Azides, which are extremely rare in biological systems, are emerging as attractive chemical handles for bioconjugation.[1–5] In particular, the CuI catalyzed 1,3-dipolar cyclization of azides with terminal alkynes to give stable triazoles[6, 7] has been employed for the tagging of a variety of biomolecules,[8–12] activity-based protein profiling,[13] and the chemical synthesis of microarrays and small molecule libraries.[14]
An attractive approach for installing azides into biomolecules is based on metabolic labeling whereby an azide-containing biosynthetic precursor is incorporated into biomolecules using the cells’ biosynthetic machinery.[15] This approach has been employed for tagging proteins, glycans, and lipids of living systems with a variety of reactive probes. These probes can facilitate the mapping of saccharide-selective glycoproteins and identify glycosylation sites.[16] Alkyne probes have also been used for cell surface imaging of azide-modified bio-molecules and a particularly attractive approach involves the generation of a fluorescent probe from a non-fluorescent precursor by a [3+2] cycloaddition.[17]
The cellular toxicity of the CuI catalyst has precluded applications wherein cells must remain viable,[18] and hence there is a great need for the development of CuI free [3+2] cycloadditions.[19–21] In this respect, alkynes can be activated by ring strain and for example constraining an alkyne within an eight membered ring creates 18 kcal mol−1 of strain, much of which is released in the transition state upon [3+2] cyclcoaddition with an azide.[19, 20] As a result, cyclooctynes such as 1 react with azides at room temperature without the need of a catalyst (Figure 1). The strain-promoted cycloaddition has been used to label biomolecules without observable cyto-toxicitiy.[20] The scope of the approach has, however, been limited due to a slow rate of reaction.[22] Appending electron-withdrawing groups to the octyne ring can increase the rate of strain-promoted cycloadditions, however, currently Staudinger ligation with phosphine 2 offers the most attractive reagent for cell surface labeling of azides.
Figure 1.
Reagents for labeling of azido-containing biomolecules.
It was envisaged that 4-dibenzocyclooctynols such as compound 3 would be ideal for the labeling of azides of living cells because the aromatic rings are expected to impose additional ring strain and conjugate with the alkyne, thereby increasing the reactivity of the alkyne in metal-free [2+3] cycloadditions with azides. The compound should, however, have excellent stability because the ortho-hydrogens of the aromatic rings shield the alkyne from nucleophilic attack. Furthermore, the hydroxyl of 3 provides a handle for the incorporation of tags such as fluorescent probes and biotin.
Compound 3 could be easily prepared from known[23, 24] 3-hydroxy-1,2:5,6-dibenzocycloocta-1,5,7-triene (4) by protection of the hydroxyl as a t-butyldimethyl silyl (TBS) ether using TBSCl in pyridine to give 5, which was brominated with bromine in chloroform to provide di-bromide 6 in a yield of 60% (Scheme 1). Although the TBS protecting group was lost during the latter transformation, the bromination was low yielding when performed on alcohol 4. Dehydro-bromination of 6 by treatment with LDA in THF at 0°C[25] gave the target cyclooctyne 3 in a yield of 45%.
Scheme 1.
Reagents and conditions. a) TBSCl, pyridine; b) Br2, CHCl3; c) LDA, THF; d) 4-nitrophenyl chloroformate, pyridine, DCM; e) DMF, Et3N.
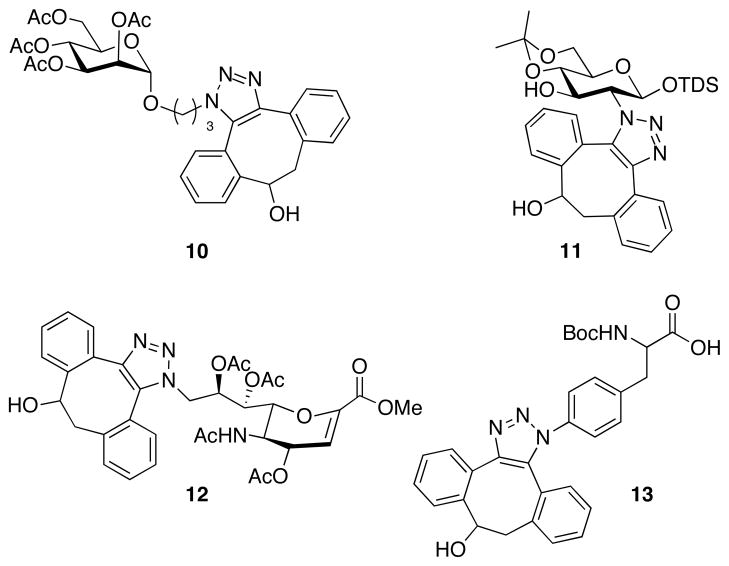
Compound 3 has an excellent shelf life and remained intact after treatment with nucleophiles such as thiols and amines. However, upon exposure to azides a fast reaction took place and gave the corresponding triazoles in high yield. For example, triazoles 10–13 were obtained in quantitative yields as mixtures of regioisomers by reaction of the corresponding azido-containing sugar and amino acid derivatives with 3 in methanol for 30 min (Figure 2). The progress of the reaction of 3 with benzyl azide in methanol and in a mixture of water/acetonitrile (1/4, v/v) was monitored by 1H NMR by integration of the benzylic proton signals and second-rate order constants of 0.17 and 2.3 M−1s−1, respectively were determined. The rate constant in acetonitrile/water is approximately three orders of magnitude faster than that of cyclooctyne 1.
Figure 2.
Metal free cycloadditons of compound 3 with azido-containing amino acid and saccharides.
Having established the superior reactivity of 3, attention was focused on the preparation of a derivative of 4-dibenzocyclooctynol (9) (Scheme 1), which is modified with biotin. Such a reagent should make it possible to visualize biomolecules after metabolic labeling cells with an azido-containing biosynthetic precursor, followed by cycloaddition with 9 and treatment with avidin modified with a fluorescence probe. Alternatively, biotinylation of glycoconjugates with 9 should make it possible to isolate these derivatives for glycocomics studies using avidin immobilized to a solid support. Compound 9 could easily be prepared by a two-step reaction involving treatment of 3 with 4-nitrophenyl chloroformate to give activated intermediate 7, followed by immediate reaction with 8.
Next, Jurkat cells were cultured in the presence of 25 μM of N-azidoacetylmannosamine (Ac4ManNAz) for 3 days to metabolically introduce N-azidoacetyl-sialic acid (SiaNAz) moieties into glycoproteins.[26] As a negative control, Jurkat cells were employed that were grown in the absence of Ac4ManNAz. The cells were exposed to 30 μM of compound 9 for various time periods and after washing, the cells were stained with avidin-FITC for 15 min at 4°C. The efficiency of the two-step cell surface labeling was determined by measuring the fluorescence intensity of the cell lysates. For comparison, the cell surface azido moieties were also labeled by Staudinger ligation with biotin-modified phosphine 2 followed by treatment with avidin-FITC. The labeling with 9 was almost complete after an incubation time of 60 min (Figure 3a). Interestingly, under identical conditions phosphine 2[22] gave significantly lower fluorescent intensities indicating that cell surface labeling by Staudinger ligation is slower and less efficient. In each case, the control cells exhibited very low fluorescence intensities demonstrating that background labeling is negligible. It was found that the two-step labeling approach with 9 had no effect on cell viability as determined by morphology and exclusion of trypan blue.
Figure 3.
Cell surface labeling with compounds 2 and 9. Jurkat cells grown for 3 days in the absence or presence of Ac4ManNAz (25 μM) were incubated (a) with compounds 2 and 9 (30 μM) for 0 – 180 min or (b) with compounds 2 and 9 (0 – 100 μM) for 1 h at room temperature. Next, cells were incubated with avidin-FITC for 15 min at 4°C, after which cell lysates were assessed for fluorescence intensity. Samples are indicated as follows: blank cells incubated with 2 (○) or 9 (□) and Ac4ManNAz cells incubated with 2 (●) or 9 (■). AU indicates arbitrary fluorescence units.
The concentration dependency of the cell surface labeling was studied by incubation cells with various concentrations of 2 and 9 followed by staining with avidin-FTIC (Figure 3b). As expected, cells displaying azido moieties showed a dose-dependent increase in fluorescence intensity. Reliable fluorescent labeling was achieved at a concentration of 3 μM of 9, however optimal results were obtained at concentrations ranging from 30 to 100 μM. No increase in labeling was observed at concentrations higher than 100 μM due to limited solubility of 9.
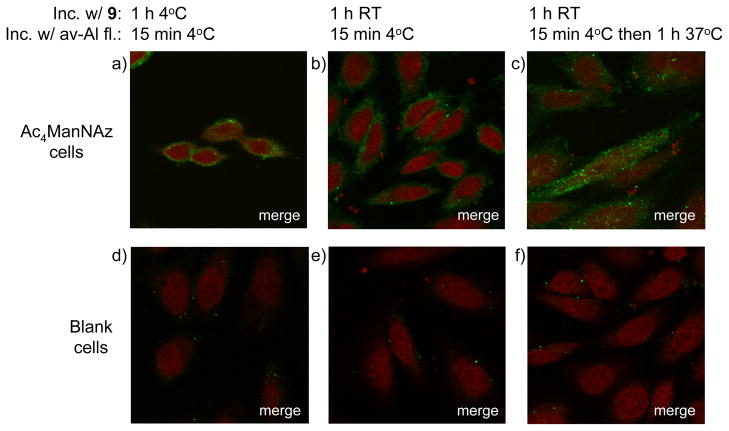
Next, attention was focused on visualizing azido-containing glycoconjugates of living cells by confocal microscopy. Thus, adherent Chinese hamster ovary (CHO) cells were cultured in the presence of Ac4ManNAz (100 μM) for three days. The resulting cell surface azido moieties were reacted with 9 (30 μM) for 1 h, and then visualized with avidin-Alexa fluor 488 for 15 min at 4°C. As expected, staining was only observed at the cell surface (Figure 4) and importantly, the labeling procedure was equally efficient when performed at ambient temperature or 4°C. Furthermore, blank cells exhibited very low fluorescence staining, confirming that background labeling is negligible.
Figure 4.
Fluorescence images of cells labeled with compound 9 and avidin-Alexa fluor 488. CHO cells grown for 3 days in the absence (d – f) or presence (a – c) of Ac4ManNAz (100 μM) were incubated with compound 9 (30 μM) for 1 h at 4°C (a, d) or room temperature (b, c, e, f). Next, cells were incubated with avidin-Alexa fluor 488 for 15 min at 4°C and, after washing, fixing, and staining for the nucleus with TO-PRO, imaged (a, b, d, e) or after washing incubated for 1 h at 37°C before fixing, nucleus staining, and imaging (c, f).
Cell surface glycoconjugates are constantly recycled by endocytosis and to monitor this process, metabolically labeled cells were reacted with 9 and avidin-Alexa fluor 488 using the standard protocol and incubated at 37°C for 1 h before examination by confocol microscopy. It was observed that a significant quantity of labeled glycoproteins had been internalized into vesicular compartments.
At the completion of these studies, Bertozzi and coworkers reported a difluorinated cyclooctyne (DIFO) that reacts with azides at almost the same reaction rate as compound 3.[27] DIFO linked to Alexa fluor was employed to investigate the dynamics of glycan trafficking. It was found that after incubation for 1 h, labeled glycans co-localized with markers for endosomes and Golgi.
4-Dibenzocyclooctynols such as 3 and 9 have several advantageous features such as ease of chemical synthesis and the possibility to further enhance the rate of cycloaddition by functionalization of the aromatic moieties. Modifying the aromatic rings may also offer an exciting opportunity to obtain reagents that become fluorescent upon [3+2] cycloaddition with azido-containing compounds, which will make it possible to monitor in real time the trafficking of glycoproteins and other biomolecules in living cells.
Supplementary Material
Footnotes
This paper is dedicated to Sir J. Fraser Stoddart in honor of his 65th birthday.
This research was supported by the Research Resource Center for Biomedical Complex Carbohydrates (P41-RR-5351). We thank Drs. Heather Flanagan-Steet and Richard Steet for assistance with confocal microscopy studies.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Kolb HC, Sharpless KB. Drug Dis Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 2.Dedola S, Nepogodiev SA, Field RA. Org Biomol Chem. 2007;5:1006–1017. doi: 10.1039/b618048p. [DOI] [PubMed] [Google Scholar]
- 3.Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 4.Nandivada H, Jiang XW, Lahann J. Adv Mater. 2007;19:2197–2208. [Google Scholar]
- 5.Wu P, Fokin VV. Aldrichim Acta. 2007;40:7–17. [Google Scholar]
- 6.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 8.Chin JW, Cropp TA, Anderson JC, Mukherji M, Zhang ZW, Schultz PG. Science. 2003;301:964–967. doi: 10.1126/science.1084772. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 10.Kho Y, Kim SC, Jiang C, Barma D, Kwon SW, Cheng JK, Jaunbergs J, Weinbaum C, Tamanoi F, Falck J, Zhao YM. Proc Natl Acad Sci. 2004;101:12479–12484. doi: 10.1073/pnas.0403413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gierlich J, Burley GA, Gramlich PME, Hammond DM, Carell T. Org Lett. 2006;8:3639–3642. doi: 10.1021/ol0610946. [DOI] [PubMed] [Google Scholar]
- 12.Link AJ, Vink MKS, Agard NJ, Prescher JA, Bertozzi CR, Tirrell DA. Proc Natl Acad Sci. 2006;103:10180–10185. doi: 10.1073/pnas.0601167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speers AE, Adam GC, Cravatt BF. J Am Chem Soc. 2003;125:4686–4687. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]
- 14.Sun XL, Stabler CL, Cazalis CS, Chaikof EL. Bioconjugate Chem. 2006;17:52–57. doi: 10.1021/bc0502311. [DOI] [PubMed] [Google Scholar]
- 15.Prescher JA, Bertozzi CR. Nat Chem Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 16.Hanson SR, Hsu TL, Weerapana E, Kishikawa K, Simon GM, Cravatt BF, Wong CH. J Am Chem Soc. 2007;129:7266–7267. doi: 10.1021/ja0724083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. Org Lett. 2004;6:4603–4606. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- 18.Link AJ, Tirrell DA. J Am Chem Soc. 2003;125:11164–11165. doi: 10.1021/ja036765z. [DOI] [PubMed] [Google Scholar]
- 19.Turner RB, Goebel P, Mallon BJ, Jarrett AD. J Am Chem Soc. 1972;95:790–792. [Google Scholar]
- 20.Agard NJ, Prescher JA, Bertozzi CR. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 21.van Berkel SS, Dirks ATJ, Debets MF, van Delft FL, Cornelissen JJLM, Nolte RJM, Rutjes FPJT. Chembiochem. 2007;8:1504–1508. doi: 10.1002/cbic.200700278. [DOI] [PubMed] [Google Scholar]
- 22.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 23.Jung ME, Mossman AB, Lyster MA. J Org Chem. 1978;43:3698–3701. [Google Scholar]
- 24.Jung ME, Miller SJ. J Am Chem Soc. 1981;103:1984–1992. [Google Scholar]
- 25.Seitz G, Pohl L, Pohlke R. Angew Chem. 1969;81:427–428. [Google Scholar]; Angew Chem Int Ed. 1969;8:447–448. [Google Scholar]
- 26.Luchansky SJ, Bertozzi CR. Chembiochem. 2004;5:1706–1709. doi: 10.1002/cbic.200400148. [DOI] [PubMed] [Google Scholar]
- 27.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.