-42 peptide.
β-Amyloid toxicity studies. Aβ1-42 peptide was obtained as a dry pellet from rPeptide (Bogart, GA) and prepared as described.50 Briefly, dry peptide was resuspended in hexafluoroisopropanol to dissolve any preformed protein aggregations, the alcohol was evaporated under vacuum and the remaining pellet stored at −80 °C. Twenty-four hours prior to use, each aliquot was resuspended in dimethyl sulfoxide at a concentration of 5 mmol/l, further diluted in F12 medium to a final concentration of 100 µmol/l and incubated at 4 °C for 24 hours to allow peptide oligomerization. Cultured cells or slices were exposed to Aβ1-42 peptide for 48 hours at a final concentration of 5 µmol/l (dissociated neuronal cultures) or 20 µmol/l (brain slices), after which time cultures were examined microscopically and counts of GFP positive cells performed.
SUPPLEMENTARY MATERIALTable S1. DNA oligomer sequence for homologous recombination and RT-PCR.
Supplementary Material
DNA oligomer sequence for homologous recombination and RT-PCR.
Acknowledgments
This work was supported by grants from the Harvard Center for Neurodegeneration and Repair, the Wellcome Trust, the Biotechnology and Biological Sciences Research Council, CurePSP and the PSP Association awarded to R.W.-M., and from the National Institutes of Health (R01 NS045961-91A1) awarded to E.A.C. R.W.-M. was a Wellcome Trust Research Career Development Fellow; S.L.S. was a Wellcome Trust Prize Student in Neuroscience. We thank Y. Saeki for help and advice on HSV-1 vector technology.
REFERENCES
- Lee VM-Y, Giasson BI., and , Trojanowski JQ. More than just two peas in a pod: common amyloidogenic properties of tau and α-synuclein in neurodegenerative disease. Trends Neurosci. 2004;27:129–134. doi: 10.1016/j.tins.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Galpern WR., and , Lang AE. Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- Goedert M., and , Spillantini MG. Tau mutations in frontotemporal dementia FTDP-17 and their relevance for Alzheimer's disease. Biochim Biophys Acta. 2000;1502:110–121. doi: 10.1016/s0925-4439(00)00037-5. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A., and , Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Pittman AM, Myers AJ, Duckworth J, Bryden L, Hanson M, Abou-Sleiman P, et al. The structure of the tau haplotype in controls and in progressive supranuclear palsy. Hum Mol Genet. 2004;13:1267–1274. doi: 10.1093/hmg/ddh138. [DOI] [PubMed] [Google Scholar]
- Pittman AM, Myers AJ, Abou-Sleiman P, Fung HC, Kaleem M, Marlowe L, et al. Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J Med Genet. 2005;42:837–846. doi: 10.1136/jmg.2005.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Melquist S, Cruts M, Theuns J, Del-Favero J, Poorkaj P, et al. High density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet. 2005;14:3281–3292. doi: 10.1093/hmg/ddi361. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Pittman AM, Zhao AS, Rohrer K, Kaleem M, Marlowe L, et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis. 2007;25:561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, et al. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann Neurol. 2000;47:374–377. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56:1702–1706. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Kaleem M, Marlowe L, Pittman AM, Lees AJ, Fung HC, et al. The H1c haplotype at the MAPT locus is associated with Alzheimer's disease. Hum Mol Genet. 2005;14:2399–2404. doi: 10.1093/hmg/ddi241. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. α-Synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. α-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Chiba-Falek O., and , Nussbaum RL. Effect of allelic variation at the NACP-Rep1 repeat upstream of the α-synuclein gene (SNCA) on transcription in a cell culture luciferase reporter system. Hum Mol Genet. 2001;10:3101–3109. doi: 10.1093/hmg/10.26.3101. [DOI] [PubMed] [Google Scholar]
- Wade-Martins R, Smith ER, Tyminski E, Chiocca EA., and , Saeki Y. An infectious transfer and expression system for genomic DNA loci in human and mouse cells. Nat Biotechnol. 2001;19:1067–1070. doi: 10.1038/nbt1101-1067. [DOI] [PubMed] [Google Scholar]
- Steele FR. Gene therapy tools for functional genomics. Genomics. 2002;80:1. doi: 10.1006/geno.2002.6790. [DOI] [PubMed] [Google Scholar]
- Wade-Martins R, Saeki Y., and , Chiocca EA. Infectious delivery of a 135 kb LDLR genomic locus leads to regulated complementation of low density lipoprotein receptor deficiency in human cells. Mol Ther. 2003;7:604–612. doi: 10.1016/s1525-0016(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Ackermann M., and , Fraefel C. One giant genomic leap for gene transfer technology. Mol Ther. 2003;7:571. doi: 10.1016/s1525-0016(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Inoue R, Moghaddam KA, Ranasinghe M, Saeki Y, Chiocca EA., and , Wade-Martins R. Infectious delivery of the 132 kb CDKN2A/CDKN2B genomic DNA region results in correctly spliced gene expression and growth suppression in glioma cells. Gene Ther. 2004;11:1195–1204. doi: 10.1038/sj.gt.3302284. [DOI] [PubMed] [Google Scholar]
- Lufino M, Manservigi R., and , Wade-Martins R. An S/MAR-based infectious episomal genomic DNA expression vector provides long-term regulated functional complementation of LDLR deficiency. Nucleic Acids Res. 2007;35:e98. doi: 10.1093/nar/gkm570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sebastian S, Gimenez-Cassina A, Diaz-Nido J, Lim F., and , Wade-Martins R. Infectious delivery and expression of a 135 kb human FRDA genomic DNA locus complements Friedreich's ataxia deficiency in human cells. Mol Ther. 2007;15:248–254. doi: 10.1038/sj.mt.6300021. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Fraefel C, Ichikawa T, Breakefield XO., and , Chiocca EA. Improved helper virus-free packaging system for HSV amplicon vectors using an ICP27-deleted, oversized HSV-1 DNA in a bacterial artificial chromosome. Mol Ther. 2001;3:591–601. doi: 10.1006/mthe.2001.0294. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM., and , McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- D'Souza I., and , Schellenberg GD. Tau exon 10 expression involves a bipartite intron 10 regulatory sequence and weak 5′ and 3′ splice sites. J Biol Chem. 2002;277:26587–26599. doi: 10.1074/jbc.M203794200. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Valerio A, Belloni M, Arrighi V, Alberici A., and , Liberini P, et al. Differential expression of fetal and mature tau isoforms in primary cultures of rat cerebellar granule cells during differentiation in vitro. Brain Res Mol Brain Res. 1995;34:38–44. doi: 10.1016/0169-328x(95)00129-g. [DOI] [PubMed] [Google Scholar]
- Duff K, Knight H, Refolo LM, Sanders S, Yu X, Picciano M, et al. Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol Dis. 2000;7:87–98. doi: 10.1006/nbdi.1999.0279. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Kas A, D'Souza I, Zhou Y, Pham Q, Stone M, et al. A genomic sequence analysis of the mouse and human microtubule-associated protein tau. Mamm Genome. 2001;12:700–712. doi: 10.1007/s00335-001-2044-8. [DOI] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP., and , Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- McMillan P, Korvatska E, Poorkaj P, Evstafjeva Z, Robinson L, Greenup L, et al. Tau isoform regulation is region- and cell-specific in mouse brain. J Comp Neurol. 2008;511:788–803. doi: 10.1002/cne.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Meyer M., and , Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Kwok JB, Teber ET, Loy C, Hallupp M, Nicholson G, Mellick GD, et al. Tau haplotypes regulate transcription and are associated with Parkinson's disease. Ann Neurol. 2004;55:329–334. doi: 10.1002/ana.10826. [DOI] [PubMed] [Google Scholar]
- Skipper L, Wilkes K, Toft M, Baker M, Lincoln S, Hulihan M, et al. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am J Hum Genet. 2004;75:669–677. doi: 10.1086/424492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah CE, Lesnick TG, Lincoln SJ, Strain KJ, de Andrade M, Bower JH, et al. Interaction of α-synuclein and tau genotypes in Parkinson's disease. Ann Neurol. 2005;57:439–443. doi: 10.1002/ana.20387. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Hutter CM, Factor SA, Nutt JG, Higgins DS, Griffith A, et al. Association analysis of MAPT H1 haplotypes and subhaplotypes in Parkinson's disease. Ann Neurol. 2007;62:137–144. doi: 10.1002/ana.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JE, Latourelle JC, Lew MF, Klein C, Suchowersky O, Shill HA, et al. Haplotypes and gene expression implicate the MAPT region for Parkinson's disease: the GenePD Study. Neurology. 2008;71:28–34. doi: 10.1212/01.wnl.0000304051.01650.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey TM, Joachim C, Paracchini S, Esiri M., and , Wade-Martins R. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet. 2006;15:3529–3527. doi: 10.1093/hmg/ddl429. [DOI] [PubMed] [Google Scholar]
- Caffrey TM, Joachim C., and , Wade-Martins R. Haplotype-specific expression of the N-terminal exons 2 and 3 at the human MAPT locus. Neurobiol Aging. 2008;29:1923–1929. doi: 10.1016/j.neurobiolaging.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey TM., and , Wade-Martins R. Functional MAPT haplotypes: bridging the gap between genotype and neuropathology. Neurobiol Dis. 2007;27:1–10. doi: 10.1016/j.nbd.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AJ, Pittman AM, Zhao AS, Rohrer K, Kaleem M, Marlowe L, et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis. 2007;25:561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J, et al. A common inversion under selection in Europeans. Nature Genet. 2005;37:129–137. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- Muyrers JPP, Zhang Y., and , Stewart AF. Techniques: recombinogenic engineering-new options for cloning and manipulating DNA. Trends Biochem Sci. 2001;26:325–331. doi: 10.1016/s0968-0004(00)01757-6. [DOI] [PubMed] [Google Scholar]
- Lesuisse C., and , Martin LJ. Long-term culture of mouse cortical neurons as a model for neuronal development, aging, and death. J Neurobiol. 2002;51:9–23. doi: 10.1002/neu.10037. [DOI] [PubMed] [Google Scholar]
- Wakimoto H, Fulci G, Tyminski E., and , Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide's enhancement of viral oncolysis. Gene Ther. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA., and , LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA oligomer sequence for homologous recombination and RT-PCR.