Abstract
Electroporation has, during the last years, proven to be a very successful delivery method for DNA vaccines and has now reached clinical evaluation. Although intramuscular electroporation is practical in animal models, intradermal electroporation might be more suitable for clinical administration. Skin is the most accessible organ of the body and has professional antigen-presenting cells in large amounts; thus, skin is an ideal target for DNA vaccine delivery. Moreover, intradermal electroporation has clear clinical benefits such as improved safety and tolerability. This article describes improvements for effective and tolerable DNA delivery to skin. The time of pulse delivery has been shortened by 90% and even pulse programs of 240-ms total duration generate robust immune responses. We show that a single vaccination using an optimized gene delivery generates (i) high and consistent protein expression in vivo, (ii) cytotoxic antigen-specific T cells expressing both IFNγ and CD107a (lysosomal-associated membrane protein 1). Furthermore, application of a topical anesthetic cream prior to vaccination does not affect the number or function of the antigen-specific T cells induced. This suggests that local anesthesia can be used to further decrease the sensation of pulse delivery in patients.
Introduction
Since the first studies of DNA vaccines in the beginning of the 1990s,1,2,3,4 over 200 clinical trials have been performed demonstrating the safety of DNA vaccination in humans. In the past few years, three DNA vaccines have been licensed for veterinary use, indicating that DNA vaccines can be effective in large animals.5,6,7 In vivo electroporation has a high potential to also render DNA vaccines effective in humans and recently this vaccine delivery method has reached clinical evaluation. Therefore, questions about patient tolerability to electroporation are now in focus. We have investigated two modifications, a shorter pulse protocol and application of a local anesthetic, of an earlier described intradermal electroporation procedure8 to improve tolerability of electroporation in patients. Although intramuscular electroporation has been the most commonly used technique in preclinical models,9,10,11,12 intradermal electroporation offers several advantages for clinical administration of DNA.
Skin (epidermis/dermis) is an ideal target for DNA vaccine delivery, as it is rich in professional antigen-presenting cells, such as Langerhans cells and dermal dendritic cells. Skin is also the most accessible organ for electroporation. As the target for intradermal electroporation/vaccination is the epidermis/dermis, which is between 1.5- and 3-mm thick in humans,13 electrode needles of only 2-mm length are sufficient for DNA delivery. The use of such short needles are expected to significantly improve the tolerability of electroporation in skin compared to muscle, where needles of 1–2 cm are commonly used in larger animals.14,15,16 Furthermore, in the case of a DNA integration event,12,17 the theoretical occurrence of a tumor due to that integration would be both easier to detect and to remove in skin compared to the muscle. Biodistribution assays have shown that the majority of plasmid DNA is confined to the vaccination site after electroporation.18,19
This article evaluates a new electroporation pulse protocol that is delivered with very high frequency. The time of pulse delivery has been shortened 10-fold compared to a previously published protocol for intradermal electroporation8 with similar results regarding gene expression and immune responses. Pulse protocols as short as 240 ms in total duration time generate robust cellular immune responses. A single DNA vaccination with this optimized gene delivery protocol generates high and consistent protein expression in vivo, as demonstrated by live imaging of vaccinated mice. Furthermore, application of a topical lidocaine–prilocaine anesthetic (emla) prior to vaccination does not affect the number or function of the antigen-specific CD8+ T cells induced. Therefore, the application of local anesthesia in combination with a short pulse protocol might be an interesting approach to limit discomfort related to electroporative DNA delivery in patients.
Results
Increasing the pulse frequency does not affect the induction of vaccine-specific CD8+ T-cell responses after intradermal electroporative DNA administration
To reduce skin sensation and discomfort during application of electroporation, we sought to optimize a previously published electroporation protocol8 with regard to shortening of pulse intervals, effectively reducing number of perceived pulses and reducing cutaneous muscle contraction. To investigate whether increasing the pulse frequency influence the induction of cellular immune responses after vaccination, a DNA vaccine encoding the prostate-specific antigen (PSA) was used. PSA-specific CD8+ T-cell responses in spleens at day 14 after a single intradermal injection of PSA DNA followed by electroporation protocols of different total pulse lengths were compared in mice. The duration of the total pulse lengths in the various electroporation protocols tested ranged between 0.24 and 2.98 seconds (Figure 1a). The pulse frequency did not affect the induction of PSA-specific CD8+ T cells; there were no significant differences in levels of IFNγ-producing CD8+ T cells in response to in vitro stimulation with the PSA-derived psa65–73 peptide between mice vaccinated with any of the electroporation protocols (Figure 1b). The levels of PSA-specific IFNγ-producing CD8+ T cells in the vaccinated groups ranged from 2.7 to 4.7% of all CD8+ T cells. No specific responses were detected in control mice vaccinated with empty vector or in splenocytes stimulated with the lymphocytic choriomeningitis virus–derived GP33 control peptide (background levels 0–0.3%, not shown). Electroporation protocol 5 (0.27 seconds) was selected for use in subsequent experiments. These results confirm our previous data8 where a robust antigen-specific T-cell response was induced by a single intradermal DNA injection with electroporation consisting of a combination of high-amplitude, short-duration and low-amplitude, long-duration pulses (electroporation condition E), here described as protocol 1. Our present data clearly demonstrate that the total electroporation pulse length (2.98 seconds) can be reduced more than 10-fold, thereby reducing the length of unpleasant sensation, without affecting the induction of a potent immune response. Furthermore, there was a clear difference in superficial muscle contraction in response to electroporation protocol 1 as opposed to protocol 2–6. Application of pulse protocol 1 resulted in 10 separate visible muscle contractions, whereas delivering pulse protocol 2–6 only resulted in one visible muscle contraction (Figure 1a).
Figure 1.
PSA-specific CD8+ T-cell responses in mice after vaccination using electroporation protocols with different pulse intervals. C57Bl/6 mice were immunized intradermally once with 20 µg pVax-PSA plasmid in 20-µl phosphate-buffered saline on each flank and electroporation was applied. Splenocytes were isolated on day 14 after immunization and cells were stimulated for 5 hours with 100 nmol/l psa65–73 or control peptide GP33. Activated CD8+ T cells were quantified using intracellular cytokine staining for IFNγ and analyzed by flow cytometry. (a) Schematic overview of electroporation protocols used in this study showing pulse (P) and interval (I) duration. (b) PSA-specific IFNγ-producing CD8+ T-cell levels induced after intradermal delivery of pVax-PSA and application of the indicated electroporation protocol. The experiment was performed twice with four mice per group and pooled data is shown. Background levels after GP33 stimulation were 0–0.15%. Bars represent mean ± SD (n = 8). Ctrl, control; PSA, prostate-specific antigen.
Application of slow and fast electroporation following DNA injection results in comparable expression of a luciferase gene in skin
Mice received one intradermal injection of plasmid DNA encoding the reporter gene luciferase (pVax-luc) followed by application of either slow (Figure 1a, protocol 1) or fast (Figure 1a, protocol 5) electroporation (from now on designated as PulseAgile (PA) slow and PA fast), and the presence of luciferase protein in skin was measured using an in vivo imaging system. Two days post plasmid injection and electroporation, all mice expressed the luciferase gene in skin regardless of the electroporation protocol used (Figure 2a). Mean photon emission was 4 × 107 and 5 × 107 photons/s in the PA slow and PA fast group respectively. Background emission in mice injected intradermally with empty vector and electroporated was <200 photons/s (Figure 2a). The luciferase expression over time did not differ between PA slow and PA fast electroporation groups and was relatively stable (Figure 2b). From day 2 to day 62 after DNA administration, the emission decreased to 1.2 × 106 and 2.8 × 106 photons/s in the PA slow and PA fast groups, respectively (Figure 2b). This demonstrates that using higher frequency electroporation pulses following intradermal injection of plasmid DNA results in stable gene expression that does not differ compared to applying pulses with lower frequency.
Figure 2.
Luciferase gene expression in skin after DNA delivery using PA slow and PA fast electroporation protocols. Balb/c mice were injected intradermally with 10 µg of pVax-luc plasmid in 20-µl PBS on each flank and were subjected to either PA slow or PA fast electroporation protocols. At day 2, 8, 30, and 62 after DNA vaccination, mice were injected intraperitoneally with 100-µl/10-g mouse body weight with 15 mg/ml of D-luciferin substrate solution. Twenty minutes after substrate injection, the in vivo luciferase expression was visualized using the IVIS 100 in vivo imaging system. (a) Representative bioluminescent image showing luciferase expression in skin 48 hours after DNA delivery. The scale shows intensity of luminescence (photons/s/cm2). The electroporation protocols used for individual mice are indicated. (b) Time course of in vivo luciferase expression after DNA delivery with PA slow (filled squares) and PA fast (filled triangles) protocols. The experiment was performed twice with 2–3 mice per group (two luciferase injection sites per mouse) and one representative experiment is shown. Error bars represent (±) SD (n = 4). PA, PulseAgile; PBS, phosphate-buffered saline; ROI, region of interest.
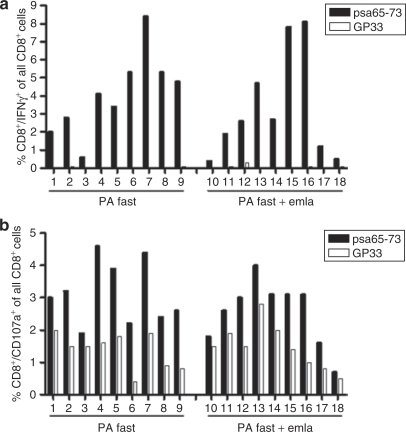
Topical application of emla cream prior to DNA injection and electroporation does not influence the induction or function of vaccine-specific CD8+ T-cell responses
After confirming that fast electroporation protocols induce similar levels of antigen-specific T cells and gene expression in skin, the influence of topical administration of the local anesthetic emla, to further reduce skin sensation and discomfort during electroporation, was investigated. The induction of PSA-specific CD8+ T cells after intradermal delivery of plasmid DNA followed by PA fast electroporation with or without pretreatment of skin with emla cream was compared. At day 14 after immunization and electroporation, all mice, pretreated with emla or not, had CD8+ T cells expressing IFNγ in response to stimulation with an immunodominant PSA peptide, psa65–73 (Figure 3a). The levels of PSA-specific T cells ranged from 0.4 to 8.4% of all CD8+ T cells (Figure 3a). There was no significant difference in IFNγ production by PSA-specific CD8+ T cells induced in groups of mice receiving emla or not. Some mice had lower levels of IFNγ-producing T cells in response to the PSA peptide (0.4–0.6%); however, their response to the GP33 control peptide was lower than 0.04%, and therefore, these mice were regarded as responding to the vaccine (Figure 3a). The function of the induced vaccine-specific CD8+ T cells after application of emla was investigated by their ability to degranulate in response to peptide stimulation. The surface expression of the CD107a (lysosomal-associated membrane protein 1) protein, which is expressed on lysosomes and transported to the cell surface upon target recognition, was analyzed. Mice in both groups clearly expressed CD107a after 5 hours of stimulation with the psa65–73 peptide, and no significant difference between groups was detected (Figure 3b). The background response to the GP33 control peptide was relatively high compared to the background response in the IFNγ assay. However, the surface expression of CD107a in the response to the PSA-derived peptide was higher in all individual mice compared to the expression of CD107a in response to the control peptide (Figure 3b). After 5 days of in vitro restimulation, the mean of cells expressing CD107a in response to psa65–73 was significantly higher than the mean of cells expressing CD107a in response to peptide GP33 (data not shown), which confirmed the specificity of the response seen after the 5-hour stimulation. Moreover, similar results for both IFNγ and CD107a were obtained when vaccinating mice with the PA slow protocol +/‐ emla (data not shown).
Figure 3.
IFNγ production and degranulation by PSA-specific CD8+ T cells after PSA DNA vaccination using PA fast protocol with and without application of anesthetic cream. C57Bl/6 mice were immunized intradermally once with 20-µg pVax-PSA plasmid in 20-µl phosphate-buffered saline on each flank and electroporation was applied. About 45–55 minutes prior to plasmid injection and electroporation, emla cream was administered topically at the injection area to one group of mice. Splenocytes were isolated on day 14 after immunization and cells were stimulated for 5 hours with 100 nmol/l psa65–73 or control peptide GP33. Activated CD8+ T cells were quantified using intracellular cytokine staining for IFNγ and surface staining for CD107a and analyzed by flow cytometry. (a) Levels of PSA-specific IFNγ production by CD8+ T cells by individual mice after intradermal delivery of pVax-PSA using PA fast protocol with and without topical addition of emla cream. Shown is specific response to psa65–73 (filled bars) and background response to GP33 (open bar). (b) Levels of PSA-specific CD8+ T cells expressing CD107a in individual mice after intradermal delivery of pVax-PSA using PA fast protocol with and without topical application of emla cream. Shown is specific response to psa65–73 (filled bars) and background response to GP33 (open bars). Pooled data from two individual experiments is shown. emla, eutectic mixture of lidocaine and prilocaine; PA, PulseAgile; PSA, prostate-specific antigen.
To investigate whether there were any differences in cytolytic function between PSA-specific CD8+ T cells induced in mice receiving emla or not, in vitro cytotoxicity assays were performed. Splenocytes were harvested and CD8+ T-cell priming was confirmed by IFNγ production in response to the psa65–73 peptide (not shown). After 5 days of in vitro restimulation, the splenocytes were co-incubated with EL4/PSA or EL4 tumor cells. There was no significant difference between splenocytes derived from mice receiving emla and from those that did not in their ability to lyse the PSA-transfected EL4 cells (Figure 4). Splenocytes isolated from mice receiving pVax-PSA plasmid in the absence of electroporation did not react to peptide stimulation, as shown before,8 and therefore, they were not able to expand and to be used for in vitro cytotoxicity studies (not shown).
Figure 4.
Cytotoxicity assay of in vitro restimulated PSA-specific CD8+ T cells after PSA DNA vaccination using PA fast protocol with and without application of anesthetic cream. Mice were immunized intradermally once with 20-µg pVax-PSA plasmid in 20-µl phosphate-buffered saline on each flank and electroporation was applied. About 45–55 minutes prior to plasmid injection and electroporation, emla cream was administered topically at the injection area to one group of mice. Splenocytes were collected 14 days after immunization and restimulated in vitro for 5 days with the psa65–73 peptide. The splenocytes were then tested for cytolytic activity against PSA expressing (filled bars) and control target cells (open bars). Shown is the lysis at 30:1 effector target (e:t) ratio. The mean of two different experiments is shown. Error bars represent SD (n = 10). emla, eutectic mixture of lidocaine and prilocaine; PA, PulseAgile; PSA, prostate-specific antigen.
Taken together, these results demonstrate that in order to reduce discomfort associated with electroporation, emla cream can be applied without affecting T-cell reactivity or function.
Discussion
In the current study, we demonstrate that skin electrovaccination can be made further tolerable to patients, by shortening the pulse protocol and by pretreatment of the vaccination site with a topical anesthetic, without affecting the induced immune response to the vaccine. In vivo electroporation is becoming increasingly popular as a DNA delivery method, and the regulatory authorities in United States and Europe have to date approved the initiation of four clinical trials investigating electroporative DNA vaccination in patients with cancer or infectious disease.20,21,22 All the approved phase 1/2 trials of electrovaccination to this point are evaluating intramuscular DNA electroporation. However, intramuscular electroporation is very invasive and results in painful muscle contractions. Due to the use of shorter needles and reduced number and severity of muscle contractions, we propose that intradermal electroporation is associated with a less unpleasant sensation. In this study, we have investigated a new pulse protocol with very short intervals.
In 2002, a study suggested that the use of pulses with a repetition frequency higher than the maximum frequency of tetanic contraction would result in reduced number of muscle contractions and associated unpleasant sensations. It was confirmed that even at pulse frequencies up to 8 kHz, the transfer of nonpermeant molecules into electropermeabilized cells in vitro remained the same as when pulse frequencies of 1 Hz was used.23 In vivo results in mice further confirmed that electrochemotherapy with higher frequency pulses (higher than the frequency of tetanic contraction) efficiently inhibited tumor growth at all pulse frequencies (1–5 kHz) examined.24 While delivering a previously published pulse protocol8 for intradermal electroporation (protocol 1, 2.98 seconds, 3.4 Hz) to mice in this study, 10 separate muscle contractions were observed. However, when new protocols with shorter time intervals between pulses (protocol 2–6, 0.53–0.24 seconds, 18.9–42 Hz) were used to deliver DNA, only one muscle contraction was observed in the mice. This is most probably due to the muscle response becoming smooth (tetanic contraction) when we deliver pulses above a certain frequency threshold. According to data published by Miklavcic et al., the frequency threshold for intramuscular electroporation lies between 20 and 100 Hz.24 A recently performed tolerance study of electroporation for electrochemotherapy in 40 cancer patients demonstrated that a train of eight 1-Hz pulses (100 µs, 600 V/cm) evoked eight painful sensations and muscle contractions, whereas the same number of pulses evoked a single muscle contraction during the delivery of 5-kHz pulses.25 The PA fast electroporation protocol (protocol 5, 0.27 seconds, 37 Hz) for DNA delivery described in the present study reduces muscle contraction and duration of unpleasant sensation, without affecting either gene expression in skin (Figure 2) or the induction of cellular immune responses (Figure 1b). Several clinical studies have concluded that muscle contractions contribute to the discomfort felt by subjects during the delivery of pulses.26,27 The proposed concept of shortening pulse protocols for more tolerable electroporative DNA vaccination is also applicable for intramuscular electroporation, especially because shortening of the duration of electroporation has been suggested to prevent electrode displacement occurring due to muscle contractions during pulse delivery.25 However, the concept of fast pulse protocols (<0.3 seconds) needs to be validated in animal models to confirm that it does not affect the induction of immune responses after intramuscular DNA delivery.
As shown in Figure 2b, luciferase protein expression can still be detected at high levels 62 days after DNA vaccination. As the half-life of firefly luciferase protein is only about 3 hours,28 the expression most likely results from continuous protein production from the plasmid. This long-term protein expression is most probably due to the relatively nonimmunogenic nature of the luciferase protein.29 We have vaccinated mice intradermally with a mixture of PSA and luciferase-encoding DNA, and in this experiment, the luciferase protein expression dropped after 15 days and was undetectable after 21 days (A.-K. Roos, F. Eriksson, J. Timmons, J. Gerhardt, U. Nyman, L. Gunmundsdotter et al., unpublished results). A similar study compared luciferase expression after intramuscular vaccination with a plasmid encoding luciferase or a plasmid encoding both luciferase and hepatitis B surface antigen and showed that the luciferase activity was significantly decreased by 14 days and continued to decline such that by 42 days, luciferase activity was only 0.1% of 3-day levels in mice vaccinated with the hepatitis B surface antigen–luciferase vector.30 In contrast, with injection of the luciferase control vector, which did not express hepatitis B surface antigen, a significant decrease in luciferase expression was only observed at 42 days, with the level of activity being 22% of 3-day levels.30 These results suggest that if an immune response is induced against the antigen used for vaccination, the majority of the cells transfected during vaccination will be eliminated.
Several clinical studies have shown that pretreatment with topical lidocaine–prilocaine (emla) significantly reduces the pain associated with immunization.31,32,33 Although previous studies have demonstrated that emla does not affect the induction of antibodies to different vaccines,32,33 there is, to our knowledge, no data describing the effect of emla on the induction of vaccine-specific T cells. Therefore, an experiment was designed to evaluate the effect of emla pretreatment before DNA vaccination on vaccine-induced CD8+ T cells. This study demonstrated that locally anesthetizing the vaccination site before pulse delivery did not affect the induction of vaccine-specific CD8+ T cells (Figure 3). The vaccine-specific T cells induced produced comparable levels of IFNγ (Figure 3a), had similar expression of the lysosomal protein CD107a (lysosomal-associated membrane protein 1) (Figure 3b) and had comparable cytolytic activity in vitro (Figure 4) as T cells induced in the absence of emla. This suggests that emla can be used in the clinic to make intradermal electroporative DNA vaccination more tolerable for patients, without affecting the induction of vaccine-specific immune responses. As the depth of analgesia is about 3 mm after a 60-minute application of emla cream (according to the product monograph provided by the manufacturer), it will most probably not serve to affect the sensation of the muscle twitch in patients. However, the emla cream will most likely increase the tolerability of the intradermal DNA injection and the insertion of the electrode.
In conclusion, we have evaluated a new fast electroporation pulse protocol and demonstrated that this, more tolerable, pulse protocol for intradermal electroporative DNA delivery induces high levels of vaccine-specific IFNγ-producing cytolytic CD8+ T cells. Furthermore, the application of a local anesthetic prior to the electroporation procedure can be used without affecting the immune response to vaccination. Therefore, we propose that this PA fast pulse protocol for intradermal electroporation is suitable for clinical testing of DNA vaccine delivery.
Materials and Methods
Animals. C57Bl/6 (H-2b) and Balb/c (H-2d) mice (6–10 weeks old) were bred and housed at the animal facility at Microbiology and Tumor Biology Center at the Karolinska Institute, Stockholm, Sweden. Mice were anesthetized with 4% isoflurane (Baxter Medical AB, Kista, Sweden) and maintained at 2–2.5% isoflurane in a mask during all intradermal injections, electroporations, and live imaging. All experiments at the Karolinska Institute were approved by the Swedish National Board for Laboratory Animals.
Plasmids. The luciferase-encoding plasmid, pVax-luc 4,663 bp, was constructed by inserting the cDNA for firefly luciferase from pGL2-Basic vector (Promega, Madison, WI) into vector pVAX1 (Invitrogen, Carlsbad, CA). Vector pVAX1 contains the human cytomegalovirus immediate/early promoter and a polyadenylation signal from the bovine growth hormone gene. Plasmid pVax-PSA (3,977 bp) was constructed by inserting the gene coding for the full-length human PSA protein (obtained from Tim Ratliff, Washington University, St Louis, MO) into vector pVAX1. Plasmids were amplified in bacteria and purified using an endotoxin-free plasmid purification kit (Qiagen, Hilden, Germany). The DNA was dissolved to 0.5 mg/ml or 1 mg/ml in sterile phosphate-buffered saline (PBS).
DNA injections and in vivo electroporation. Intradermal injections with 10–20 µg DNA/20 µl PBS were made on the lower back, near the base of the tail, using a 29G insulin grade syringe (Micro-Fine U-100; BD Consumer Healthcare, Franklin Lakes, NJ). Immediately after intradermal DNA administration, a needle array electrode was placed over the raised skin area of injection and voltage was applied (2 pulses, 1,125 V/cm, 50 µs + 8 pulses, 275 V/cm, 10 ms). The number, amplitude, and length of pulses were always the same, only the interval between the pulses varied (described in Figure 1a). Needle array electrodes consisted of two parallel rows of four 2-mm pins (1.5 × 4 mm gaps) (Cyto Pulse Sciences, Glen Burnie, MD). Electroporation was performed using the DERMA VAX Clinical DNA vaccine delivery system (Cyto Pulse Sciences). Negative control mice received empty vector, pVAX1, or PBS injections followed by electroporation. For cytotoxicity studies, control mice received pVax-PSA plasmid without electroporation.
Topical application of lidocaine–prilocaine (emla). Emla emulsion (lidocaine (25 mg/g) and prilocaine (25 mg/g); AstraZeneca, Södertälje, Sweden) was applied on the lower back, near the base of the tail of the mice. About 45–55 minutes after emla application, the mice were anesthetized with isoflurane, and intradermal vaccination/electroporation on the site where emla had been applied was performed as described above.
Live imaging of protein expression after DNA vaccination. Balb/c mice were injected intradermally with 20 µl PBS or 10 µg pVax-luc/20 µl PBS and immediately electroporated. To monitor in vivo luciferase protein expression, mice were injected i.p. with 100 µl/10 g mouse body weight of a 15 mg/ml solution of D-luciferin potassium salt (Xenogen, Alameda, CA) in PBS and anesthetized with 2.3% isoflurane. Assessment of photonic emissions (photons/s/cm2) was performed 20 minutes after injection of D-luciferin with an in vivo imaging system 100 (IVIS 100; Xenogen). Overlay of images and luminescence measurements were made using Living Image software (version 2.50.1; Xenogen). A region of interest was manually selected over the signal intensity. The area of the region of interest was kept constant and the intensity of luminescence (photons/s/cm2) was recorded within a region of interest. Background luminescence (<200 pixels/s/cm2) was determined by measuring luminescence from mice injected with empty vector DNA followed by electroporation.
Lymphocyte preparation and T-cell stimulation. For analysis of cytotoxic T-lymphocyte response in the spleen, mice were euthanized and spleens harvested 13–14 days after immunization. Single-cell suspensions were obtained by homogenizing spleens and passing cells through a 70-µm cell strainer. The red blood cells were lysed by ammonium chloride lysing reagent (BD Biosciences, San Jose, CA). The splenocytes were used directly for ex vivo assays (5-hour peptide stimulation) in complete Dulbecco's modified Eagle's medium (supplemented with 10 mmol/l HEPES, 5 × 10-5 mol/l 2-mercaptoethanol, 25 µg/ml gentamicin, 2 mmol/l L-glutamine, 1% nonessential amino acids, and 10% fetal calf serum). Splenocytes were stimulated with 100 nmol/l of the synthetic peptide psa65–73 (HCIRNKSVI) or 100 nmol/l of the H-2Db-restricted lymphocytic choriomeningitis virus–derived control peptide GP33 (KAVYNFATC) (both from ProImmune, Oxford, UK). The peptide psa65–73 represents an immunodominant H-2Db-restricted cytotoxic T-lymphocyte epitope of human PSA.34 For in vitro restimulation, splenocytes were stimulated with 1 nmol/l of the psa65–73 peptide for 5 days.
Intracellular cytokine staining for IFNγ. Staining for intracellular IFNγ in splenocytes was performed as follows. Briefly, splenocytes (1 × 106/well) were cultured for 5 hours in 96-well plates with peptides, psa65–73 or irrelevant peptide GP33. After 2 hours of incubation at 37 °C in a 5% CO2 incubator, GolgiPlug reagent (BD Biosciences) was added to the cells and incubation continued for another 3 hours. The splenocytes were then stained for the surface marker CD8 (rat IgG2a-FITC labeled anti-mouse CD8a; BD Biosciences), fixed and permeabilized with a CytoFix/CytoPerm Plus kit (BD Biosciences) according to the manufacturer's instructions, and then stained for intracellular IFNγ (rat IgG1-PE labeled anti-mouse IFNγ; BD Biosciences). Purified rat IgG (Sigma-Aldrich, Stockholm, Sweden) was added to both staining steps to block unspecific binding. Samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and CELLQuest software (BD Biosciences). Proper compensation during data collection was set using lymphocytes stimulated with PMA and ionomycin (Sigma-Aldrich).
CD107a degranulation assay. Splenocytes were stimulated with peptides directly after isolation and then analyzed for surface exposure of CD107a. Briefly, lymphocytes (1 × 106/well) were seeded in U-bottom 96-well plates with 100 nmol/l of synthetic peptides, psa65–73 or the irrelevant peptide GP33. A conjugated antibody (rat IgG2a-FITC labeled anti-mouse CD107a; BD Biosciences) to the granular membrane protein CD107a (lysosomal-associated membrane protein 1) was added to the cells prior to stimulation. The cultures were incubated for 1 hour at 37 °C in a 5% CO2 incubator, followed by an additional 4 hours in the presence of the secretion inhibitor monensin (GolgiStop reagent; BD Biosciences). The lymphocytes were then stained for the surface marker CD8 (rat IgG2a-APC labeled anti-mouse CD8a; BD Biosciences). Purified rat IgG (Sigma-Aldrich) was added to the CD8 staining step to block unspecific binding. Samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and CELLQuest software (BD Biosciences).
51Cr-release assay. Splenocytes were collected after 5 days of in vitro restimulation and tested for their cytolytic capacity against PSA-transfected targets. Target cells (EL4/PSA or EL4) were labeled with 1–2 µCi/µl of Na51CrO4 (PerkinElmer, Upplands Väsby, Sweden) at 37 °C for 2 hours. After labeling, the targets (1 × 104/well) were washed extensively and incubated with effector cells at 30:1 effector:target ratio in U-bottom 96-well plates for 5 hours. Following incubation, 100 µl of supernatant was collected and the amount of released 51Cr was measured using a gamma counter (Wallac, Upplands Väsby, Sweden). Specific lysis was calculated using the formula [(E‐S) / (T‐S)] × 100% where E represents the experimental release, S the spontaneous release, and T the total release. Total release was accomplished by incubating the targets in 5% Triton X-100.
Statistics. Comparison of data from different groups was performed using two-way ANOVA and Kruskal–Wallis tests with a significance level of at least P ≤ 0.05.
Acknowledgments
This study was supported in part by the Cancer Society in Stockholm, the Karolinska Institutes Fund, the Swedish Cancer Society, the EU 6-FP ALLOSTEM (LSHB-CT-2004-502219), and US Department of Defense Prostate Cancer Research Program (PC030958). D.C.W. and A.D.K. are employees of Cyto Pulse Sciences, Inc. and A.-K.R. is a paid consultant to Cyto Pulse Sciences, Inc.
REFERENCES
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo Science 19902471465–1468.Pt 1 [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC., and , Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DC, DeVit M., and , Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- Kurath G. Biotechnology and DNA vaccines for aquatic animals. Rev Sci Tech. 2008;27:175–196. [PubMed] [Google Scholar]
- Press Release Fort Dodge Animal Health announces approval of West Nile virus DNA vaccine for horses . < http://www.wyeth.com/news/archive >. 18 July 2005
- Press Release USDA grants conditional approval for first therapeutic vaccine to treat cancer . < http://us.merial.com/merial_corporate/news/press_releases/ >. 26 March 2007
- Roos AK, Moreno S, Leder C, Pavlenko M, King A., and , Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006;13:320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mendiratta SK, Thai G, Eslahi NK, Thull NM, Matar M, Bronte V, et al. Therapeutic tumor immunity induced by polyimmunization with melanoma antigens gp100 and TRP-2. Cancer Res. 2001;61:859–863. [PubMed] [Google Scholar]
- Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- Selby M, Goldbeck C, Pertile T, Walsh R., and , Ulmer J. Enhancement of DNA vaccine potency by electroporation in vivo. J Biotechnol. 2000;83:147–152. doi: 10.1016/s0168-1656(00)00308-4. [DOI] [PubMed] [Google Scholar]
- Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007;81:5257–5269. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent A, Mistretta F, Bottigioli D, Dahel K, Goujon C, Nicolas JF, et al. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine. 2007;25:6423–6430. doi: 10.1016/j.vaccine.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004;22:2489–2493. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- Tsang C, Babiuk S, van Drunen Littel-van den Hurk S, Babiuk LA., and , Griebel P. A single DNA immunization in combination with electroporation prolongs the primary immune response and maintains immune memory for six months. Vaccine. 2007;25:5485–5494. doi: 10.1016/j.vaccine.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Tjelle TE, Salte R, Mathiesen I., and , Kjeken R. A novel electroporation device for gene delivery in large animals and humans. Vaccine. 2006;24:4667–4670. doi: 10.1016/j.vaccine.2005.08.068. [DOI] [PubMed] [Google Scholar]
- Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, Barnum AB, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11:711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- Long YC, Jaichandran S, Ho LP, Tien SL, Tan SY., and , Kon OL. FVIII gene delivery by muscle electroporation corrects murine hemophilia A. J Gene Med. 2005;7:494–505. doi: 10.1002/jgm.683. [DOI] [PubMed] [Google Scholar]
- Ahlén G, Söderholm J, Tjelle T, Kjeken R, Frelin L, Höglund U, et al. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol. 2007;179:4741–4753. doi: 10.4049/jimmunol.179.7.4741. [DOI] [PubMed] [Google Scholar]
- Inovio Biomedical Corporation Home Page 2007 ). < http://www.inovio.com >.
- NIH Clinical Trials Website—Identifier: NCT00250419 2007 ). < http://www.ClinicalTrials.gov >.
- NIH Clinical Trials Website—Identifier: NCT00471133 2007 ). < http://www.ClinicalTrials.gov >.
- Pucihar G, Mir LM., and , Miklavcic D. The effect of pulse repetition frequency on the uptake into electropermeabilized cells in vitro with possible applications in electrochemotherapy. Bioelectrochemistry. 2002;57:167–172. doi: 10.1016/s1567-5394(02)00116-0. [DOI] [PubMed] [Google Scholar]
- Miklavcic D, Pucihar G, Pavlovec M, Ribaric S, Mali M, Macek-Lebar A, et al. The effect of high frequency electric pulses on muscle contractions and antitumor efficiency in vivo for a potential use in clinical electrochemotherapy. Bioelectrochemistry. 2005;65:121–128. doi: 10.1016/j.bioelechem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Zupanic A, Ribaric S., and , Miklavcic D. Increasing the repetition frequency of electric pulse delivery reduces unpleasant sensations that occur in electrochemotherapy. Neoplasma. 2007;54:246–250. [PubMed] [Google Scholar]
- Mir LM, Glass LF, Sersa G, Teissié J, Domenge C, Miklavcic D, et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer. 1998;77:2336–2342. doi: 10.1038/bjc.1998.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R, Gilbert R., and , Jaroszeski MJ. Clinical applications of electrochemotherapy. Adv Drug Deliv Rev. 1999;35:119–129. doi: 10.1016/s0169-409x(98)00067-2. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Hayes LS., and , Lloyd DB. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991;103:171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Murakami T., and , Kobayashi E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation. 2006;81:1179–1184. doi: 10.1097/01.tp.0000203137.06587.4a. [DOI] [PubMed] [Google Scholar]
- Payette PJ, Weeratna RD, McCluskie MJ., and , Davis HL. Immune-mediated destruction of transfected myocytes following DNA vaccination occurs via multiple mechanisms. Gene Ther. 2001;8:1395–1400. doi: 10.1038/sj.gt.3301534. [DOI] [PubMed] [Google Scholar]
- Dubus JC, Mely L., and , Lanteaume A. Use of lidocaine-prilocaine patch for the mantoux test: influence on pain and reading. Int J Pharm. 2006;327:78–80. doi: 10.1016/j.ijpharm.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Halperin BA, Halperin SA, McGrath P, Smith B., and , Houston T. Use of lidocaine-prilocaine patch to decrease intramuscular injection pain does not adversely affect the antibody response to diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b conjugate and hepatitis B vaccines in infants from birth to six months of age. Pediatr Infect Dis J. 2002;21:399–405. doi: 10.1097/00006454-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Halperin SA, McGrath P, Smith B., and , Houston T. Lidocaine-prilocaine patch decreases the pain associated with the subcutaneous administration of measles-mumps-rubella vaccine but does not adversely affect the antibody response. J Pediatr. 2000;136:789–794. [PubMed] [Google Scholar]
- Pavlenko M, Leder C, Roos AK, Levitsky V., and , Pisa P. Identification of an immunodominant H-2D(b)-restricted CTL epitope of human PSA. Prostate. 2005;64:50–59. doi: 10.1002/pros.20221. [DOI] [PubMed] [Google Scholar]