Abstract
Lentiviral vectors (LVs) are tools for in vivo gene delivery, to correct genetic defects or to deliver antigens for vaccination. It was reported that systemic injection of LVs in mice transduced cells in liver and spleen. Here we describe the reasons for, and consequences of, persistent gene expression in spleen. After 5 days of intravenous injection, a green fluorescence protein (GFP)-expressing LV was detected in lymphocytes, macrophages and all subsets of dendritic cells (DCs) in spleen. In the case of macrophages and DCs, the percentage of transduced cells increased between 5 and 30 days after injection. We used bromodeoxyuridine (BrdU) incorporation to show that the macrophages were largely nondividing, whereas the transduced DCs arose from dividing precursor cells and could be detected in spleen 2 months after injection. Expression of ovalbumin (OVA) in the LV reduced the number of transduced DCs in spleen after 30 days. However, the remaining transduced cells stimulated proliferation and activation of OVA-specific CD8+ T cells transferred 2 months after LV injection. The mice also maintained cytolytic activity against OVA-pulsed targets. These results show that LVs transduce DC precursors, which maintain transduced DCs in spleen for at least 2 months, leading to prolonged antigen presentation and effective T-cell memory.
Introduction
Lentiviral vectors (LVs) are potentially useful tools for in vivo gene delivery. Intravenous injection of LVs carrying the vesicular stomatitis virus glycoprotein envelope results in transduction of cells predominantly in liver and spleen.1,2,3 This property has led to the exploitation of LVs for the correction of genetic disorders which result from single gene defects that can be cured by gene expression in hepatocytes. Indeed, promising results have been obtained in a number of animal models using this approach, for example, hemophilias, mucopolysaccharidoses, Crigler-Najjar, and Wilson diseases.4,5,6,7 However, antigen-presenting cells in liver (Kupfer cells) and spleen (macrophages, B cells and dendritic cells) are also transduced after intravenous injection.1,8,9 This could explain the immune responses to antigens expressed in LVs that are observed after intravenous injection.9,10,11 Indeed, we have shown that restricting antigen expression to macrophages and DCs in spleen using the dectin-2 promoter improves intravenous immunization.12 Conversely, restricting therapeutic transgene expression to hepatocytes inhibits immune responses and leads to improved transgene persistence.13,14
We were intrigued by the reported long-term gene expression in antigen-presenting cells in spleen, which lasted several months after injection.1,9 Splenic macrophages in the mouse are mainly nonproliferating, long-lived cells.15 However, splenic DCs turn over rapidly. The average half-life of CD11c+ cells in spleen is 3 days;16 whereas some DC subsets turnover faster even the relatively long-lived plasmacytoid DC (pDC) subset turnover within 14 days.17 This raises the question of how the population of LV-modified DC is maintained in spleen for several months.
LVs are now regarded as promising vaccine vector candidates for the treatment of infectious disease or cancer.18,19 We therefore also questioned the role of long-term gene expression in DCs during LV immunization. The length of time that antigen needs to be presented in order to trigger optimal CD8+ and CD4+ T-cell expansion has been examined in transgenic mice where the duration of antigen expression in antigen-presenting cells can be regulated. For CD8+ T cells, less than a 24-hour exposure to antigen is sufficient,20 whereas CD4+ T cells require continuous presentation of antigen throughout a 5-day expansion period.21 However, antigen presentation for several months is clearly beyond the time frame of an optimal primary immune response. Memory T-cell responses can also persist in the absence of antigen presentation.22 Indeed, persistent antigen presentation, particularly by quiescent DCs, is generally believed to result in tolerance.23
These data might suggest that a vaccine vector would only express antigen for sufficient time to stimulate an optimal primary response. However, it has also been proposed that persistence of vectors including vesicular stomatitis virus,24 adenovirus,25 and LVs26 is important for their vaccine function. Here we demonstrate that LVs transduce proliferating DC precursors after systemic administration, explaining the persistent pool of modified DC in spleen. Transduced DCs express and present an antigen for several months after immunization; this prolonged antigen presentation does not impair the effective immune response induced by LV administration.
Results
Transduced antigen-presenting cells in the spleen at least 2 months after LV injection
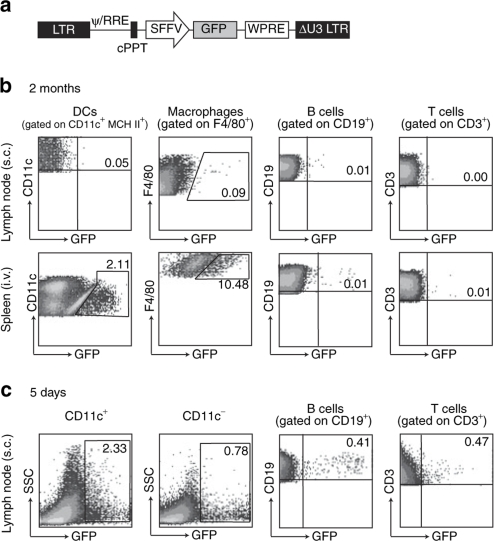
We first compared persistence of transduced cells 2 months after LV injection subcutaneously (s.c.), analyzing the draining lymph node (LN), or intravenously, analyzing the spleen. After injection of a LV encoding green fluorescence protein (GFP) (Figure 1a), DCs were purified using magnetic cell sorting of CD11c+ cells from LN or spleen and cells were also stained with antibodies to identify macrophages, B cells, and T cells. The percentage of GFP+ cells was determined for DCs, macrophages, and lymphocytes by fluorescence-activated cell sorter (Figure 1b). Transduced cells were detected in the spleen but not in the LN at this 2 months' time point following intravenous injection. In a control experiment we detected a significant percentage of transduced DCs and lymphocytes in the LN 5 days after subcutaneous LV injection (Figure 1c). We have previously shown that these transduced DCs are both skin-derived and resident DCs.12 Some of these transduced cells may subsequently exit the LN as we could no longer detect them there after 10 days, but we could detect antigen presentation using adoptive T-cell transfer, for 3 weeks.27 It is also possible that presentation by other cells at the injection site directly or indirectly present antigen at later times. Thus, transduced cells in spleen were only detected after intravenous injection. Our detection of transduced DCs and macrophages in spleen 2 months after intravenous LV injection was in agreement with previous reports.1,9
Figure 1.
Biodistribution of transduced cells. (a) Mice (2–3 per group) were injected either subcutaneously (s.c.) or intravenously (i.v.) with phosphate buffered saline or 108 IU GFP LV. At different time points, cells from spleen and lymph nodes (inguinal and popliteal) were collected, pooled, separated with anti-CD11c magnetic microbeads and stained with antibodies for flow cytometric analysis. The percentage of GFP+ DCs, macrophages, B cells and T cells was determined in (b) the lymph nodes and spleen 2 months after s.c. or i.v. injection, and (c) in lymph nodes 5 days after s.c. injection. DC, dendritic cell; GFP, green fluorescence protein; LV, lentiviral vector.
Long-term GFP expression in all DC subsets in spleen
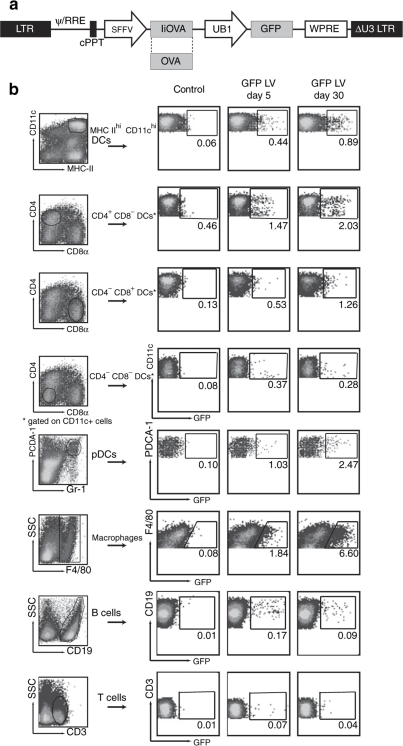
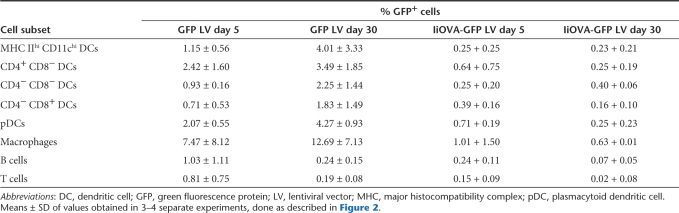
We then determined which DC subsets in mouse spleen were transduced following intravenous LV injection. In these experiments we used the LV expressing GFP and a second IiOVA-GFP LV expressing GFP and ovalbumin fused to the invariant chain11 (IiOVA, Figure 2a). We then examined GFP expression both in the short term (5 days) and in the long term (30 days). Figure 2b shows results with the GFP LV; although all the DC subsets present in the spleen were transduced, the percentage was highest in the CD4+CD8− subpopulation, which is also the most abundant in the spleen.28 Transduction of pDC, macrophages, and B cells was also detected. The same transduced cell populations were detected at both 5 and 30 days, although the percentage of transduced cells differed. Table 1 shows that, while there was a considerable variation between experiments, the mean percentage of transduction of DCs and macrophages increased with time using the GFP LV, whereas the percentage of transduced B and T cells decreased. The percentage of GFP-expressing cells that we could detect was lower when we used the IiOVA-GFP LV, though the pattern of transduction was similar. In this case the percentage transduced cells in most DC subsets and macrophages decreased with time, similarly to the B cells (Table 1).
Figure 2.
In vivo tracking of transduced cell subsets in the spleen after systemic injection of LVs. (a) Mice (2–3 per group) were injected intravenously with phosphate buffered saline or 108 IU of GFP LV (Figure 1) or IiOVA-GFP LV. Five or 30 days later, splenocytes from each group were pooled and DCs purified with anti-CD11c magnetic microbeads and stained with antibodies to analyze transduction of different cell subpopulations by flow cytometry. (b) The plots show the results of one representative experiment using the GFP LV. DC, dendritic cell; GFP, green fluorescence protein; LV, lentiviral vector.
Table 1.
Summary of in vivo tracking of transduced cell subsets in spleen after intravenous injection
Antigen expression affects long-term LV transduction in spleen
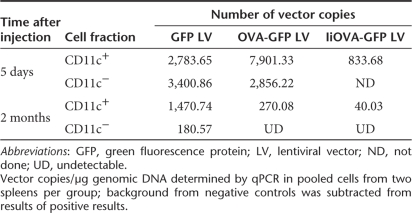
These data suggested that the presence of IiOVA in the LV, perhaps because of its immunogenicity, reduced LV persistence in DC in spleen. We tested this hypothesis in a further experiment using the GFP LV, the IiOVA-GFP LV and an OVA-GFP LV coexpressing secreted OVA and GFP (Figure 2a). In each case transduced DC were detected; with the GFP LV the percentage again increased over time, whereas with the IiOVA-GFP LV and the OVA-GFP LV the percentage of GFP-expressing DC decreased considerably (Supplementary Table S1 online). We then analyzed the number of LV integrants present by quantitative PCR in either the CD11c+ or the CD11c− populations. In the case of the GFP vector there was a small reduction (50%) in the number of LV genomes in the CD11c+ population, but there was a substantial decrease (95%) in the CD11c− population (Table 2). The increase in the percentage of GFP+ cells in the presence of a 50% reduction in GFP LV genomes is probably explained by GFP protein accumulation, as the level of GFP fluorescence in some cell populations is close to background (Figure 2b). The decrease in GFP LV genomes in the CD11c− population presumably occurs in the B- and T-cell population, which constitute ~80% of splenocytes compared to the 5% of F4/80+ macrophages (Supplementary Table S1). In agreement with the results observed by fluorescence-activated cell sorter, the number of copies of IiOVA-GFP LV and OVA-GFP LV also decreased substantially (95–96%) in the CD11c+ population (Table 2).
Table 2.
Vector copies in different fractions of spleen cells determined by qPCR after intravenous injection of LVs
LVs transduce dividing DC precursors
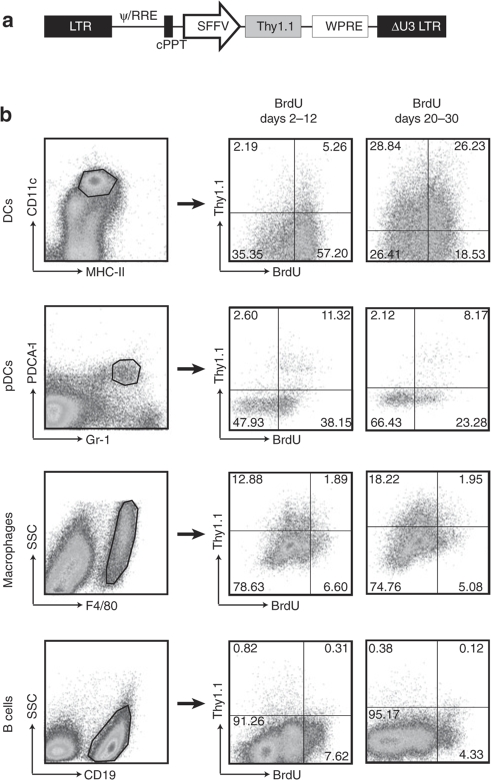
One of the possible explanations for the persistence of antigen presentation after immunization with LVs is the transduction of DC precursors. To test this hypothesis, we evaluated the incorporation of bromodeoxyuridine (BrdU) in transduced DCs. BrdU is incorporated into dividing DC precursors whose progeny renew the DC population in different lymphoid organs.16 To avoid the loss of fluorescence of GFP after intranuclear staining of BrdU, we tracked transduced cells with the surface marker Thy1.1 (CD90.1) (Figure 3a). In the first experiment mice were injected intravenously with LV. Then after 48 hours BrdU was administered intraperitoneally to ensure immediate access of the label to dividing precursors. BrdU was then administered in drinking water for 10 days, as longer term administration of BrdU is toxic to the mice, and then splenocytes were analyzed for BrdU incorporation and LV transduction.
Figure 3.
BrdU incorporation by transduced cells. (a) A LV-encoding Thy1.1 was used to track transduced cells by antibody staining and flow cytometry. Mice (n = 3–5 per group) were injected intravenously with phosphate buffered saline or Thy1.1 LV in the tail vein. Two days later, they received 200 µg of BrdU intraperitoneally followed by provision of BrdU 0.8 mg/ml in the drinking water from days 2 to 12. The experiment was repeated but BrdU was started at day 20 and given up to day 30 after injection of the LV. Incorporation of BrdU was analyzed by flow cytometry in different subpopulations of CD11c+ and CD11c− splenocytes. (b) Similar results were obtained for both periods of administration, representative plots are shown. BrdU, bromodeoxyuridine; DC, dendritic cell; GFP, green fluorescence protein; LV, lentiviral vector; MHC II, major histocompatibility complex 2; pDC, plasmacytoid dendritic cell.
Figure 3b shows that the majority of splenic DCs and pDCs had proliferated after 10 days, as previously reported.16 In contrast, a small fraction of splenic macrophages and B cells had divided at this time point (Figure 3b). Strikingly, a large proportion of DCs and pDCs had divided and LV transduction was similar in the BrdU labeled and BrdU unlabeled subsets of DCs and higher in the BrdU labeled subset of pDCs (Table 3). Some macrophages had divided, and the LV transduced BrdU positive and negative populations at a similar rate. The vast majority of B cells had not divided; however, LV transduction was much more efficient in the population that had incorporated BrdU. We then performed a second experiment in which BrdU was administered between day 20 and day 30 after intravenous LV injection and splenocytes were then analyzed on day 30. A similar difference between the transduced cells was observed, with the DCs and pDCs having recently proliferated and the macrophages and B cells largely remaining nondividing during this period. These data demonstrate that LVs can transduce progenitor cells that give rise to DCs and pDCs after intravenous injection; such transduced progenitors continue to repopulate the DC and pDC populations in spleen for at least 30 days after LV injection. The persistent macrophage transduction in spleen may also arise from transduction of precursors, although these divide less.
Table 3.
BrdU incorporation in transduced cells after intravenous injection of Thy1.1-LV
Persistence of antigen presentation in spleen after immunization with LVs
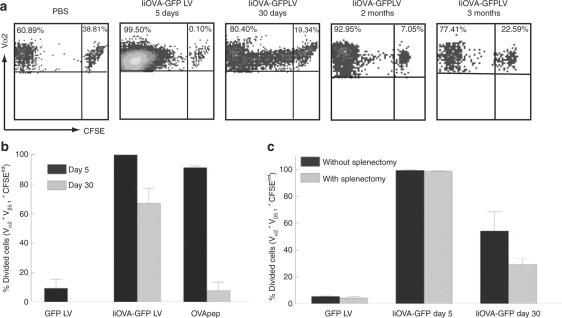
We then wanted to know whether the persistence of transduced antigen-presenting cells in the spleen also resulted in sustained antigen presentation. To test this we used adoptive transfer of OVA-specific H2Kb-restricted T-cell receptor transgenic lymphocytes from OT-1 mice, which recognize the CD8+ epitope OVA257–264, as a readout. Prior to their transfer into naive or immunized mice, these cells were labeled with carboxyfluorescein succinimydil ester (CFSE) to monitor their expansion and then tracked by fluorescence-activated cell sorter in the spleen. When transferred 5 days after immunization, almost all the OT-1 cells underwent division; interestingly, there was still proliferation of OT-1 cells transferred 30 days after mice were immunized (Figure 4a). The lower stimulation at day 30 may be explained by subtle differences of modified DC present at this time point (Table 1) or differences in the activation status of the DC. Furthermore, residual antigen presentation could still be detected 2 months after immunization, although it progressively decreased and little stimulation of OT-1 division was detected after 3 months (Figure 4a). In contrast, persistent antigen presentation was not observed when mice were injected intravenously with OVA257–264 plus monophosphoryl lipid A adjuvant (Figure 4b) or the lytic vaccinia virus expressing OVA (data not shown).
Figure 4.
Persistence of antigen presentation. Mice received intravenous injection of 5 × 106 CFSE-labeled cells from OT-1 mice at different time points after intravenous injection of PBS, GFP or IiOVA-GFP LVs. Five days later, transferred cells were tracked in the spleen and their expansion was assessed by dilution of CFSE by flow cytometry. The plots show events gated in (a) Vα2+ Vβ5.1+ cells. (b) Results from five experiments are summarized. (c) The experiment was repeated but 2 days before the adoptive transfer, spleens were removed and the OT-1 cells were tracked in peripheral blood. CFSE, carboxyfluorescein succinimydil ester; GFP, green fluorescence protein; LV, lentiviral vector; OVApep, OVA peptide; PBS, phosphate buffered saline.
To determine the site of prolonged antigen presentation, we performed splenectomy on immunized mice before OT-1 cell transfer, and then assessed their expansion in peripheral blood by flow cytometry, 5 days after adoptive transfer. As shown in Figure 4c, almost all of the OT-1 cells divided in the mice that were immunized 5 days before the adoptive transfer, with no difference between the mice with or without spleen. Therefore after intravenous injection of the LVs, there is a systemic transduction of antigen-presenting cells in various anatomical sites. In contrast, in the mice that received adoptively transferred T cells 30 days after immunization, there was an ~50% reduction in the percentage of OT-1 cells that proliferated when the spleen was removed (Figure 4c). It is unlikely that this difference is due to a homing problem of the transferred cells in the absence of spleen, as there are no differences in the levels of proliferation in the short-term group between mice with or without spleen (Figure 4c). This means that the spleen plays a significant role in long-term antigen presentation in LV immunized mice, although it is not exclusive and they are also antigen-presenting cells in other tissues, probably liver.1
The consequences of long-term antigen presentation
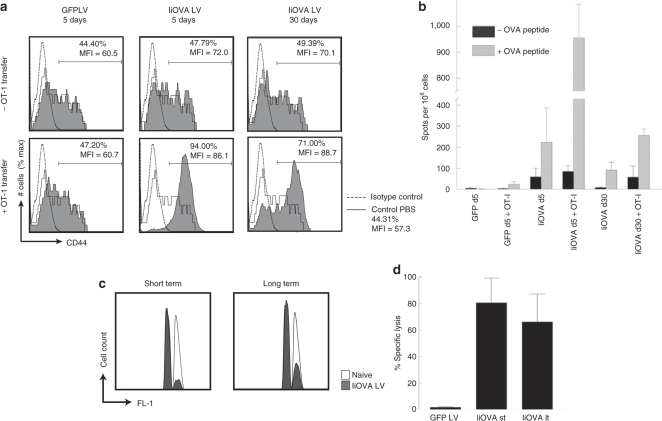
Proliferation of CD8+ T cells stimulated by antigen presentation in the periphery can lead to tolerance rather than immunization.29 We therefore examined the phenotype and function of the OT-1 cells that were transferred into mice that were presenting antigen via LV transduced cells. Figure 5a shows that the T-cell activation marker CD44 was upregulated in the Vα2+Vβ5+ cell population of the transferred OT-1 cells. More activated cells were observed when the OT-1 cells were transferred 5 days after immunization, but a significant population of activated cells was also detected when transfer was delayed until day 30. We also examined the ability of splenocytes to produce interferon-γ in response to OVA257–264 (Figure 5b). In the immunized mice with no OT-1 cells transferred, we observed an average of ~200 spots/106 cells which decreased to 100 spots/106 cells after 30 days, corresponding to a long-lived endogenous CD8+ T-cell response. Upon OT-1 adoptive transfer, these numbers increased to 950 and 250, respectively, showing that the transferred cells were also functionally activated.
Figure 5.
In vivo immune responses. OT-1 cells were adoptively transferred into mice that had been injected intravenously with IiOVA-GFP LV at different times. (a) Five days later, the spleen cells from these mice were harvested and analyzed by flow cytometry for expression of CD44 in Vα2+Vβ5.1+ cells. (b) They were also cultured overnight to detect secretion of IFN-γ upon restimulation with OVA257–264 peptide. Controls were mice injected with GFP LV or phosphate buffered saline (PBS). The results are compared with those of mice that did not receive OT-1 cells. (c) In vivo killing assay. Mice were injected either with PBS, GFP LV or IiOVA-GFP LV intravenously. After 10 days (short term, st) or 10 weeks (long term, lt), they were injected with a 1:1 mix of unpulsed (CFSElow) and OVA257–264-pulsed (CFSEhigh) splenocytes. (c) The specific lysis of target cells was assessed by flow cytometry in individual spleens (5 per group) 18 hours later, comparing immunized (filled curves) and control mice (unfilled curves). (d) Averages of mice analyzed individually (n = 6) are shown. CFSE, carboxyfluorescein succinimydil ester; GFP, green fluorescence protein; IFN, interferon; LV, lentiviral vector; OVA, ovalbumin.
Finally, we examined cytolytic activity in the mice that had been immunized intravenously with LV. Figure 5c,d shows that OVA257–264-pulsed targets, injected into mice 10 weeks after LV immunization, were still killed; the efficiency was only slightly decreased from that observed 10 days after LV immunization. We have previously reported that mice immunized intravenously with a LV have a strong T-cell memory response when challenged after 1 year.11 Together these data show that after intravenous LV immunization long-term antigen presentation leads to a prolonged and effective CD8+ T-cell response. We see no indication that long-term antigen presentation by LV transduced cells is tolerogenic.
Discussion
Our data shows that intravenous LV injection results in efficient transduction of proliferating cells that give rise to a long-term supply of DCs in spleen. The fact that DCs are the only predominantly BrdU-labeled, transduced population argues for the transduction of a committed DC progenitor. Such cells have been detected in bone marrow, which could be the target.30 However, the most likely explanation for our data is therefore that, upon intravenous injection, LVs transduce the resident DC precursors that are present in spleen,31 which have recently been shown to give rise to both conventional DC subsets and pDCs32. The fact that we observe recently divided transduced cells at least 30 days after LV injection, suggests either that the DC precursors proliferate for up to 30 days after transduction, or that transduced, dormant precursors are activated to proliferate throughout at least this period.
Our experiments have been performed in immune-competent C57BL/6 mice, using a LV expressing GFP, which could potentially be immunogenic. DCs are protected to some extent against immune killing.33 This can explain the ability of GFP, a potential antigen, to be persistently expressed in splenic DCs in immunocompetent mice. However, we know that intravenous injection of a LV encoding OVA stimulates a robust immune response against OVA.11 This was likely sufficient to eliminate many OVA-expressing DCs, leading to our observed reduction in vector genome copies, when OVA epitopes were expressed. It is also possible that the interaction of CD40 on DC with CD40L on cognate T cells promotes DC cell survival.34 This could in turn be playing a role in the persistence of GFP-expressing DC. However our very similar results, at least up to 30 days after immunization, with the nonimmunogenic Thy1.1-expressing LV argue against this.
Despite the elimination of some antigen-expressing DCs, we observed long-term antigen presentation in mice immunized intravenously with LVs encoding OVA. Transduction of immature DCs by a LV has been reported to trigger toll-like receptors, recognizing components of the vector, which leads to DC activation.35 However, transduction of a DC precursor would not necessarily result in effective T-cell stimulation by its progeny, as they might not be activated. Indeed, antigen presentation by resting DCs can induce peripheral CD8+ T-cell tolerance.36 However, we found that the consequence of persistent antigen presentation by DCs derived from transduced progenitors was immunization rather than tolerance. This was demonstrated both by activation of adoptively transferred OT-1 T cells and by detection of a sustained endogenous CD8+ T-cell response.
There is considerable interest in the use of LVs as vaccine vectors, as they are ideally adapted to deliver and express antigen genes in DCs in vivo.26,37 Our data suggest that LV targeting of an equivalent DC precursor in the human would allow sustained, potentially life-long, T-cell stimulation. It may not, however, be simple to achieve systemic LV immunization in a clinical vaccine. Our previous study using subcutaneous injection showed that antigen presentation persisted for at most 3 weeks. In this case integrating and nonintegrating LVs were used and similar persistence of presentation was detected, arguing against transduction of proliferating precursor cells following this route of injection.27 Other systemic immunization routes, such as intramuscular injection, need to be tested. We have also recently shown that LVs engineered to coexpress activated MEK1 or interferon regulatory factor 3 alleles with an antigen can inhibit the immune response, by inducing “tolerizing” DCs.38 Thus, delivery of a self-antigen to appropriate DC precursors could provide life-long therapy for autoimmunity. However, the longevity of these DC precursors and their progeny might prove an obstacle to systemic gene delivery for gene therapy using LVs, or any other vector with the potential to integrate. In this case DC precursor transduction could result in a sustained, and even delayed immune response against the therapeutic transgene.
Materials and Methods
Lentivectors. The pHRSIN-CSGW construct is described in.39 This vector contains a GFP insert driven by a spleen focus-forming virus promoter. The pIiOVA-GFP construct contains a spleen focus-forming virus-driven cassette with a fusion of the C-terminal end of the invariant chain and amino acids 242–353 of chicken OVA, and the human ubiquitin promoter driving the expression of GFP. The pOVA-GFP contains the wild-type OVA (amino acids 1–386), which is secreted, under control of the spleen focus-forming virus promoter.11
For the pHRSIN-Thy1.1 vector, a Thy1.1 insert was amplified by PCR from pMiT vector, kindly provided by P. Marrack using the following primers: GGATCCGCCACCATGAACCCAGCCATCAGCG and GCGGCCGCTCACAGAGAAATGAAGTCCAGGG
The PCR product was subcloned into the BamHI-NotI restriction site to replace GFP in the single-promoter vector.
Viruses were produced by transient co-transfection of 293T cells with the transfer vector, a human immunodeficiency virus-1 derived packaging plasmid (p8.91) and a plasmid encoding for the vesicular stomatitis virus glycoprotein-derived envelope (pMD.G), as described before.11 Supernatants were collected 72 hours later and viral particles were concentrated 100–200-fold by a two-round ultracentrifugation, then resuspended in phosphate buffered saline and kept at −80 °C until usage.
Viral titers were determined by infecting 293T cells with different dilutions of the vectors and measuring GFP expression by flow cytometry. They were also determined by measuring the concentration in ng/µl of reverse transcriptase in each viral stock determined by a colorimetric assay using a commercial kit (Roche, Mannheim, Germany) according to the manufacturer's instructions.
Immunizations. C57BL/6 mice were bred in house and handled according to institutional guidelines. Six- to eight-week-old mice were injected intravenously (i.v.) in the tail vein or s.c. in the footpads with 108 IU or 500 ng reverse transcriptase LVs, or with phosphate buffered saline in negative controls. Some mice were also injected with 50 µg of OVA class I peptide (SIINFEKL; Proimmune, Oxford, UK) plus 30 µg of monophosphoryl lipid A (MPL; Sigma-Aldrich, St Louis, MO) as adjuvant, both i.v.
In vivo tracking of transduced cells. Spleens of immunized and control mice (2–3 per group) were harvested and pooled at different time points after injection with LVs. They were incubated for 20 minutes at 37 °C with in a solution containing collagenase IV 2 mg/ml (Worthington, Lakewood, NJ) and 10 mmol/l Hepes buffer (Gibco, Grand Island, NY) in hanks balanced salt solution. Then they were mashed through a 70 µm nylon mesh, washed and resuspended in red blood cell lysis buffer for 5 minutes. After washing, Fc receptors were blocked with an anti-mouse FcR (CD16/32) antibody obtained from a rat hybridoma cell line.
DCs were purified by positive selection using magnetic CD11c (N418) microbeads (Miltenyi Biotec, Auburn, CA) according to manufacturer's instructions. Positive and negative fractions were stained with the following antibodies for flow cytometry analysis of different DC subsets, lymphocytes and macrophages: anti-CD11c-bio (BD Pharmingen, San Diego, CA), anti-MHCII-PE (eBiosciences, San Diego, CA), anti-PDCA-1-PE (Miltenyi Biotec), anti-Gr-1-APC (eBiosciences), anti-CD8-PE (eBiosciences), anti-CD4-PECy7 (eBiosciences), anti-F4/80-PE (eBiosciences), anti-CD19-PE (eBiosciences), anti-CD3-antigen-presenting cell (eBiosciences). Data were collected using FACSCalibur or BD LSR flow cytometers (BD Biosciences, San Diego, CA) and the data analyzed with CellQuest and FlowJo.
Quantitative PCR. Genomic DNA was obtained from CD11c+ and CD11c− fractions after magnetic cell sorting using a DNeasy Tissue Kit (Qiagen, Hilden, Germany). For the TaqMan PCR, a probe (CAGTGGCGCCCGAACAGGGA) and primers (TGTGTGCCCGTCTGTTGTGT and GAGTCCTGCGTCG AGAGAGC, all from Sigma Genosys) specific for the strong stop region of the LV were used.
OT-1 adoptive transfer. OT-1 transgenic mice were bred in the Windeyer Institute. Spleen cells were harvested and labeled for 10 minutes with 5 µM CFSE (Invitrogen, Carlsbad, CA) at 37 °C. Up to 107 CFSE-labeled splenocytes from OT-1 mice were adoptively transferred i.v. into immunized and control C57BL/6 mice. After 5 days they were tracked in peripheral blood or spleens by flow cytometry, staining them with antibodies against the Vα2 (Caltag, Buckingham, UK) and Vβ5.1, 5.2 (BD Pharmingen) chains of the T-cell receptor. Cell proliferation was determined by dilution of CFSE.
ELISpot assays. Enzyme-linked immunospot (ELISpot) plates (Millipore, Billerica, MA) were covered overnight with purified anti-interferon-γ (BD Pharmingen). Different numbers of splenocytes (3 × 106 to 5 × 105 per well) from immunized and control mice were incubated in duplicates either in the presence of 50 ng/ml OVA257–264 peptide (SIINFEKL) or medium. After 24 hours they were developed according to manufacturer's instructions. Spots were counted using AID ELISPOT counter and software.
Splenectomy. Mice were anesthetized with 5% isoflurane and maintained with 1–3% of the same anesthetic. In aseptic conditions, spleens were removed through a subcostal incision. The animals were recovered at 37 °C and closely monitored immediately and the days after the procedure. For pain control, 0.1 mg buprenorphine was injected s.c. 30 minutes before anesthesia and every 12 hours thereafter or upon distress signs.
In vivo BrdU labeling. Groups of mice (n = 3–5) were injected with 500 ng reverse transcriptase of Thy1.1 LV or phosphate buffered saline as control. One group was injected intraperitoneally with 200 µg of 5-bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich) in hanks balanced salt solution on day 2 after injection of the LV and then continuously given BrdU (0.8 mg/ml) in drinking water that was changed daily for 10 days. In another group, the BrdU was started on day 20 after injection of LV. In both cases, the spleens were harvested after 10 days of BrdU administration and CD11c+ cells were purified with magnetic microbeads as described above. Cells were stained for surface markers and nuclear BrdU using a BrdU Flow Kit (BD Pharmingen) according to the manufacturer's instructions. In brief, after surface antigen staining, cells were fixed and permeabilized. Then they were treated with DNase I (300 µg/ml in Dulbecco's phosphate-buffered saline buffer) for 1 hour at 37 °C, washed, reblocked in 10% mouse serum and stained with an APC-labeled anti-BrdU antibody for flow cytometry analysis.
In vivo killing assay. Spleen cells from naive mice were resuspended in hanks balanced salt solution at 5 × 106 cells/ml and pulsed with OVA257–264 peptide at 5 µg/ml during 2 hours at room temperature and then labeled with CFSE at 10 µmol/l as described before. They were mixed in a 1:1 ratio with non pulsed cells that were labeled with CFSE at 3 µmol/l. This mixture was injected in control and immunized mice at different time points. Specific lysis of the pulsed cells was analyzed 18 hours later by flow cytometry of cells from the spleen. The percentage of killing was calculated with the following formula: 1-[(%CFSEhigh/%CFSElow)immunized/(%CFSEhigh/%CFSElow)non immunized].
SUPPLEMENTARY MATERIALTable S1. Transduction of cell subsets in the spleen after iv injection of different LVs.
Supplementary Material
Transduction of cell subsets in the spleen after iv injection of different LVs.
REFERENCES
- VandenDriessche T, Thorrez L, Naldini L, Follenzi A, Moons L, Berneman Z.Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo Blood 2002100813–822.et al [DOI] [PubMed] [Google Scholar]
- Pan D, Gunther R, Duan W, Wendell S, Kaemmerer W, Kafri T.Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow Mol Ther 2002619–29.et al [DOI] [PubMed] [Google Scholar]
- Peng KW, Pham L, Ye H, Zufferey R, Trono D, Cosset FL.Organ distribution of gene expression after intravenous infusion of targeted and untargeted lentiviral vectors Gene Ther 200181456–1463.et al [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Aubert D, Bellodi-Privato M, Flageul M, Pichard V, Jaidane-Abdelghani Z.Critical assessment of lifelong phenotype correction in hyperbilirubinemic Gunn rats after retroviral mediated gene transfer Gene Ther 2007141270–1277.et al [DOI] [PubMed] [Google Scholar]
- Di Domenico C, Di Napoli D, Gonzalez Y Reyero E, Lombardo A, Naldini L., and , Di Natale P. Limited transgene immune response and long-term expression of human alpha-L-iduronidase in young adult mice with mucopolysaccharidosis type I by liver-directed gene therapy. Hum Gene Ther. 2006;17:1112–1121. doi: 10.1089/hum.2006.17.1112. [DOI] [PubMed] [Google Scholar]
- Merle U, Encke J, Tuma S, Volkmann M, Naldini L., and , Stremmel W. Lentiviral gene transfer ameliorates disease progression in Long-Evans cinnamon rats: an animal model for Wilson disease. Scand J Gastroenterol. 2006;41:974–982. doi: 10.1080/00365520600554790. [DOI] [PubMed] [Google Scholar]
- van der Wegen P, Louwen R, Imam AM, Buijs-Offerman RM, Sinaasappel M, Grosveld F.Successful treatment of UGT1A1 deficiency in a rat model of Crigler-Najjar disease by intravenous administration of a liver-specific lentiviral vector Mol Ther 200613374–381.et al [DOI] [PubMed] [Google Scholar]
- Follenzi A, Sabatino G, Lombardo A, Boccaccio C., and , Naldini L. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum Gene Ther. 2002;13:243–260. doi: 10.1089/10430340252769770. [DOI] [PubMed] [Google Scholar]
- Kimura T, Koya RC, Anselmi L, Sternini C, Wang HJ, Comin-Anduix B.Lentiviral vectors with CMV or MHCII promoters administered in vivo: immune reactivity versus persistence of expression Mol Ther 2007151390–1399.et al [DOI] [PubMed] [Google Scholar]
- Palmowski MJ, Lopes L, Ikeda Y, Salio M, Cerundolo V., and , Collins MK. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J Immunol. 2004;172:1582–1587. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- Rowe HM, Lopes L, Ikeda Y, Bailey R, Barde I, Zenke M.Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene Mol Ther 200613310–319.et al [DOI] [PubMed] [Google Scholar]
- Lopes L, Dewannieux M, Gileadi U, Bailey R, Ikeda Y, Whittaker C.Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses J Virol 20088286–95.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P.A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice Blood 20071104144–4152.et al [DOI] [PubMed] [Google Scholar]
- Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG., and , Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- Wijffels JF, de Rover Z, Beelen RH, Kraal G., and , van Rooijen N. Macrophage subpopulations in the mouse spleen renewed by local proliferation. Immunobiology. 1994;191:52–64. doi: 10.1016/s0171-2985(11)80267-6. [DOI] [PubMed] [Google Scholar]
- Kamath AT, Henri S, Battye F, Tough DF., and , Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- O'Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM.Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus J Exp Med 20021961307–1319.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckpot K, Emeagi PU., and , Thielemans K. Lentiviral vectors for anti-tumor immunotherapy. Curr Gene Ther. 2008;8:438–448. doi: 10.2174/156652308786848058. [DOI] [PubMed] [Google Scholar]
- He Y, Munn D., and , Falo LD. Recombinant lentivector as a genetic immunization vehicle for antitumor immunity. Expert Rev Vaccines. 2007;6:913–924. doi: 10.1586/14760584.6.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MJ., and , Fink PJ. The CD8 response on autopilot. Nat Immunol. 2001;2:381–382. doi: 10.1038/87676. [DOI] [PubMed] [Google Scholar]
- Obst R, van Santen HM, Mathis D., and , Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., and , Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Redmond WL., and , Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Simon ID, Publicover J., and , Rose JK. Replication and propagation of attenuated vesicular stomatitis virus vectors in vivo: vector spread correlates with induction of immune responses and persistence of genomic RNA. J Virol. 2007;81:2078–2082. doi: 10.1128/JVI.02525-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D.Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines Blood 20071101916–1923.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang J, Donahue C., and , Falo LD. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwacz K, Mukherjee S, Apolonia L, Blundell MP, Bouma G, Escors D.Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy J Virol 2009833094–3103.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., and , Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Hernandez J, Aung S, Redmond WL., and , Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR.A clonogenic bone marrow progenitor specific for macrophages and dendritic cells Science 200631183–87.et al [DOI] [PubMed] [Google Scholar]
- Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M.Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes Nat Immunol 20067663–671.et al [DOI] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A.Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo Nat Immunol 200781217–1226.et al [DOI] [PubMed] [Google Scholar]
- Medema JP, Schuurhuis DH, Rea D, van Tongeren J, de Jong J, Bres SA.Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells J Exp Med 2001194657–667.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan A, Heldmann M, Terbeck G, Weih F, Linden C, Bröcker EB.MHC class II and CD40 play opposing roles in dendritic cell survival Eur J Immunol 2000302612–2619.et al [DOI] [PubMed] [Google Scholar]
- Breckpot K, Emeagi P, Dullaers M, Michiels A, Heirman C., and , Thielemans K. Activation of immature monocyte-derived dendritic cells after transduction with high doses of lentiviral vectors. Hum Gene Ther. 2007;18:536–546. doi: 10.1089/hum.2007.006. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC., and , Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L.Engineered lentivector targeting of dendritic cells for in vivo immunization Nat Biotechnol 200826326–334.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escors D, Lopes L, Lin R, Hiscott J, Akira S, Davis RJ.Targeting dendritic cell signaling to regulate the response to immunization Blood 20081113050–3061.et al [DOI] [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C.High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter Hum Gene Ther 200213803–813.et al [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transduction of cell subsets in the spleen after iv injection of different LVs.