Abstract
Surface acoustic waves (SAWs) have been used as a rapid and efficient technique for driving microparticles into a three-dimensional scaffold matrix, raising the possibility that SAW may be effective in seeding live cells into scaffolds, that is, if the cells were able to survive the infusion process. Primary osteoblast-like cells were used to specifically address this issue: To investigate the effects of SAW on the cells’ viability, proliferation, and differentiation. Fluorescence-labeled osteoblast-like cells were seeded into polycaprolactone scaffolds using the SAW method with a static method as a control. The cell distribution in the scaffold was assessed through image analysis. The cells were far more uniformly driven into the scaffold with the SAW method compared to the control, and the seeding process with SAW was also significantly faster: Cells were delivered into the scaffold in seconds compared to the hour-long process of static seeding. Over 80% of the osteoblast-like cells were found to be viable after being treated with SAW at 20 MHz for 10–30 s with an applied power of 380 mW over a wide range of cell suspension volumes (10–100 μℓ) and cell densities (1000–8000 cells∕μℓ). After determining the optimal cell seeding parameters, we further found that the treated cells offered the same functionality as untreated cells. Taken together, these results show that the SAW method has significant potential as a practical scaffold cell seeding method for tissue and orthopedic engineering.
INTRODUCTION
Cell seeding is a crucial step in the process of forming viable tissue,1 from the fabrication of suitable scaffolds, proper isolation, and culturing of the desired cells to the deposition of these cells in the scaffolding material with a final round of culturing prior to use. The key requirement in seeding such scaffolds with cells is to retain the cells’ viability and functionality, which, in turn, depend on how they are handled. For example, the cells’ adhesive, proliferation, and differentiation processes may be adversely affected by exposure of the cells to air, humidity, and temperature changes and various forms of radiation.2, 3, 4, 5 If possible, rapidly infusing cells into implantable scaffolds would reduce their exposure to these environmental changes, potentially improving the quality of the tissue engineered from the cells. Further, reducing the seeding time makes it possible to perform this task at the point of care, i.e., in the surgical theater, for example. The success of tissue engineering with seeded scaffold structures, whether performed rapidly or not, is strongly correlated with a uniform distribution of the cells in the scaffold due to the homogeneity of the subsequent cultured tissue. Using other techniques, cells are merely deposited on the surfaces of scaffolds.2, 3, 4, 5, 6 As a result, the distribution of the new cultured tissue in the scaffold is not uniform and the tissue repair process is consequently delayed after implantation.4, 6
Rapid and uniform cell seeding—while maintaining cell viability and functionality—is the paragon for enabling tissue engineering.2, 3, 4, 5, 6 Conventional static seeding methods,2, 3, 4, 5, 6, 7, 8, 9 which seed cells into a scaffold through gravity-driven perfusion, are unable to meet these requirements. Under gravity alone, the perfusion of a cell suspension into a scaffold is very slow due to the large capillary forces that arise in implantable scaffolds due to a combination of the hydrophobicity of the polymers used in such structures and the typically small 100 μm order pore size. In addition to the lengthy process of infusion using this method, uniform cell distributions are difficult to achieve.4, 6 Although the static seeding method is still widely used due to its simplicity, other methods have been proposed to improve both the seeding efficiency and the homogeneity of the cell distribution. In these methods, the scaffold is usually fixed in place and immersed in a cell suspension. Agitating the cell suspension forms a relative velocity gradient between the advected cells and the stationary scaffold, driving the cells into it. The methods are also called shearing seeding methods as there exists a shear force between the cells and the cell culture medium during the seeding process. However, low seeding efficiencies and nonuniform cell distributions have been reported with these methods as well.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Alvarez-Barreto and co-workers10, 11 developed a more refined approach from these ideas using flow perfusion; a cell suspension is first poured on top of the prewetted scaffold and then the flow is drawn into the scaffold by oscillatory pumping across it, driving the fluid with the suspended cells into the scaffold matrix. Compared to past methods, this technique has been reported to deliver superior cell adhesion and seeding efficiency. However, even this perfusion method is slow, requiring 1–2 h. Moreover, large fluid pumps are required to compensate for the large pressure drops associated with the high capillary stresses resisting the flow of the fluid through the scaffold, a notable limitation in miniaturizing the technology to dimensions appropriate for point-of-care use.10, 11
We reported in a previous work another method13 using surface acoustic wave (SAW) radiation14 to provide the perfusion force. In that work, we demonstrated that small (10 μℓ) suspensions of microparticles may be rapidly driven into a porous polycaprolactone (PCL) scaffold in 10 s, some three to four orders of magnitude faster than the hour-long process required using the static and perfusion methods described above. Furthermore, the method uniformly distributed the particles throughout the scaffold. However, the work used particles, not live cells, let alone the kinds of cells that would be typically used in tissue engineering, a distinct shortcoming as the deleterious effect of ultrasound on cells is well known. Though in a later work15 we reported the infusion of yeast cells with some cursory results on the viability of primary-like osteoblast cells postexposure to the SAW radiation, we did not consider their viability in detail, nor did we consider the far more important issue of whether such cells remain functional postexposure and are able to form useful tissue. This is the focus of this work.
In what is reported in the remainder of this paper, osteoblast-like cells were first seeded into scaffolds by both SAW and static methods, the latter as a control. The distribution of the cells was analyzed to demonstrate that the osteoblast-like cells may be rapidly and uniformly delivered into the scaffolds by SAW. Next, different SAW radiation conditions were applied to thoroughly study the effects of the SAW on the cells’ viability and to ascertain the appropriate conditions to use for effective infusion of the cells using SAW irradiation. The effects of the SAW irradiation under such conditions on the cells’ proliferation and differentiability were then investigated. We close the paper with a discussion of the results in the context of the intended application of the technology.
MATERIALS AND METHODS
Scaffolds
PCL (MW=65 000, MP=65 °C) (Sigma Chemical Co., USA) scaffolds were prepared using a conventional solvent casting and particulate leaching method.16 Briefly, a PCL solution with a concentration of 10% (w∕v) was prepared by dissolving solid PCL particles in chloroform. Sodium chloride (NaCl) particles, sieved as porogens (100–150 μm in size), were then added into the solution to prepare a NaCl suspension at a weight:weight ratio of NaCl to polymer of 1:9, which was then cast into a Teflon mold. The samples were air dried and then vacuum dried to remove any remaining solvent and, subsequently, immersed in a large amount of de-ionized water for at least 72 h. The water was refreshed every 3 h in the first 24 h to leach the porogens from the PCL-salt composite structure to leave behind a porous PCL structure. The samples were finally vacuum dried to obtain a set of sponge-like PCL scaffolds with pore sizes of 150–300 μm and a thickness of 2 mm. Before seeding, the scaffolds were carefully cut with a razor blade into 4×4 mm2 and stored in a desiccator. The porosity of the scaffold was about 90%±1.5%, determined by using Archimedes’ principle as described by Yang et al.17
Seeding experiments
Cell isolation and fluorescence labeling
To examine the viability of mammalian cells, in particular, primary osteoblast-like cells were isolated using standard techniques. Long bones or calvaria were isolated from 6–8 week old C57bl∕6 mice, crushed∕minced using bone crunchers into smaller particles (the calvaria were minced as described) with the marrow flushed using a normal saline solution. They were then subjected to serial collagenase digestions to isolate the bone and stromal cell populations. Collagenase activity was stopped by the addition of 15% fetal calf serum (FBS) and the cells were collected at the end of each digestion. Subsequently, the cells were strained through a cell strainer, centrifuged, washed, and resuspended in alpha-modified Eagle’s medium supplemented with 10%–15% FBS and plated in a tissue culture flask for 4 days to permit the recovery of the cells. At this stage, the cells were removed from the tissue culture flask using trypsin∕ethylenediamine tetra-acetic acid, collected, washed and centrifuged, resuspended in a freeze medium (1×106 cells∕mℓ), and frozen under liquid nitrogen vapor until required.
Both nonfluorescent and fluorescent cells [expressing red fluorescent protein (RFP), isolated from RFP+transgenic mice] were used, with nonfluorescent cells used for the assessment of cell’s viability, proliferation, and differentiation, while RFP+cells were used for facilitating the observation of cell distributions within the scaffold.
Cell seeding and measurement of their distribution
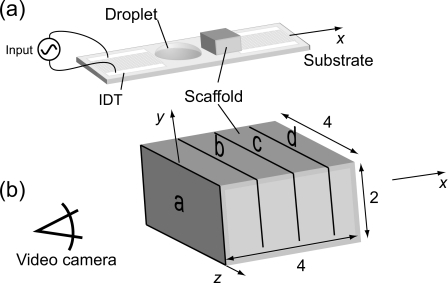
A SAW is generated by applying a radio frequency (RF) signal to an interdigital transducer (IDT) fabricated onto a SAW substrate. The nature of the SAW substrate and the IDTs was described in our previous studies.13, 15 Figure 1a shows a schematic of the experimental setup as seen from above [see EPAPS, Ref. 18, enhanced]. Situated on the left side is the input IDT. A RF signal generator (SML-04 Signal Generator, Rohde and Schwarz, Germany) and an amplifier (model 10W1000C, 0.5–1000 MHz, Amplifier Research, USA) were used to generate the electrical signal input via bus bars to the IDT electrodes to efficiently convert the electrical signal into a traveling SAW propagating away from the IDT along the x axis.
Figure 1.
(a) Schematic of the experimental setup for cell seeding. (b) Four 1-mm-thick sections were cut through the scaffold to evaluate the distribution of the cells within it. We observed the slices from the entry side.
For SAW-driven cell seeding, a scaffold was first placed on the surface of the SAW device. A 10 μℓ primary osteoblast-like cell suspension with a density of 50 000 cells∕μℓ was then pipetted between the IDT and the scaffold, as shown in Fig. 1a, and subjected to SAW irradiation at 20 MHz and 380 mW for 10 s (the optimal working condition was set according to the results in our previous studies) [Fig. 1a].
Once the SAW forms on the substrate, approximately one-third of the acoustic energy propagates into the droplet19 and acts to generate acoustic streaming within the droplet, moving it and the cells within toward and into the scaffold. Three 10 μℓ droplets were used, one immediately after the other, to infuse 30 μℓ of the cell suspension into the scaffold. The 10 μℓ droplets were chosen rather than a single 30 μℓ droplet to avoid the solution escaping around the sides of the scaffold. In each run, only 10 s were required for the cell suspension to be completely driven into the scaffold. For the static seeding control, a 30 μℓ droplet comprising the same concentration of primary osteoblast-like cells was placed in contact with the scaffold’s side surface. Once the cell suspension contacted the scaffold surface, it slowly diffused into the scaffold under the action of capillary forces. The time required for cell suspension diffusion was around 30 min, much longer than for SAW seeding.
After seeding, the scaffolds were cut into four 1-mm-thick slices [Fig. 1b]. The cross section of each slice (a–d) was observed under a reflection fluorescence microscope system (Olympus BXFM, Tokyo, Japan) and images of the exposed cross-sectional surfaces were captured using an attached high-speed color video camera (Olympus iSpeed, Tokyo, Japan). The excitation maxima wavelength for these cells is 542 nm (green) and the emission maxima wavelength is 612 nm (red). For both the static control and SAW irradiation methods, the cell suspension was introduced into the scaffold into slice a [Fig. 1b] and penetrated onward through slices b–d to perfuse the entire scaffold. Photoshop CS (Adobe Systems Software, Ltd., Ireland) was used to measure the red-color pixel intensities of each image. In analyzing the cell distribution through the entire scaffold, the pixel intensity value of each image was normalized against the intensity of the image of slice a. To analyze the cell distribution in each slice, the standard deviation (SD) of the pixel intensity in red was also recorded. The lower the SD of the red-color pixel intensity, the more uniform the cell distribution.
Post-SAW treatment cell assessment
Experimental setup
Two kinds of SAW devices with different IDT designs were fabricated in order to generate different SAWs. One IDT that was designed to deliver SAW with a resonance frequency of 10 MHz was constructed with 25 straight finger pairs, a 12-mm-wide aperture, and a wavelength of λ=440 μm that defines the strip and gap widths to both be 110 μm. The other IDT was designed to provide SAW radiation at 20 MHz with 60 straight finger pairs, a 8-mm-wide aperture, and strip and gap widths of 49 μm. The SAW devices were then sterilized by exposure to ultraviolet radiation overnight. A drop of cell suspension was subsequently placed onto the surface of the device and irradiated with SAW using the same setup illustrated in Fig. 1a, though without the scaffold. The cell suspension was then collected and diluted in an appropriate amount of culture media for further culturing and analysis. The SAW frequency, applied RF power, treatment time, and the volume and density of the cell suspension were all varied to determine the optimal conditions for manipulating the cells while leaving their viability and functionality intact.
Cell viability
A specialized flow cytometer [Becton Dickinson fluorescence-activated cell sorting (FACS) Vantage SE-DiVa, San Jose, CA] and a Beckman Coulter analyzer (FC500-CXP, Fullerton, CA) were used to rapidly assess the viability of the cells after being treated by SAW via FACS. Propidium iodide dye was used to assess the total cell viability, being taken up only by nonviable cells whose membrane was disrupted. After determining the optimal operating condition, subsequent functional assessments were carried out using this specific configuration and the results were compared to control cells that had not been subjected to the SAW radiation.
Cell proliferation
Cell proliferation was measured using AlamarBlue reagent (BioSource, Camarillo, CA). The assay incorporates a specially selected oxidation-reduction indicator, which both fluoresces and undergoes colorimetric change in response to cellular metabolic reduction in the growth medium resulting from cell proliferation. Thus, the system measures cell proliferation as a function of the cellular metabolic activity: The greater the metabolic activity, the more the AlamarBlue reagent is reduced, leading to the observed color change. The growth-related reduction causes the indicator to change from an oxidized nonfluorescent form to a reduced fluorescent form. This reagent was added to cells in culture and the fluorescence was measured on a plate reader (Fluostar Optima, BMG LabTech, Offenburg, Germany), and readings were taken over time, thus providing a measure of cell proliferation over time.
Cell differentiation and mineralization
Alkaline phosphatase (ALP) activity, which is a marker of cell differentiation ability, was assessed at day 10 through the use of a commercial kit (Takara Bio, Inc., USA). Briefly, cells in each well were washed and lysed using the provided lysis buffer containing 0.1% NP-40. A volume of the subsequent lysate was used for ALP activity assessment, and the activity was assayed through the cleavage of the p-nitrophenol phosphate substrate yielding the p-nitrophenol product. The level of the product was determined through absorbance at 405 nm on a FluoStar Optima plate reader. To eliminate the contribution of the serum found in the original cell culture medium toward the total protein in each lysate, the albumin in the serum was removed using Affi-Gel blue sepharose columns. Following dialysis of each sample overnight, the albumin was removed through incubation with the blue sepharose beads and subsequent washing. The total protein in each sample was measured using the Bradford assay, and the ALP activity in each sample was normalized to the level of protein in each sample.
To demonstrate osteogenic differentiation, the accumulation of calcium phosphate in the cells was observed by von Kossa staining. Mineralization was assessed after 18 days of culturing; spicules were seen in the cultures at this time due to mineral deposition. Each culture was washed and fixed using formalin. These fixed, air-dried cultures were then stained with silver nitrate and exposed to sunlight∕UV radiation for 15–30 min, leading to calcium reduction and replacement with silver deposits, which could be seen as metallic silver staining dark brown or black.
Statistical analysis
All data were expressed as a mean ± standard deviation (SD) for n=3 and were analyzed using the two tail standard Student’s t-test analysis.
RESULTS
Scaffold cell distribution
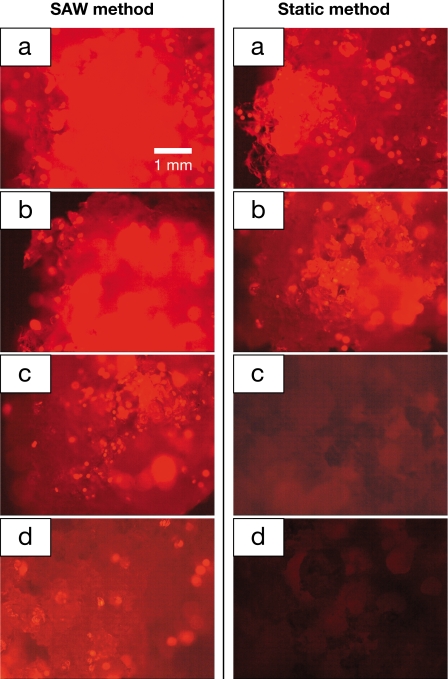
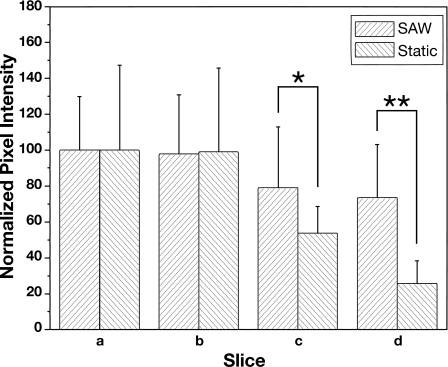
The spatial distribution of the cells within the scaffold and the uniformity of the cell distribution were determined by observing the images of the cross-sectional scaffold slices under the fluorescence microscope, as shown in Fig. 2. The images in the left column show the distribution of cells in the scaffold seeded by the SAW method. For comparison, the distribution of cells in the scaffold seeded by the static method is shown by the images in the right column of Fig. 2. The corresponding cell distribution in the entire scaffold and in each slice, obtained via pixel intensity analysis of the fluorescent microscopic images in Fig. 2, is shown in Fig. 3. Using SAW irradiation, the pixel intensity remained above 75 throughout. Compared to the static method, which dropped to less than 25 in the last slice, the distribution of cells is evidently more homogeneous. The SD of pixel intensity across the image of each slice is also provided and indicates the distribution of cells across each slice.20 It is important to note here that a comparison between the SD and the mean values at each slice is not possible and is not an indication of the accuracy of the measurement because the finite size of each cell and each pixel interact to always deliver a nonzero SD of the pixel intensity across an image regardless of the actual distribution of the cells in the image. What can be determined, however, is that in contrast to the static method, the SD is nearly constant for the SAW method, indicating a more uniform distribution of cells across each slice when infused via SAW. When the cells were seeded by the SAW method, there were still a significant number of cells in the slice farthest from the entry side, i.e., slice d (Fig. 2), confirmed by the high value (74%) of the normalized pixel intensity of slice d compared to slice a [Fig. 3a]. However, cells seeded by the static method were only able to penetrate about 40% of the overall width of the entire scaffold and most of the cells were only present in the surface layer (slice a). As a result, the pixel intensity dropped dramatically from slice b to slice c. With SAW, the cells were also more uniformly distributed as seen in Fig. 3b; the pixel intensity SD (∼30) of each scaffold slice using the SAW method was much lower than with the static method (∼50).
Figure 2.
Fluorescent images of cross sections of the scaffold at the positions corresponding to slices a–d, as shown in Fig. 1b. Slice a is the first slice where the cell suspension enters the scaffold. The left column shows the scaffold slices seeded by the SAW method. Significant penetration of the cells to the last slice is observed. The right columns are images of the static-loaded slices, showing little particle penetration beyond the first two slices. All images are at 20× magnification. In both cases, the scaffold dissection was carried out immediately after the entire suspension entered the scaffold, after an elapsed time of ten seconds for the SAW-driven samples and 30 min for the static seeding samples.
Figure 3.
Image analysis of the cell distribution in the scaffold. The pixel intensity reported for each slice is normalized to slice a (thus, slice a has an arbitrary pixel intensity of 100) (*P<0.05, **P<0.01).
Cell viability evaluation
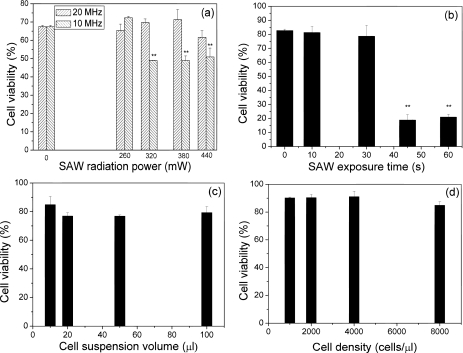
Figure 4 shows the viability of cells treated with SAW under different conditions by varying the SAW frequency, applied RF power, exposure time, and the volume and density of the cell suspension. First, SAW devices with two different resonant frequencies of 10 and 20 MHz were employed, while the SAW exposure time and the volume and density of the cell suspension were fixed to be 10 s and 10 μℓ and 5000 cells∕μℓ, respectively. It can be seen from Fig. 4a that the cell viability did not decrease significantly when subjected to 20 MHz SAW, showing no difference in comparison with cells that were not exposed to the SAW treatment (control). Over 80% of the cells maintained their viability after being treated for 10 s at an applied power over 380 mW at 20 MHz. However, only 50% cells were still alive after being treated by 10 MHz SAW for 10 s at an applied power of 380 mW. The viability of the cells subjected to 10 MHz SAW decreased significantly as the applied RF power was increased, indicating that the cell viability was more sensitive to the applied RF power at the lower frequency, likely an effect of the larger displacement at the lower frequency for a given input power. The vibration velocity is proportional to the input power over most of the frequency range that may be used with the SAW device. The amplitude of the displacement, however, is inversely proportional to the frequency used and, therefore, cell structure displacement and strain are also inversely proportional to the frequency. Based on this result, SAW with a frequency of 20 MHz was employed in subsequent assessments in which 380 mW was employed since the cell viability was observed to decrease when the RF power was 440 mW. Above 440 mW, the intense SAW radiation resulted in the atomization19 of the droplet containing the suspension before it reached the scaffold.
Figure 4.
Viability assessments of primary osteoblast-like cells after being treated with SAWs under different conditions. (a) Viability of cells treated by the SAW for 10 s at different frequencies and RF powers. The cell density is 5000 cells∕μℓ and the volume of cell suspension is 10 μℓ. (b) Viability of cells treated by the SAW for different exposure times at 20 MHz and 380 mW. The cell density is 5000 cells∕μℓ and the volume of the cell suspension is 10 μℓ. (c) Viability of cells treated by the SAW at 20 MHz and 380 mW for 10 s. The cell suspension is changed from 10 to 100 μℓ while maintaining the same cell density of 5000 cells∕μℓ. (d) Viability of cells treated by the SAW at 20 MHz and 380 mW for 10 s. The cell density is changed from 1000 to 8000 cells∕μℓ using the same 10 μℓ volume. The symbol “∗∗” indicates that there is significant difference between these data to the control group.
Figure 4b shows that the cell viability sharply decreased from 80% to 20% as the radiation time exceeded 40 s on a 10 μℓ cell suspension with a density of 5000 cells∕μℓ. Therefore, 40 s appears to be the exposure time limit for seeding a 10 μℓ cell suspension without affecting its viability. However, since the perfusion occurs in only 10 s, this would not pose considerable concern for the purposes of practical SAW cell seeding. Furthermore, increasing the cell suspension volume from 10 to 100 μℓ and the cell density from 1000 to 8000 cells∕μℓ did not appear to affect the cell viability [Figs. 4c, 4d]. This is convenient if a larger number of cells are to be seeded. In all following cell experiments, a 20 MHz SAW was used at an applied RF power of 380 mW. The exposure time of the cells to the SAW was held constant at 10 s.
In summary, we have investigated the viability of cells treated by SAW under a variety of configurations. It was found that the cell viability was as high as 89% when 10 μℓ of cell suspension was exposed to a 20 MHz SAW for 10 s. In addition, a wide range of cell suspension volumes and densities were shown to be equally effective, allowing flexibility in the use of this approach to cell seeding.
Cell functionality assessment
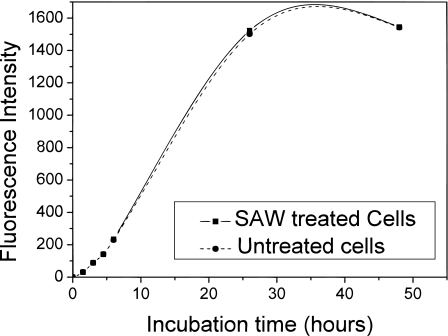
Investigations were then carried out to evaluate the effects of the SAW radiation on the cells’ functionality, i.e., the proliferation, and differentiation abilities after exposure to the SAW. Briefly, treated and untreated cells were transferred separately into culture plates and cultured for another 50 h, 37 °C, 5% CO2, and 5% O2. The proliferation ability was measured at different time points. There was no significant difference in the proliferation of the treated and untreated cells over the 50 h incubation, as shown in Fig. 5.
Figure 5.
Cell proliferation ability of both treated and untreated cells as a function of time measured via AlamarBlue uptake. The cells were treated by SAW at 20 MHz and 380 mW for 10 s, and the volume of the cell suspension and the cell density are 10 μℓ and 5000 cells∕μℓ, respectively.
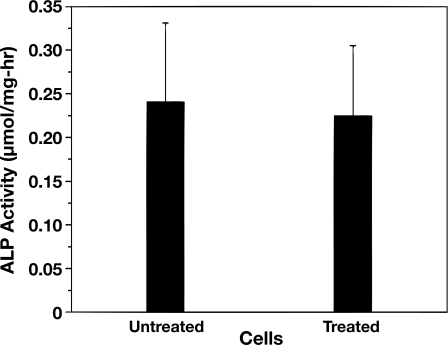
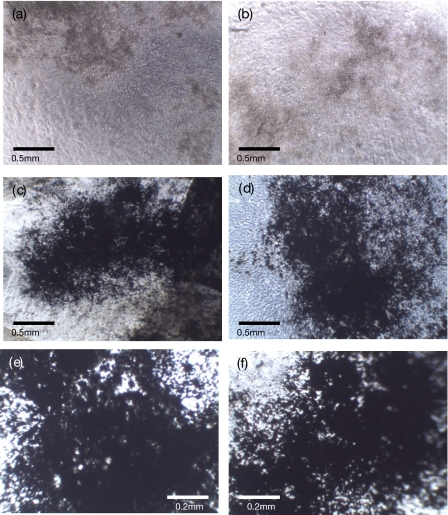
Cell differentiation ability was assessed through ALP activity and the results indicate little difference between the treated and untreated cells’ ALP activity after being cultured for 10 days, as shown in Fig. 6. This functionality was further assessed by culturing both treated and untreated cells in mineralization-promoting medium for 18 days and the osteogenic differentiation was observed by von Kossa staining. Figure 7 shows the images of cells with or without SAW treatment both before von Kossa staining [cells in culture, Figs. 7a, 7b] and after von Kossa staining [Figs. 7c, 7d, 7e, 7f]. Calcium phosphate deposits seen as spicules were visible in both of the cell cultures before von Kossa staining. The black silver deposits could be seen after von Kossa staining, indicating calcium phosphate deposition by the cells.
Figure 6.
Cell ALP ability of treated and untreated cells as a function of time. Cells were treated with 20 MHz SAW at 380 mW for 10 s. The cell suspension volume and concentration are 10 μℓ and 1000 cells∕μℓ, respectively. There is no significant difference between the ALP abilities of treated and untreated cells.
Figure 7.
Calcium phosphate deposits without [brown (a) and (b)] or with von Kossa staining [black (c)–(f)] of treated and untreated cells after being cultured for 18 days. Images of (a) untreated and (b) SAW-treated cells after being cultured for 18 days without von Kossa staining. The magnification is 40×. Images of (c) untreated and (d) SAW-treated cells after being cultured for 18 days with von Kossa staining. The magnification is 40×. Images of (e) untreated and (f) SAW-treated cells after being cultured for 18 days with von Kossa staining. The magnification is 100×. The cells were treated by the SAW at 20 MHz and 380 mW for 10 s. The volume of the cell suspension and cell density are 10 μℓ and 1000 cells∕μℓ, respectively.
DISCUSSION
With the SAW method, the entire scaffold was seeded within 10 s, far more rapidly than with the conventional static method (30 min). As compared to the methods described in the literature,4, 6, 7, 8, 9, 10, 11, 12 where the seeding process usually takes hours to days, the present SAW method significantly accelerates the cell seeding process. The fast seeding time also means that multiple drops containing the cells can be successively driven into the scaffold to achieve greater cell loading. In addition, the SAW method only requires a SAW device, much simpler and compact than the large and complicated systems involving pumps and bioreactors required with conventional techniques. In addition to accelerating and simplifying the process, we have also demonstrated that the level of seeding uniformity can be greatly improved by using SAW perfusion. The static method resulted in a statistically lower uniformity, consistent with the nonuniform spatial cell distributions reported by several groups.4, 6, 21, 22 This low uniformity may be explained by the difficulty in evenly distributing the small volume of cell suspension over the scaffold surface and by the intrinsically weak mechanism of infusion (i.e., via gravity and capillary action).
CONCLUSIONS
When we described a novel method for controlled driving particle suspensions through three-dimensional scaffolds with SAW in our previous paper, we proposed that this method might be applied in tissue engineering as a new cell seeding method with the advantage of providing a rapid and effective seeding process.13 In the present study, the SAW-driven cell seeding method was further proved to be feasible for tissue engineering by investigating the effects of the SAW energy on the viability, proliferation, and differentiation of primary osteoblast-like cells. As we mentioned before, there are various cell seeding methods being developed and most of these studies investigate the cell viability and functionality.4, 6, 7, 8, 9, 10, 11, 12 Undoubtedly, cell viability and functionality are critical to the usefulness of any new technique for tissue engineering.5
Therefore, in addition to high cell viability, the proliferation and differentiation ability of the treated cells showed no difference from those of the untreated cells, indicating that the SAW has no deleterious effects on the cells’ functionality. Although it is difficult to compare these results to the data in literature as different cells and measurement methods were used, the results demonstrate that the SAW method is a safe method for seeding cells into scaffolds. We studied the feasibility of the SAW method for cell seeding by seeding osteoblast-like cells into scaffolds with SAW and investigating the osteoblast-like cells’ viability and functionality after being treated by SAW throughout using a control sample for comparison that has not been exposed to SAW. Osteoblast-like cell seeding experiments confirmed that the SAW method is able to rapidly seed cells (10 s for 10 μℓ) uniformly throughout the culture scaffold. The cell experiments showed that the post-treatment cells maintain over 85% viability and are able to proliferate and differentiate like untreated cells. Based on these results, and the ease in using this technique, we conclude that the SAW method has considerable potential in improving the future of tissue and orthopedic engineering by providing an efficient means for rapid and efficient scaffold cell seeding.
References
- Langer R. and Vacanti J., Science 260, 920 (1993). 10.1126/science.8493529 [DOI] [PubMed] [Google Scholar]
- Soletti L., Nieponice A., Guan J., Stankus J., Wagner W., and Vorp D., Biomaterials 27, 4863 (2006). 10.1016/j.biomaterials.2006.04.042 [DOI] [PubMed] [Google Scholar]
- Zhao F. and Ma T., Biotechnol. Bioeng. 91, 482 (2005). 10.1002/bit.20532 [DOI] [PubMed] [Google Scholar]
- Dong J., Uemura T., Shirasaki Y., and Tateishi T., Biomaterials 23, 4493 (2002). 10.1016/S0142-9612(02)00193-X [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G., Obradovic B., Martin I., Bursac P., Langer R., and Freed L., Biotechnol. Prog. 14, 130 (1998). 10.1021/bp970120j [DOI] [PubMed] [Google Scholar]
- McFetridge P., Daniel J., Bodamyali T., Horrocks M., and Chaudhuri J., J. Biomed. Mater. Res. 70, 224 (2004). [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Yamaoka T., Iwase R., and Murakami A., Biotechnol. Bioeng. 93, 947 (2006). 10.1002/bit.20797 [DOI] [PubMed] [Google Scholar]
- Burg K., W.Holder, Jr., Culberson C., Beiler R., Greene K., Loebsack A., Roland W., Eiselt P., Mooney D., and Halberstadt C., J. Biomed. Mater. Res. 51, 642 (2000). [DOI] [PubMed] [Google Scholar]
- Kim B., Putnam A., Kulik T., and Mooney D., Biotechnol. Bioeng. 57, 224 (1998). [PubMed] [Google Scholar]
- Alvarez-Barreto J., Linehan S., Shambaugh R., and Sikavitsas V., Ann. Biomed. Eng. 35, 429 (2007). 10.1007/s10439-006-9244-z [DOI] [PubMed] [Google Scholar]
- Alvarez-Barreto J. and Sikavitsas V., Macromol. Biosci. 7, 579 (2007). 10.1002/mabi.200600280 [DOI] [PubMed] [Google Scholar]
- Wendt D., Marsano A., Jakob M., Heberer M., and Martin I., Biotechnol. Bioeng. 84, 205 (2003). 10.1002/bit.10759 [DOI] [PubMed] [Google Scholar]
- Li H., Friend J. R., and Yeo L. Y., Biomaterials 9, 647 (2007). [Google Scholar]
- Yeo L. Y. and Friend J. R., Biomicrofluidics 3, 012002 (2009). 10.1063/1.3056040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok M., Li H., Yeo L., and Friend J., Biotechnol. Bioeng. 103, 387 (2009). 10.1002/bit.22243 [DOI] [PubMed] [Google Scholar]
- Li H. and Chang J., J. Mater. Sci.: Mater. Med. 15, 1089 (2004). 10.1023/B:JMSM.0000046390.09540.c2 [DOI] [PubMed] [Google Scholar]
- Yang J., Shi G., Bei J., Wang S., Cao Y., Shang Q., Yang G., and Wang W., J. Biomed. Mater. Res. 62, 438 (2002). [DOI] [PubMed] [Google Scholar]
- See EPAPS supplementary material at http://dx.doi.org/10.1063/1.3194282 E-BIOMGB-3-002903for the videos of droplet motion under irradiation by SAW.
- Qi A., Yeo L., and Friend J., Phys. Fluids 20, 074103 (2008). 10.1063/1.2953537 [DOI] [Google Scholar]
- Wang S., Lai Y., Ben Y., and Chang H., Ind. Eng. Chem. Res. 43, 2902 (2004). 10.1021/ie030689r [DOI] [Google Scholar]
- Li Y., Ma T., Kniss D., Lasky L., and Yang S., Biotechnol. Prog. 17, 935 (2001). [DOI] [PubMed] [Google Scholar]
- Ishaug-Riley S., Crane-Kruger G., Yaszemski M., and Mikos A., Biomaterials 19, 1405 (1998). 10.1016/S0142-9612(98)00021-0 [DOI] [PubMed] [Google Scholar]