Abstract
Bio-electrosprays (BESs) provide a means of precisely manipulating cells and thus have the potential for many clinical uses such as the generation of artificial tissues∕organs. Previously we tested the biological safety of this technology with a variety of living cells and also embryos from the vertebrate model organisms Danio rerio (zebrafish) and Xenopus tropicalis (frog). However, the viability and fertility of the treated embryos could not be fully assessed due to animal licensing laws. Here we assay the viability and fertility of Drosophila melanogaster (fruit fly) embryos in conjunction with the bio-electrospray procedure. Bio-electrosprayed Drosophila embryos developed into fully fertile adult flies that were indistinguishable from wild-type. Thus, we demonstrate that the bio-electrospray procedure does not induce genetic or physical damage that significantly affects the development or fertility of a multicellular organism. This study along with our previous investigations demonstrates the potential of this approach to be developed for the precise manipulation of sensitive biological materials.
INTRODUCTION
Bio-electrospraying1, 2, 3 is an electric field driven noncontact jetting biotechnology that was pioneered in 2005 for the direct handling living cells. This technique evolved from electrosprays,4, 5 also known as electrohydrodynamic atomization, and is driven by high intensity electric fields. The electric field assists in accelerating a volume of liquid between two electrodes. The liquid exiting the needle is placed several millimeters centrally above a grounded electrode. In this study, we test a ring-shaped ground electrode that can be altered in geometry to become either a plate or point. The plate configuration is tested in aerosol science studies and applications, while the point is tested for precision deposition. The ring electrode assists in forming a three-dimensional conical spray useful for controlled large area-based deposition. Previous studies using this configuration reported that larger droplets pass through the center, while finer droplets recirculate on the extremities of the electrode. In addition to the variation in ground electrode geometries, the flow rate of the media to the needle inlet and its consistency, together with the media properties, play a critical role in jet stability and continuity. The liquid behavior at the needle exit has also undergone extensive studies.6, 7
The cone-jet mode, also known as the Taylor cone, is the method that has been explored for the widest range of applications.8, 9 In cone-jet mode, the morphology of the liquid at the needle exit transforms into a three-dimensional cone, from which a jet evolves at the apex. In controlled conditions, the emerging jet undergoes jet breakup resulting in a near-monodistribution of droplets. To obtain this result, the three controlling parameters must be in agreement; otherwise we observe other jetting modes, the vast majority of which are unstable. To date, we have been able to demonstrate that bio-electrospraying of a wide range of living cells does not lead to genetic or physiological changes. Our goal is to develop this technology as a novel biological tissue fabrication approach, which could be exploited for tissue repair and replacement.
Although we have demonstrated that both homogeneous and heterogeneous cell suspensions can be manipulated using bio-electrosprays (BESs) without detriment using several techniques including flow cytometry, reverse transcription-polymerase chain reaction, gene chip micro arrays, and cytogenetic studies, we wanted to test this unique technology further with whole multicellular organisms. Animal development requires the precise coordination of many different processes including gene expression, cell-cell communication, cell division, cell death, cell movement, and cell shape changes; disruption of any one of these usually leads to death or gross malformation of the embryo. Thus, embryonic development is particularly sensitive to adverse environmental conditions that cause mechanical or genetic damage. The survival and fertility of treated embryos would also support the use of this technology as an approach for handling large quantities of embryos. For example, if proven safe, bio-electrospraying could form the basis of technology for sorting embryos to study development under variable conditions.
Our preliminary studies involved passing either Danio rerio or Xenopus tropicalis embryos through the BES apparatus.10, 11 Although these studies demonstrated that the process had no deleterious effects on developing embryos, we were unable to assess the viability of these organisms to full maturity or assess their fertility due to animal handling regulations. Therefore in this study of BES, we have assessed the entire life cycle and fertility of embryos from Drosophila melanogaster (fruit fly) since Drosophila is not subject to stringent animal handling regulations. Drosophila is a well-established model organism that has been used productively to study many different biological processes including genetics, development, aging, learning, and behavior.12 In particular, the study of Drosophila has led to many key insights into the embryonic development of all animals, including humans.
We have subjected Drosophila at embryonic and larval stages to a range of jetting conditions and followed their development and fertility and found no significant differences from controls. Thus, the BES procedure does not damage the genetic or cellular processes in developing Drosophila embryos and does not disrupt the genetic integrity of the germline. This lends further support for the biological safety of BES and their potential use as a biomedical tool.
EXPERIMENTAL SECTION
BES equipment setup and operation
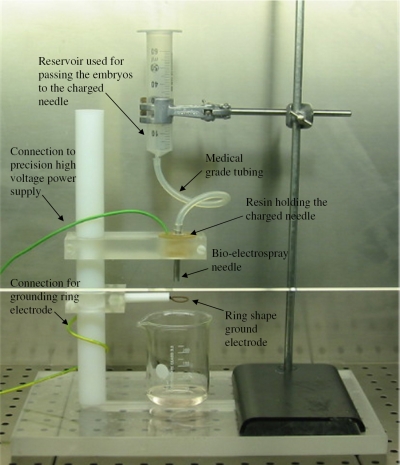
The bio-electrospraying of Drosophila melanogaster embryos was carried out with a stainless steel needle with internal and external bore diameters of ∼6 and ∼8 mm, respectively. The BES needle was held firmly in resin and was connected to a precision high voltage power supply (FP-30, Glassman Europe, Ltd., Tadley, United Kingdom), capable of applying a voltage of up to ±30 kV, with a drawing current of ∼4 mA. The inlet of the needle was connected to medical grade silicone tubing with a similar internal bore diameter. The inlet end of the silicone tube was connected to a reservoir of Dulbecco’s phosphate buffered saline (DPBS) containing Drosophila melanogaster embryos. The flow of suspended embryos was controlled by way of gravitational flow and could be varied between ∼10−5 and 10−8 m3 s−1. Centrally below the needle exit was a ring-shaped ground electrode with an internal diameter of ∼18 mm (Fig. 1). The needle and ground electrode configuration explored in these studies allowed the distance between the electrodes to be varied as required. The BES setup was housed within a laminar flow class II safety hood.
Figure 1.
Digital optical image of the BES equipment setup explored within a class II safety cabinet for jetting Drosophila melanogaster embryo suspensions.
Embryo suspension preparation
0–3 h wild-type (Oregan-R, kept at 25 °C) embryos were collected on apple juice agar plates and transferred into PBS. These embryos were split into three groups of roughly equal size: (1) embryos that were not subjected to any form of physical stress, (2) embryos passed through the BES needle without the application of an applied potential difference between the electrodes (“needle control”), and (3) embryos bio-electrosprayed at varied applied voltages to flow rates. Similarly, suspension aliquots were prepared with Drosophila embryos that had reached the second instar larval stage at ∼50 h.
Suspension characterization
The BES jet processing properties of the embryo suspensions were characterized in terms of their electrical conductivity, viscosity, surface tension, relative permittivity, and density. Measurements were made using a conductivity meter (HACH SensION 156 probe), Visco-Easy rotational viscometer, Kruss Tensiometer K9 (Du Novy’s ring and plate method), a calibrated cell connected to a high precision multimeter, and a standard density bottle, respectively.
Embryo collection
Needle control and bio-electrosprayed embryos were collected directly into sterile 50 ml tubes by placing the tube directly in-line and below the exit of the jetting needle.
Optical microscopy
Microscopy was carried out using Leica optical microscopes (Leica MZ10F, DMIL and DM2500).
Assays for viability and fertility
The hatch rate of treated and control embryos was determined by counting out batches of 100 embryos on to apple juice agar and leaving them to age for at least 24 h at 25 °C. The number of embryos that hatched into larvae was noted. Fifty freshly hatched first instar larvae of each group were collected into vials containing standard fly culture medium and allowed to develop into adult flies. The number of adult flies that emerged was recorded before matings were set up of single experimental flies with three wild-type flies to determine fertility. Crosses that produced adult progeny were deemed to be fertile. Samples containing larvae at second instar stages were studied for viability and fertility in the same manner as eggs.
RESULTS AND DISCUSSION
We explored gravitational based flow rates and a precision high voltage power supply. The latter applied a voltage to the needle or the potential difference between the electrodes (Fig. 1). This equipment was set up in a laminar flow safety cabinet such that we could vary the distance between the electrodes while varying the flow rate. This allowed us to use large bore needles, which guaranteed minimal shear stresses on the embryos. In this study we assessed a wide range of applied voltages to flow rates within the remit of our equipment constraints, ranging from ∼1 to 30 kV and from ∼10−5 to 10−8 m3 s−1 for the applied voltage and flow rate to the needle, respectively. Preliminary studies indicated the best operational conditions to explore for a given system setup. We set the distance between the needles to ∼10 mm, then applied a voltage between the electrodes, and recorded the jetting behavior in real time by way of a high-speed digital camera (Phantum V7.3, which is capable of imaging 500 000 fps on loan from the Engineering and Physical Sciences Research Council loan pool), in conjunction with a monozoom lens and a GE light source. Our initial studies did not achieve stable jetting conditions during the bio-electrospraying of the embryos. This is perhaps not surprising as the embryo suspension had high conductivity and low viscosity, in contrast to the two parameters required for promoting jet stability and continuity, low conductivity, and high viscosity (Table 1). However achieving jet stability and continuity was not a major issue, as testing for embryo viability was our primary aim in this study. If jet stability and continuity are required, the use of the coaxial or concentric BES needle system can be explored.13, 14
Table 1.
Measured and calculated characteristics of Drosophila embryo suspensions.
| Electrical conductivity (Sm−1) | Viscosity (mPa s) | Surface tension (mN m−1) | Relative permittivity | Density (kg m−3) |
|---|---|---|---|---|
| ∼10−2 | ∼28 | ∼55 | ∼33 | ∼975 |
The results from our preliminary studies indicated that our apparatus required rearranging. Thus, we placed the electrodes ∼5 cm apart and used voltages starting at ∼1 kV, while the microdripping of the embryo suspension was initiated at a flow rate of ∼106 m3 s−1. Maintaining the same flow rate, we increased the applied voltage to ∼2 kV and beyond, which increased the frequency of microdripping. Increasing the applied voltage to ∼10 kV resulted in unstable jets (Fig. 2), while increasing the voltage beyond this lead to discharging effects. At ∼11 kV we observed the crossing of sparks between the electrodes (Fig. 3). We were able to apply a voltage greater than 11 kV without discharging effects by increasing the distance between the electrodes, which essentially weakened the electric field.
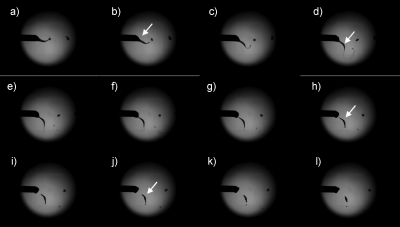
Figure 2.
Representative high-speed image sequences depicting the jetting of the Drosophila melanogaster embryo suspensions. The white arrow in panels B, D, H, and J demonstrate the event known as Coulomb fission taking place. These digital image sequences were captured at ∼100 000 frames∕s.
Figure 3.
High-speed image depicting the sparking of the BES system at elevated applied voltages (>11 kV) for a given flow rate (∼106 m3 s−1).
Figure 2 depicts a high-speed camera sequence of the jetting process in action at ∼10 kV. Panels B, D, H, and J demonstrate the embryo suspension in contact with the jetting needle, losing contact and afterward breaking into several droplets. This occurrence is well known as Coulomb fission when the surface charge of the droplet has overcome the surface tension of the liquid, promoting the explosion of the droplet into several smaller droplets. In our studies the liquid fragmented into several smaller droplets. Nevertheless, this event had no effects on the viability of treated embryos when compared to controls over both short and long time points.
To asses the viability of Drosophila embryos subjected to the BES procedure, we collected 0–3 h embryos into PBS and split into these three groups: (1) untreated (control), (2) needle control, and (3) bio-electrosprayed. Similarly, larvae at the second instar stage were aliquoted into these three groups to test for any effects on later development when the animal has a nervous system and muscles. The viability of treated embryos and controls was ascertained in five separate trials. Embryos collected before suspension in PBS are similar to those depicted in Figs. 4a, 4b. Embryos collected post-bio-electrospraying were indistinguishable from untreated wild-type embryos (Fig. 4).
Figure 4.
High-magnification optical micrographs of bio-electrosprayed Drosophila at time points (a) ∼2 h (embryos/eggs), (b) ∼50 h (second instar), (c) 180 h (pupa), and (d) fully developed Drosophila. Panels (a)–(d) depict animals that have undergone the treatment process and are indistinguishable from the controls at the corresponding time points.
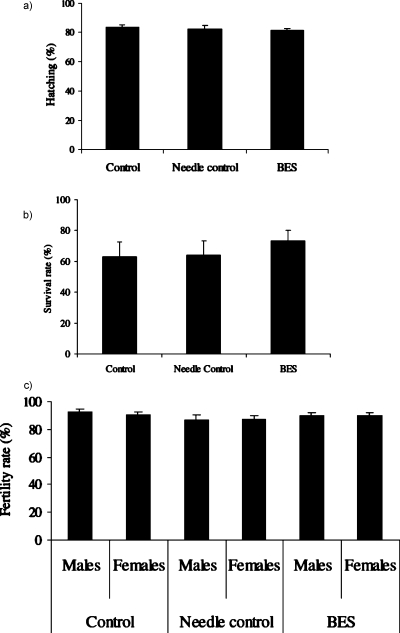
The effect of bio-electrospraying was assayed by scoring for effects on viability and fertility (Fig. 5). Bio-electrosprayed embryos (at the preblastoderm or blastoderm stage) developed and hatched into first instar larvae at the same rate as control embryos [Fig. 5a; n=500 and p=0.9051], and later developed into adult flies that were indistinguishable from wild-type [Figs. 4d, 5b, 5c]. The number of larvae that survived to adulthood was near identical between samples [Fig. 5b; n=250 with a “p” value of 0.4015]. The bio-electrosprayed animals did not demonstrate reduced fertility [Fig. 5c; “n” was 75 with a “p” values of 0.5501 and 0.5015 for males and females, respectively] in comparison to the controls, indicating that genetic integrity of the germline had been maintained. Similarly, needle control and treated samples of larvae continued to develop and were indistinguishable from untreated controls. In all of our assays we obtained a p-value of >0.05 (student t-test), indicating no significant difference between the treated and control samples.
Figure 5.
Statistics on the experiments for assessing (a) hatching of the embryos into larvae (five batches of 100 embryos were used to assess the hatching; results represent the average, i.e., n=500), (b) survival of hatched larvae to adulthood (five batches of 50 larvae were used to assess development, i.e., n=250), and finally (c) fertility (five batches of 15 adult male flies and 15 adult female flies were used to assess fertility, i.e., n=75).
CONCLUSIONS
We have demonstrated that bio-electrospraying has no adverse effects on the development, viability, or fertility of Drosophila embryos. We have also passed similar numbers of embryos through aerodynamically assisted bio-jetting and demonstrated no harmful effects on the embryos. The uniqueness of these two approaches over other direct embryo handling systems is their ability to handle highly concentrated embryo suspensions through large bore needles, thus maintaining viability. Now that we have demonstrated the safety of this procedure, we aim to exploit the underlying mechanism to both sort and distribute embryos with precision. Finally, these studies further establish BES and aerodynamically assisted bio-jetting as having potential for a wide range of applications spanning biology and medicine.
ACKNOWLEDGMENTS
S.N.J. gratefully acknowledges The Royal Society (United Kingdom) and the Engineering and Physical Sciences Research Council (United Kingdom) equipment pool for both funding the BioPhysics Group via a seed corn research grant and for loaning the high-speed imaging equipment used in these investigations, respectively. B.H.J. is funded by a MRC Career Development Award.
References
- Jayasinghe S. N., Qureshi A. N., and Eagles P. A. M., Small 2, 216 (2006). 10.1002/smll.200500291 [DOI] [PubMed] [Google Scholar]
- Jayasinghe S. N., Eagles P. A. M., and Qureshi A. N., Biotechnology Journal 1, 86 (2006). 10.1002/biot.200500025 [DOI] [PubMed] [Google Scholar]
- Odenwälder P. K., Irvine S., McEwan J. R., and Jayasinghe S. N., Biotechnology Journal 2, 622 (2007). 10.1002/biot.200700031 [DOI] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., and Whitehouse C. M., Science 246, 64 (1989). 10.1126/science.2675315 [DOI] [PubMed] [Google Scholar]
- Kuil M. E., Abrahams J. P., and Marijnissen J. C. M., Biotechnology Journal 1, 969 (2006). 10.1002/biot.200600062 [DOI] [PubMed] [Google Scholar]
- Jaworek A. and Krupa A., J. Aerosol Sci. 30, 873 (1999). 10.1016/S0021-8502(98)00787-3 [DOI] [Google Scholar]
- Cloupeau M. and Prunet-Foch B., J. Aerosol Sci. 25, 1021 (1994). 10.1016/0021-8502(94)90199-6 [DOI] [Google Scholar]
- Taylor G. I., Proc. R. Soc. London, Ser. A 280, 383 (1964). 10.1098/rspa.1964.0151 [DOI] [Google Scholar]
- Camelot D. M. A., Hartman R. P. A., Marijnissen J. C. M., Scarlett B., and Brunner D., J. Aerosol Sci. 30, 976 (1999). 10.1016/S0021-8502(98)00060-3 [DOI] [Google Scholar]
- Clarke J. D. W. and Jayasinghe S. N., Biomedical Materials 3, 011001 (2008). 10.1088/1748-6041/3/1/011001 [DOI] [PubMed] [Google Scholar]
- Geach T., Mongkoldhumrongkul N., Zimmerman L. B., and Jayasinghe S. N., Analyst (Cambridge, U.K.) 134, 743 (2009). 10.1039/b817827e [DOI] [PubMed] [Google Scholar]
- Ashburner M. D., Golic K. G., and Hawley R. S., Drosophila: A Laboratory Handbook, 2nd ed. (Cold Spring Harbor Laboratory, New York, 2004). [Google Scholar]
- Jayasinghe S. N. and Townsend-Nicholson A., Lab Chip 6, 1086 (2006). 10.1039/b606508m [DOI] [PubMed] [Google Scholar]
- Jayasinghe S. N. and Townsend-Nicholson A., Biotechnology Journal 1, 1018 (2006). 10.1002/biot.200600128 [DOI] [PubMed] [Google Scholar]