Abstract
Rexin-G, a pathotropic nanoparticle bearing a cytocidal cyclin G1 construct was tested in a phase I/II study for chemotherapy-resistant sarcomas and a phase II study for chemotherapy-resistant osteosarcoma. Twenty sarcoma patients and 22 osteosarcoma patients received escalating doses of Rexin-G intravenously from 8 × 1011 to 24 × 1011 colony forming units (cfu)/cycle. Treatment was continued if there was ≤ grade 1 toxicity. No dose-limiting toxicity (DLT) was observed, and no vector DNA integration, replication-competent retrovirus (RCR) or vector-neutralizing antibodies were noted. In the phase I/II study, 3/6 patients had stable disease (SD) at the lowest dose; median progression-free survival (PFS) was 1.2 months, and overall survival (OS), 3.3 months. At higher doses, 10/14 patients had SD; median PFS was 3.7 months and median OS, 7.8 months. In this phase I/II study, a dose–response relationship with Rexin-G dosage was observed for progression-free and OS times (P = 0.02 and 0.005, respectively). In the phase II study, 10/17 evaluable patients had SD, median PFS was ≥3 months and median OS, 6.9 months. These studies suggest that Rexin-G is safe, may help control tumor growth, and may possibly improve survival in chemotherapy-resistant sarcoma and osteosarcoma.
Introduction
Osteosarcoma is a rare malignant tumor of bone usually affecting adolescents and young adults.1 Current combination chemotherapy, radiation therapy, and surgery, have significantly improved the survival of affected persons. Effective drugs include doxorubicin, cisplatin, methotrexate, and ifosfamide.2 However, no standard second line therapy exists for those who relapse or fail to achieve a second remission with the best reported overall survival (OS) of 0.6 years.3 Additionally, the long-term sequelae and secondary malignancies associated with toxic chemotherapy in children and adolescents augment the need for more effective and less toxic therapies.3
Soft tissue sarcoma is also a rare cancer of mesenchymal tissues.4 Current treatment for soft tissue sarcoma includes surgical resection, radiotherapy, and chemotherapy.5 Despite improvements in the control of local disease, a significant number of patients ultimately die of metastatic disease following radical surgery due to a lack of effective adjuvant treatments.6,7,8,9,10 Only three drugs—doxorubicin, dacarbazine, and ifosfamide—are consistently associated with response rates of ≥20%, and after failure of these drugs, patients with advanced soft tissue sarcoma have few therapeutic options.11
Among the leading alternatives to traditional chemotherapeutics, both cancer immunotherapy and cancer gene therapy strategies are currently under active clinical investigation.12,13,14 Rexin-G, the first and, so far, only targeted gene therapy vector bearing a cytocidal dominant negative cyclin G1 construct,15 is currently being tested simultaneously in three phase I/II clinical trials for chemotherapy-resistant metastatic sarcoma, pancreatic cancer, and breast cancer, and in one phase II study for chemotherapy-resistant metastatic osteosarcoma in the United States.
In this article, we report on the results of two independent studies (i) evaluating the overall safety and potential efficacy of Rexin-G in chemotherapy-resistant sarcoma in a phase I/II study, and (ii) confirming the efficacy and overall safety of Rexin-G in chemotherapy-resistant osteosarcoma in a phase II study.
Results
Patient demographics
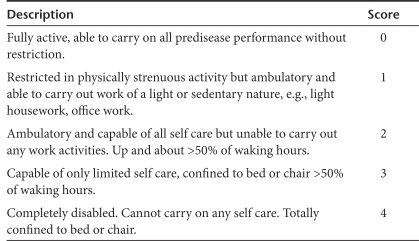
Table 1 shows the patient demographics for the phase I/II sarcoma study and the phase II osteosarcoma study. There were nine different types of sarcomas enrolled in the phase I/II study, including leiomyosarcoma (n = 5), osteosarcoma (n = 3), synovial cell sarcoma (n = 3), liposarcoma (n = 3), mixed malignant Mullerian tumor of ovary (n = 2), Ewing's sarcoma (n = 1), chondrosarcoma (n = 1), malignant fibrous histiocytoma (n = 1), and malignant spindle cell sarcoma (n = 1). Ninety-five percent of patients in the phase I/II sarcoma study had metastatic disease and a median of four previous chemotherapy regimens, whereas 100% of patients in the phase II osteosarcoma study had metastatic disease and a median of four previous chemotherapy regimens. Eastern Cooperative Oncology Group score was 0–1. Table 2 shows the performance scoring system employed.
Table 1.
Patient demographics
Table 2.
Eastern Cooperative Oncology Group performance scoring system
Analysis of safety
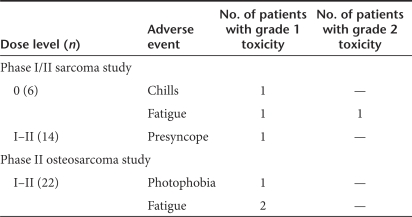
Treatment-related adverse events are listed in Table 3. There was no dose-limiting toxicity (DLT) or organ-related toxicity. In the phase I/II study, at dose level 0, study drug-related adverse events included grade 1 chills in one patient, and grade 1–2 fatigue in two patients, whereas at dose level I–II, one patient had grade 1 presyncope. In the phase II study, one patient experienced grade 1 photophobia, and two patients had grade 1 fatigue which was considered possibly study drug-related.
Table 3.
Treatment-related adverse events
Correlative analysis showed no neutralizing antibodies detected in the patients' sera. However, 3/14 patients who received dose level II developed weakly positive antibodies against the gp70 envelope protein by western blot analysis, 3–4 months after treatment initiation. Further, all DNA samples from patients' peripheral blood lymphocytes (PBLs) were found to be negative for replication-competent retrovirus (RCR), and no vector DNA integration was detected.
Analysis of efficacy
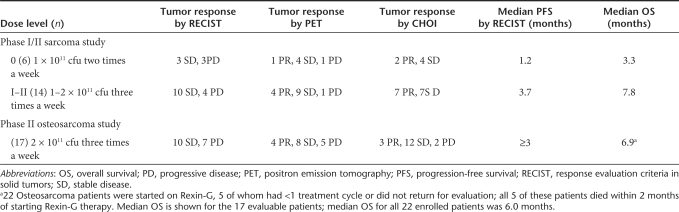
In the phase I/II study, six patients were treated at dose level I and 14 patients at dose level I–II (combined). Table 4 (Sarcoma study) shows the tumor responses using standard response evaluation criteria in solid tumors (RECIST), International positron emission tomography (PET), and CHOI criteria for each dose level.
Table 4.
Effects of Rexin-G on tumor response by RECIST, PET, and CHOI criteria, on PFS and OS
In the phase I/II sarcoma study, at dose level 0, 3/6 patients had stable disease (SD) whereas at dose level I–II, 10/14 patients had SD by standard RECIST criteria. Interestingly, using the International PET criteria, at dose level 0, 1/6 patients had a partial response (PR), and 4/6 patients had SD, and at dose level I–II, 4/14 patients had PRs, and 9/14 patients had SD. Similarly, using CHOI criteria, at dose level 0, 2/6 patients had PRs, and 4/6 patients had SD, and at dose level I–II, 7/14 patients had PRs and 7/14 patients had SD. These findings suggest that the International PET and CHOI criteria may be more sensitive indicators of tumor responses to Rexin-G treatment than the standard RECIST.
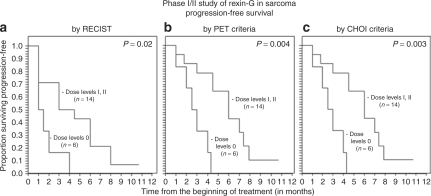
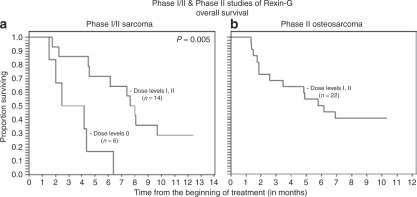
The median progression-free survival (PFS) times by RECIST was 1.2 months for dose level 0 versus 3.7 months for dose levels I–II (combined), and the corresponding median overall survival times were 3.3 months for dose level 0 versus 7.8 months for dose levels I–II (Table 4). Using the Cox regression model and Kaplan–Meier analysis,16 a dose–response relationship was observed between Rexin-G dosage and both PFS and OS (P = 0.02 for PFS by RECIST, 0.004 by PET and 0.003 by CHOI criteria; P = 0.005 for OS; Figures 1a–c and 2a).
Figure 1.
Analyses of progression-free survival (PFS) of patients in the phase I/II sarcoma study using various radiologic imaging criteria. Kaplan-Meier analyses show a dose–response relationship between progression-free survival and Rexin-G dosage, as determined (a) by RECIST, (b) by International PET criteria, and (c) by CHOI criteria.
Figure 2.
Analyses of overall survival of patients in the phase I/II sarcoma study and the phase II osteosarcoma study. Kaplan-Meier analyses show the survival curves of patients in (a) the phase I/II sarcoma study and (b) the phase II osteosarcoma study.
In the phase II study, 17 of 22 patients completed at least one treatment cycle and were considered evaluable. Five patients were taken off study without completing one treatment cycle due to disease-related complications or a personal decision to discontinue participation and return home. The tumor responses based on RECIST, International PET criteria and CHOI criteria are shown in Table 4. Using standard RECIST, 10/17 (59%) evaluable patients had SD, whereas using International PET criteria, 4/17 patients had PRs, and 8/17 patients had SD, totaling 70% of patients having PRs or SD. Using CHOI criteria, 3/17 had PRs and 12/17 had SD totaling 88% of patients having PRs or SD. Therefore, tumor responses were significantly higher in the Rexin-G treated group compared to those expected of historical controls (with ≤5% having a positive response if untreated; P < 0.025).3 Median PFS was ≥3 months, and OS was 6.9 months (6 months for all 22 enrolled patients).
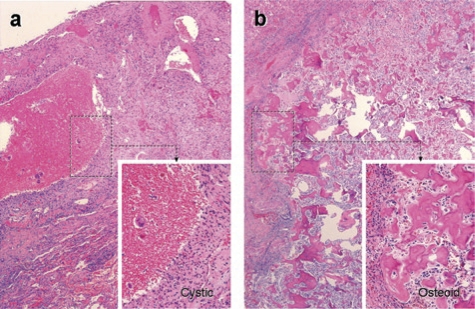
Two patients are disease-free after surgical resection of residual tumors, with three cycles of Rexin-G given before and 3–4 cycles after surgery. These patients enjoy sustained surgical remissions 10.7 and 9.7 months after treatment initiation, and >6 months after surgery. In one patient, histopathologic examination of two resected tumor nodules showed a 2.5 cystic nodule and rare osteosarcoma cells and a 0.9 cm calcified nodule and residual osteosarcoma cells with rare mitosis (Figure 3). Of note, the 2.5 cm tumor had increased in size and was diagnosed as progressive disease (PD) by RECIST but a PR by PET criteria. This finding suggests that RECIST criteria may not be a reliable indicator of tumor response to Rexin-G treatment in osteosarcoma.
Figure 3.
Histopathological findings in the lung metastases of a patient with chemotherapy-resistant osteosarcoma after Rexin-G treatment. Representative H&E stained tissue sections from resected residual lung nodules in a Rexin-G treated patient. (a) 2.5 cm cystic mass with organized clot and rare osteosarcoma cells; (b) 0.9 cm calcified tumor mass with reparative fibrosis, immune infiltrate and osteosarcoma cells with rare mitosis.
Discussion
Rexin-G is a pathotropic nanoparticle incorporating a collagen-matrix binding motif on its surface and bearing a dominant negative cyclin G1 construct as its genetic payload. When injected intravenously, Rexin-G accumulates in cancerous lesions wherein collagen is exposed by tumor invasion, neoangiogenesis or extracellular remodeling, thus increasing the effective local concentration in tumors in the vicinity of target cancer cells. Rexin-G causes cell death by blocking the cell cycle in G1 phase and inducing apoptosis of proliferative cancer cells and associated neovasculature in preclinical studies.17,18
In the phase I/II sarcoma study, tumor control was suggested by longer PFS and OS in the higher dose cohorts compared to the low dose cohort. In the phase II osteosarcoma study, a significant proportion of patients had SD by RECIST or PRs/SD by PET and CHOI criteria, suggesting increased sensitivity of PET and CHOI criteria for early detection of tumor responses to Rexin-G treatment. No DLT or organ-related toxicity was observed with prolonged use of Rexin-G. Correlative analysis showed no vector-neutralizing antibodies detected in serum, and no evidence of vector DNA integration nor RCR in PBLs, further attesting to the overall safety of Rexin-G. Surgical resection of residual tumors following Rexin-G treatment—which enabled histological examination, as well as strategic tumor debulking—followed by the administration of additional postoperative treatment, serves to underscore the potential clinical benefit of Rexin-G in neoadjuvant/adjuvant protocols.
In conclusion, the objectives of the phase I/II Study have been met. The overall safety of Rexin-G administered within the defined dose ranges was clearly established by the absence of DLT or vector safety concerns. Further, the objectives of the phase II study for osteosarcoma have also been met, wherein tumor responses by RECIST of 10 SD of 17 evaluable patients (59%; 95% confidence interval, 33–82%) and a median PFS of ≥3 months and a median OS of 6.9 months in patients treated with at least 1 cycle, and 6 months in all enrolled patients, were demonstrated. Taken together, the results of these two independent well-defined phase I/II and phase II studies suggest that Rexin-G may help control tumor growth, and may possibly improve progression-free and OS times in chemotherapy-resistant sarcoma and osteosarcoma, thus hopefully providing the required elements for consideration of accelerated approval for osteosarcoma by the US Food and Drug Administration.19,20
Materials and Methods
Study design. The phase I/II sarcoma study employed a modified cohort of three design.21 Three patients were treated at each dose level with expansion to six patients per cohort if DLT was observed in one of the three first patients at each dose level. Each cohort was also expanded to six or seven patients if significant biologic activity was noted. Maximum tolerated dose was defined as the highest safely tolerated dose, where ≤1 patient experienced DLT, with the next higher dose level having at least two patients who experienced DLT. DLT was defined as any grade 3, 4, or 5 adverse event considered possibly, probably, or definitely related to the study drug, excluding grade 3 absolute neutrophil count lasting <72 hours, grade 3 alopecia, or any grade 3 or worse nausea, vomiting, or diarrhea where the patient did not receive maximal supportive care.22
Adaptive Trial Design: A phase II efficacy component was incorporated in the phase I/II sarcoma study by allowing additional treatment cycles to be given if the patient had ≤grade 1 toxicity. Further, across the board dose escalation was allowed by the Food and Drug Administration when the safety of preceding dose levels had been documented. The principal investigator, S.P.C., was also allowed to recommend surgical resection/debulking after at least one treatment cycle had been administered.
For the phase II osteosarcoma study, the optimal dose was derived from the results of the phase I/II sarcoma study. The number of patients enrolled was deduced from a statistical projection of 25% of patients achieving SD or better by International PET criteria and/or RECIST, assuming that ≥95% of patients with chemo-resistant osteosarcoma will progress if untreated.3 Clinical toxicity was also reported according to severity and relatedness to the study drug.
Clinical objectives/endpoints. The primary objective of the phase I/II study for sarcoma is to determine the clinical toxicity of escalating doses of Rexin-G as defined by patient performance status, toxicity assessment score, hematologic, and metabolic profiles. The secondary objectives include (i) evaluation of the potential of Rexin-G for evoking an immune response, recombination events and/or unwanted vector integration in nontarget organs, and (ii) identification of an antitumor response to Rexin-G.
The primary objective of the phase II study for osteosarcoma is to confirm the efficacy of Rexin-G in terms of tumor responses (complete response (CR), PR, or SD) by International PET criteria and by RECIST. A favorable tumor response was recorded if the patient had a CR, PR, or SD. The secondary objectives are (i) to confirm the efficacy of Rexin-G in terms of a median PFS of ≥1 month and a median OS of ≥6 months, and (ii) to evaluate the safety/toxicity of continued use of Rexin-G and the potential of Rexin-G for evoking an immune response, recombination events and/or unwanted vector integration in nontarget organs (PBLs).
Patient population. The phase I/II study included patients with a pathologic diagnosis of sarcoma who were refractory to standard chemotherapy, and the phase II study, patients with recurrent or metastatic osteosarcoma who were considered refractory to known therapies. Histologic or cytologic confirmation at diagnosis or recurrence was required. Inclusion criteria consisted of an Eastern Cooperative Oncology Group performance score of 0–1 and adequate hematologic, hepatic, and kidney function. Exclusion criteria included human immunodeficiency virus, hepatitis B virus, or hepatitis C virus positivity, clinically significant ascites, medical, or psychiatric conditions that could compromise successful adherence to the protocol, and unwillingness to employ effective contraception during treatment with Rexin-G and for 6 weeks following treatment completion. The clinical protocols (C07-103 and C07-110) were reviewed and approved by the Western Institutional Review Board, Olympia WA 98502 and the Biosafety Committee of Epeius Clinical Research Unit, San Marino CA 91108.
Patient recruitment and assignment. The phase I/II and phase II studies using Rexin-G for sarcoma and osteosarcoma were registered on www.clinicaltrials.gov (NCT00505713 and NCT00572130, respectively) within 1 week of study initiation, and patients were recruited on a first come first served basis after appropriate screening procedures were conducted. Written informed consent, in accordance with the Declaration of Helsinski protocols, was obtained from each patient at the time of enrollment. The phase I/II sarcoma study is an open label study using escalating doses of Rexin-G. Six patients were enrolled at dose level 0 followed by a dose escalation in seven patients at dose level I and another seven patients at dose level II when safety/toxicity data in at least three patients at lower dose levels had been recorded and no DLT was encountered. The phase II osteosarcoma study enrolled 22 patients at dose level II. Table 1 shows the demographics of all enrolled patients.
Treatment. Rexin-G is a nonreplicative “pathotropic” (pathology-targeted) retroviral vector bearing a collagen-binding motif on its envelope protein23 and encoding an N-terminal deletion mutant construct of human cyclin G124 under the control of the moloney murine leukemia virus long terminal repeat promoter. The Rexin-G vector is produced by transient co-transfection of three separate plasmids in 293T cells (human kidney 293 cells transformed with the SV40 large T antigen) maintained as a fully validated master cell bank.17,25,26 The final product exhibits a viral titer of 5 × 109 colony forming units (cfu) per milliliter, a biologic potency of 50–70% growth inhibitory activity in A375 melanoma cells, <550 bp residual DNA, no detectable E1A or SV40 large T antigen, and no detectable RCR.27 The clinical vector is stored in volumes of 23 ml in 30 ml vials or 40 ml in 150 ml cryobags in a −80 ± 10 °C freezer marked Biohazard. Preparation of the Rexin-G vector for patient administration consisted of rapid thawing of the vector in the vial or cryobag in a 34 °C waterbath. The vector was thawed 15–30 minutes prior to infusion into the patient, and was infused within 1 hour of thawing, intravenously over 5–10 minutes or at 4 ml/min. All personnel who handled and disposed of the vector observed Biosafety Level 2 compliance in accordance with the National Institutes of Health Guidelines for Research Involving Recombinant DNA molecules.
The phase I/II sarcoma study enrolled 20 patients who received escalating doses of Rexin-G. Briefly, each treatment cycle was 6 weeks, consisting of 4 weeks treatment and 2 weeks rest period. The following two dose levels were employed: dose level 0 = 1 × 1011 cfu two times a week for 4 weeks (cumulative dose per cycle: 8 × 1011 cfu); dose level I = 1 × 1011 cfu three times a week for 4 weeks (cumulative dose per cycle: 12 × 1011 cfu); and dose level II = 2 × 1011 cfu three times a week (cumulative dose per cycle: 24 × 1011 cfu). The treatment cycles were repeated if the patient had grade 1 or less toxicity.
The phase II osteosarcoma study enrolled 22 patients who received dose level II or 2 × 1011 cfu three times a week for 4 weeks (cumulative dose per cycle: 24 × 1011 cfu), considered an optimal dose of Rexin-G for sarcoma. Some patients were started at 1 × 1011 cfu three times a week but the dose was eventually escalated to dose level II when safety at dose level I was documented in the phase I/II sarcoma study. The treatment cycles were repeated if the patient had grade 1 or less toxicity.
Safety and efficacy evaluation. Pretreatment evaluation included history, physical exam, complete blood count with differential and platelet count, serum chemistry panel including aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, creatinine, and total bilirubin, assessment of coagulation including prothrombin time, international normalized ratio, and activated partial thromboplastin time, testing for human immunodeficiency virus, hepatitis B virus, and hepatitis C virus, imaging evaluation to include a whole body fludeoxyglucose/PET computed tomography (CT) scan, electrocardiogram and chest X-ray. All patients had a complete blood count and serum chemistry panel performed weekly during treatment.
Safety analysis. Toxicity was assessed before and after each vector infusion, and before beginning an additional treatment cycle. Patients had serum collected for vector antibody detection and peripheral blood mononuclear cells collected for assessment of vector DNA integration and RCR at the end of 4 weeks, at 6 weeks or before the start of a treatment cycle.
Toxicity was graded using the National Cancer Institute Common Terminology Criteria Version 3.0.22 Overall evaluation of safety/toxicity was conducted by the principal investigator and associate (S.P.C. and V.S.C., respectively).
Correlative analysis. Correlative laboratory analysis was performed in the Epeius Biotechnologies Quality Control Unit, using standard operating procedures in compliance with good laboratory practices.28
Detection of antivector antibodies in patients' serum: Testing for the presence of antivector antibodies was performed on serum samples obtained preinfusion and at 4 weeks and thereafter, at 6 weeks following treatment initiation or before each treatment cycle.
Vector neutralization assay: A375 cells (ATCC, Manassas, VA), a human amelanotic melanoma cancer cell line, are incubated in 12-well plates, at a plating density of 0.8 × 104 cells per well, in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (D10). After overnight attachment, the cells are exposed to 1 ml of a mixture of Rexin-G vector and patient's pre and post-treatment serum, fetal bovine serum and D10 (as controls) in the presence of Polybrene (8 µg/ml) for 2 hours at 37 °C, 5% CO2, with periodic rocking. Then, 1 ml fresh D10 is added, and the cultures are further incubated at 37 °C, 5% CO2. The medium is replaced with 1 ml fresh D10 the next day. To assess the dominant negative cyclin G gene product potency of the Rexin-G vector, the transduced cells are evaluated for their reduced proliferative potential by counting the number of viable cells in triplicate cultures at serial intervals (4 and 5 days) after transduction. This is our standard vector growth inhibition assay calculated as follows:
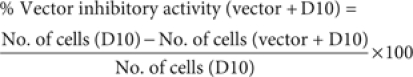 |
To obtain the vector inhibitory activity in serum-treated and control cultures, the mean cell numbers in cultures transduced with mixtures of vector + D10 were compared with vector + serum-treated cultures and expressed as % vector inhibitory activity, using the following formula:
 |
The presence of vector-neutralizing activity is detected by comparing the % inhibitory activity in mixtures of vector + D10 versus vector + serum using the following formula:
% Vector-neutralizing activity = % inhibitory activity (vector + serum) − % inhibitory activity (patient baseline or fetal bovine serum)
A negative value indicates a drop in inhibitory activity. When the value is ≤10 no vector neutralization has occurred, and >10 neutralization has occurred.
Testing for antibodies against the gp70 envelope protein: Detection of serum antibodies against gp70 is conducted by western slot blot analysis using gp70 containing Rexin-G retroviral vector as a polyacrylamide gel electrophoresis resolved capture antigen incubated with either serum from treated patients or positive control gp70 murine monoclonal antibody.32 The appearance of an immunoreactive band on the Rexin-G slot blot at 70 kDa (gp70) in slot lanes of patient's postinfusion serum indicates presence of patient postinfusion antibodies against the gp70 envelope protein.
Testing for the presence of RCR in patient PBLs: Testing for the presence of RCR was performed on DNA extracted from PBLs of treated patients, obtained at baseline (before vector infusion) and at the end of 4 weeks (after start of treatment). The assay was designed to detect the presence of RCR DNA sequences postvector infusion using PCR. In this study, real-time PCR with an iCycler, Master mix reagents and optimized moloney murine leukemia virus envelope primers is used to amplify a small portion of the 2001 bp moloney murine leukemia virus envelope gene (164 bp fragment from 411 to 574 bp) present in our Rexin-G retroviral vector. Serial tenfold dilutions of control moloney murine leukemia virus envelope plasmid bovine epitope-retroviral envelope DNA in the picogram to femptogram range is diluted into control samples of human genomic DNA to establish a standard curve down to the limit of detection of the assay. Syber green DNA intercalation dye is used as a probe to monitor the progress of increased DNA quantities generated in the PCR reaction. MyIQ software records the real-time PCR reaction data and subsequently calculates the standard curve as well as the value for test patient samples. The real-time PCR results for amplification of the moloney murine leukemia virus envelope gene from 3 µg DNA (less if noted) patient lymphocyte genomic must be below the lowest result on the standard curve and or below preinfusion values to be considered “Negative” for RCR.
Vector DNA integration studies: Testing for presence of vector DNA integration was performed on DNA extracted from PBLs obtained preinfusion, 1 week, and at the end of 4 weeks or before the start of each treatment cycle. Testing for vector DNA integration in PBLs was performed by centrifuging patient blood samples in a CPT collection tube to separate white blood cells from RBCs and serum. White blood cell DNA is isolated using a Qiagen blood DNA isolation kit, then quantified with a UV spectrophotometer. Real-time PCR with an iCycler, Master mix reagents and optimized Neo primers are used to amplify a small portion of the 795 bp Neomycin Phosphotransferase gene (75 bp fragment from 382 to 456 bp) present in our dnG1-Erex retroviral vector. Serial tenfold dilutions of Rexin-G retroviral vector control plasmid dnG1-Erex DNA in the pico to femptogram range is diluted into control human genomic DNA to establish a standard curve down to the limit of detection of the assay. Syber green DNA intercalation dye is used as a probe to monitor the progress of increased DNA quantities generated in the PCR reaction. MyIQ software records the real-time PCR reaction data and subsequently calculates the standard curve as well as the value for test patient samples. The real-time PCR results for amplification of the neomycin phosphotransferase gene from patient lymphocyte genomic DNA must fall below the lowest result on the standard curve and or below preinfusion values to be considered “Negative” for vector integration.
Efficacy analysis. Efficacy assessment with fludeoxyglucose PET/CT scan was also performed at the end of 4 weeks, and at the end of 6 weeks or before starting an additional treatment cycle up to 12 weeks, and every 12 weeks thereafter. All PET/CT images were performed and reviewed by independent radiologists of the Medical Imaging Center of Southern California, Santa Monica, who are experts at nuclear and PET imaging and who were blinded to the Rexin-G dose levels. Tumor response was evaluated using the standard National Cancer Institute RECIST criteria,29 the International PET criteria,30 and the CHOI criteria,31 according to Food and Drug Administration-approved protocols for tumor response assessment.
The modified International PET Criteria defines a CR as disappearance of fludeoxyglucose avid uptake in target and nontarget lesions with no new lesions; PR as a decrease in maximum standardized uptake value of >25% from baseline with no new lesions and no obvious progression of nontarget lesions; SD as not meeting the criteria for CR, PR or PD and no symptomatic deterioration attributed to tumor progression; and PD as an increase in maximum standardized uptake value of >25% from baseline, any new lesions, and obvious progression of nontarget lesions.30
The modified CHOI Criteria defines CR as the disappearance of all disease and no new lesions; PR as a decrease in size of ≥10% or a decrease in CT density (HU) ≥15% with no new lesions and no obvious progression of nonmeasurable disease; SD as not meeting the criteria for CR, PR or PD and no symptomatic deterioration attributed to tumor progression; and PD as an increase in unidimensional tumor size of >10% and did not meet criteria for PR by CT density, any new lesions, including new tumor nodules in a previously cystic tumor.31
Overall evaluations of tumor responses, PFS and OS were conducted by the principal investigator and associate (S P C and V S C, respectively).
Statistical analysis. Frequency tables, graphs, and summary statistics were used to describe patient characteristics and outcome data for both phase I/II and phase II studies. Clinical data from 1 August, 2007 to 29 October, 2008 were analyzed. Kaplan-Meier methodology16 was used to describe graphically the distribution of OS. OS time was calculated in days and divided by 30.4 to convert to months. PFS time was approximated, using the times of patient evaluations. OS and PFS times were compared in groups of patients treated at different dose levels, using permutation tests on the logrank statistic with at least 10,000 replications. Tumor response data by different specific criteria (RECIST, PET or CHOI criteria) were reported. Reported P-values are two-sided, and P < 0.05 was considered statistically significant. Analysis was done using NCSS software (Number Cruncher Statistical Systems, Kaysville, Utah). Statistical analysis was performed by a biostatistician not otherwise involved in the study (W.C.B.).
Acknowledgments
We are grateful to John P. Levy, Rebecca A. Reed, W. Nina Petchpud, and Liqiong Liu (Epeius Quality Control Unit) for quality control testing of product and patient samples, to Antonette C. Balais, R.N. and Jun de Guzman (Epeius Clinical Research Unit) for data management. S.P.C., principal investigator, V.S.C., L.F., A.S., and D.Q., who participated in the design, patient recruitment, conduct and evaluation of the clinical safety and efficacy of Rexin-G, and W.C.B, who performed the statistical analysis, have no conflict of interest. F.L.H and E.M.G, who designed the studies and conducted the correlative analysis are compensated employees of Epeius Biotechnologies Corporation. The studies were supported by Epeius Biotechnologies Corporation, San Marino, CA 91108.
REFERENCES
- Surveillance, Epidemiology, and End Results (SEER) Program Prevalence database: “US estimated 29-year LD counts on prevalence on 1/1/2004 by duration” for Bones and Joints Cancer. National Cancer Institute, DCCPS, Surveillance Research Program, Statistical Research and Applications Branch, based on the November 2006 SEER data submission 2007 . < www.seer.cancer.gov >.
- Longhi A, Errani C, De Paolis M, Mercuri M., and , Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Kempf-Bielack B, Bielack SS, Jürgens H, Branscheid D, Berdel WE, Exner GU, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology, and End Results (SEER) Program (2007) Prevalence database: “US estimated 29-year LD counts on prevalence on 1/1/2004 by duration” for Soft Tissue including Heart CancerNational Cancer Institute, DCCPS, Surveillance Research Program, Statistical Research and Applications Branch, based on the November 2006 SEER data submission . < www.seer.cancer.gov >.
- Milano A, Apice G, Ferrari E, Fazioli F, de Rosa V, de Luna AS, et al. New emerging drugs in soft tissue sarcoma. Crit Rev Oncol Hematol. 2006;59:74–84. doi: 10.1016/j.critrevonc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Rossi CR, Brandes A., and , Nitti D. Adult soft tissue sarcomas: conventional therapies and molecularly targeted approaches. Cancer Treat Rev. 2006;32:9–27. doi: 10.1016/j.ctrv.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Geer RJ, Woodruff J, Casper ES., and , Brennan MF. Management of small soft-tissue sarcoma of the extremity in adults. Arch Surg. 1992;127:1285–1289. doi: 10.1001/archsurg.1992.01420110027007. [DOI] [PubMed] [Google Scholar]
- Karakousis CP., and , Zografos GC. Radiation therapy for high grade soft tissue sarcomas of the extremities treated with limb-preserving surgery. Eur J Surg Oncol. 2002;28:431–436. doi: 10.1053/ejso.2002.1264. [DOI] [PubMed] [Google Scholar]
- Pisters PW, Leung DH, Woodruff J, Shi W., and , Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- Cormier JN., and , Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- Demetri GD. Major developments in the understanding and treatment of soft-tissue sarcomas in adults. Curr Opin Oncol. 1998;10:343–347. doi: 10.1097/00001622-199807000-00011. [DOI] [PubMed] [Google Scholar]
- Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children's Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- Mori K, Rédini F, Gouin F, Cherrier B., and , Heymann D. Osteosarcoma: current status of immunotherapy and future trends (Review) Oncol Rep. 2006;15:693–700. [PubMed] [Google Scholar]
- Witlox MA, Lamfers ML, Wuisman PI, Curiel DT., and , Siegal GP. Evolving gene therapy approaches for osteosarcoma using viral vectors: review. Bone. 2007;40:797–812. doi: 10.1016/j.bone.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehler R, Russell SJ., and , Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., and , Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Skotzko M, Wu L, Anderson WF, Gordon EM., and , Hall FL. Retroviral vector-mediated gene transfer of antisense cyclin G1 (CYCG1) inhibits proliferation of human osteogenic sarcoma cells. Cancer Res. 1995;55:5493–5498. [PubMed] [Google Scholar]
- Gordon EM, Chen ZH, Liu L, Whitley M, Liu L, Wei D, et al. Systemic administration of a matrix-targeted retroviral vector is efficacious for cancer gene therapy in mice. Hum Gene Ther. 2001;12:193–204. doi: 10.1089/104303401750061258. [DOI] [PubMed] [Google Scholar]
- Oncologic “Threshold of Credibility” Paradigm being explored at FDA (2004): “The Pink Sheet” Prescription Pharmaceuticals and Biotechnology 66: p. 15
- Guidance for industry clinical trial endpoints for the approval of cancer drugs and biologics DHHS, FDA, CDER, CBER. 2007. pp. 1–19.
- Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- The NCI Common Terminology Criteria for Adverse Events Version 3 (2003) Cancer Therapy Evaluation Program DCTD, NCI, NIH, DHHS 1–72. , ( http://ctep.cancer.gov ):
- Hall FL, Liu L, Zhu NL, Stapfer M, Anderson WF, Beart RW, et al. Molecular engineering of matrix-targeted retroviral vectors incorporating a surveillance function inherent in von Willebrand factor. Hum Gene Ther. 2000;11:983–993. doi: 10.1089/10430340050015293. [DOI] [PubMed] [Google Scholar]
- Xu F, Prescott MF, Liu PX, Chen ZH, Liau G, Gordon EM, et al. Long term inhibition of neointima formation in balloon-injured rat arteries by intraluminal instillation of a matrix-targeted retroviral vector bearing a cytocidal mutant cyclin G1 construct. Int J Mol Med. 2001;8:19–30. doi: 10.3892/ijmm.8.1.19. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Cornelio GH, Lorenzo CC, Levy JP, Reed RA, Liu L, et al. First clinical experience using a ‘pathotropic' injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int J Oncol. 2004;24:177–185. [PubMed] [Google Scholar]
- Gordon EM, Lopez FF, Cornelio GH, Lorenzo CC, Levy JP, Reed RA, et al. Pathotropic nanoparticles for cancer gene therapy Rexin-G IV: three-year clinical experience. Int J Oncol. 2006;29:1053–1064. [PubMed] [Google Scholar]
- Guidance for Industry: Supplemental guidance on testing for replication competent retrovirus in retroviral vector based gene therapy products and during follow-up of patients in clinical trials using retroviral vectors (2006) UUSPHS, FDA, CBER; pp. 1–13. [DOI] [PubMed] [Google Scholar]
- Galanis E, Carlson SK, Foster NR, Lowe V, Quevedo F, McWilliams RR, et al. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol Ther. 2008;16:979–984. doi: 10.1038/mt.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1752–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- Tabata N, Miyazawa M, Fujisawa R, Takei YA, Abe H., and , Hashimoto K. Establishment of monoclonal anti-retroviral gp70 autoantibodies from MRL/lpr lupus mice and induction of glomerular gp70 deposition and pathology by transfer into non-autoimmune mice. J Virol. 2000;74:4116–4126. doi: 10.1128/jvi.74.9.4116-4126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]