Abstract
The success of gene therapy strategies to cure disease relies on the control of unwanted immune responses to transgene products, genetically modified cells and/or to the vector. Effective treatment of an established immune response is much harder to achieve than prevention of a response before it has had a chance to develop. However, preventive strategies are not always effective in avoiding immune responses, thus the use of drugs to induce immunosuppression (IS) is required. The growing discovery of novel drugs provides a conceptual shift from using generalized, moderately intensive immunosuppressive regimens towards a refined approach to attain the optimal balance of naive cells, effector cells, memory cells, and regulatory cells, harnessing the natural tolerance mechanisms of the body. We review several strategies based on transient IS coupled with gene therapy for sustained immune tolerance induction to the therapeutic transgene.
Introduction
Over the past decades the gene therapy field has rapidly evolved from an initial focus on the efficacy of several viral and nonviral gene-transfer systems to the safety of these strategies, and this has culminated in the initiation of large numbers of early-phase clinical trials. The major safety issues identified from these preclinical and clinical studies include the risk of insertional mutagenesis, inadvertent germline transmission of vector sequences, and unwanted immune responses to the vector and to the therapeutic transgene.
Two of the central safety issues in using gene-based strategies to treat disease are tolerance induction to the transgene and avoiding any unwanted immune responses to the vector. Most gene therapy trials for genetic diseases are aimed at sustained expression of therapeutic genes by introducing the vector into the target tissue with minimal or no tissue damage. Transduced cells and/or the expression of the therapeutic transgene following delivery of vectors are potentially able to trigger alloimmune responses involving both naive and memory lymphocytes, including lymphocytes specific for viral antigens.1 This scenario creates, to a certain extent, a clinical parallel to the immune responses following organ transplantation in which neoantigens in the graft are presented to the host-immune system. To avoid allograft rejection, immunosuppression (IS) is required during the induction phase followed by a long-term maintenance regimen. There are major differences between gene therapy and organ transplantation, such as the amounts of antigen presented, nature of antigen and number of antigen-specific T cells. Thus, the intense IS that is required for organ transplantation is unlikely needed for gene-transfer based strategies. It is well known that avoiding immune responses such as allograft rejection is more successful than attempting to eradicate an already established antiallograft B- or T-cell–mediated response. Similarly, in gene therapy every effort should be made to avoid immune responses prophylactically.
In this review, we will focus on drug-based strategies to avoid immune responses to the vector and/or the transgene following in vivo delivery of recombinant vectors. Most of immune suppression strategies described in this review directed at avoiding adaptive immune response will also have an affect on the innate response to the gene delivery vector (viral proteins or CpG DNA, etc.) by decreasing inflammatory responses. The use of vector-modified hematopoietic stem cell therapy in which myelocytotoxic and IS drugs are given to the host to create space in the bone marrow for the homing and expansion of gene-corrected cells will not be reviewed.
Mechanism of Immune Responses and Tolerance Induction
The immune systems reaction to antigen depends on (i) the relative frequencies of responding T and B cells and on the thresholds of binding affinity that their receptors display, (ii) the levels of antigen present, and (iii) the period during which the antigen remains in secondary lymphoid tissue, where primary immune responses are initiated.
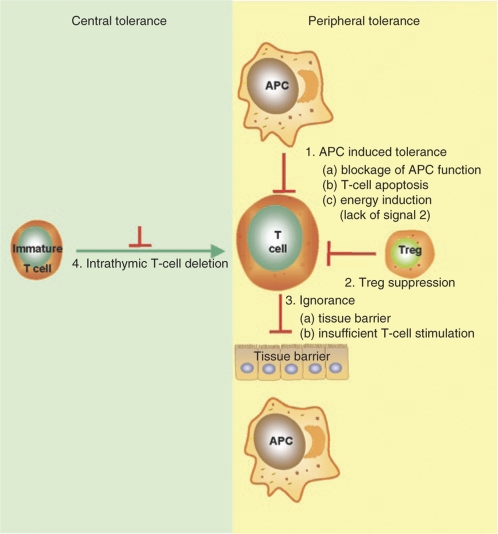
Tolerance induction is the process by which the immune system is able to adapt to exogenous antigens and is characterized by an antigen-specific nonreactivity (Figure 1). T- and B-cell tolerance can be established or disrupted either centrally, at the site of primary lymphocyte development in the thymus or bone marrow; or peripherally in the lymphoid tissue where antigen recognition and processing occur. In the peripheral immune system the key mechanisms that induce and maintain tolerance include clonal deletion, anergy, ignorance, and suppression. Ignorance describes the situation whereby T cells fail to respond to a specific antigen. This can be due to (i) low levels of antigen that are insufficient to activate T cells, (ii) antigens that are physically separated from T cells (such as blood-brain barrier). Antigens that are presented in the absence of co-stimulation signaling can induce anergy, characterized by state of T-cell unresponsiveness. Deletion of T cells can occur when the cell is activated in the absence of co-stimulation, or due to a lack of growth factors. Tolerance induction by suppression is an active process by which a regulatory subset of T cells (Tregs, Tr1, etc.) specifically suppresses the activity of T cells.2,3
Figure 1.
Mechanisms of T-cell regulation. APC, antigen-presenting cell.
Strategies to Prevent Immune Responses in the Context of Gene Transfer
In an effort to avoid immune responses during gene transfer, viral gene therapy vectors have been designed to contain few or no viral coding genes and avoid expression of pathogenic genes.4 Factors influencing the host-immune response against the vector, such as route of vector administration, dose of vector, choice of promoter/enhancer, alterations to vector genome sequence and/or structure, the status and the nature of the target tissue (e.g., underlying disease or immune-privileged sites), and patient-related factors (age, gender, immune status, drug intake, comorbid pathology and others) are all critical to the development of a clinically relevant gene-based strategy to treat human diseases.5 For some clinical conditions, fetal or neonatal therapy are critical for the treatment of the disease and in these strategies the immune responses to the vector and/or transgene may be minimized.6
Transgene expression restricted to the target tissue by using tissue-specific promoters has been extensively exploited to avoid immune responses to the transgene. One important strategy to avoid an immune response is to prevent transgene expression within antigen-presenting cells (APCs), such as dendritic cells, B cells, or macrophages. However, the uptake of exogenous protein by APC and presentation in the context of major histocompatibility complex class I or class II does not require direct transduction of APCs by the recombinant vectors. For muscle-restricted expression, plasmid DNA appears to generate cytotoxic CD8+ lymphocytes using a cross-priming mechanism whereby APCs take up, process and present exogenous antigen and present it on major histocompatibility complex class I molecules.7 Therefore the use of muscle-specific promoters would not prevent immune responses if cross priming is involved, even if the vectors do not transduce APCs. That being said, it is still preferable to avoid expressing in APCs as direct transduction of APCs can exacerbate immune responses.8,9 It should be noted that there have been some examples of tolerance induction by expressing peptide-immunoglobulin fusion proteins in B cells. The exact mechanism of this tolerance induction is unclear, however it appears to involve T-regulatory epitopes encoded in the immunoglobulin (Ig) G molecule.10,11
The liver is an attractive target for gene transfer as it has long been known as tolerogenic organ.12 Studies in mice have shown that tolerance induction by liver-specific expression of the transgene is an active suppresive mechanism involving the induction of Treg cells (for detailed review see LoDuca et al.13). Liver-specific promoters are successful in inducing long-term, sustained expression of the therapeutic transgene in large animal models following delivery of adeno-associated virus (AAV) vectors to adult animals14,15 or murine Moloney leukemia virus-based retroviral vectors to neonatal dogs.16 Interestingly, the use of a liver-specific promoter was not sufficient to completely prevent an immune response in the context of lentiviral vectors delivered to liver of adult mice,17 nor to prevent the generation of inhibitory antibodies using nonviral vectors encoding human factor VIII.18 In order to overcome these limitations, Brown et al. described a gene-transfer system that exploits the endogenous microRNA machinery for transgene regulation. They have shown the incorporation of the microRNA mir-142-3p target sequence suppresses the expression of the transgene in hematopoietic lineages, thus avoiding neutralizing antibodies against the transgene product.19 Similar studies have been carried out using hydrodynamic delivery of plasmid under the control of tissue-specific (liver or skeletal muscle) promoters and mir-142-3p. Although incorporation of the microRNA sequence did decrease antitransgene antibody titers, transgene-specific immune tolerance was not achieved.20 Therefore, in some systems the use of tissue-specific promoters will be enough to avoid immune responses, whereas in a different context (vector type, tissue target, route of administration, etc.) additional strategies may be required.
Regulated expression of the transgene is another strategy that can be used to minimize the risk of unwanted immune responses. In this approach a regulated promoter is used to delay transgene expression until the tissue has recovered from underlying inflammation and/or trauma that can be associated with vector administration. This prevents the immune system from first encountering the transgene in the context of a “danger signal,” one that is likely to prompt an immune response. Several systems have been exploited for such an immunoevasion strategy, such as Tet-On tetracycline regulatable system. However, nonhuman primate (NHP) studies have shown humoral and cytotoxic immune response against the nonspecies specific transactivator. Novel regulated expression systems based on human transcription factors are in development and probably are likely less immunogenic.21,22
Delivering vector to tissue and/or a space considered to be immune-privileged is a logical option to evade unwanted immune responses in gene therapy. These areas include the brain, eye, testis, and uterus among others. Therefore, gene transfer at these tissues may avoid or minimize immune responses to both vector and transgene. Lowenstein et al. reviewed a series of studies on viral vector delivery into the brain of naive and previously vector-immunized animal models demonstrate that the immunologic protection of the naive brain could be hampered by the local of the injection, vector dose and vector type.23 Thus, it is likely that perturbations of the “immune-privileged sites” may compromise the anatomical integrity of these natural barriers and change local immune responses.24
Immune Suppression Strategies
Preventive strategies are not always sufficient to avoid immune responses to transgenes and/or vectors, thus the use of more potent alternatives is necessary. One of these alternatives is the use of drug-induced IS, a very well established strategy for organ transplantation that has been recently translated to the gene therapy field.
Tolerance induction or IS are possible strategies to enhance the efficacy and the duration of gene expression without major safety concerns. Some factors need to be taken into consideration for IS drug therapy coupled with gene therapy. The safety aspects of this combination need to be addressed in preclinical studies and from epidemiological clinical studies in other settings requiring long-term IS. The main considerations for the use of IS therapy are described below:
1. Interference with vector internalization, stability, and transduction efficiency.
2. Modification of the biodistribution of vector to target and nontarget tissues.
3. Compromising host-defense mechanisms required to prevent infection by opportunistic pathogens and potentially altering vector transduction efficacy and/or immunological profile.
4. Higher susceptibility of tissue damage by the combination of vector and/or drug, especially in strategies targeting tissues with underlying diseases. Examples include primary or iatrogenic liver disease, renal diseases, and inflammatory status of skeletal muscle in patients with muscular dystrophy, inflammatory joint diseases, and bone marrow failure.
5. Drug side effects that can exacerbate underlying metabolic complications such as lipid disorders, glucose metabolism and others.
6. Long-term complications such as increased risk of malignancies, impairing gonadal function and infertility.
Immune Suppression Versus Tolerance Induction
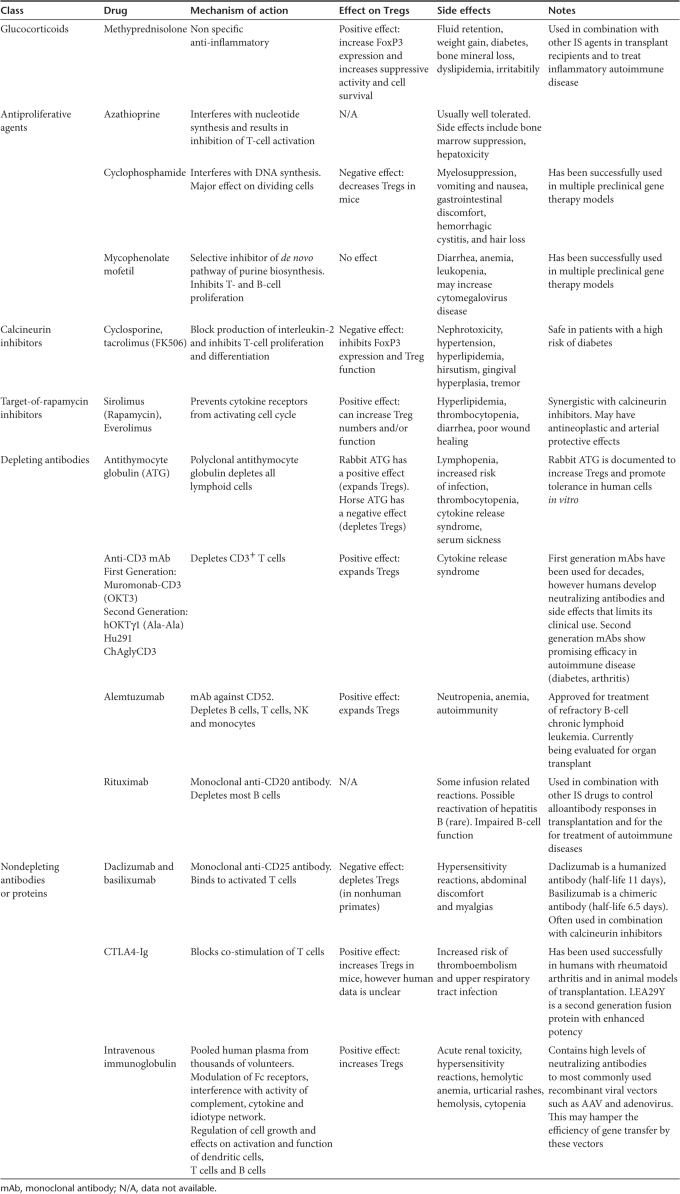
IS involves blocking the activity or efficacy of the immune system. Since the introduction of IS therapy in the 1950s, IS has been an integral part of organ transplant protocols. Much progress has been made in the prevention of acute immune responses to organ transplants; however, chronic allograft rejection is still a major problem. This demands the re-evaluation of early concepts focused mainly on aggressive IS rather than balanced IS and tolerance induction. IS protocols involve the use of a wide range of drugs (see Table 1), each having side effects, and most protocols require the patient to stay on IS agents for many years. The combination of different classes of drugs have allowed a more sophisticated application of IS. There has been a shift from high intensity ablative therapy to less intense, more refined use of IS that can tip the balance from total immune suppression to a setting more prone to induce tolerance.
Table 1.
Immunosuppression drugs: mechanism of action and side effects
In gene therapy applications, the ultimate goal is to achieve long-term antigen-specific tolerance to the transgene product. There is a delicate balance between immune suppression and tolerance induction. The identification and characterization of T-regulatory cells has enabled the design of effective strategies to control immune responsiveness. The mechanisms by which Tregs control immune responses are complex and variable, but there is a consensus that Treg-mediated immune regulation plays crucial roles in both the induction and maintenance of tolerance.25 IS strategies that block activation/proliferation of Tregs or completely deplete them from circulation are predicted to hamper tolerance induction, necessitating the long-term use of IS. Thus, intensive IS may prevent the achievement of the ultimate goal of IS regimens, which is induction of tolerance to the foreign antigens.
Immunosuppressive Drugs
Current treatment for immunological disorders are nearly all empirical in origin, using immunosuppressive drugs identified by screening large numbers of natural and synthetic compounds. In the majority of IS protocols for organ transplants, IS drugs are given in combination because many of the classes of IS drugs act synergistically. This allows greater efficacy from lower doses of drug, an important consideration when trying to avoid unwanted dose-dependent side effects.1 IS can be achieved by depleting lymphocytes, blocking lymphocyte response pathways, or diverting lymphocyte traffic. IS drugs include glucocorticoids, small-molecule drugs, depleting and nondepleting protein drugs (polyclonal and monoclonal antibodies), fusion proteins, and intravenous IgG (Table 1 and Figure 2). Table 1 summarizes the different classes of immunomodulatory drugs and includes information as to the mechanism of action, possible side effects, and other pertinent information on the use of these drugs in IS regimens. Of note, drugs are also classified according with their ability to interfere with Treg cell population and/or function.
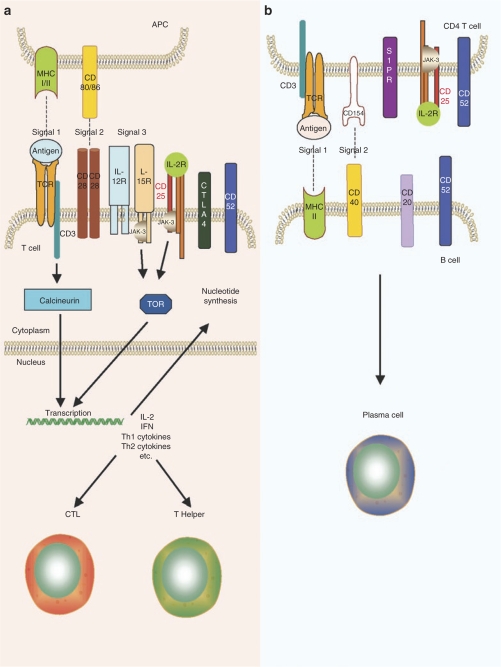
Figure 2.
T- and B-cell activation. Overview of the major proteins and pathways involved in (a) T-cell activation by antigen-presenting cells (APCs) and (b) B-cell activation by CD4 T helper cells. CTL, cytotoxic T lymphocyte; IFN, interferon; IL, interleukin; TOR, target-of-rapamycin.
There is not a single IS regimen that is largely used in organ transplant even within an organ-specific group. Ongoing and planned trials consist of heterogeneous drug combinations. Therefore, it is prudent to consider all major characteristics of the underlying disease to be treated by gene therapy in the light of the organ transplantation experience to evaluate both efficacy and side effects of all available drugs. In organ transplantation models, the unusually large number of T cells that are responsive to transplant tissues (5 in 20 T cells) as compared with the response to a foreign protein (one in one million) is remarkable.26 Thus, the pharmacological IS regimens to induce successful immune modulation most likely required in gene-transfer protocols may be less intense than for those to control organ transplant rejection.27 This may argue against the need for intensive induction therapy with monoclonal or polyclonal antibodies in a gene therapy setting.
Notably, most of these IS drugs have been used in the context of other alloimmune-mediated, primary autoimmune and benign diseases. For example, the efficacy of mycophenolate mofetil (MMF), tacrolimus and cyclosporine in various regimens has been extensively tested in solid organ transplantation including liver, kidney, lung, heart among adults1,28,29,30 and in pediatric patients.31 Unlike cyclosporine, tacrolimus does not inhibit the absorption of MMF. Thus the combination of tacrolimus and MMF requires a lower dose of the drugs, which improves the safety of this regimen. The safety of these drugs is also evident by the long-term follow up of patients receiving tacrolimus or MMF for the treatment of benign diseases such as psoriasis, rheumatoid arthritis, lupus nephritis, and autoimmune gastrointestinal disorders.32,33 Because of the growing tendency to enroll patients with relative long life expectancy in gene therapy clinical studies, the safety outcome of a given IS therapy needs to be established not only in organ transplant recipients (short life expectancy) but preferentially in patients with chronic diseases.
Preclinical Animal Models
The choice of animal model is critical for the assessment of the safety and efficacy of an IS regimen to prevent or control immune responses. The use of immunocompetent large animal models of the target disease provides the ideal model where immune responses to the neo transgene and/or vector can be properly monitored. However, for several diseases only rodent models are available and the relevance of immune responses in inbred species is likely to be of limited utility in predicting human responses. Thus, the use of large animals models without underlying disease is acceptable to address specific safety and efficacy concerns of the IS drug regimen, and general parameters of gene transfer, expression and toxicity. The use of NHP is desirable when drugs such as monoclonal antibodies or small molecules are developed for specific human targets. But this model also has limitations, an example of which is the recent data on the interruption of a clinical trial in which healthy human volunteers became severely ill upon receiving an anti-CD28 monoclonal antibody.34 This drug was tested in NHP at doses >100-fold higher than used in humans and proved safe. The failure to predict the “cytokine storm” observed in humans in response to the anti-CD28 antibody administration provides strong evidence of the limitations of NHP studies. The use of great apes such as chimpanzees is restricted due to high cost and low numbers of available animals for many researchers. In addition, some promising IS drugs are not effective in NHP models, such as anti-CD3 and Campath (anti-CD52 monoclonal antibody that targets leukocytes); thus preclinical tests in the context of gene therapy have been hampered. Overall, preclinical studies in relevant animal models are critical to the development of IS and gene transfer, but the translation of the results of preclinical studies may not always be direct.
IS Regimen in Gene Therapy
The regimen and the duration of IS required to prevent or to ameliorate undesirable immune responses following gene therapy is not yet defined. There is evidence in several large animal models of disease suggesting that transient (short-term) immune modulation would allow sustained transgene expression and correction of the disease phenotype. Table 2 is an overview of several preclinical gene therapy studies coupled with transient IS carried out in small and large animal models. For diseases without an available animal model, data obtained in nondiseased animal models are informative in terms of safety and toxicity of a given gene-based strategy.
Table 2.
Preclinical gene therapy studies coupled with transient IS
In a mucopolysaccharidosis I feline model, intravenous injection of a canine α-L-iduronidase–expressing retroviral vector resulted in the development of a cytotoxic T lymphocyte (CTL) response against the nonspecies specific transgene. In this stringent immunological model the addition of transient IS using CTLA4-Ig was effective in blocking CTL and allowing long-term transgene expression.35 In another models, a short-duration protocol based on CTLA4-Ig in combination with anti-CD40L was the most effective strategy to prevent immune responses to the nonspecies specific transgenes following liver delivery of nonviral or retroviral vectors in murine models of hemophilia A or mucopolysaccharidosis I.36,37
Intravascular delivery of AAV2 vectors to skeletal muscle has been successfully achieved in hemophilia B dogs [clotting factor IX (FIX) deficiency] and sustained transgene expression (>5 years) has been achieved at levels greater than tenfold higher than delivery by the direct intramuscular route (IM).38 In these experiments, immune responses to the neo transgene (canine FIX) were prevented by transient IS with weekly doses of cyclophosphamide (once a week and the last dose injected at day 42). This regimen was also effective in preventing the formation of antibodies to canine FIX following IM injection of AAV-FIX in another model of hemophilia B with a high risk of developing FIX antibody.39 Notably, cyclophosphamide was ineffective in inducing tolerance to FIX once the antibody to FIX was already present after IM injection of AAV-FIX in the noninhibitor prone canine hemophilia B model.40 This reinforces the idea that preventive, rather than therapeutic immunosuppressive strategies, are desired to control immune responses following gene transfer. Moreover, this IS strategy was only partially effective in feline models of lipoprotein lipase deficiency following IM injection of AAV1 vector encoding a nonspecies specific transgene.41 Thus, the use of cyclophosphamide alone may be not sufficient to effective immunotolerance induction in all disease models.
Studies using cell or gene-based therapy coupled with IS are encouraging for the treatment of muscular dystrophy. A study using the golden retriever muscular dystrophy model demonstrated T cell-mediated immune responses to the vector capsid and/or transgene following IM injection of AAV2 or AAV6 in naive normal dogs. This prompted the authors to use short-term IS (up to 3 months post vector injection) to prevent immune responses.42 The regimen, containing cyclosporine, MMF and rabbit antithymocyte globulin was effective in sustaining expression of canine µ-dystrophin after discontinuation of the drugs without local T-cell infiltrates. Data from a recent study on the use of mesangioblast stem cells in the golden retriever muscular dystrophy model also reinforce the importance of method of delivery and IS for Duchenne muscular dystrophy.43 Following delivery of the mesangioblasts by intra-arterial (femoral) injection, dystrophin expression was associated with remarkable improvement of both muscle morphology and function. It is possible that IS (cyclosporine or rapamycin) required for the use of heterologous mesangioblasts was playing a coadjuvant role in the improvement of the disease phenotype.
In these two canine models using AAV vectors for skeletal muscle transduction, hemophilia B and golden retriever muscular dystrophy, very different intensities of IS regimens were required to achieve long-term sustained transgene expression. These models provide examples of the complexity of immune responses when the target tissue is prone to inflammatory responses such as the skeletal muscle of golden retriever muscular dystrophy dogs in contrast to healthy muscle of hemophilia B dogs. In the former model a less aggressive IS regimen was not effective and immune responses prevent long-term expression of the therapeutic transgene.
Recently, three studies on the subretinal delivery of AAV2 to subjects with Leber congenital amaurosis with mutation in the RPE65 gene demonstrate no local or systemic toxicity.44,45,46 Notably, evidence of vision improvement was detected in some patients, as was predicted from preclinical studies in dogs and NHP. At least two of the trials used short-course of high dose steroids, a common practice for the surgery procedure itself that was not modified for gene delivery. The delivery of vector to immune-privileged organs such as the eye and the brain23 often requires invasive procedures to reach the target tissue, therefore it is possible that changes in the vector or in the environmental conditions may also affect the immune status of these sites and anti-inflammatory or immunosuppressive therapies may be transiently required. However, subretinal injection of lentiviral vectors expressing enhanced green fluorescent protein required IS with methylprednisolone and cyclosporine to prevent immune responses.24 Thus, this study illustrates that even in immune-privileged sites (such as the eye), immune responses can be triggered if the environment is perturbed or if the transgene product is sufficiently foreign (nonmammalian in this case).
The ability of adenoviral vectors to direct long-term transgene expression has been hampered by both the host-immune response to the vector and the nonimmune mediated loss of vector genomes. Several strategies to overcome innate and adaptive immune responses have been proposed such as transient depletion of tissue macrophages by clodronate liposomes,47 the use of adenoviral vectors of alternate serotype,48 or transient immunosuppressive therapy49,50,51,52,53,54 have shown to inhibit humoral and cell-mediated responses in the context of in vivo delivery of adenoviral vectors. Recently a simple protocol was described involving a single dose of dexamethasone that demonstrated decreased innate and adaptive immune responses, while at the same time avoiding adenovirus stimulated thrombocytopenia and leukocyte infiltration.55 Systemic administration of helper-dependent vector is still further complicated by the potential liver toxicity and transient thrombocytopenia as observed in canine models of hemophilia.56 This toxicity can be minimized by local delivery using balloon occlusion catheters as has been shown in a NHP model.57
Immune Responses in Early-Phase Clinical Trials Using AAV Vectors
Recent findings in a clinical trial in which an AAV vector expressing human FIX was introduced into the liver of hemophilia B subjects58 revealed an unanticipated rejection of transduced hepatocytes mediated by AAV2 capsid-specific CD8+ T cells. Notably, neither a CD8+ T-cell response nor formation of antibody to FIX were ever detected. In contrast to several preclinical animal models, studies in healthy subjects showed that humans carry a population of antigen-specific memory CD8+ T cells probably originating from wild-type AAV2 infections59 that expand upon exposure to AAV capsid and trigged immune rejection of the target cells. Several possible solutions for this problem include the administration of a short-term IS regimen, using alternate serotypes of AAV vectors, and/or engineering of the capsid proteins to escape immune recognition. Cellular immune responses to the AAV capsid were also observed in another clinical trial for lipoprotein lipase deficiency based on IM injection of AAV1-lipoprotein lipase. In one subject of the high dose cohort, CD8+ T-cell responses to the vector capsid were associated with transient transgene expression in the absence of immuno responses to the transgene.60,61 In an attempt to avoid vector capsid-mediated immune responses, a short-course of MMF and cyclosporine was administered for 12 weeks. In this study, transient IS was safe and effective in preventing or delaying antivector T-cell responses.62
To date, preclinical studies in several species failed to predict and to reproduce the findings of vector-capsid cellular immune responses. Thus, the efficacy of a IS regimen to prevent this complication cannot be properly addressed in preclinical studies. However, the overall safety of the IS coupled with AAV vectors is feasible, notably in data obtained in NHP models. Two studies on IS regimens consisted of MMF with tacrolimus (for AAV8 vector) or MMF and rapamycin (for AAV2 vector) over a period of 10 weeks.59,63 Collectively, these studies showed that these IS regimens do not interfere with parameters of gene transfer, vector biodistribution and transgene expression following delivery of vector to the hepatic artery of NHP. However, studies in NHP treated with an AAV2 vector expressing human FIX showed that adding daclizumab to a regimen consisting of MMF and rapamycin resulted in a boost of the anti-AAV2 antibody titer and formation of neutralizing antibodies to the FIX transgene,59 a serious complication in the treatment of hemophilia. In this study, the monitoring of peripheral blood mononuclear cells of AAV-injected NHP revealed that following daclizumab injection the population of CD4+CD25+FoxP3+ Treg cells diminished to almost undetectable levels and returned to baseline levels after week 11. Thus, it is probable that the pool of Treg cells involved in inducing and/or sustaining immune tolerance to FIX was severely affected by the anti-CD25 regimen. This hypothesis is supported by data demonstrating that sustained transgene expression by AAV-mediated, liver-directed gene transfer induces antigen-specific tolerance, and in mice this effect is mediated by a subset of CD4+ CD25+ Treg cells.64 The role of T reg cells in other tissue targets by AAV vectors is not yet determined. However, it is possible to induce transgene-specific T-regulatory cells by liver-restricted expression that suppress cellular immune responses in strategies that otherwise are hampered by strong immune responses.65
Further evidence on the importance of selecting IS drugs with minimal or no downregulation of the Treg compartment was derived from work using the nonobese diabetes murine model. It was shown that administration of anti-CD3 antibody alone was sufficient to induce tolerance. However when anti-CD3 was coadministered with cyclosporine (a calcineurin inhibitor that can prevent transcription of cytokines, including those that regulate Treg development), tolerance induction was prevented.66 Thus these data also highlight another important consideration, that different therapeutic outcomes can derive from the use of IS regimens by modifying just one of the drugs, even in the same clinical setting.
Effect of Neutralizing Antivector Antibodies
The presence of neutralizing antibodies to the wild-type viruses common among humans is another limitation of in vivo transduction efficacy using the cognate recombinant vector. The use of AAV vectors in NHPs with neutralizing antibodies to AAV capsid proteins at titers ≥1:5 failed to permit sufficient vector transduction and transgene expression in comparison with animals with low or undetectable antibody titers.63 In humans, AAV2 hepatic gene expression was prevented in the presence of neutralizing antibodies against the AAV2 capsid at titers of 1:17.58 In contrast, the presence of neutralizing antibodies to AAV2 did not prevent local FIX gene transfer and transgene expression following IM injection of AAV2 encoding human FIX in human subjects with hemophilia B.67
The use of drugs targeting B cells prior to vector delivery to subjects with high-titer antibodies to the vector has not been tested yet. One possibility is the removal of circulating specific IgG by extracorporeal absorption into affinity columns associated with transient IS or anti-CD20 monoclonal antibody as has been carried out for the treatment of autoimmune diseases. However, the limited capacity of IgG removal and the high cost of this approach are the major obstacles to widespread use of this approach.
Novel Immunomodulatory Agents
There are several other targets of therapeutic interest to induce effective IS that in combination with other drugs are highly attractive for immune tolerance induction (Figure 2).
FTY720 is a novel drug which induces lymphopenia due its ability to sequester T and B cells into peripheral and mesenteric lymph nodes by a mechanism involving sphingosine-1-phosphate receptor on lymphocytes.68 FTY720 has been tested in clinical trials in phase III studies in humans undergoing kidney transplantation and has proven safe and efficacious.1
Janus kinase 3 is a tyrosine kinase associated with the cytokine receptor γ chain, which participates in the signaling of many cytokine receptors (interleukin-2, 4, 7, 9, 12, and 21, Figure 2). Novel strategies based on inhibition of the Janus kinase 3 pathway are currently being investigated as potential specific immunosuppressive regimens. The compounds PF-956980 and CP-690550, are currently undergoing preclinical and clinical investigations, respectively. CP-690550 has been tested in clinical trials for rheumatoid arthritis and prevention of allograft rejection.69 Interestingly, another tyrosine kinase inhibitor (imatinib mesylate), which is now the first line treatment of chronic myeloid leukemia, also plays a role in cell receptor signaling.70 Studies in a lymphocytic choriomeningitis virus model demonstrated that imatinib efficiently targets the memory CTLs post re-exposure to lymphocytic choriomeningitis virus infection without compromising responses to other viruses, a highly desirable safety feature of immunosuppressive drug. In addition, the use of imatinib also delayed the onset of diabetes in a CTL-induced diabetes model.70
Th17 cells are a novel T cell of distinct lineage has recently been described. These proinflammatory cells express interleukin-17 and interleukin-21 and play an important role in inflammatory and autoimmune diseases. Interesting, these cells appear to be reciprocally regulated with Tregs (for more information regarding Th17 cells see a review by Dong).71 Recent work has found a crucial role for retinoic acid in promoting FoxP3 expression (Treg) and inhibiting Th17 development.72 Therefore, drugs such as all-trans retinoic acid may be useful for immune tolerance induction in the context of gene therapy by inducing Tregs and decreasing Th17 cells. All-trans retinoic acid is currently used in humans to treat acute promyelocytic leukemia (caused by translocation and fusion of the retinoic acid receptor gene with the promyelocytic leukemia gene). Although there have been no clinical studies using all-trans retinoic acid in a transplant setting, it has been used to treat emphysema in rats73 and clinical trials for the treatment of emphysema in humans showed that it was well tolerated.74
FoxP3 protein is a lineage specification factor for the development and function of Tregs, and histone deacetylase inhibitor treatment is known to increase acetylation of FoxP3, enhancing its expression and boosting the number and function of Foxp3+CD4+CD25+ Tregs.75 This class of drug (e.g., SAHA) has already been used for anticancer therapy and has shown promise in decreasing graft-versus-host disease in animal models of allogenic bone marrow transplantation,76 and thus may be a new candidate for manipulation of Tregs towards clinical tolerance.
One alternative to avoiding CTL responses against the vector is to transiently deplete CD8+ T cells, thus blocking the cell-mediated responses to the vector. In a NHP model of allograft kidney transplant, anti-CD8 was effective in depleting CD8+ memory T cells and allowed for successful mixed chimerism and tolerance.77 However, CD8+ T cells play a major role in the innate immune response to viral infections,78,79,80 and different models have shown that the loss of CD8+ T cells can result in increased viremia of AIDS in simian immunodeficiency virus infection,81 hepatitis B and C virus,82,83 cytomegalovirus,84 and Epstein-Barr virus.85
Proteasome inhibitors are a novel class of pharmaceutical agent that is currently being used for the treatment of multiple myeloma (bortezomib).86,87 Proteasome inhibitors have been found to be well tolerated in humans and there is some emerging evidence that they might have efficacy as immunosuppressive agents.88 Proteasome inhibitors have been shown to induce apoptosis in activated and proliferating (but not resting) T-cells,89,90 as well as suppress the function and inhibit the activation of human CD4+ T cells and dendritic cells.91 In mouse models of heart and islet transplants proteasome inhibitors have been efficacious at prolonging allograft function and immune tolerance induction.92,93 In addition, the use of proteasome inhibitors in AAV-mediated gene-transfer protocols is highly attractive, as these compounds have also been shown to enhance AAV-mediated gene expression in vitro and in vivo.94,95,96,97,98
Short- and Long-Term Complications of IS
The most common risk of IS therapy is increased susceptibility to opportunistic infection.99 For those gene therapy studies requiring invasive procedure (surgery, intravascular catheterization) for vector delivery to the target organ, a higher risk of nosocomial infection within the first weeks is expected when compared to minimally or noninvasive approaches. Proper screening and implementation of prophylactic therapeutics could also minimize the risk of activation of latent infections such as cytomegalovirus, Pneumocystis carinii, herpes simplex virus, hepatitis B virus, Mycobacterium tuberculosis, and others. These complications most frequently occur during, but are not restricted to, the first month of immunosuppressive therapy. The main determinants of the risk of infection are the dose, duration, and sequence of immunosuppressive therapies. This complication can be minimized by monitoring drug levels and by using a short-duration of IS.
The main long-term complications following organ transplant include cardiovascular disease and cancer. Because sirolimus has been clinically associated with a protective effect on the development of occlusive arterial disease and antitumor effects, its use is an attractive option for late maintenance IS regimens.100,101 As in many gene therapy strategies IS will be employed only transiently, the long-term complications related to the drugs are expected to be minimal.
Conclusion
Gene therapy is an emerging medical technology that has the promise to treat many genetic and acquired diseases. While considerable advances have been made in animal and human studies, the host-immune response remains a formidable barrier to the effective translation of gene-transfer studies from the bench to the clinic. The wealth of information using immunosuppressive agents that has been gained over the past 60 years from the organ transplant field can be used to help guide the use of IS in gene-transfer protocols. To date there are no guidelines for the use or duration of a specific IS regimen. It is likely that different IS therapeutic strategies will require different combinations of drugs over distinct periods of time depending on the vector, disease, target tissue, and as the therapeutic outcome necessitates. The development of preclinical models is imperative to address the safety profile of such IS regimens in a specific context. Furthermore, a careful evaluation of the data has to take into consideration the evolutionary level of the immune system of the model (rodent versus NHP versus humans) and the disease-specific model availability (normal animals versus animals with underlying-specific disease relevant to humans).
Recent advances in the development of immunosuppressive therapy and regimens have had a beneficial effect on morbidity and mortality in transplantation and immune-mediated diseases. Immunosuppressive therapy shows promise as an effective strategy to prevent immune responses against the transgene and vectors in gene therapy.
Acknowledgments
We thank Katherine A High and J Fraser Wright for the helpful discussion and Junwei Sun for expert assistance. This work is supported by grants from NIH-NHLBI (HL084220 and P01 HL078810) and Hemophilia Association of New York.
REFERENCES
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- Kamradt T., and , Mitchison NA. Tolerance and autoimmunity. N Engl J Med. 2001;344:655–664. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW., and , Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A., and , Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Bessis N, GarciaCozar FJ., and , Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11 Suppl 1:S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- Ponder KP. Immunology of neonatal gene transfer. Curr Gene Ther. 2007;7:403–410. doi: 10.2174/156652307782151434. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Deck RR, Dewitt CM, Donnhly JI., and , Liu MA. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology. 1996;89:59–67. doi: 10.1046/j.1365-2567.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin KQ, Mizukami H, Urabe M, Toda Y, Shinoda K, Yoshida A, et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J Virol. 2006;80:11899–11910. doi: 10.1128/JVI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- Su Y, Carey G, Maric M., and , Scott DW. B cells induce tolerance by presenting endogenous peptide-IgG on MHC class II molecules via an IFN-gamma-inducible lysosomal thiol reductase-dependent pathway. J Immunol. 2008;181:1153–1160. doi: 10.4049/jimmunol.181.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112:3303–3311. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG., and , Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:194–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- Loduca PA, Hoffman BE., and , Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R, Mucci M, Addya S, Tetreault R, Bellinger DA, Nichols TC, et al. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum Gene Ther. 2006;17:427–439. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Nichols TC, Sarkar R, McCorquodale S, Bellinger DA., and , Ponder KP. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc Natl Acad Sci USA. 2005;102:6080–6085. doi: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG., and , Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- Ye P, Thompson AR, Sarkar R, Shen Z, Lillicrap DP, Kaufman RJ, et al. Naked DNA transfer of Factor VIII induced transgene-specific, species-independent immune response in hemophilia A mice. Mol Ther. 2004;10:117–126. doi: 10.1016/j.ymthe.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P, et al. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- Wolff LJ, Wolff JA., and , Sebestyén MG. Effect of tissue-specific promoters and microRNA recognition elements on stability of transgene expression after hydrodynamic naked plasmid DNA delivery. Hum Gene Ther. 2009;20:374–388. doi: 10.1089/hum.2008.088. [DOI] [PubMed] [Google Scholar]
- Ye X, Rivera VM, Zoltick P, Cerasoli F, Schnell MA, Gao G, et al. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- Yang W, Keenan TP, Rozamus LW, Wang X, Rivera VM, Rollins CT, et al. Regulation of gene expression by synthetic dimerizers with novel specificity. Bioorg Med Chem Lett. 2003;13:3181–3184. doi: 10.1016/s0960-894x(03)00707-8. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K., and , Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7:347–360. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Kong J, Hargitai J, Goff SP., and , Gouras P. Transient immunosuppression stops rejection of virus-transduced enhanced green fluorescent protein in rabbit retina. J Virol. 2004;78:11327–11333. doi: 10.1128/JVI.78.20.11327-11333.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T., and , Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Strom TB. 2006 Homer W. Smith Lecture: taming T cells. J Am Soc Nephrol. 2007;18:2824–2832. doi: 10.1681/ASN.2007070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama K, Hatsushika K, Ando T, Sakuma M, Wako M, Kato R, et al. Imatinib mesylate both prevents and treats the arthritis induced by type II collagen antibody in mice. Mod Rheumatol. 2007;17:306–310. doi: 10.1007/s10165-007-0592-9. [DOI] [PubMed] [Google Scholar]
- Mueller XM. Drug immunosuppression therapy for adult heart transplantation. Part 2: clinical applications and results. Ann Thorac Surg. 2004;77:363–371. doi: 10.1016/j.athoracsur.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Perry I., and , Neuberger J. Immunosuppression: towards a logical approach in liver transplantation. Clin Exp Immunol. 2005;139:2–10. doi: 10.1111/j.1365-2249.2005.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurohr C, Huppmann P, Zimmermann G, Leuchte H, Baumgartner R, Hatz R, et al. Tacrolimus and mycophenolate mofetil as first line immunosuppression after lung transplantation. Transpl Int. 2009;22:635–643. doi: 10.1111/j.1432-2277.2009.00843.x. [DOI] [PubMed] [Google Scholar]
- Kari JA., and , Trompeter RS. What is the calcineurin inhibitor of choice for pediatric renal transplantation. Pediatr Transplant. 2004;8:437–444. doi: 10.1111/j.1399-3046.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- Yocum DE, Furst DE, Bensen WG, Burch FX, Borton MA, Mengle-Gaw LJ, et al. Safety of tacrolimus in patients with rheumatoid arthritis: long-term experience. Rheumatology (Oxford) 2004;43:992–999. doi: 10.1093/rheumatology/keh155. [DOI] [PubMed] [Google Scholar]
- Chang GJ, Mahanty HD, Vincenti F, Freise CE, Roberts JP, Ascher NL, et al. A calcineurin inhibitor-sparing regimen with sirolimus, mycophenolate mofetil, and anti-CD25 mAb provides effective immunosuppression in kidney transplant recipients with delayed or impaired graft function. Clin Transplant. 2000;14:550–554. doi: 10.1034/j.1399-0012.2000.140606.x. [DOI] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Ponder KP, Wang B, Wang P, Ma X, Herati R, Wang B, et al. Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-Ig. Mol Ther. 2006;14:5–13. doi: 10.1016/j.ymthe.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Miao CH, Ye P, Thompson AR, Rawlings DJ., and , Ochs HD. Immunomodulation of transgene responses following naked DNA transfer of human factor VIII into hemophilia A mice. Blood. 2006;108:19–27. doi: 10.1182/blood-2005-11-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Liu Y, Tittiger M, Hennig A, Kovacs A, Popelka S, et al. Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation. Mol Ther. 2007;15:889–902. doi: 10.1038/sj.mt.6300112. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CJ, Twisk J, Bakker AC, Miao F, Verbart D, Rip J, et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther. 2006;17:487–499. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Phase i trial of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results. Hum Gene Ther. 2008;19:970–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah MK, Schiedner G, Thorrez L, Brown B, Johnston M, Gillijns V, et al. Therapeutic factor VIII levels and negligible toxicity in mouse and dog models of hemophilia A following gene therapy with high-capacity adenoviral vectors. Blood. 2003;101:1734–1743. doi: 10.1182/blood-2002-03-0823. [DOI] [PubMed] [Google Scholar]
- Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JM, Lochmüller H, O'Hara A, Fletcher S, Kakulas BA, Massie B, et al. High-level dystrophin expression after adenovirus-mediated dystrophin minigene transfer to skeletal muscle of dystrophic dogs: prolongation of expression with immunosuppression. Hum Gene Ther. 1998;9:629–634. doi: 10.1089/hum.1998.9.5-629. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Nagahama M, Obuchi Y, Taira K, Tomori H, Sugawa H, et al. Successful gene transfer to the porcine liver in vivo with an adenoviral vector. J Surg Res. 1998;76:105–110. doi: 10.1006/jsre.1998.5302. [DOI] [PubMed] [Google Scholar]
- Ilan Y, Jona VK, Sengupta K, Davidson A, Horwitz MS, Roy-Chowdhury N, et al. Transient immunosuppression with FK506 permits long-term expression of therapeutic genes introduced into the liver using recombinant adenoviruses in the rat. Hepatology. 1997;26:949–956. doi: 10.1002/hep.510260422. [DOI] [PubMed] [Google Scholar]
- Guerette B, Gingras M, Wood K, Roy R., and , Tremblay JP. Immunosuppression with monoclonal antibodies and CTLA4-Ig after myoblast transplantation in mice. Transplantation. 1996;62:962–967. doi: 10.1097/00007890-199610150-00015. [DOI] [PubMed] [Google Scholar]
- Jooss K, Turka LA., and , Wilson JM. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998;5:309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- Kay MA, Meuse L, Gown AM, Linsley P, Hollenbaugh D, Aruffo A, et al. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T, et al. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR. Toward gene therapy for hemophilia A with novel adenoviral vectors: successes and limitations in canine models. J Thromb Haemost. 2006;4:1215–1217. doi: 10.1111/j.1538-7836.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, Zuo Y, et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui JD, Basner-Tscharkarjan E, Hasbrouck NC, Edmonson S, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells Blood 2009(epub ahead of print) [DOI] [PMC free article] [PubMed]
- Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- Hui JD, Mingozzi F, Kleefstra A, Meulenberg JM, Edmonson S, Morin D, et al. Immunosuppression modulates immune responses to AAV capsid in human subjects undergoing intramuscular gene transfer for lipoprotein lipase deficiency. Blood. 2008;112:822. [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adeno associated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L., and , Herzog RW. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci USA. 2006;103:4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L, Primo J., and , Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Schwab SR., and , Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Changelian PS, Moshinsky D, Kuhn CF, Flanagan ME, Munchhof MJ, Harris TM, et al. The specificity of JAK3 kinase inhibitors. Blood. 2008;111:2155–2157. doi: 10.1182/blood-2007-09-115030. [DOI] [PubMed] [Google Scholar]
- Mumprecht S, Matter M, Pavelic V., and , Ochsenbein AF. Imatinib mesylate selectively impairs expansion of memory cytotoxic T cells without affecting the control of primary viral infections. Blood. 2006;108:3406–3413. doi: 10.1182/blood-2006-04-018705. [DOI] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro GD., and , Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med. 1997;3:675–677. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- Mao JT, Goldin JG, Dermand J, Ibrahim G, Brown MS, Emerick A, et al. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165:718–723. doi: 10.1164/ajrccm.165.5.2106123. [DOI] [PubMed] [Google Scholar]
- Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- Reddy P, Maeda Y, Hotary K, Liu C, Reznikov LL, Dinarello CA, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci USA. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama A, Ng CY, Millington TM, Boskovic S, Murakami T, Wain JC, et al. Comparison of lung and kidney allografts in induction of tolerance by a mixed-chimerism approach in cynomolgus monkeys. Transplant Proc. 2009;41:429–430. doi: 10.1016/j.transproceed.2008.08.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A, Costelloe EO, Johnson BJ, Groves P, Buttigieg K., and , Fitzpatrick DR. The genes for perforin, granzymes A-C and IFN-gamma are differentially expressed in single CD8(+) T cells during primary activation. Int Immunol. 2002;14:605–613. doi: 10.1093/intimm/dxf028. [DOI] [PubMed] [Google Scholar]
- Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Madden LJ, Zandonatti MA, Flynn CT, Taffe MA, Marcondes MC, Schmitz JE, et al. CD8+ cell depletion amplifies the acute retroviral syndrome. J Neurovirol. 2004;10 Suppl 1:58–66. doi: 10.1080/753312754. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Rehermann B, Chang KM, McHutchison JG, Kokka R, Houghton M., and , Chisari FV. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Invest. 1996;98:1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest. 1997;99:1472–1477. doi: 10.1172/JCI119308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sester M, Sester U, Gärtner BC, Girndt M, Meyerhans A., and , Köhler H. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J Am Soc Nephrol. 2002;13:2577–2584. doi: 10.1097/01.asn.0000030141.41726.52. [DOI] [PubMed] [Google Scholar]
- van Baarle D, Hovenkamp E, Callan MF, Wolthers KC, Kostense S, Tan LC, et al. Dysfunctional Epstein-Barr virus (EBV)-specific CD8(+) T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2001;98:146–155. doi: 10.1182/blood.v98.1.146. [DOI] [PubMed] [Google Scholar]
- Adams J. Proteasome inhibition in cancer: development of PS-341. Semin Oncol. 2001;28:613–619. doi: 10.1016/s0093-7754(01)90034-x. [DOI] [PubMed] [Google Scholar]
- Bladé J, Cibeira MT., and , Rosiñol L. Bortezomib: a valuable new antineoplastic strategy in multiple myeloma. Acta Oncol. 2005;44:440–448. doi: 10.1080/02841860510030002. [DOI] [PubMed] [Google Scholar]
- Wu J. On the role of proteasomes in cell biology and proteasome inhibition as a novel frontier in the development of immunosuppressants. Am J Transplant. 2002;2:904–912. doi: 10.1034/j.1600-6143.2002.21006.x. [DOI] [PubMed] [Google Scholar]
- Cui H, Matsui K, Omura S, Schauer SL, Matulka RA, Sonenshein GE, et al. Proteasome regulation of activation-induced T cell death. Proc Natl Acad Sci USA. 1997;94:7515–7520. doi: 10.1073/pnas.94.14.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo H, Chen H, Duguid W., and , Wu J. Role of proteasomes in T cell activation and proliferation. J Immunol. 1998;160:788–801. [PubMed] [Google Scholar]
- Berges C, Haberstock H, Fuchs D, Miltz M, Sadeghi M, Opelz G, et al. Proteasome inhibition suppresses essential immune functions of human CD4+ T cells. Immunology. 2008;124:234–246. doi: 10.1111/j.1365-2567.2007.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Wu Y, Qi S, Wan X, Chen H., and , Wu J. A proteasome inhibitor effectively prevents mouse heart allograft rejection. Transplantation. 2001;72:196–202. doi: 10.1097/00007890-200107270-00005. [DOI] [PubMed] [Google Scholar]
- Wu Y, Han B, Luo H, Shi G., and , Wu J. Dipeptide boronic acid, a novel proteasome inhibitor, prevents islet-allograft rejection. Transplantation. 2004;78:360–366. doi: 10.1097/01.tp.0000128855.10397.db. [DOI] [PubMed] [Google Scholar]
- Duan D, Yue Y, Yan Z, Yang J., and , Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douar AM, Poulard K, Stockholm D., and , Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75:1824–1833. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denby L, Nicklin SA., and , Baker AH. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Ther. 2005;12:1534–1538. doi: 10.1038/sj.gt.3302564. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Cochrane M, McIntosh J, Ng CY, Zhou J, Gray JT, et al. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16:60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS., and , Samulski RJ. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN, et al. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001;4:201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Mount JD, Arruda VR, High KA., and , Lothrop CD. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- Wang L, Nichols TC, Read MS, Bellinger DA., and , Verma IM. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- Toromanoff A, Chérel Y, Guilbaud M, Penaud-Budloo M, Snyder RO, Haskins ME, et al. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Schiedner G, Gilchrist SC, Kochanek S., and , Clemens PR. CTLA4Ig delivered by high-capacity adenoviral vector induces stable expression of dystrophin in mdx mouse muscle. Gene Ther. 2004;11:1453–1461. doi: 10.1038/sj.gt.3302315. [DOI] [PubMed] [Google Scholar]
- Schowalter DB, Meuse L, Wilson CB, Linsley PS., and , Kay MA. Constitutive expression of murine CTLA4Ig from a recombinant adenovirus vector results in prolonged transgene expression. Gene Ther. 1997;4:853–860. doi: 10.1038/sj.gt.3300466. [DOI] [PubMed] [Google Scholar]
- Shen WY, Lai MC, Beilby J, Barnett NL, Liu J, Constable IJ, et al. Combined effect of cyclosporine and sirolimus on improving the longevity of recombinant adenovirus-mediated transgene expression in the retina. Arch Ophthalmol. 2001;119:1033–1043. doi: 10.1001/archopht.119.7.1033. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Negri T, Cozzi F, Colpo R, Andreetta F, Croci D, et al. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer. Gene Ther. 2003;10:750–757. doi: 10.1038/sj.gt.3301941. [DOI] [PubMed] [Google Scholar]
- Puppi J, Guillonneau C, Pichard V, Bellodi-Privato M, Cuturi MC, Anegon I, et al. Long term transgene expression by hepatocytes transduced with retroviral vectors requires induction of immune tolerance to the transgene. J Hepatol. 2004;41:222–228. doi: 10.1016/j.jhep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Peng B, Ye P, Blazar BR, Freeman GJ, Rawlings DJ, Ochs HD, et al. Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice. Blood. 2008;112:1662–1672. doi: 10.1182/blood-2008-01-128413. [DOI] [PMC free article] [PubMed] [Google Scholar]