Abstract
Feasibility, amount of sample aliquots, processing time and cost are critical considerations for optimizing and conducting assays for large-population based studies. Well designed statistical approaches that quickly identify optimal conditions for a given assay could assist efficient completion of the laboratory assays for such studies.
For example, assessment of the profile of secreted cytokines is important in understanding the immune response after vaccination. To characterize the cytokine immune response following smallpox vaccination, PBMC obtained from recently vaccinated subjects were stimulated with varying doses of live or UV-inactivated vaccinia virus and cultured for up to 8 days.
In this paper, we describe a novel statistical method to identify optimal operating conditions for length in culture and virus MOI in order to measure a panel of secreted Th1, Th2, and inflammatory cytokines. This statistical method is comprised of two components. It first identifies a subset of the possible time in culture by virus MOI combinations to be studied. It then utilizes response surface analysis techniques to predict the optimal operating conditions for the measurement of each secreted cytokine. This method was applied, and the predicted optimal combinations of length in culture and virus MOI for maximum vaccinia-specific cytokine secretion were identified. The use of the response surface methodology can be applied to the optimization of other laboratory assays; especially when the number of PBMC available limits the testing of all possible combinations of parameters.
Keywords: Smallpox, Vaccinia, ELISA, Cytokine, Response surface
1. Introduction
Cytokines play an important role in the host immune response to smallpox by regulating the activation, proliferation and differentiation of immune cells via multifaceted and complex pathways (Esteves et al., 2007; Rubins et al., 2004; Smith and Kotwal, 2002). Limited literature evaluating the cellular immune response to the smallpox vaccine suggests that it is primarily driven by both CD4+ and CD8+ T cell populations, and the immune response microenvironment is modulated by the interplay of type 1 helper (Th1), type 2 helper (Th2) and inflammatory cytokines (Kennedy et al., 2004; Combadiere et al., 2004; Ennis et al., 2002; Demkowicz et al., 1996; McKinney et al., 2006; Hammarlund et al., 2003; Rock et al., 2006). Cytokines orchestrate the overall immune response by cross-regulating each other, and variations in cytokine levels can have a profound impact on the outcome of vaccination or disease. Therefore, cytokine profile assessment is important to characterize immune responses to vaccines, including the smallpox (vaccinia) vaccine.
Since cytokines are actively released and rapidly consumed at the site of a local immune reaction, most of the cytokines are present in low or undetectable levels in peripheral blood. Variations in cytokine synthesis and secretion can be best studied in vitro by stimulating cells with the antigen of interest at an optimum stimulation dose and then analyzing the resulting secretion profiles (Cavaillon et al., 1990). Since each cytokine has a unique peak response that varies with time, antigen type and dose, and the assay used for detection, it is important to optimize all of the above conditions for each cytokine individually (Rostaing et al., 1999; Reddy et al., 2004; Listvanova et al., 2003). Several studies have adopted multiplex cytokine analysis technologies as an effective tool for cytokine profiling (Lagrelius et al., 2006; Bozza et al., 2007; Kurkjian et al., 2006). However, multiplexing of cytokines generates a single time-based set of data points and ignores the fact that each cytokine has a different secretion profile (for example “early” vs. “late” cytokine secretion) making interpretation complex. In addition, multiplexing of cytokines is expensive and not readily available in smaller laboratory settings.
In the present study, we chose to use a conventional ELISA as the technique for cytokine profiling in a large study (n = 1076) of the immune response to smallpox vaccine. Here we describe a simple approach to identify and validate the optimal operating conditions for cytokine profiling by a statistical method based on determining the response surface curves of a panel of secreted Th1, Th2, and inflammatory cytokines individually.
2. Materials and methods
2.1. Study subjects
We enrolled six recent smallpox vaccinees (Dryvax®, Wyeth Laboratories, Inc., Marietta, PA) for the optimization phase of the study. All subjects were vaccinated in February and March of 2003 and blood samples were obtained three years after vaccination. Of these six, three were ultimately chosen for the response surface optimization experiment, based on their IFN-γ cytokine secretion levels.
The optimal parameters for each individual cytokine from the optimization phase were then verified in a subset (n = 256) of healthy adults, 18–40 years of age, who were recruited as part of a large (n = 1076), population-based study examining variations in immune response after smallpox vaccination. All subjects received one dose of Dryvax® smallpox vaccine between 2002 and 2006. The Institutional Review Board of Mayo Clinic granted approval for the study and peripheral blood samples were drawn after written informed consent was obtained from each subject.
2.2. Isolation of peripheral blood mononuclear cells (PBMC)
100 mL of whole blood was collected from each participant in heparinized tubes and PBMC were isolated within 24 h by density gradient centrifugation using Accuspin (Sigma, St. Louis, MO) tubes containing HISTOPAQUE®-1077 (Sigma) according to standard protocol. Isolated PBMC were resuspended at a concentration of 1×107 cells/mL in RPMI 1640 media containing L-Glutamine (Invitrogen, Carlsbad, CA) supplemented with 10% dimethyl sulfoxide (Protide Pharmaceuticals, St. Paul, MN) and 20% fetal calf serum (FCS; Hyclone, Logan, UT), frozen overnight at −80 °C in a controlled-rate freezing container, and transferred to liquid nitrogen for storage until thawed and cultured.
2.3. Growth of vaccinia virus
The New York City Board of Health (NYCBOH) vaccine strain of vaccinia virus was obtained from ATCC (Manassas, VA) and was used to make a master stock of the virus. In order to eliminate a potential source of variability, we used a single batch of vaccinia virus for all assays. HeLa S3 (ATCC) cells were maintained in Dulbecco's Modified Eagle Medium containing high glucose and l-glutamine (DMEM, Invitrogen) supplemented with 10% FCS (Hyclone), 1% non-essential amino acids (Mediatech, Inc.), 100 U/mL penicillin (Sigma), and 100 µg/mL streptomycin (Sigma). Susceptible HeLa S3 cells were infected at a multiplicity of infection (MOI) of 0.05 with NYCBOH vaccinia virus for 2 to 3 days. The virus was purified according to established protocols with slight modifications (Earl et al., 2001a,b). Briefly, infected cells were collected into a minimal volume of media and spun at 500 ×g for 10 min at 4 °C. The cellular pellet was resuspended in 10 mM Tris–Cl (Sigma), pH 9.0, and the cells were lysed by three sonication cycles in 20 s bursts. Cellular debris was removed by centrifugation at 2000 ×g for 10 min at 4 °C and the supernatant containing virus was layered onto a cushion of 36% sucrose (Sigma). Following ultracentrifugation at 33,000 ×g for 80 min at 4 °C, the supernatant was removed and the pellet containing virus was resuspended in 1 mM Tris–Cl, pH 9.0. The purified virus was sonicated as described above to fully resuspend the virus and stored at 4 °C until it was titered.
The vaccinia virus was titered at least three times using a standard plaque assay on confluent monolayers of Vero cells (ATCC) according to established protocols (Earl et al., 2001a,b; Newman et al., 2003). The titered vaccinia virus stock was diluted to 1×108 pfu/mL with 1× Hanks' balanced salt solution (HBSS, Mediatech) containing 0.1% bovine serum albumin (BSA, Sigma). Psoralen (Sigma,) was added to the diluted stock (final concentration of 5 µg/mL) and the virus was incubated for 10 min at room temperature. One milliliter of the viral mixture was added to each well of a 6 well tissue culture plate and UV-irradiated for 60 s at 365 nm in a UV crosslinker (Spectrolinker™, Spectronics Corporation, Westbury, NY). The inactivated virus stock was titered by plaque assay to ensure inactivation. The virus stock was divided into single use aliquots and stored at −80 °C until use.
2.4. Thawing and resting PBMC
After cryopreservation, PBMC were rapidly thawed as we have previously described (Ovsyannikova et al., 2005a,b). The final cell pellet was resuspended in RPMI 1640 culture media containing l-glutamine (Invitrogen) supplemented with 5% FCS (Hyclone), 100 U/mL penicillin (Sigma), 100 µg/mL streptomycin (Sigma), and 1 mM sodium pyruvate (Mediatech). PBMC were counted, adjusted to a concentration of 2×106 cells/mL with the same media, and cultured (4×106 cells/well) in 24 well plates in the presence of 50 IU/mL of IL-2 (Proleukin®, Chiron, Emeryville, CA) for 18 h at 37 °C. The media containing the PBMC was removed from the plates and any adherent cells were detached by the addition of 0.25% Trypsin-EDTA (Invitrogen) for 10 min at 37 °C. PBMC were pelleted and resuspended in the above media at a concentration of 2×106 cells/mL for use in the cytokine secretion assays.
2.5. Vaccinia-specific IFN-γ ELISA assay to choose optimization subjects
After resting, 2×105 PBMC (100 µL/well) from each of the six optimization subjects were added to a round bottom tissue culture plate. PBMC were stimulated in triplicate with a MOI of 0.1 of either live or UV-inactivated vaccinia virus diluted in RPMI 1640 culture media containing 5% FCS (Hyclone), 100 U/mL penicillin (Sigma), 100 µg/mL streptomycin (Sigma), and 1 mM sodium pyruvate (Mediatech) or RPMI 1640 culture media containing 5% FCS (Hyclone), 100 U/mL penicillin (Sigma), 100 µg/mL streptomycin (Sigma), and 1 mM sodium pyruvate (Mediatech) (negative control). One well of PBMC from each subject was stimulated with 5 µg/mL PHA (Sigma) as a positive control. Cell-free supernatants were removed after 3 days in culture at 37 °C. Secretion of vaccinia-specific IFN-γ was determined by ELISA (BD Pharmingen, San Diego, CA) following the manufacturer's protocol. Plates were read at 450 nm using a microplate reader (Molecular Devices, Sunnyvale, CA).
We assessed the IFN-γ secretion levels in triplicate from the six optimization subjects, and defined secretion as the difference between the median of the stimulated cells and the median of the unstimulated cells. We reviewed the IFN-γ secretion levels from the six optimization subjects and chose the three that best represented the entire spectrum of values (one subject with high levels, one with moderate levels, and one with low levels) to optimize the MOI and time in culture parameters for the cytokines of interest.
2.6. Design of optimization studies
We designed an optimization experiment in order to identify operating conditions for three primary factors that can be controlled in the lab and that were expected to have major effects on the measured ELISA values. These factors were: use of live versus inactivated vaccinia virus, virus MOI concentrations of 0.05, 0.1, 0.5, 1.0 or 5.0, and length of time in culture of 6, 12, 24, 48, 72, or 96 h. Cytokines not attaining optimum secretion values at these time points were subsequently re-evaluated at 5, 6, 7, and 8 days. Due to limitations in the number of isolated PBMC available for culture, evaluation of all three subjects at all possible combinations of the MOI and times was not feasible.
Determination of the optimum time and MOI combinations was carried out using response surface methodology (Greenwood, 2005). This approach assumes that the predicted value of the response variable y for all possible combinations of the values of two factor variables x1 and x2 can be modeled through the quadratic response function
where the βs represent the regression coefficients of the linear effect terms for x1 and x2, their corresponding quadratic effects, and , and their cross-product, x1x2 and ε represent the residual error not captured by the regression model. The ideal response surface resembles a three-dimensional convex “hill” with a single peak occurring at the estimated point of maximum response. Given the design matrix X, with columns containing the x variables and their cross-product and quadratic terms, and with rows containing the observed operating settings, the variance of the predicted value of y is given by the values along the diagonal of the matrix σ2X(XTX)−1XT. In this expression, σ2 is the variance of the observed measurements from a specific secreted cytokine, and ‘T’ and ‘−1’ represent the matrix transpose and inverse operations, respectively. A resulting property of this linear models result is that the prediction variance is a function of the variability in the assay, combined with the settings chosen under which the laboratory assays will be conducted.
A strength of response surface methodology is its ability to predict secretion levels, and the variance of these predictions, at unmeasured values of the x variables. Coupling this interpolation property with the fact that the prediction variance is a function of the selected combinations of experimental conditions that will be examined, we were able to design an optimization experiment to identify the ultimate operating conditions to be used in our studies. This optimization study used only a subset of the candidate virus MOI and time in culture settings that were of interest (see Table 1). This subset was required because of the limited availability of isolated PBMC available for the optimization study.
Table 1.
Matrix used for assay optimization to determine the optimal time in culture a and MOI of live and inactive vaccinia virus
| Time→ MOI ↓ | 6 h | 12 h | 24 h | 2 days | 3 days | 4 days | 5 days | 6 days | 7 days | 8 days |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 | x | x | x | x | x | |||||
| 0.1 | x | x | x | |||||||
| 0.5 | x | x | x | x | ||||||
| 1.0 | x | x | x | |||||||
| 5.0 | x | x | x | x | x |
Marked cells indicate the combinations of MOI and time in culture examined for each of the selected subjects. Experiments were carried out separately for both live and inactive virus at each of these combinations.
All cytokines were evaluated at time points 6 h through 4 days as marked. IL-4, IL-6 and IL-10 were further evaluated at time points 5 days through 8 days as marked because optimum values were not attained after 4 days.
In order to identify the subset of experimental conditions, we collected all possible combinations of virus MOI and time in culture that were of primary interest and derived the X matrix for the fully factorial design. Because of the unequal spacing of the desired settings to be studied, we used log-transformed values of the culture time and virus MOI levels, and computed the quadratic and cross-product terms using these log-transformed measures. The sub-selection of the specific collection of factor combinations to be used then proceeded in a sequential fashion. That is, each of the candidate factor combinations was then removed, one at a time, to define alternative experimental designs. Each of these experimental designs was then coded into its own design matrix, X−i, where i represented the combination of the factors that was excluded. The variance of the predicted level of the quadratic response surface was estimated for each of the target factor combinations and the maximum value of these predicted variances was extracted. The specific factor combination whose maximum predicted variance was the closest to that predicted in the design that included all factor combinations was then removed from the design. In the case of a tie, the factor combination to be removed was selected at random from the tied set. This process was repeated until only 6 experimental combinations among the original 30 remained. The final design that was selected was chosen such that the standard error of the predicted response surface was no larger than one-half of the standard deviation of the secreted cytokine measure when data from three individuals were combined in the analyses. The time and MOI combinations chosen for the experiment are provided in Table 1. For each subject, vaccinia-specific secretion of each cytokine was measured after stimulation with live and inactive virus at each of these combinations.
2.7. Culturing of PBMC for optimization of cytokine ELISAs
PBMC for the three optimization experiment subjects were thawed and rested in the presence of IL-2 as described above. After resting, 2×105 PBMC (100 µL/well) from each of the three subjects were added to round bottom tissue culture plates and stimulated in triplicate with the appropriate MOI of live and UV-inactivated vaccinia virus according to the matrix in Table 1. The virus was diluted as described above. At each time point, 2×105 PBMC were also cultured in triplicate with RPMI 1640 culture media containing 5% FCS, 100 U/mL penicillin (Sigma), 100 µg/mL streptomycin (Sigma), and 1 mM sodium pyruvate (Mediatech) (negative control). One well of PBMC (2×105) from each subject was also stimulated with 5 µg/mL PHA as a positive control. For the detection of secreted IL-4, PBMC were cultured as above with the addition of 2 µg/mL of monoclonal anti-human IL-4R antibody (R&D Systems, Minneapolis, MN) as we have previously described (Dhiman et al., 2004).
Cell-free supernatants were removed at each specified time point between 6 h and 4 days and vaccinia virus-specific IL-1β,IL-2,IL-12p40,IL-12 p70, TNF-α, and IFN-γ (all from BD Pharmingen), IFN-α(PBL Biomedical Laboratories, Piscataway, NJ), IFN-β (PBL Biomedical Laboratories) and IL-18 (MBL International, Woburn, MA) responses were quantitatively determined by ELISA following the manufacturer's protocol. The matrix was extended to 8 days for the determination of vaccinia-specific IL-4, IL-6, and IL-10 (all from BD Pharmingen) because optimum secretion of these cytokines was not attained after 4 days. The supernatant from one plate was used to detect secretion of three different cytokines.
The optimized culture conditions were validated in 256 recently vaccinated subjects. PBMC were thawed, rested and cultured as described above. PBMC (2×105/subject) were cultured in the presence of the optimal amount of inactive vaccinia virus (Table 2) and cell-free supernatants were removed at the optimal time point between 24 h and 8 days (Table 2) and frozen at −80 °C until the time of the ELISA assay. Cell culture supernatants were appropriately diluted and ELISA assays were performed according to the manufacturer's protocol. The optical density of each plate was measured at 450 nm on a microplate reader (Molecular Devices Corporation, Sunnyvale, CA) and the concentration of each cytokine was determined from reference standards used in each assay.
Table 2.
Optimal cell culture conditions for infection of PBMC for detection of vaccinia-specific cytokine secretion
| Cytokine | Inactive vaccinia MOI | Length in culture |
|---|---|---|
| IFN-β, IL-2, IL-18 | 5 | 24 h |
| IL-12 p40, IL-12 p70 | 0.5 | 24 h |
| TNF-α, IL-1β | 0.5 | 24 h |
| IFN-α, IFN-γ | 0.05 | 4 days |
| IL-4, IL-10 | 0.05 | 7 days |
| IL-6 | 5 | 8 days |
2.8. Statistical analysis
2.8.1. Response surface analyses
Upon selection of the factor combinations to be directly assessed, we obtained triplicate assessments of cytokine secretion with and without stimulating with vaccinia virus. From these assessments, we calculated standardized cytokine secretion values by computing the difference between the mean of the stimulated cells and the mean of the unstimulated cells, divided by a pooled standard error estimate. This is in contrast to the previous definition used for selecting the optimization subjects, and was computed to reflect the magnitude of the stimulation effect relative to the variability in the ELISA assay. This standardized mean difference in secretion levels resembles a t-statistic and thus provides a convenient interpretation in the context of peak response; cytokines with maximum response values of approximately 2 or larger can be considered to have demonstrated a statistically significant response to vaccinia virus.
For each cytokine and each virus type (live and UV-inactivated), we fit a quadratic response surface to the observed data, and from the resulting estimates determined the time in culture and MOI that resulted in the highest point on the response surface. In addition to estimating the value and location of the maximum value on the response surface, we calculated the standard error of the response at this point. All combinations of time and MOI that yielded an estimated response falling within one standard error of the peak value were considered statistically equivalent to the peak. Specific values of the factor combinations were then selected for each of the cytokines being studied.
2.8.2. Validation analyses
ELISA assays were performed on supernatants from 256 additional subjects using the optimized conditions determined from the response surface analyses in order to validate these time and MOI choices. For the validation phase of the study, we analyzed the data as previously described for the optimization experiment by determining the standardized difference in the means divided by their common standard error. In addition, cytokine secretion was also defined as the difference between the median of the stimulated values and the median of the unstimulated values and is reported as pg/mL. Secretion values were summarized across all such subjects using medians and inter-quartile ranges (IQRs).
3. Results
3.1. Selection of subjects to use for ELISA optimization
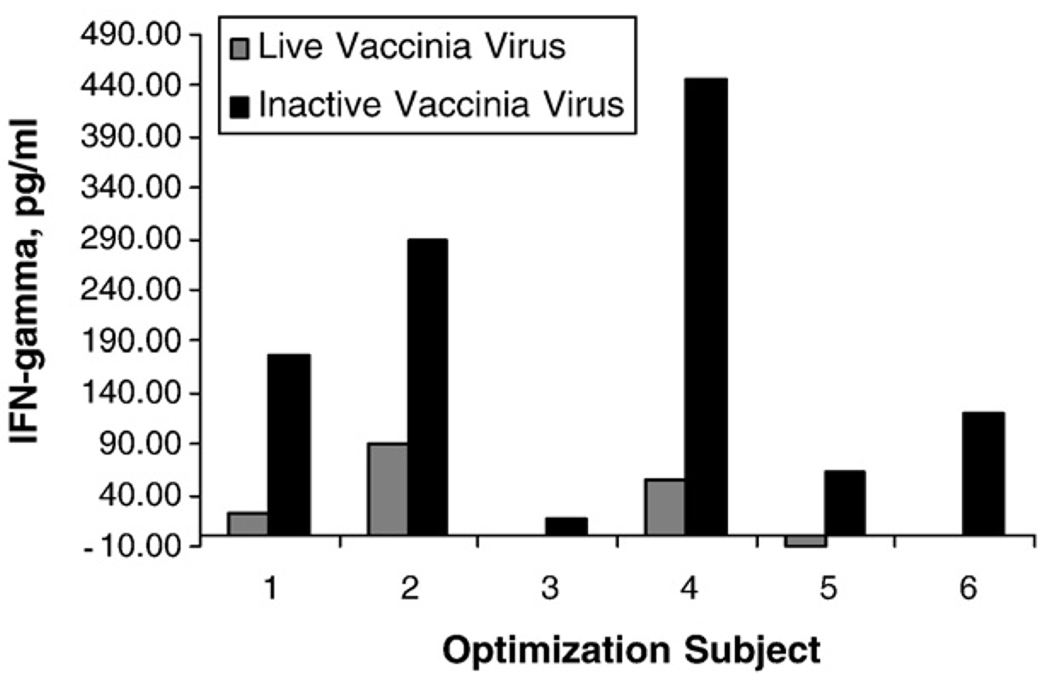
Six recently vaccinated subjects were recruited in the optimization study in order to identify three subjects with a range in vaccinia-specific IFN-γ secretion (high, medium, and low) to use to optimize the culture conditions for the remaining cytokines of interest. Fig. 1 shows the range in IFN-γ secretion for the six vaccinated subjects after stimulation of PBMC with both live and inactivated vaccinia virus. Stimulation of PBMC with inactivated vaccinia virus resulted in higher IFN-γ secretion by PBMC from recently vaccinated subjects. Subjects 2, 4, and 5 were chosen for the optimization of the optimal time in culture and vaccinia virus MOI for the remaining cytokines.
Fig. 1.
Vaccinia-specific IFN-γ secretion from PBMC of six optimization subjects. After resting overnight in the presence of 50 IU/mL IL-2, 2×105 PBMC were stimulated in triplicate with either live or UV-inactivated vaccinia virus at an MOI of 0.1. Cell-free supernatants were removed after 3 days in culture at 37 °C and secretion of IFN-γ was determined by ELISA. Bars represent the difference between the median of the stimulated and the median of the unstimulated wells.
3.2. Predicted cytokine secretion
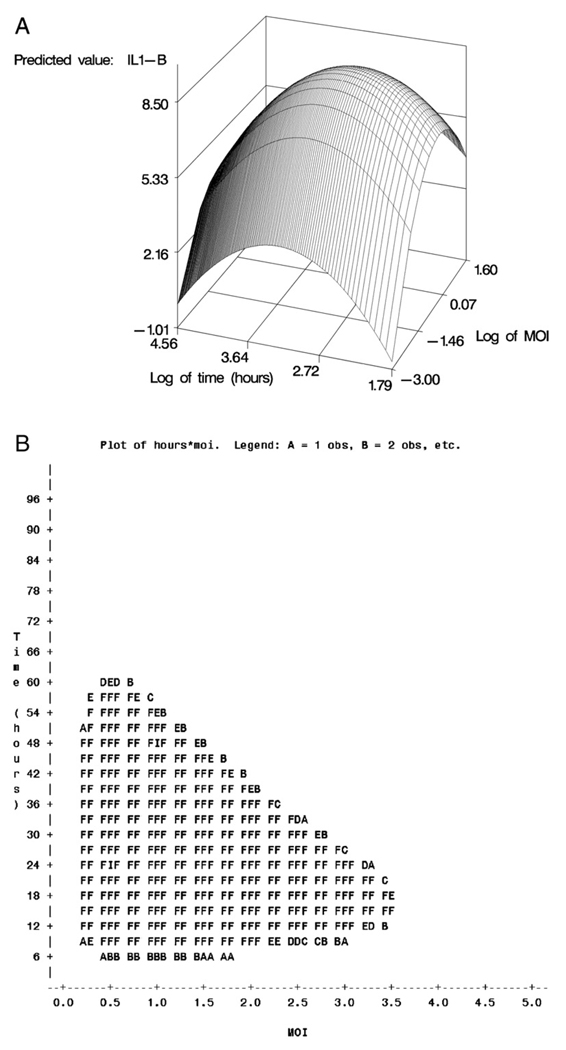
Stimulation of PBMC with both the live and inactivated vaccinia virus induced secretion of IL-2,IL-6, IL-10,TNF-α, IL-1β, IFN-α, and IFN-γ. In general, PBMC stimulated with inactivated vaccinia virus had a higher predicted value of secretion compared to stimulation with the live virus. For each cytokine, the length of culture and viral MOI that maximized the vaccinia-specific response was determined using response surface methods. An example response surface curve for secretion of vaccinia-specific IL-1β is shown in Fig. 2A and B. Fig. 2A depicts the three-dimensional response surface of IL-1β secretion after stimulation with inactive vaccinia virus. Any combination of time and MOI within the filled area of Panel B is statistically equivalent to the estimated maximum cytokine secretion. The combination of length in culture and MOI of both live and inactive vaccinia virus that maximized the predicted value is shown for each cytokine in Table 3. Also shown is the approximate range in time and MOI of the virus for each cytokine that maximized the response. After stimulation with inactivated vaccinia virus, maximum secretion of IL-2, TNF-α, and IL-1β occurred after less than 25 h in culture. The maximum predicted secretion of IL-6 and IL-10 occurred after culture of PBMC in the presence of inactivated vaccinia virus for 192 h. Vaccinia-specific secretion of both IFN-α and IFN-γ from PBMC was highest after 96 h in culture with an MOI of inactivated virus of 0.05. As shown in Table 2, the predicted value of IL-4,IL-12p40,IL-12p70,IL-18 and IFN-β secretion was low. The vaccinia-specific secretion of these cytokines was below the detectable limit of the ELISA assay (data not shown).
Fig. 2.
A). Response surface for vaccinia-specific IL-1β response of PBMC stimulated with inactive vaccinia virus. Panel A depicts the three-dimensional response surface, plotting time and MOI on the log scale, as originally included in the regression analysis. The maximum response value for this surface occurred at a time of 19 h and an MOI of 0.7. B). Response surface for vaccinia-specific IL-1β response of PBMC stimulated with inactive vaccinia virus. The shaded region of Panel B displays all combinations of time and MOI with resulting responses falling within one standard error of the maximum. All such values can be considered statistically equivalent to the maximum. For ease of interpretation, time and MOI values for Panel B are reported in their original (that is, back-transformed) sampling units.
Table 3.
Response surface results: time (in hours) and MOI that maximizes the predicted value of cytokine secretion for both live and inactive vaccinia virus
| Cytokine | Virus | Predicted valuea | Standard error | Optimal time | Optimal MOI | Range of time b | Range of MOI b |
|---|---|---|---|---|---|---|---|
| IL-2 | Live | 5.67 | 1.21 | 11 | 5 | 8–24 | 4–5 |
| IL-2 | Inactive | 5.96 | 1.64 | 16 | 5 | 12–36 | 3.5–5 |
| IL-4 | Live | 1.42 | 0.99 | 192 | 5 | 145–192 | 0.05–5 |
| IL-4 | Inactive | 2.01 | 1.30 | 168 | 0.05 | 96–192 | 0.05 |
| IL-6 | Live | nd c | nd c | nd c | nd c | nd c | nd c |
| IL-6 | Inactive | 22.98 | 3.65 | 192 | 5 | 192 | 3–5 |
| IL-10 | Live | 3.10 | 1.61 | 192 | 0.05 | 156–192 | 0.05 |
| IL-10 | Inactive | 3.88 | 1.69 | 192 | 0.05 | 175–192 | 0.05 |
| IL-12p40 | Live | −0.41 | 0.69 | 96 | 0.05 | 96 | 0.05 |
| IL-12p40 | Inactive | 0.26 | 0.67 | 24 | 0.05 | 8–32 | 0.05 |
| IL-12p70 | Live | 0.99 | 0.51 | 23.5 | 0.2 | 24 | 0.2–0.5 |
| IL-12p70 | Inactive | 1.88 | 0.78 | 22 | 0.7 | 16–36 | 0.2–3 |
| IL-18 | Live | 1.69 | 0.63 | 96 | 0.05 | 96 | 0.05 |
| IL-18 | Inactive | 1.74 | 0.61 | 24 | 0.05 | 24 | 0.05 or 5.0 |
| TNF-α | Live | 5.32 | 1.56 | 17.5 | 0.5 | 8–42 | 0.2–1.0 |
| TNF-α | Inactive | 7.21 | 2.41 | 25 | 0.4 | 12–65 | 0.2–1.0 |
| IL-1β | Live | 4.59 | 1.26 | 96 | 1.3 | 10–96 | 0.5–5 |
| IL-1β | Inactive | 8.50 | 2.35 | 19 | 0.7 | 8–45 | 0.3–1.8 |
| IFN-α | Live | 11.76 | 2.27 | 96 | 0.2 | 80–96 | 0.05–0.8 |
| IFN-α | Inactive | 24.71 | 3.48 | 96 | 0.05 | 96 | 0.05 |
| IFN-β | Live | 1.75 | 0.54 | 14.5 | 5 | 12–24 | 1.5–5 |
| IFN-β | Inactive | 2.30 | 0.49 | 30.5 | 5 | 24–36 | 3–5 |
| IFN-γ | Live | 4.51 | 1.60 | 96 | 0.05 | 60–96 | 0.05 |
| IFN-γ | Inactive | 4.11 | 1.49 | 96 | 0.05 | 96 | 0.05 |
Maximum predicted value of cytokine secretion, defined as the difference between the mean of the stimulated cells and the mean of the unstimulated cells, divided by their common standard error.
Approximate range of time and MOI values yielding secretion levels considered statistically equivalent to the maximum predicted value.
Not determined.
3.3. Optimal cell culture conditions for detection of vaccinia-specific cytokine secretion
Since the number of isolated PBMC available from each subject in the larger study population was limited, we wanted to minimize the number of cells needed to perform the assay. The cell-free supernatant removed from each culture plate was used to monitor the secretion of multiple cytokines. Up to three different cytokine ELISA assays can be performed using the supernatant removed from each culture plate. By looking at the range in time and MOI predicted to maximize secretion (Table 3), we were able to assay multiple cytokines from the supernatant removed from each culture plate. This reduced the number of PBMC needed by half. Since stimulation of PBMC with inactivated virus produced the highest predicted value of secretion, the optimal culture conditions were chosen based on the predicted secretion after stimulation with inactivated vaccinia virus. The supernatant removed from each plate was used to detect multiple cytokines each having the same culture conditions as outlined in Table 2. Cell-free supernatants were removed after 24 h, 4, 7, and 8 days in culture with inactivated vaccinia for the detection of the panel of cytokines.
3.4. Verification of the optimal cell culture conditions for stimulation of PBMC
The assay optimization described above was performed on three subjects, so it was important to verify the predicted optimal culture conditions on a larger number of subjects. ELISA assays were performed on supernatants from 256 smallpox vaccinated subjects to verify the predicted optimal culture conditions outlined in Table 2 according to the standard operating procedure developed. Using the optimized conditions determined from the response surface methodology approach we detected vaccinia-specific secretion of eight out of the twelve cytokines of interest (Table 4). We detected release from PBMC following culture with inactivated vaccinia virus of IFN-α, IFN-γ, TNF-α, IL-1β, and IL-6. In the optimization experiment we could not measure secretion of IL-12p40 above the detection limit of the kit. However, in the larger subset of subjects we were able to detect vaccinia-specific release of this cytokine. We also detected lower, but detectable, secretion of vaccinia-specific IL-2 and IL-10 from vaccinia-stimulated PBMC. There was no detectable release of vaccinia-specific IL-4, IL-12p70, IL-18, and IFN-β above the detectable limits of the assay from PBMC of smallpox vaccinated subjects, although positive controls elicited secretion indicating that the assay worked.
Table 4.
Validation of optimal cell culture conditions: Secretion of vaccinia-specific cytokines by PBMC infected with UV-inactivated vaccinia virus
| Cytokine | Standardized mean difference a |
Difference in median secretion level b |
|
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| IL-1β | 36.9 (17.2, 79.7) | 45.1 (23.2, 99.7) | |
| IL-2 | 3.8 (0.6, 12.9) | 22.8 (5, 44.7) | |
| IL-4 | 0.3 (−1.3, 3.0) | 1 (−3.8, 5.4) | |
| IL-6 | 40.5 (16.5, 87.7) | 1011.8 (517.2, 2026.2) | |
| IL-10 | 2.6 (−0.2, 9.0) | 6.1 (−0.3, 22.8) | |
| IL-12p40 | 9.0 (3.7, 21.6) | 68.9 (33.6, 133.8) | |
| IL-12p70 | 1.9 (−0.8, 5.2) | 1.9 (−1.6, 5.7) | |
| IL-18 | 0.7 (−0.7, 3.0) | 0.8 (−1.6, 3.8) | |
| IFN-α | 51.0 (16.7, 120.7) | 109.3 (45.4, 192) | |
| IFN-β | 0.7 (−1.1, 3.6) | 1.6 (−3.2, 6.2) | |
| IFN-γ | 5.9 (0.6, 16.2) | 208.8 (26.1, 898.4) | |
| TNF-α | 39.5 (19.3, 80.1) | 175.1 (101.9, 299.6) | |
Cytokine secretion defined as the difference between the mean of the vaccinia-stimulated cells and the mean of the unstimulated cells, divided by their common standard error. Cytokine secretion is reported as standard error units away from zero.
Cytokine secretion defined as the difference in the median of the vaccinia-stimulated cells and the median of the unstimulated cells. Cytokine secretion is reported as pg/mL.
4. Discussion
Assays for the detection of humoral and cellular immune responses using plaque neutralization, antibody-based ELISA, IFN-γ ELISPOT, and intracellular cytokine staining have been used to monitor the immune response to the smallpox vaccine (Hammarlund et al., 2003; Viner and Isaacs, 2005; Combadiere et al., 2004; Kennedy et al., 2004; Ennis et al., 2002; Treanor et al., 2006; Rock et al., 2006). To our knowledge, there have been limited reports of ELISA assays used to determine human vaccinia-specific cytokine responses after smallpox vaccination for the panel of cytokines of interest in our study. For cost and feasibility reasons, it was necessary to optimize the conditions for culturing the PBMC to detect secretion of the cytokines of interest by ELISA.
The response surface approach described can also be easily applied to the optimization of other laboratory assays, including the ELISPOT assay, and can be used in the optimization of other virus-specific cytokine responses. The advantage of this methodology is that it uses fewer cells than testing each time point/MOI combination of potential interest. PBMC used for the assay can be obtained from each subject at one point in time eliminating any variability in cytokine secretion associated with multiple blood draws, including day-to-day and diurnal variation, which would influence the baseline secretion of each cytokine, as well as any variation associated with the handling of the whole blood and isolating and freezing the PBMC (Ray et al., 2006). If every time point/MOI combination was tested in triplicate with both live and inactive virus, the ELISA optimization phase of the study alone would require 173.6×106 rested PBMCs. A conservative estimate for recovery of cells after resting overnight in the presence of IL-2 is 50%. Therefore, in order to test all possible combinations for each of the twelve cytokines, including having a single well of PHA as a positive control, 347.2×106 cells from each subject would be required increasing the cost and labor of the study and impacting the feasibility of conducting such a study. The number of cells needed to measure all cytokines when utilizing the response surface approach was 37.8×106 cells after resting (75.6×106 before resting).
In order to minimize the volume of blood drawn from each subject and to reduce the number of isolated PBMC needed from each subject for the ELISA assays, we utilized the supernatant removed from each culture plate for more than one cytokine ELISA assay. Up to three different cytokine ELISA assays can be performed with the supernatant removed from one culture plate. This is especially important as the maximum number of isolated cells obtained from each subject from a single blood draw often limits the number of in vitro assays that can be performed.
Another common method for analyzing multiple cytokines from cell-culture supernatants is the multiplex bead array assay. An advantage of the multiplexing assay over ELISA is that it is a high-throughput method requiring a small volume of sample for the simultaneous detection of multiple cytokines (Young et al., 2008). However, multiplexing of cytokines can be expensive, especially when the cost of purchasing the specialized instrumentation and software needed to analyze the sample is considered (Osuchowski et al., 2005). Further, since each cytokine has a unique pattern of secretion, multiplexing of cytokines does not allow for the detection of each cytokine at the optimal time point for maximum secretion of that cytokine. The ELISA assay allows for the quantification of antigen-specific release of each cytokine since each cytokine is detected at the optimal time of secretion.
Using the response surface methodology, we identified the predicted combination of length in culture and virus MOI for maximum vaccinia-specific secretion. The predicted optimal parameters were then further assessed in a subset of our study population to verify that the predicted time and MOI of vaccinia virus were correct. We detected release of vaccinia-specific IFN-α, IFN-γ, TNF-α, IL-1β, IL-2, IL-6,IL-10, and IL-12p40 above the detectable limit of the ELISA assay. As part of the optimization, we compared vaccinia-specific secretion of the cytokines following stimulation with either the replicating (live) NYCBOH strain or the virus inactivated with psoralen and long wavelength UV light. The vaccinia-specific secretion of the cytokines in the panel was higher when using the inactivated vaccinia virus. In this context, inactivated vaccinia virus retains the ability to infect cells but cannot replicate in the host cell (Tsung et al., 1996). Since there is limited information in the literature regarding the vaccinia-specific secretion of these cytokines after smallpox vaccination, we chose the twelve cytokines in the panel based on their broad role in poxvirus infections.
There are several limitations to this study. First, the response surface methodology relies on the assumption that the secretion of each cytokine of interest has a single maximum. A decrease in secretion is expected as the combination of MOI and length in culture move away from the optimal culture conditions that result in maximum secretion. As long as this assumption is accurate, the response surface approach can be used to accurately predict the optimal conditions. Another limitation of this methodology is that if the maximum cytokine secretion value is close to zero, then one may just be modeling biological noise. The response surface methodology can be used to predict the culture conditions that result in maximum secretion; however, the maximum predicted secretion might be below the detectable limit of the ELISA assay. Due to the large number of cells needed to perform other assays on these subjects, we were limited in the number of isolated PBMC that could be used for the ELISA assays. Perhaps doubling the number of PBMC used in culture to 4×105/well, specifically for IL-4 as previously demonstrated (Dhiman et al., 2004), would increase secretion of the cytokines that we were unable to detect.
In conclusion, the optimized culture conditions described will be used to determine the cytokine secretion profile after smallpox vaccination in the entire population of subjects (n = 1076) enrolled in the study. The response surface approach can also be easily applied to the optimization of other laboratory assays, including the ELISPOT assay, and can also be used in the optimization of other viral-specific cytokine responses. The value of this technique is in allowing a limited number of subject samples to be tested across multiple conditions/variables; while minimizing use of sample, time required, and overall cost. Further, an examination of each response graph allows for the logical grouping of cytokines that can be measured at the same time and same conditions. Such individualization improves scientific accuracy of the results obtained.
Acknowledgements
We thank the subjects who participated in this study as well as the technicians and fellows in the Mayo Vaccine Research Group. We thank Richard B. Kennedy, PhD for growing the vaccinia virus used in this study. We thank Cheryl A. Hart for editorial assistance and David A. Watson for statistical analysis. This work was supported by NIH grants AI40065, AI48793 and AI33144.
Abbreviations
- PBMC
peripheral blood mononuclear cells
- ELISA
enzyme-linked immunosorbent assay
- IL
interleukin
- IFN
interferon
- TNF
tumor necrosis factor
- NYCBOH
New York City Board of Health
- HBSS
Hanks' balanced salt solution
- BSA
bovine serum albumin
- MOI
multiplicity of infection
- PHA
phytohemagglutinin
- IQR
inter-quartile range
- PPVO
parapox virus ovis
References
- Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit. Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon JM, Fitting C, Haeffner-Cavaillon N, Kirsch SJ, Warren HS. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect. Immun. 1990;58:2375. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, Bricaire F, Debre P, Autran B. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J. Exp. Med. 2004;199:1585. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demkowicz WE, Jr, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 1996;70:2627. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman N, Ovsyannikova IG, Howe RC, Ryan JE, Jacobson RM, Poland GA. Interleukin-4 induced by measles virus and measles-derived peptides as measured by IL-4 receptor-blocking ELISA. J. Immunol. Methods. 2004;287:217. doi: 10.1016/j.jim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Earl PL, Cooper N, Wyatt LS, Moss B, Carroll MW. Preparation of cell cultures and vaccinia virus stocks. Curr. Protoc. Mol. Biol. 2001a;16 doi: 10.1002/0471142727.mb1616s43. Unit16. [DOI] [PubMed] [Google Scholar]
- Earl PL, Moss B, Wyatt LS, Carroll MW. Generation of recombinant vaccinia viruses. Curr. Protoc. Mol. Biol. 2001b;16 doi: 10.1002/0471142727.mb1617s43. Unit16. [DOI] [PubMed] [Google Scholar]
- Ennis FA, Cruz J, Demkowicz WE, Jr, Rothman AL, McClain DJ. Primary induction of human CD8+ cytotoxic T lymphocytes and interferon-g-producing T cells after smallpox vaccination. J. Infect. Dis. 2002;187:1657. doi: 10.1086/340517. [DOI] [PubMed] [Google Scholar]
- Esteves GH, Simoes AC, Souza E, Dias RA, Ospina R, Venancio TM. New insights about host response to smallpox using microarray data. BMC Syst. Biol. 2007;1:38. doi: 10.1186/1752-0509-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. Interpreting vaccine efficacy. Clin. Infect. Dis. 2005;40:1519. doi: 10.1086/429833. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9:1131. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Kennedy JS, Frey SE, Yan L, Rothman AL, Cruz J, Newman FK, Orphin L, Belshe RB, Ennis FA. Induction of human T cell-mediated immune responses after primary and secondary smallpox vaccination. J. Infect. Dis. 2004;190:1286. doi: 10.1086/423848. [DOI] [PubMed] [Google Scholar]
- Kurkjian KM, Mahmutovic AJ, Kellar KL, Haque R, Bern C, Secor WE. Multiplex analysis of circulating cytokines in the sera of patients with different clinical forms of visceral leishmaniasis. Cytometry Part A. 2006;69:353. doi: 10.1002/cyto.a.20256. [DOI] [PubMed] [Google Scholar]
- Lagrelius M, Jones P, Franck K, Gaines H. Cytokine detection by multiplex technology useful for assessing antigen specific cytokine profiles and kinetics in whole blood cultured up to seven days. Cytokine. 2006;33:156. doi: 10.1016/j.cyto.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Listvanova S, Temmerman S, Stordeur P, Verscheure V, Place S, Zhou L, Locht C, Mascart F. Optimal kinetics for quantification of antigen-induced cytokines in human peripheral blood mononuclear cells by realtime PCR and by ELISA. J. Immunol. Methods. 2003;281:27. doi: 10.1016/s0022-1759(03)00267-9. [DOI] [PubMed] [Google Scholar]
- McKinney BA, Reif DM, Rock MT, Edwards KM, Kingsmore SF, Moore JH, Crowe JE., Jr Cytokine expression patterns associated with systemic adverse events following smallpox immunization. J. Infect. Dis. 2006;194:444. doi: 10.1086/505503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman FK, Frey SE, Blevins TP, Mandava M, Bonifacio A, Jr, Yan L, Belshe RB. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J. Clin. Microbiol. 2003;41:3154. doi: 10.1128/JCM.41.7.3154-3157.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuchowski MF, Siddiqui J, Copeland S, Remick DG. Sequential ELISA to profile multiple cytokines from small volumes. J. Immunol. Methods. 2005;302:172. doi: 10.1016/j.jim.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Ryan JE, Vierkant RA, Pankratz VS, Jacobsen SJ, Poland GA. HLA class II alleles and measles virus-specific cytokine immune response following two doses of measles vaccine. Immunogenetics. 2005a;56:798. doi: 10.1007/s00251-004-0756-0. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Ryan JE, Vierkant RA, Pankratz SV, Jacobson RM, Poland GA. Immunologic significance of HLA class I genes in measles virus-specific IFN-gamma and IL-4 cytokine immune responses. Immunogenetics. 2005b;57:828. doi: 10.1007/s00251-005-0061-6. [DOI] [PubMed] [Google Scholar]
- Ray CA, Dumaual C, Willey M, Fill J, O'Brien PJ, Gourley I, Devanarayan V, Konrad RJ. Optimization of analytical and pre-analytical variables associated with an ex vivo cytokine secretion assay. J. Pharm. Biomed. Anal. 2006;41:189. doi: 10.1016/j.jpba.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J. Immunol. Methods. 2004;293:127. doi: 10.1016/j.jim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Rock MT, Yoder SM, Talbot TR, Edwards KM, Crowe JE., Jr Cellular immune responses to diluted and undiluted aventis pasteur smallpox vaccine. J. Infect. Dis. 2006;194:435. doi: 10.1086/505506. [DOI] [PubMed] [Google Scholar]
- Rostaing L, Tkaczuk J, Durand M, Peres C, Durand D, de Preval C, Ohayon E, Abbal M. Kinetics of intracytoplasmic Th1 and Th2 cytokine production assessed by flow cytometry following in vitro activation of peripheral blood mononuclear cells. Cytometry. 1999;35:318. doi: 10.1002/(sici)1097-0320(19990401)35:4<318::aid-cyto4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rubins KH, Hensley LE, Jahrling PB, Whitney AR, Geisbert TW, Huggins JW, Owen A, LeDuc JW, Brown PO, Relman DA. The host response to smallpox: analysis of the gene expression program in peripheral blood cells in a nonhuman primate model. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15190. doi: 10.1073/pnas.0405759101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Kotwal GJ. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 2002;28:149. doi: 10.1080/1040-840291046722. [DOI] [PubMed] [Google Scholar]
- Treanor J, Wu H, Liang H, Topham DJ. Immune responses to vaccinia and influenza elicited during primary versus recent or distant secondary smallpox vaccination of adults. Vaccine. 2006;24:6913. doi: 10.1016/j.vaccine.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Tsung K, Yim JH, Marti W, Buller RM, Norton JA. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J. Virol. 1996;70:165. doi: 10.1128/jvi.70.1.165-171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner KM, Isaacs SN. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 2005;7:579. doi: 10.1016/j.micinf.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Young SH, Antonini JM, Roberts JR, Erdely AD, Zeidler-Erdely PC. Performance evaluation of cytometric bead assays for the measurement of lung cytokines in two rodent models. J. Immunol. Methods. 2008;331:59. doi: 10.1016/j.jim.2007.11.004. [DOI] [PubMed] [Google Scholar]