Abstract
Zn2+ is critical for the functional and structural integrity of cells and contributes to a number of important processes including gene expression. It has been shown that NO exogenously applied via NO donors resulting in nitrosative stress leads to cytoplasmic Zn2+ release from the zinc storing protein metallothionein (MT) and probably other proteins that complex Zn2+ via cysteine thiols. We show here that, in cytokine-activated murine aortic endothelial cells, NO derived from the inducible NO synthase (iNOS) induces a transient nuclear release of Zn2+. This nuclear Zn2+ release depends on the presence of MT as shown by the lack of this effect in activated endothelial cells from MT-deficient mice and temporally correlates with nuclear MT translocation. Data also show that NO is an essential but not sufficient signal for MT-mediated Zn2+ trafficking from the cytoplasm into the nucleus. In addition, we found that, endogenously via iNOS, synthesized NO increases the constitutive mRNA expression of both MT-1 and MT-2 genes and that nitrosative stress exogenously applied via an NO donor increases constitutive MT mRNA expression via intracellular Zn2+ release. In conclusion, we here provide evidence for a signaling mechanism based on iNOS-derived NO through the regulation of intracellular Zn2+ trafficking and homeostasis.
Multiple biological functions have been ascribed to NO as a molecule serving signaling or regulating tasks or acting as a cytotoxic molecule, depending on its mode of enzymatic synthesis, its local concentration, and its chemical reactions with other molecules. Nanomolar concentrations of NO are synthesized by constitutively expressed NO synthases (cNOS) in a tightly regulated and pulsative fashion, which typically serve to activate the guanylyl cyclase to synthesize the second messenger cGMP (1). In this case, the target of the radical NO is the heme group with its central iron atom. Besides the two cNOS, also an inducible NO synthase (iNOS) exists that is expressed only after induction by proinflammatory cytokines and/or bacterial products like lipopolysaccharide (LPS). Expression of iNOS is found in a variety of acute or chronic diseases (2, 3) and, once expressed, synthesizes NO for prolonged periods of time, which may result in micromolar NO concentrations (4). Under these conditions, NO may react with molecular oxygen in a reaction mainly depending on the NO concentration to yield higher reactive nitrogen oxides (NOx such as N2O3, etc.), which display a much broader chemical reaction spectrum than NO itself (5). Among the amino acids present in proteins, preferentially cysteines are modified by NOx yielding S-nitrosothiols (6). Prominent targets within cells are proteins containing Fe-S or Zn-S clusters. Although Fe-S clusters are essential components of many active sites in enzymes, Zn-S clusters mainly serve as structural elements of proteins mediating specific DNA or RNA binding as well as protein–protein interactions.
Under aerobic conditions, NO via thiolate-nitrosation induces the release of Zn2+ from the zinc-storing protein metallothionein (MT) in vitro (7–11). By using the fluorescent resonance energy transfer technique and cells transfected with MT fused to green fluorescent protein, NO was found to induce an intracellular conformational change of MT, suggesting intracellular Zn2+ release from this protein (12). In addition, exogenously added NO has been shown to induce S-nitrosation of intracellular MT (13, 14). Moreover, nitrosative stress induces Zn2+ release in various types of cells predominantly within the cytoplasm (15–19). The absence of an NO-mediated increase in labile Zn2+ in endothelial cells from MT-1 and MT-2 knockout mice inferred a critical role for MT in the regulation of Zn2+ homeostasis by NO (20).
To date, all experiments showing increased intracellular labile zinc after nitrosative stress have been performed by using chemical compounds that generate NO. Therefore, we were interested, whether NO derived from iNOS activity may also have an impact on the intracellular zinc homeostasis. To our surprise, we found that iNOS-derived NO induces a transient release of Zn2+ almost exclusively within nuclei of cells. Moreover, we identified MT as the essential carrier molecule necessary for this nuclear Zn2+ release. In addition, we show that nitrosative stress increases MT gene expression via intracellular Zn2+ release. In conclusion, iNOS-derived NO and the ensuing nuclear Zn2+ release seem to represent a mechanism for NO-based signaling under inflammatory conditions.
Materials and Methods
Materials. N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylene diamine (TPEN) was purchased from Sigma. l-N5-(1-iminoethyl)-ornithine-dihydrochloride (l-NIO) and Zinquin ethyl ester (Zinquin) were from Alexis (Grünberg, Germany), and the recombinant cytokines human IL-1β, human tumor necrosis factor (TNF)-α, or murine IFN-γ were from Strathmann Biotec (Hannover, Germany). (Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)-amino]diazen-1-ium-1,2-diolate (DETA/NO) was synthesized as described (21). Decomposed DETA/NO (DETA/NO–NO) was prepared by incubating a 50-mM stock solution for 72 h at 37°C.
Cells. WT murine aortic endothelial cells (WT MAEC) were isolated from C57BL/6 mice, from iNOS-deficient C57BLBL/6 mice (MAEC iNOS–/–) (22), from 129S7/SvEvBrd-Mt1tm1BriMt2tm1Bri mice (MEAC MT–/–), or from the respective WT control strain 129S3/SvImJ (The Jackson Laboratory) by outgrowth from aortic rings as described (23). Cell characterization was performed by using endothelium characteristic surface markers like anti-vWF antiserum (Sigma) and the endothelium-specific mAb against mouse CD106/VCAM1 (ImmunoKontact, Frankfurt, Germany). Only cells of passage 6–12 were used. Activation for up to 72 h by a mixture of proinflammatory cytokines did not result in cell death. Murine erythrocytes were collected from heparinized blood of C57BL/6 mice as described (24). Apoptotic cell death was determined after incubation with 15 μM of the DNA stain Hoechst 33342 for 5 min at 37°C and subsequent analysis by fluorescence microscopy. Necrosis was detected by Trypan exclusion.
Labeling of Cells. For detection of intracellular labile Zn2+, 105 MAEC were cultured in six-well flat-bottom plates (Greiner Bio-One, Frickenhausen, Germany) and incubated with 25 μM Zinquin for 45 min at 37°C. Cells were then examined under a fluorescence microscope (Axioplan, Zeiss, Oberkochen, Germany) using the Zeiss filter set 02 (excitation 365 nm, emission LP 420 nm). For micrographs, a constant exposure time of 7 s was chosen. For immunohistochemical detection of MT, 1.5 × 104 MAEC were cultured in chamber slides (Permanox, Nalge Nunc), fixed in acetone for 10 min, and washed twice with PBS. Endogenous peroxidase activity was inhibited by 0.3% H2O2 in methanol for 10 min and washed with H2O. All subsequent steps were performed at 4°C in a humid chamber. After blocking with 0.5% cationized BSA (Aurion, Washington, DC) for 20 min, cells were washed with PBS and incubated with a mAb (IgG1) specific for the isoforms MT-1 and MT-2 (E9; DAKO) for 12 h. As a negative control, we used a mAb (IgG1) specific for Aspergillus niger glucose oxidase (DAKO), an enzyme neither present nor inducible in mammalian tissues. Slides were washed with PBS and processed by using a biotin-labeled anti-mouse antibody and horseradish peroxidase-coupled streptavidin (Vectastain ABC kit PK-4000; Vector Laboratories) followed by diaminobenzidine staining.
Semiquantitative RT-PCR. Total cellular RNA was isolated by using the RNeasy Mini Kit and the RNase-free Dnase Set (Qiagen, Hilden, Germany) following the manufacturer's protocol with 1 μg of RNA each for cDNA synthesis (25). Reverse transcription was carried out at 37°C for 1 h by using the Omniscript RT Kit and oligo(dT)16 primers (Qiagen). For semiquantification, PCR was performed with the isolated cDNA by using the TaqPCR Core Kit (Qiagen) and primers out of exon boundary 1 and 2 and out of exon 3, respectively: murine MT-1 cDNA (GenBank accession no. BC036990), sense, E1/2 5′-GCTCCTGCTCCACCGGCGGC-3′ (bases 81–100), antisense, E3 3′-CAGGCACAGCACGTGCAC-5′ (bases 235–252); murine MT-2 cDNA (GenBank accession no. BC031758), sense, E1/2 5′-GCTCCTGTGCCTCCGATGGA-3′ (bases 61–80), antisense, E3 3′-CAGGCACAGCACGTGCAC-5′ (bases 235–252). Expression of the housekeeping gene GAPDH was analyzed by using primers for murine GAPDH (GenBank accession no. M32599), sense, 5′-CAACTACATGGTTTACATGTTCC-3′ (bases 160–184), antisense, 3′-GGACTGTGGTCATGAGCCCT-5′ (bases 556–575). PCR was carried out following standard procedures (26). To ensure that amplification conditions were within the linear phase, PCR was performed by using 15–23 cycles with RNA isolated from MAEC treated with or without 100 μM Zn2+. The following cycle profiles were found to be suitable: 17 cycles at 94°C/30 s, 55°C/30 s, 72°C/30 s for MT-1; 17 cycles at 94°C/30 s, 60°C/30 s, 72°C/30 s for MT-2; or 17 cycles at 94°C/30 s, 58°C/45 s, 72°C/45 s for GAPDH-2 mRNA amplification. As a control, PCR was performed with all additives but without cDNA or with all additives but only with RNA, respectively, to exclude unspecific amplifications. Equal amounts of DNA were electrophoresed on 1.8% agarose gels. The MT/GAPDH ratios were obtained by densitometric analysis of ethidium bromide-visualized amplification product bands. Data derived from several individual experiments (n = 3–6) are reported as mean ± SD. Analysis was performed with Student's t test (two-tailed for independent samples) with P < 0.05 considered as significant.
Determination of Nitrite. MAEC (104) were cultured for 24 h in the absence or presence of proinflammatory cytokines and l-NIO. Nitrite accumulating in the culture supernatants was determined by using the diazotization reaction and NaNO2 as standard (23).
Results
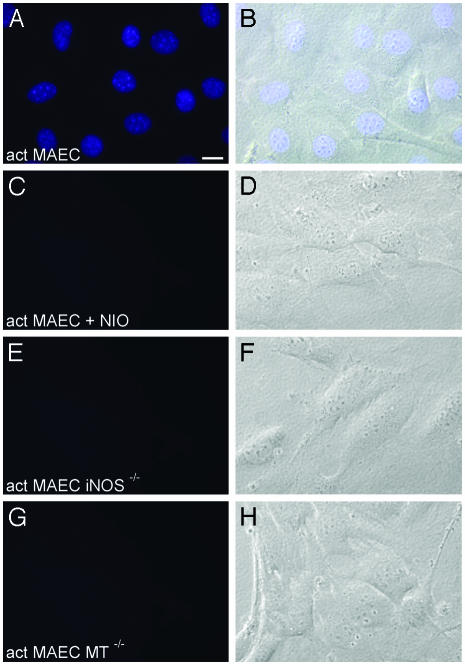
iNOS Activity Induces Intranuclear Zn2+ Release. Nitrosative stress induced by exogenously applied NO donors has repeatedly been found to induce Zn2+ release in various types of cells predominantly in their cytoplasm (15–19). To investigate whether NO synthesized endogenously by iNOS is sufficient to induce Zn2+ release within cells, primary MAEC from C57BL/6 mice (WT MAEC) were activated with a proinflammatory cytokine mixture (CM) of IL-1β, TNF-α, and IFN-γ in the absence or presence of the iNOS-specific inhibitor l-NIO. After various time intervals, the cells were stained with the Zn2+-specific fluorophore Zinquin and investigated under a fluorescence microscope. Resting, nonactivated WT MAEC did not show any Zn2+-specific fluorescence activity at any time point (not shown). Six, 12, or 18 h after cytokine addition, also no zinc-specific fluorescence activity could be found. However, 24 h after cytokine addition, a strong Zn2+-specific fluorescent signal was detected located in the nuclei of all activated WT MAEC (Fig. 1 A and B). Interestingly, this Zn2+-specific fluorescence activity showed a punctate (“speckled”) staining pattern against a more diffuse nucleoplasmic background staining (Fig. 2). The number of these highly fluorescent nuclear spots was extremely variable, ranging from 15 to 45 with a mean value of 25 and from ≈0.1 μm to ≈0.5 μm in diameter. Culture in the presence of the CM plus 250 μM l-NIO completely abolished the Zn2+-specific fluorescence signal (Fig. 1C). In addition, MAEC isolated from iNOS-deficient mice (MAEC iNOS–/–) and activated by the CM also lacked a Zn2+-specific fluorescence signal after staining with Zinquin (1E). Quantifying the NO production of WT MAEC in the absence or presence of the CM or l-NIO showed a strong positive correlation between NO synthesis (Table 1) and the Zn2+-specific fluorescence activity and showed that only quite high iNOS-derived NO concentrations induce an intranuclear Zn2+ release. As a further control, WT MAEC were incubated for 24 h with 50 μM ZnSO4 and subsequently stained with Zinquin. A slightly increased cytoplasmic Zn2+-specific fluorescence signal was found, indicating a rise in cytoplasmic labile zinc concentrations (not shown). However, no nuclear Zn2+-specific fluorescence activity could be detected.
Fig. 1.
NO derived from iNOS activity induces intranuclear Zn2+ release in MAEC. MAEC from WT (A–D), iNOS-deficient (E and F), or MT-deficient mice (G and H) were activated with IL-1β + TNF-α + IFN-γ for 24 h in the absence or presence of 250 μM of the iNOS-specific inhibitor l-NIO. Cells were then labeled with Zinquin, which specifically becomes fluorescent after reaction with labile, but not protein-bound, Zn2+. In activated WT MAEC, a bright Zn2+-specific fluorescence signal is located almost exclusively in the nuclei, with a punctate-staining pattern against a more diffuse nuclear background staining (A and B). In contrast, no Zn2+-specific fluorescence signal can be detected in activated WT MAEC cultured in the presence of l-NIO (C and D)or in MAEC deficient for either iNOS (E and F)orfor MT-1 + MT-2 (G and H). Thus, iNOS-derived NO, as well as MT, is essential for intranuclear Zn2+ release. (Scale bar = 5 nm.)
Fig. 2.
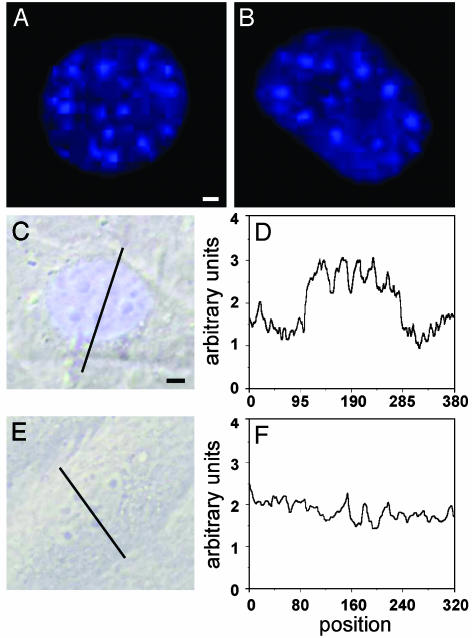
NO derived from iNOS activity induces a punctate nuclear Zn2+-specific fluorescence activity. Fluorescence and transillumination micrographs of nuclei of cytokine-activated WT MAEC cultured in the absence (A–C) or presence (E)of250 μM of the iNOS-specific inhibitor l-NIO. A line scan through the nuclei was performed at 500 nm (D and F), confirming the nuclear punctate-staining pattern against a more diffuse nuclear background. [Scale bars represent 0.5 nm (A and B) or 0.75 nm (C and E).]
Table 1. NO synthesis of WT MAEC and MAEC MT-/-.
| WT MAEC, μM nitrite | MAEC MT-/-, μM nitrite | |
|---|---|---|
| Resting | 0.8 ± 0.3 | 0.6 ± 0.2 |
| Activated | 13.0 ± 2.0 | 10.2 ± 1.1 |
| Activated + l-NIO | 2.3 ± 0.3 | 2.1 ± 0.2 |
Characterization of the Nuclear Zn2+ Release Induced by iNOS-Derived NO. To elucidate whether nuclear Zn2+ release induced by iNOS activity is a reversible effect, WT MAEC were again activated with the CM. After 24 h, the cell culture medium was exchanged, and the cells were cultured in the absence of cytokines for an additional 24-h time period. Subsequent incubation with Zinquin did not result in a Zn2+-specific fluorescence activity. This strongly indicates that iNOS-induced nuclear Zn2+ release is a transient and completely reversible phenomenon.
To investigate whether iNOS-derived NO is acting intracellularly or leaves the cells before inducing nuclear Zn2+ release, WT MAEC were again activated with the CM. Two hours later, increasing numbers (1 × 104 to 1 × 107) of freshly isolated syngeneic erythrocytes were added as a trap for extracellular NO. After a total culture time of 24 h, the WT MAEC were washed free of erythrocytes and stained with Zinquin. Although culture in the presence of 1 × 104 erythrocytes did not result in a decreased nuclear Zn2+-specific fluorescence activity, 1 × 105 erythrocytes reduced, and 1 × 106 as well as 1 × 107 erythrocytes completely abolished, the Zn2+-specific fluorescence activity. This result suggests that iNOS-derived NO diffuses out of the cells and acts in an autocrine or in a paracrine manner.
MT Is Essential for iNOS-Mediated Intranuclear Zn2+ Release. MT has been found to be essential for cytoplasmic Zn2+ release after treating cells with nitrosative stress via exogenous applied NO donors (19). To investigate whether MT also is essential for the intranuclear Zn2+ release mediated by iNOS activity, MAEC were isolated from 129S7/SvEvBrd-Mt1tm1BriMt2tm1Bri mice (MAEC MT–/–), where the MT1 and MT2 genes had been deleted (27). As a control, primary MAEC isolated from the control inbred strain 129S3/SvImJ mice were used. Although MAEC from 129S3/SvImJ mice activated for 24 h with the CM showed intranuclear Zn2+ release identical to WT MAEC from C57BL/6 mice (not shown), no intracellular Zn2+ release could be detected in MAEC MT–/– (Fig. 1G). Comparing the capacity to synthesize NO after cytokine activation, no significant differences could be found by using MAEC isolated from C57BL/6 mice or from MT–/– mice (Table 1). These results show that MT is essential for the transient intranuclear Zn2+ release induced by iNOS activity.
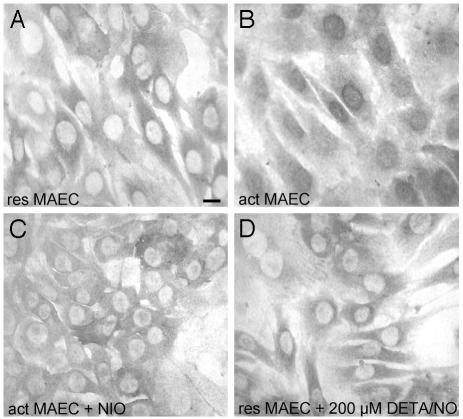
Nuclear Translocation of MT Is Cytokineas Well as NO-Dependent. Because MT is essential for the observed nuclear Zn2+ release, we next investigated whether activation by cytokines and iNOS activity affect the subcellular localization of MT. Wt MAEC or MAEC MT–/– were activated with the CM in the presence or absence of 250 μM l-NIO. After 0, 6, 12, 18, or 24 h, cells were fixed and stained with a monoclonal antibody (E9) specific for MT-1 and MT-2. As expected, MAEC MT–/– did not show any positive staining (not shown). In contrast, resting WT MAEC showed a cytoplasmic MT staining (Fig. 3A), which did not change after cytokine-activation for 6, 12, or 18 h (not shown). However, 24 h after addition of the CM, all WT MAEC showed a strong nuclear staining for MT (Fig. 3B). This nuclear MT staining was absent when cells were activated and cultured in the presence of l-NIO (Fig. 3C). Culture of resting WT MAEC in the presence of up to 200 μM of the NO donor DETA/NO did not result in an increased nuclear MT staining (Fig. 3D). These results show that both cytokines, as well as iNOS-derived NO, are essential for inducing the translocation of MT into the nucleus and in addition a direct functional as well as temporal correlation between intranuclear iNOS-mediated Zn2+ release and nuclear MT translocation.
Fig. 3.
Both proinflammatory cytokines as well as iNOS-derived NO are essential for MT nuclear translocation. Micrographs of resting (A) or cytokine-activated WT MAEC cultured for 24 h in the absence (B) or presence (C)of250 μM of the iNOS-specific inhibitor l-NIO or resting WT MAEC cultured for 24 h in the presence of 200 μM of the NO donor DETA/NO (D). Cells were labeled with a mAb specific for MT-1 as well as MT-2, and staining was visualized with the peroxidase-diaminobenzidine method. (Scale bar = 5 nm.)
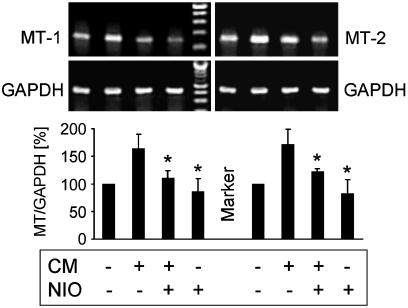
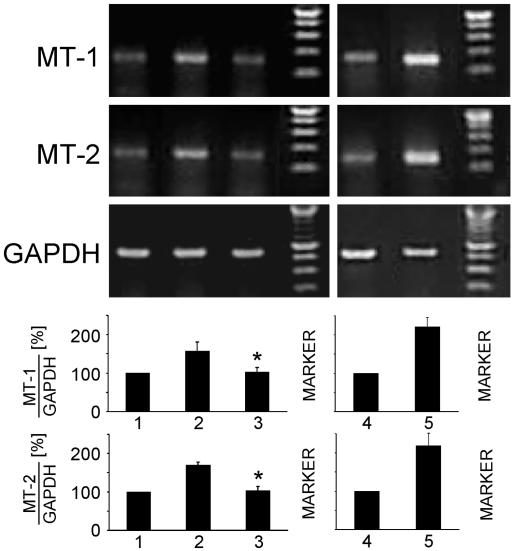
Proinflammatory Cytokines Increase MT Gene Expression via NO. MT-1 and MT-2 are constitutively expressed proteins. However, cytokines like IL-6 or IL-1β, as well as heavy metals, have been shown to increase the gene expression of MTs (for reviews, see refs. 28 and 29). To investigate effects of proinflammatory cytokines on the expression of MT mRNA, WT MAEC were cultured for up to 24 h in the presence of the CM. Subsequently, total RNA was isolated, and RT-PCR using MT-1- as well as MT-2-specific primers was performed. Results confirmed that MT-1, as well as MT-2, are constitutively expressed in WT MAEC (Fig. 4). The CM increased the expression of both MT mRNAs in a time-dependent fashion, with a maximal 1.6-fold increased MT-1 mRNA expression at 4–8 h and a maximal 1.7-fold increased MT-2 mRNA expression at 2–6 h (not shown). Therefore, the 6-h time point was used for all subsequent experiments. To investigate the role of iNOS-derived NO on cytokine-induced MT-mRNA expression, WT MAEC were cultured for 6 h in the absence or presence of the CM with or without 250 μM l-NIO. Fig. 4 shows that l-NIO significantly reduced the cytokine-increased expression of MT-1 as well as of MT-2 mRNA. This result indicates that iNOS-derived NO significantly contributes to the increased gene expression of MT-1 and MT-2 induced by proinflammatory cytokines.
Fig. 4.
NO derived from iNOS activity increases constitutive MT-1 and MT-2 mRNA expression. Wt MAEC were cultured for6hinthe absence or presence of IL-1β + TNF-α + IFN-γ (CM) with or without 250 μM of the iNOS-specific inhibitor l-NIO. Total cellular RNA was isolated, and MT-1-, MT-2-, and GAPDH-specific RT-PCR were performed. The CM in an NO-dependent manner increases constitutive MT-1 and MT-2 mRNA expression. Values are mean ± SD of six independent experiments. *, P < 0.005 as compared with cytokine-treated cells.
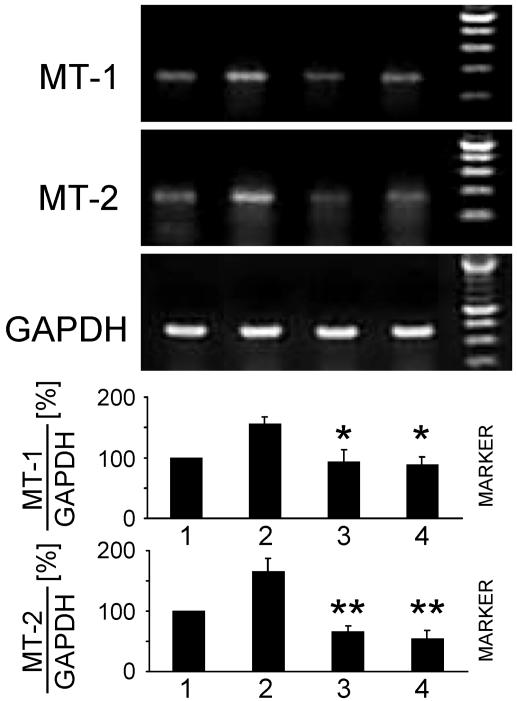
Exogenously Applied NO Increases MT Gene Expression. To investigate whether exogenously applied NO also increases the expression of MT-1 or MT-2 mRNA, WT MAEC were cultured in the presence of various subtoxic concentrations of the NO donor DETA/NO for 6 h. DETA/NO at a concentration of 200 μM increased the mRNA expression of both constitutively expressed MT-1 and MT-2 ≈1.7-fold (Fig. 5, lane 2). In contrast, the decomposed control compound DETA/NO–NO did not show any effect (Fig. 5, lane 3). To compare these results with effects of zinc on MT gene expression, we first determined the subtoxic Zn2+ concentrations. Culture of WT MAEC for 24 h in the presence of up to 100 μMZn2+ did not result in a significant rate of cell death whereas higher zinc concentrations proved to be cytotoxic. To quantify MT expression increased by subtoxic Zn2+ concentrations, cells were cultured in the presence of up to 100 μM Zn2+ for 6 h. Results showed that 100 μM Zn2+ increased the mRNA expression of MT-1 or MT-2 up to 2.2-fold (Fig. 5, lane 5). These results show that NO exogenously applied via an NO donor increases constitutive MT mRNA expression to a similar extent when compared with treatment with proinflammatory cytokines. However, Zn2+ increased constitutive MT gene expression even more strongly than NO.
Fig. 5.
NO exogenously applied by an NO donor as well as Zn2+ increase constitutive MT-1 and MT-2 mRNA expression. Wt MAEC were cultured for 6 h in the absence (lanes 1 and 4) or presence (lane 2) of 200 μM of the NO donor DETA/NO or of decomposed DETA/NO (DETA/NO–NO) (lane 3), or of 100 μM Zn2+ (lane 5). Total cellular RNA was isolated, and MT-1-, MT-2-, and GAPDH-specific RT-PCR were performed. NO derived from DETA/NO as well as Zn2+ increase constitutive MT-1 and MT-2 mRNA expression. Values are mean ± SD of three independent experiments. *, P < 0.02 as compared with lane 2.
Intracellular Zn2+ Released After Exogenously Applied NO Up-Regulates MT Gene Expression. To investigate whether intracellular Zn2+ released after exogenously applied NO is involved in the up-regulation of MT mRNA expression, we treated WT MAEC with DETA/NO in the presence of the intracellular Zn2+-chelator TPEN, which is highly diffusible and complexes Zn2+ with a high specificity. The maximally tolerizable dose of TPEN for WT MAEC was found to be 5 μM whereas higher TPEN concentrations induced apoptosis. Cultures of resting WT MAEC were treated with 200 μM DETA/NO in the presence or absence of 5 μM TPEN for 6 h. Fig. 6 shows that 5 μM TPEN inhibits the DETA/NO-mediated increased MT-1 as well as MT-2 mRNA expression down to constitutively expressed mRNA levels. Thus, increased MT gene expression after exogenously applied NO is mediated by intracellularly released Zn2+.
Fig. 6.
Intracellular Zn2+ released after exogenously applied NO upregulates constitutive MT-1 as well as MT-2 mRNA expression. Wt MAEC were cultured in the absence (lane 1) or presence (lanes 2 and 3) of 200 μM DETA/NO with or without 5 μM of the intracellular Zn2+-specific chelator TPEN (lanes 3 and 4). Total cellular RNA was isolated, and MT-1-, MT-2-, and GAPDH-specific RT-PCR were performed. DETA/NO increases MT-1 and MT-2 mRNA expression, which is inhibited by TPEN, whereas TPEN alone has no effect. Values are mean ± SD of three independent experiments. *, P < 0.01; **, P < 0.005 as compared with lane 2.
Discussion
Whereas the total zinc concentration in eukaryotic cells (≈200 μM) is quite high (30), cytoplasmic free zinc concentrations have been estimated to be in the range of 100 pM or lower (31–33). Thus, under physiological conditions, the majority of Zn2+ within cells is complexed by proteins. To date, MT is the only protein that has been implicated in cellular Zn2+ storage, and usually a significant percentage of the total cellular zinc is complexed by MT (34). Several functions have been designated to MT (35). It has been shown to protect cells against oxidative damage and to exert chaperone-like activities donating Zn2+ to target apometalloproteins, e.g., zinc finger transcription factors. In addition, MT has been suggested to play a central role in heavy metal metabolism and detoxification and in the management of various forms of stresses (for reviews see refs. 36 and 37).
Under normal conditions MT is located in the cellular cytoplasm. During proliferation and regeneration, however, MT has been found in nuclei and suggested to be closely linked to the requirement of Zn2+ for nuclear metalloenzymes and transcription factors (for reviews, see refs. 38 and 39). In addition, translocation of MT from the cytoplasm into the nucleus has been described after irradiation of keratinocytes with UVB or solar-simulated light (40, 41). We here show that, in cells activated with proinflammatory cytokines, NO endogenously produced via iNOS leads to a nuclear translocation of MT, which positively correlates with a nuclear appearance of labile Zn2+. These results indicate that, after cytokine activation and subsequent iNOS induction, MTs participate in Zn2+ trafficking from the cytoplasm into the nucleus. Both cytokines, as well as NO, are essential for this effect, which can first be detected 24 h after cell activation. It is suggested that first Zn2+-loaded MT is translocated to the nucleus and then, as the intracellular NO concentration increases, Zn2+ is released from this nuclear MT. That erythrocytes used as an extracellular sink for NO inhibit nuclear Zn2+ release shows that, at least, a considerable amount of NO acts in an autocrine or paracrine fashion, i.e., it diffuses out of the endothelial cells into the extracellular space before diffusing back or into neighboring cells. A similar effect has previously been found with activated hepatocytes where intracellular iron-nitrosyl complex formation could be inhibited by coculture with erythrocytes (42).
By using the Zn2+-specific fluorophore Zinquin, a speckled Zn2+-staining pattern against a more diffuse nuclear staining can be seen 24 h after cytokine activation (see Fig. 2). It has been described (43) that loading cells with zinc plus the zinc ionophore pyrithione results in a Zn2+-specific Zinquin fluorescence activity, which is evenly distributed throughout the entire cell including the nucleus. In addition, under normal cell culture conditions, the nuclear Zn2+ has been found to be bound tightly to proteins and is not detectable by Zinquin (43). These findings, together with the absence of a nuclear zinc staining after treating resting cells with Zn2+, indicate that Zinquin, indeed, detects only labile zinc (i.e., Zn2+ that is free or only loosely bound to proteins or small molecules).
Many nuclear factors are localized either partially or completely in distinct subnuclear compartments, producing a punctate staining pattern when analyzed by indirect immunofluorescence. These supramolecular assemblages variably are termed dots, speckles, or bodies. Despite differences, all of these structures are roughly spherical, 0.1–1 μm in diameter. Examples are coiled bodies, promyelotic leukemia (PML) nuclear bodies, BRCA1 nuclear dots, centromeric foci, splicing speckles, and others (for review, see ref. 44). Some of these structures have been implicated in essential processes, such as transcription and splicing. These structures seem to be dynamic and to undergo cycles of assembly and disassembly. To date, it is not known whether these supramolecular assemblies are involved in the organization of descrete sets of biochemical reactions or are merely nonfunctional aggregates or storage sites. It remains to be determined whether the spots of increased labile nuclear Zn2+ identified in our study are of any functional relevance. Several of the proteins present in nuclear bodies, dots, or speckles contain RING domains (45), small zinc-binding domains that coordinate two Zn2+ by cysteines mostly by using a cross-brace topology (for review, see ref. 46). RING proteins have been shown to assemble in bodies that act structurally as polyvalent scaffolds, thermodynamically by amplifying activities of partner proteins, or catalytically by spatiotemporal coupling of enzymatic reactions. RING assembly may be essential for normal cellular function, e.g., to organize, control, and integrate networks of biochemical reactions (45). Because proteins containing cysteine–Zn2+ complexes are molecular targets for NO (47), it is conceivable that iNOS-derived NO induces Zn2+ release from proteins containing RING domains present in nuclear speckles.
The iNOS-induced increase in nuclear labile Zn2+ represents a transient phenomenon, suggesting that, after a recovery period, cells are capable to again complex the nuclear labile Zn2+. NO induces disulfide formation in MT (7), which can be reduced by cells, whereas H2O2 oxidizes MT cysteines beyond the levels of disulfides (48). This result is in line with previous findings, showing that cells exposed to nitrosative in contrast to oxidative stress are able to repair disrupted zinc fingers of transcription factors after the stress has declined (49).
Zn2+ may serve as an intracellular regulator that operates at concentrations far below those of Ca2+ and hence coordinates different sets of biochemical processes (50). The thiolate ligands in MT confer redox activity on zinc clusters (51), and Zn2+ release from MT after nitrosative stress could be a mechanism to increase the intracellular free Zn2+ concentration to exert signaling functions. Indeed we found that intracellular Zn2+ released during nitrosative stress up-regulates MT gene expression (see Fig. 6).
Culturing cells in the presence of Zn2+ at a concentration of 10 μM results in inhibition of iNOS expression induced by proinflammatory cytokines (52). Moreover, slightly higher Zn2+ concentrations are capable of inhibiting iNOS activity (53). This finding indicates that, during inflammatory reactions, NO-induced intracellular Zn2+ release will inhibit further iNOS expression and iNOS-derived NO production via a feedback loop. This effect may, at least in part, explain the well known anti-inflammatory properties of Zn2+.
In conclusion, we here provide evidence that iNOS-derived NO mediates signaling within cells via Zn2+ by regulating its intracellular trafficking and homeostasis.
Acknowledgments
We thank Dr. V. Burchardt (Diabetes Research Center Düsseldorf) for providing iNOS–/– mice.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: iNOS, inducible NO synthase; MAEC, murine aortic endothelial cells; MT, metallothionein; Zinquin, Zinquin ethyl ester; TNF, tumor necrosis factor; DETA/NO, (Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)amino]diazen-1-ium-1,2-diolate; l-NIO, l-N5-(1-iminoethyl)-ornithine-dihydrochloride; CM, cytokine mixture; TPEN, N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylene diamine.
References
- 1.Ignarro, L. J. (1999) Biosci. Rep. 19, 51–71. [DOI] [PubMed] [Google Scholar]
- 2.Kröncke, K. D., Fehsel, K. & Kolb-Bachofen, V. (1998) Clin. Exp. Immunol. 113, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamora, R., Vodovotz, Y. & Billiar, T. R. (2000) Mol. Med. 6, 347–373. [PMC free article] [PubMed] [Google Scholar]
- 4.Laurent, M., Lepoivre, M. & Tenu, J. P. (1996) Biochem. J. 314, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wink, D. A. & Mitchell, J. B. (1998) Free Radical Biol. Med. 25, 434–456. [DOI] [PubMed] [Google Scholar]
- 6.Wink, D. A., Nims, R. W., Darbyshire, J. F., Christodoulou, D., Hanbauer, I., Cox, G. W., Laval, F., Laval, J., Cook, J. A., Krishna, M. C., et al. (1994) Chem. Res. Toxicol. 7, 519–525. [DOI] [PubMed] [Google Scholar]
- 7.Kröncke, K. D., Fehsel, K., Schmidt, T., Zenke, F. T., Dasting, I., Wesener, J. R., Bettermann, H., Breunig, K. D. & Kolb-Bachofen, V. (1994) Biochem. Biophys. Res. Commun. 200, 1105–1110. [DOI] [PubMed] [Google Scholar]
- 8.Misra, R. R., Hochadel, J. F., Smith, G. T., Cook, J. C., Waalkes, M. P. & Wink, D. A. (1996) Chem. Res. Toxicol. 9, 326–332. [DOI] [PubMed] [Google Scholar]
- 9.Aravindakumar, C. T., Ceulemans, J. & De Ley, M. (1999) Biochem. J. 344, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montoliu, C., Monfort, P., Carrasco, J., Palacios, O., Capdevila, M., Hidalgo, J. & Felipo, V. (2000) J. Neurochem. 75, 266–273. [DOI] [PubMed] [Google Scholar]
- 11.Zangger, K., Oz, G., Haslinger, E., Kunert, O. & Armitage, I. M. (2001) FASEB J. 15, 1303–1305. [DOI] [PubMed] [Google Scholar]
- 12.Pearce, L. L., Gandley, R. E., Han, W., Wasserloos, K., Stitt, M., Kanai, A. J., McLaughlin, M. K., Pitt, B. R. & Levitan, E. S. (2000) Proc. Natl. Acad. Sci. USA 97, 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, S., Kawai, K., Tyurin, V. A., Tyurina, Y. Y., Borisenko, G. G., Fabisiak, J. P., Quinn, P. J., Pitt, B. R. & Kagan, V. E. (2001) Biochem. J. 354, 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabisiak, J. P., Borisenko, G. G., Liu, S. X., Tyurin, V. A., Pitt, B. R. & Kagan, V. E. (2002) Methods Enzymol. 353, 268–281. [DOI] [PubMed] [Google Scholar]
- 15.Berendji, D., Kolb-Bachofen, V., Meyer, K. L., Grapenthin, O., Weber, H., Wahn, V. & Kröncke, K. D. (1997) FEBS Lett. 405, 37–41. [DOI] [PubMed] [Google Scholar]
- 16.Cuajungco, M. P. & Lees, G. J. (1998) Brain Res. 799, 118–129. [DOI] [PubMed] [Google Scholar]
- 17.Haase, H. & Beyersmann, D. (1999) Biometals 12, 247–254. [DOI] [PubMed] [Google Scholar]
- 18.Tartler, U., Kröncke, K. D., Meyer, K. L., Suschek, C. V. & Kolb-Bachofen, V. (2000) Nitric Oxide 4, 609–614. [DOI] [PubMed] [Google Scholar]
- 19.St Croix, C. M., Wasserloos, K. J., Dineley, K. E., Reynolds, I. J., Levitan, E. S. & Pitt, B. R. (2002) Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L185–L192. [DOI] [PubMed] [Google Scholar]
- 20.Tang, Z. L., Wasserloos, K. J., Liu, X., Stitt, M. S., Reynolds, I. J., Pitt, B. R. & St. Croix, C. M. (2002) Mol. Cell. Biochem. 234–235, 211–217. [PubMed] [Google Scholar]
- 21.Hrabie, J. A., Arnold, E. V., Citro, M. L., George, C. & Keefer, L. K. (2000) J. Org. Chem. 65, 5745–5751. [DOI] [PubMed] [Google Scholar]
- 22.Laubach, V. E., Shesely, E. G., Smithies, O. & Sherman, P. A. (1995) Proc. Natl. Acad. Sci. USA 92, 10688–10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suschek, C., Rothe, H., Fehsel, K., Enczmann, J. & Kolb-Bachofen, V. (1993) J. Immunol. 151, 3283–3291. [PubMed] [Google Scholar]
- 24.Wiegand, F., Kröncke, K. D. & Kolb-Bachofen, V. (1993) Transplantation 56, 1206–1212. [DOI] [PubMed] [Google Scholar]
- 25.Gubler, U. & Hoffman, B. J. (1983) Gene 25, 263–269. [DOI] [PubMed] [Google Scholar]
- 26.Saiki, R. K., Gelfand, D. H., Stoffel, S., Scharf, S. J., Higuchi, R., Horn, G. T., Mullis, K. B. & Erlich, H. A. (1988) Science 239, 487–491. [DOI] [PubMed] [Google Scholar]
- 27.Masters, B. A., Kelly, E. J., Quaife, C. J., Brinster, R. L. & Palmiter, R. D. (1994) Proc. Natl. Acad. Sci. USA 91, 584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghoshal, K. & Jacob, S. T. (2001) Prog. Nucleic Acid Res. Mol. Biol. 66, 357–384. [DOI] [PubMed] [Google Scholar]
- 29.Coyle, P., Philcox, J. C., Carey, L. C. & Rofe, A. M. (2002) Cell Mol. Life Sci. 59, 627–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmiter, R. D. & Findley, S. D. (1995) EMBO J. 14, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peck, E. J., Jr., & Ray, W. J., Jr. (1971) J. Biol. Chem. 246, 1160–1167. [PubMed] [Google Scholar]
- 32.Simons, T. J. B. (1991) J. Membr. Biol. 123, 63–71. [DOI] [PubMed] [Google Scholar]
- 33.Atar, D., Backx, P. H., Appel, M. M., Gao, W. D. & Marban, E. (1995) J. Biol. Chem. 270, 2473–2477. [DOI] [PubMed] [Google Scholar]
- 34.Kägi, J. H. & Schäffer, A. (1988) Biochemistry 27, 8509–8515. [DOI] [PubMed] [Google Scholar]
- 35.Palmiter, R. D. (1998) Proc. Natl. Acad. Sci. USA 95, 8428–8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis, S. R. & Cousins, R. J. (2000) J. Nutr. 130, 1085–1088. [DOI] [PubMed] [Google Scholar]
- 37.Maret, W. (2000) J. Nutr. 130, 1455S–1458S. [DOI] [PubMed] [Google Scholar]
- 38.Cherian, M. G. & Apostolova, M. D. (2000) Cell Mol. Biol. 46, 347–356. [PubMed] [Google Scholar]
- 39.Beyersmann, D. & Haase, H. (2001) Biometals 14, 331–341. [DOI] [PubMed] [Google Scholar]
- 40.Hanada, K., Tamai, K., Sawamura, D., Hashimoto, I. & Muramatsu, T. (1998) J. Invest. Dermatol. 110, 98–100. [DOI] [PubMed] [Google Scholar]
- 41.Jourdan, E., Emonet-Piccardi, N., Didier, C., Beani, J. C., Favier, A. & Richard, M. J. (2002) Arch. Biochem. Biophys. 405, 170–177. [DOI] [PubMed] [Google Scholar]
- 42.Stadler, J., Bergonia, H. A., Di Silvio, M., Sweetland, M. A., Billiar, T. R., Simmons, R. L. & Lancaster, J. R. (1993) Arch. Biochem. Biophys. 302, 4–11. [DOI] [PubMed] [Google Scholar]
- 43.Haase, H. & Beyersmann, D. (2002) Biochem. Biophys. Res. Commun. 296, 923–928. [DOI] [PubMed] [Google Scholar]
- 44.Lamond, A. I. & Earnshaw, W. C. (1998) Science 280, 547–553. [DOI] [PubMed] [Google Scholar]
- 45.Kentsis, A., Gordon, R. E. & Borden, K. L. (2002) Proc. Natl. Acad. Sci. USA 99, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borden, K. L. (2000) J. Mol. Biol. 295, 1103–1112. [DOI] [PubMed] [Google Scholar]
- 47.Kröncke, K. D. (2001) FASEB J. 15, 2503–2507. [DOI] [PubMed] [Google Scholar]
- 48.Quesada, A. R., Byrnes, R. W., Krezoski, S. O. & Petering, D. H. (1996) Arch. Biochem. Biophys. 334, 241–250. [DOI] [PubMed] [Google Scholar]
- 49.Kröncke, K. D., Klotz, L. O., Suschek, C. V. & Sies, H. (2002) J. Biol. Chem. 277, 13294–13301. [DOI] [PubMed] [Google Scholar]
- 50.Fischer, E. H. & Davie, E. W. (1998) Proc. Natl. Acad. Sci. USA 95, 3333–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maret, W. & Vallee, B. L. (1998) Proc. Natl. Acad. Sci. USA 95, 3478–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaoka, J., Kume, T., Akaike, A. & Miyachi, Y. (2000) J. Dermatol. Sci. 23, 27–35. [DOI] [PubMed] [Google Scholar]
- 53.Abou-Mohamed, G., Papapetropoulos, A., Catravas, J. D. & Caldwell, R. W. (1998) Eur. J. Pharmacol. 341, 265–272. [DOI] [PubMed] [Google Scholar]