Abstract
Axonemal complexes in flagella are largely pre-packaged in the cell body. As such, one mutation often results in the absence of the co-assembled components and permanent motility deficiencies. For example, a Chlamydomonas mutant defective in RSP4 in the radial spoke (RS), which is critical for bend propagation, has paralyzed flagella that also lack the paralogue RSP6 and three additional RS proteins. Intriguingly, recent studies showed that several mutant strains contain a mixed population of swimmers and paralyzed cells despite their identical genetic background. Here we report a cause underlying these variations. Two new mutants lacking RSP6 swim processively and other components appear normally assembled in early log phase indicating that, unlike RSP4, this paralogue is dispensable. However, swimmers cannot maintain the typical helical trajectory and reactivated cell models tend to spin. Interestingly the motile fraction and the spokehead content dwindle during stationary phase. These results suggest that 1) intact RS is critical for maintaining the rhythm of oscillatory beating and thus the helical trajectory; 2) assembly of the axonemal complex with subtle defects is less efficient and the inefficiency is accentuated in compromised conditions, leading to reversible dyskinesia. Consistently, several organisms only possess one RSP4/6 gene. Gene duplication in Chlamydomonas enhances RS assembly to maintain optimal motility in various environments.
Introduction
Various cell types utilize motile cilia and flagella to propel surrounding fluid. The movement is highly regulated via the signaling of 2nd messengers and a network of regulatory proteins. This versatile motility enables cells to respond to changing environments. The machinery for oscillatory beating and regulation is built into evolutionarily conserved 9+2 axonemes comprised of a microtubule-based structural scaffold and associated macromolecular complexes, such as axonemal dynein motors, radial spokes (RS) and the central pair apparatus (CP). Genetic defects in this machinery of Chlamydomonas reinhardtii result in a spectrum of aberrant motilities. Defects in the motors result in reduced beat frequency or amplitude (Kamiya, 2002). Mutants that lack a major component of the RS or CP are entirely paralyzed or assume a jerky motion, as these two structural complexes work together to orchestrate the sequential activation of dynein motors to generate seamless oscillatory beating (Randall et al., 1964; Piperno et al., 1977; Witman et al., 1978; Dutcher et al., 1984; Yokoyama et al., 2004; Yang et al., 2008). The RS and CP are also intimately involved in 2nd-messenger-dependent motility changes (Smith and Sale, 1992; Smith, 2002; Smith and Yang, 2004) as well as nucleotide metabolism (Mitchell and Sale, 1999; Patel-King et al., 2004; Zhang and Mitchell, 2004; Mitchell et al., 2005).
The anomalies of most flagellar mutants are described and perceived as irreversible and irreparable. The only means known to partially restore the motility of live mutant cells is through extragenic suppressor mutations (Huang et al., 1982) or direct mechanical stimulation (Hayashibe et al., 1997). Yet the phenotypes of a few Chlamydomonas mutant strains are not so cut-and-dried. The vegetative cells of dynein arm mutant pf13 are paralyzed while the gametic ones are motile (Huang et al., 1979; Brokaw and Kamiya, 1987). Some strains with subtle biochemical and morphological deficiencies in RS contain mixed populations of swimmers and paralyzed cells (Huang et al., 1982; Frey et al., 1997; Gaillard et al., 2006; Yang and Yang, 2006). Notably, it was demonstrated that in pf25, which was defective in two radial spoke proteins (RSP), the ratio of swimmers and paralyzed cells fluctuates according to the culture conditions. Cells in a log phase culture can appear WT-like but become entirely paralyzed in spent media. After being cultured in fresh media for 1–2 days, the culture was teeming with swimmers again. Thus, certain types of dyskinesia appear reversible. It is unknown if a specific regulatory mechanism or an additional deficiency that occurred in a certain condition compounded the original axonemal defect. However, biochemical differences were not evident in motile or immotile populations of pf13 or pf25 strains (Luck and Piperno, 1988; Yang and Yang, 2006).
In vitro studies have shown that phosphorylation can modulate the motility level of axonemes. Inhibition of cAMP-dependent protein kinase (PKA) increases the reactivation rate of WT axonemes (Hasegawa et al., 1987). In the same line, a motile mutant with two a. a. residues replaced in spoke protein 3 (RSP3) has an equal ratio of swimmers and paralyzed cells. A similar ratio is found in the reactivated cell model while inhibition of cAMP dependent protein kinase (PKA) increases the motile fraction of cell models (Gaillard et al., 2006). However, inhibition of kinase activity cannot mobilize the paralyzed pf25 cells in the spent media (Yang and Yang, 2006), suggesting that distinct mechanisms influence the reversible paralysis of pf25 in vivo.
To explore the underlying mechanisms, we investigated two independent isolates that also display this mercurial motility change. Interestingly, both strains lack RSP6, one of the two paralogues in the head module of the radial spoke (Curry et al., 1992). Their motility levels as well as the degree of the RS deficiency are both influenced by media conditions, albeit at a slightly different rate. These results shed critical insight into the precise arrangement and the disorder of 9+2 axonemes.
Materials and methods
Strains and culture conditions
Chlamydomonas reinhardtii wild-type strain cc124 (-) and radial spoke mutant pf26ts were acquired from the Chlamydomonas Resource Center (Duke University, Durham, NC). All cells were maintained on Tris-acetate-phosphate (TAP) agar plates for 6–7 days. A small fraction was inoculated into ~ 300 ml Sager’s liquid medium and cultured under aerated photoheterotrophic growth in 14/10 light/dark cycle (Harris, 2009) unless indicated otherwise. The cultures reached stationary phase in 4 days. The 1C12 strain was recovered from screening for motility mutants following exposure to ultraviolet radiation. The 45G7 strain was identified from a screen of insertional mutagenesis using pMN24 plasmid with the NIT1 gene for metabolic selection (Mitchell and Sale, 1999).
Molecular biology and genetics
Transformation rescue
The BAC clone #7P14 (Clemson University, Clemson, SC) was purified with Phase Prep BAC kit (Sigma-Aldrich, St. Louis, MO) and digested with NotI restriction enzyme. A ~5.5kb fragment containing the RSP6 gene was band-purified and cloned into pBluescript KS vector (Stratagene). The construct was then co-transformed with the pSI103 plasmid that confers paromomycin resistance into the cells of 1C12 and 45G7 strains using the glass bead method as described previously (Yang and Yang, 2006). For screening transformants, cells from single colonies were resuspended in liquid medium in 96-well plates for motility analysis.
Characterization of mutations
Genomic DNA for sequencing was prepared by transferring RSP6 mutant cells with a toothpick to an aliquot of 50 µl 10 mM EDTA. The cell suspension was boiled for 5 min followed by brief vortex and centrifugation. The supernatant was used as template for PCR reactions to amplify the overlapping regions in the RSP6 genes. The PCR products were cloned and sequenced by Iowa State University DNA sequencing facility. The sense and antisense primer pairs for PCR are as followed.
#1:cagtcatttctggacagaatcgctgc; gtcaggcaggctgcaggtacgtgag.
#2: ctcacgtacctgcagcctgcctgac; cctcgcactcgaactggattaagcacg.
#3: cgtgcttaatccagttcgagtgcgagg; cctctagcaccgctgtaggcctaagc.
#4: gcttaggcctacagcggtgctagagg; ccactcgtcgttggcggacagctcg.
#5: cgagctgtccgccaacgacgagtgg; gctcctcagcctcagctaaccacacc.
#6: cagctgctgctcgagtgcaacgacctgc: gctccatgctgctgtgcgcagcttatgg.
The primer pair used to amplify the region that was disrupted by pMN24 plasmid in 45G7 strain was: #4 sense in RSP6 gene and antisense primer in NIT1 gene of pMN24: gtcaaagcacctgtgtacctcgcgag.
The RSP4/6 homologues and the phylogenic trees were obtained using BLASP program at National Center for Biotechnology Information (NCBI).
Phenotype assessment
Motility analysis
The percentage of swimming cells was determined by observing aliquots of the cell culture at 200X magnification with an Olympus BH-2 compound microscope. The light source was filtered through a 62-mm filter with a 625-nm cutoff (HOYA, Japan) to prevent light induced motility anomalies. At least 200 cells from more than 6 randomly selected fields were counted. Swimmers were defined as cells that actively move away from the original spot. Immotile cells that were obviously stuck to glass surfaces were excluded from calculations. As motile cells move in and out of fields quickly, the percentage of swimmers was an average of estimates and standard deviations were not included. Cell densities were determined by counting cell numbers in a hemacytometer chamber (Hausser Scientific Co., PA)
For tracing the trajectories of cells, light-filtered time-lapse images were taken under 200 X magnification with a CoolSnap CCD camera (Yang and Yang, 2006) at a rate of 20 frames/sec for 5 seconds. Individual cells were tracked by MetaMorph software.
Reactivation of cell models
Reactivation of NP-40-permeabilized cells was performed as previously described (Gaillard et al., 2006) except that a final concentration of 0.5 mM ATP was used for the reactivation.
Biochemistry
Axonemal preparation and velocity sedimentation of KI axonemal extract on sucrose gradients were performed as described previously (Yang, et al., 2001). Western blots using polyclonal antibodies for individual radial spoke proteins have been described previously (Yang et al., 2006).
Electron microscopy
The procedure was described previously (Yang et al., 2008)
Results
RSP6 is a nonessential paralogue in the radial spokehead
Screening of chemical mutagenesis and insertional mutagenesis respectively recovered two strains, 1C12 and 45G7, with abnormal motility. The cells, when resuspended from agar plates into liquid medium, were largely paralyzed with a few cells moving locally. To test if the severe motility deficiency was due to defective RS and CP, mutants were crossed with the suppressor mutant sup-pf1. The paralysis was rescued, suggesting that the strains were CP or RS mutants.
To test if the mutants were defective in RS, axonemes for western blots were harvested from 45G7, 1C12 and wild type (WT) cells as usual from aerated stationary phase liquid cultures. Curiously, some mutant cells swam quite well. Among the RSPs tested, only RSP6 was absent in 1C12 axonemes but other RS proteins including RS head proteins were present (Fig. 1A). Although the spokehead proteins (RSP1, 9 and 10) and the spoke chaperone in the stalk (RSP16) of 1C12 were less abundant than those in the WT control as shown in Fig. 1A, the reduction was not due to flagellar demembranations and for most preparations was not evident. Identical results were obtained from the 45G7 strain. This finding was unexpected because the only other spokehead mutants, pf1, defective in RSP4 (an RSP6 paralogue), and pf17, defective in RSP9, lacked all 5 proteins in the RS head and were entirely paralyzed (Table I). On the other hand, in the axonemes of the RSP6 temperature sensitive mutant pf26ts, spokehead components, including the mutated RSP6 were present. This variable reduction also occurred in pf26ts (Huang et al. 1981).
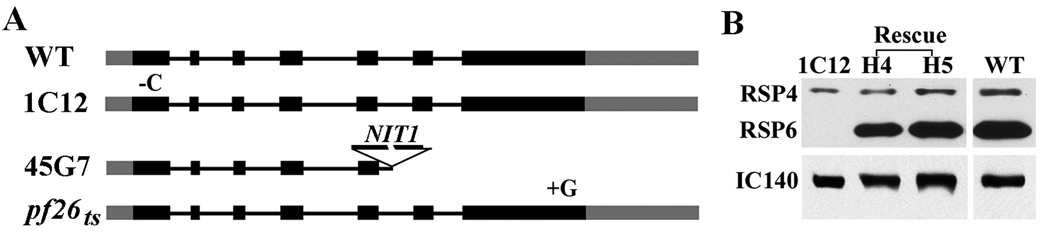
Fig. 1.
Characterization of a new mutant strain 1C12. (A) Western analyses of axonemes revealed that RS in 1C12 axonemes lacked RSP6 only. Each protein was revealed by antibodies raised against individual molecules. Anti-RSP6 antibody cross-reacted with RSP4 and hence RSP4 band appeared weaker than RSP6. In this particular preparation, the spokehead proteins, RSP 1, 9 and 10 and the stalk chaperone HSP40 (RSP16) were less abundant in the 1C12 axonemes than those in the wild type control. This difference was not evident in most preparations. (B) Transmission electron microscopy of axoneme cross sections. Compared to WT, no obvious morphological defect can be found in every RS in the 1C12 axoneme. The samples were prepared from the early stationary phase liquid culture
Table 1.
The molecules in the head module of Chlamydomonas radial spoke and the available mutants
| Molecular Domaina | MW(kDa)b | Mutantc | Spokeheadc | Motilityc | |
|---|---|---|---|---|---|
| RSP1d | 123 | N/A | |||
| RSP10d | 24 | N/A | |||
| RSP4 | 76 | pf1 | − | − | |
| RSP6 | 67 | pf26ts | + | +/− | |
| RSP9 | 26 | pf17 | − | − |
Each box represents a MORN (MN) motif.
The RSP4/6 molecules are much bigger than RSP9 and 10, two of the other three spokehead components. We reasoned that if the bulbous spokehead only consisted of the 5 molecules (Table I) as postulated (Curry and Rosenbaum, 1993), morphological defects due to loss of RSP6 should be visible by electron microscopy (EM). To test this, axonemes from liquid cultures were processed for EM. However, the bulbous spokehead did not always appear smaller but seemed less defined in cross sections (Fig. 1B) or longitudinal sections. The presence of spokehead is consistent with the motile RSP6-minus cells, yet missing RSP6 did not result in definitive deformed RS. This could be due to the insufficient resolution of EM for RS or that the bulbous spokehead actually consists of more than the 5 RSPs defined based on mutants (Curry and Rosenbaum, 1993).
To test if the RSP6 gene was defective in the two mutants, RSP6 genomic DNA was amplified by PCR. Sequencing of the amplified RSP6 gene fragments from 1C12 showed that AC at #141–142 coding sequence in the 1st exon was replaced by T (supplemental Fig. S1, Fig. 2A). The elimination of 1 bp caused a frame shift after 47th codon and a premature termination after codon #58. Considering that the entire molecule is 459 a.a. long and is highly homologous to RSP4, the new polypeptide of only the first 47 a.a. is most likely nonfunctional. In terms of the insertional mutant 45G7, the region after the fifth intron of the RSP6 gene cannot be amplified, suggesting that insertion of pMN24 plasmid occurred around this region and was accompanied by the deletion of the entire 3’ end sequence. To determine the precise defect, we amplified a 300 bp fragment at the insertional region using a sense primer upstream to the fifth intron of the RSP6 gene and the second primer from the NIT1 gene in the pMN24. Sequencing revealed that the NIT1 gene was connected in a reverse orientation to the fifth intron of the RSP6 gene. Since the 3’ end of the gene including three fifths of the coding sequences was deleted, the truncated polypeptide at best would terminate in an in-frame stop codon in the fifth intron upstream of the insertional site. The MW and isoelectric point of the predicted polypeptide would be 21 kDa and 4.44 respectively, comparable to RSP10 and 11 (Yang et al., 2006). However, no new peptides were visible around this region in the 2-D gel of 45G7 axonemes. Thus, truncated RSP6 polypeptides are likely absent in the axoneme and nonessential for the assembly of the spokehead. The RSP6 gene in pf26ts was also sequenced. An additional G was found in the last exon of the RSP6 gene. The frame shift resulted in the replacement of the last 31 a.a. of pI 3.8 with foreign 55 residues of pI 11.8. This finding was consistent with the basic shift of the mutated protein in the 2-D gel (Huang et al., 1981) and suggested that the altered C-termini interfere with the conformation of the spokehead at the restrictive temperature.
Fig. 2.
The deficiencies of 1C12 and 45G7 flagella were caused by mutations in the RSP6 gene. (A) A schematic diagram depicting the mutations in the RSP6 genes in three RSP6 mutants. For 1C12, a single-nucleotide deletion in the first exon resulted in premature termination after 58 amino acids. In the case of 45G7, insertion of pMN24 plasmid containing nitrate reductase gene (NIT1) in the fifth intron resulted in deletion of three fifth of the 3' end. An addition of one base pair in the last exon in the pf26ts RSP6 gene caused frame shift of the C-terminus and the presence of the mutated RSP6 in pf26 axoneme (Huang et al., 1981). The results were obtained by PCR of genomic DNA, followed by cloning in some cases and sequencing. The mutations were confirmed by repeated PCR and sequencing. The black bars represent exons and the two gray bars represent 5' and 3' UTR respectively. The lines between black bars represent introns. (B) Western analyses revealed that RSP6 was restored in two representative transformants (H4 and H5) to the WT level. The positive and negative controls were WT and 1C12 respectively. The protein loading control was dynein intermediate chain, IC140.
To test whether the phenotype of the 1C12 strain was due to the mutated RSP6 gene, transformation rescue of 1C12 and 45G7 strains with the RSP6 gene was carried out. To recover RSP6 genomic DNA, we first analyzed the DNA end sequences of BAC clones that were mapped to the region of the RSP6 gene in Chlamydomonas genome v.2.0 (http://genome.jgi-psf.org/Chlre2/Chlre2.home.html). From all of the clones that included the entire RSP6 gene, clone #7P14 was chosen for its shortest BAC DNA. A 5.5-kb fragment that included the untranslated regions and additional ~ 1 kB flanking sequence was released by NotI restriction digestion of the purified BAC DNA. The band-purified fragment was cloned into a pBluescript vector and the plasmid was co-transformed into 1C12 cells with the plasmid pSI103 that confers paromomycin (PMM) resistance (Yang et al., 2008). PMM-resistant transformants were resuspended from agar plates into liquid medium. Among 126 colonies examined, 12 strains appeared rescued to mostly motile cells. In contrast, the parental 1C12 cells were paralyzed. Axonemes were prepared from two representative transformants (H4 and H5, Fig. 2B), 1C12 and WT control for western blot analysis. As expected, RSP6 level in transformants’ flagella was restored to the WT level. Similarly, the RSP6 gene was also able to rescue the deficiencies of the 45G7 strain. H4 and H5 transformants were used as wild type control (WT*) for motility analysis (see below). Both RSP6 mutant strains were used in the following experiments with identical results and only the results for 1C12 will be described. For the record, 1C12 and 45G7 strains will be renamed as pf26-2 and pf26-3 respectively.
Taken together, these data showed that although RSP4 and RSP6 are highly homologous in sequence and length, only RSP4 is essential for the assembly of RS head and motility whereas the requirement for RSP6 is not as absolute.
Duplicated RSP4/6 genes are not universal
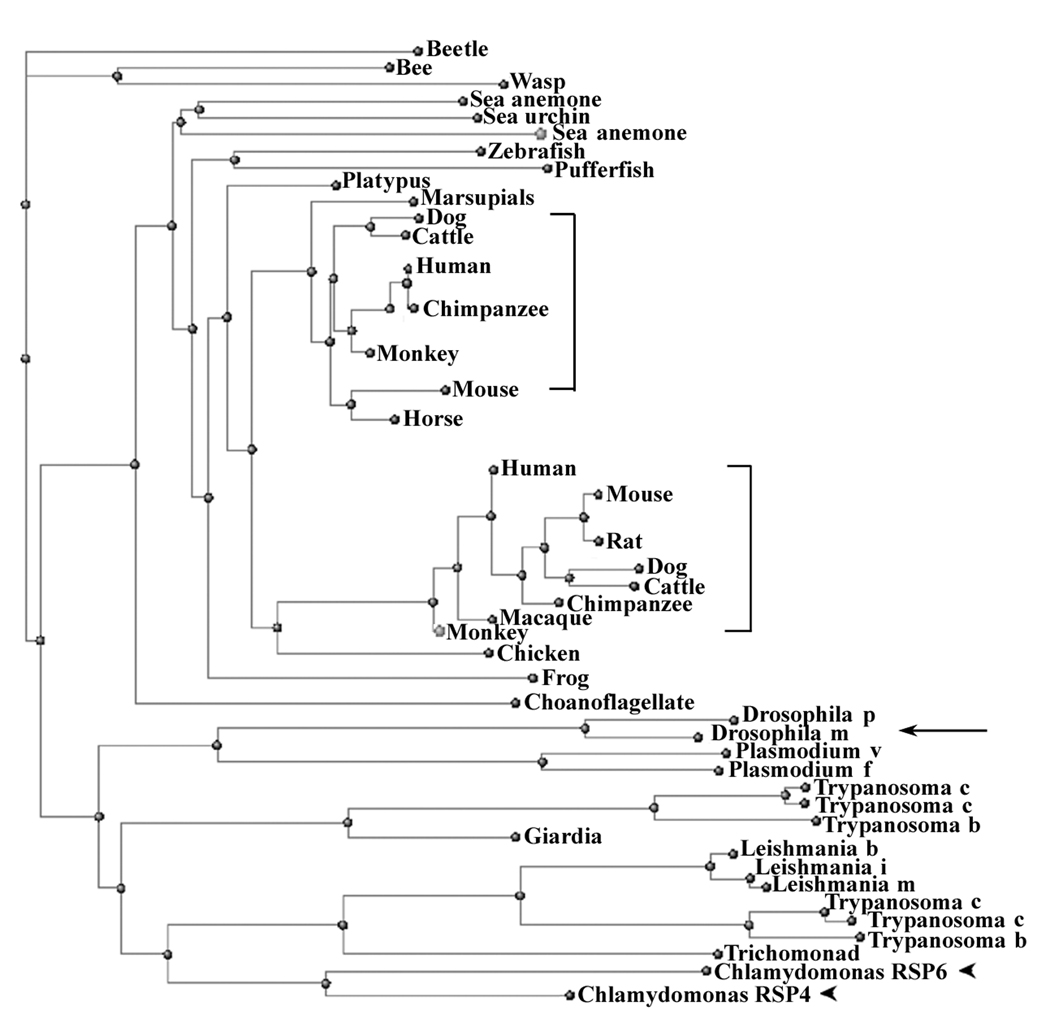
Chlamydomonas RSP4 and RSP6 are encoded by two duplicated genes tandemly aligned in linkage group V, sharing 48% identical a.a. residues distributed throughout the molecules (Curry et al., 1992). Two copies of highly conserved RSP4/6 genes are also present in human and mouse (Eriksson et al., 2001; Castleman et al., 2009). As the significance of the two duplicated genes appeared different, we questioned if duplicated RSP4/6 is universal after all. To test this, BLASTP was conducted using the essential Chlamydomonas RSP4 as a query. Although only the genomes of selected organisms were analyzed, it is evident that mammals (brackets, Fig. 3) and many protists harbor two or more copies. The branches of phylogeny trees and multiple sequence alignment showed that the two genes from Chlamydomonas (arrowheads) were much more homologous to each other than to any one of the human orthologues. Additional database analyses (not shown) showed that duplication of the ancestral RSP4/6 gene appears to have occurred once in the ancestors of birds and mammals after they diverged from other tetrapods (with the second copy only retained in mammals), once in the ancestors of excavates, chromalveolates and viridaplantae (with the second copy only retained in excavates), once more recently in the Chlamydomonas lineage, prior to the separation of Chlamydomonas reinhardtii and Volvox carteri, and two or three times in the ciliate lineage leading to Paramecium tetraurelia. Crucially, only one RSP4/6 gene was found in other branches of eukaryotes, including Fungi (Batrachochytrium dendrobatidis), Choanoflagellates (Monosig brevicolis) and invertebrate metazoans such as Drosophila (Fig. 3. arrow). Consistently, only a single RSP4/6 band is present in the purified Ciona RS complex (Satouh et al., 2005).
Fig. 3.
Phylogeny tree reveals that two duplicated genes for RSP4 and RSP6 are not present in every organism with motile cilia and flagella. For example, Drosophila melanogaster (arrow) only contains one RSP4/6 gene, while the two RSP4/6 orthologous genes in mammals form two distinct sub-trees (brackets). Notably, Chlamydomonas RSP4 and RSP6 (arrowheads) are most homologous to each other rather than to either of the mammalian sub-trees. The tree was part of the output from BLASTP available in NCBI webpage using Chlamydomonas RSP4 as a query. For clarity, redundant sequences were deleted and scientific names for organisms were replaced by common names.
Thus the ancestral eukaryote only had a single RSP4/6 gene, and many cilia still function adequately with a single gene product, but gene duplications have also frequently resulted in retention of both copies and divergence into two proteins with distinct significance.
The motility of RSP6 mutants is overtly sensitive to culture conditions
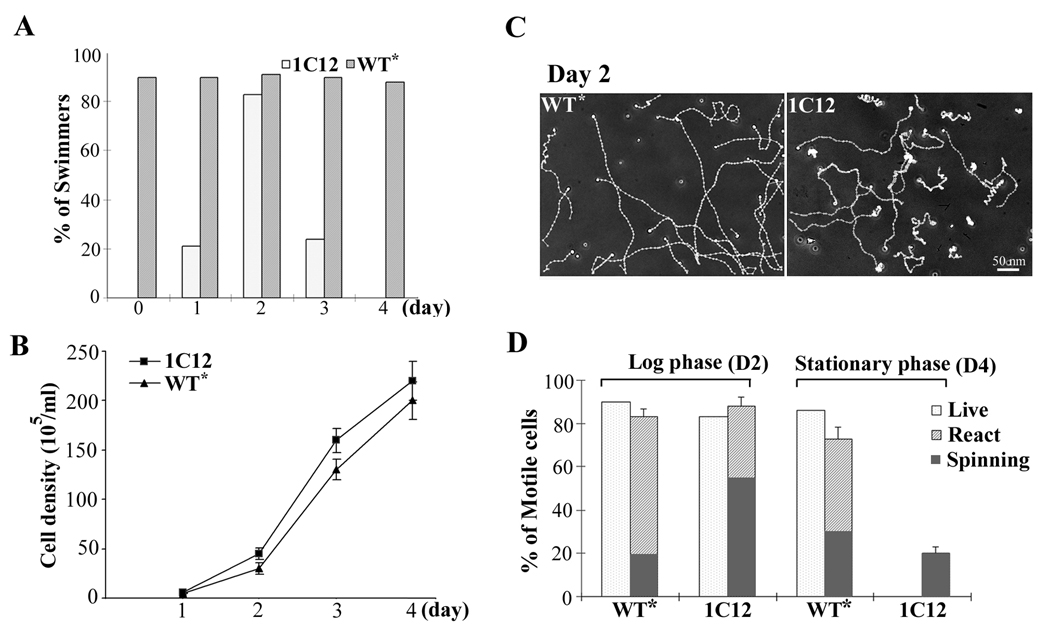
Liquid cultures for axoneme preparation and EM were usually harvested from early stationary phase with a density of ~2 X 107 cells/ml 4 days after inoculation from agar plates. A small number of RSP6-minus cells swam processively. Strikingly, the culture harvested two days earlier for transformation appeared almost like WT culture, unless carefully examined for immotile cells. We postulated that the motility of RSP6-minus cells was overtly sensitive to the culture condition, like pf25, which is only deficient in two RS proteins (Yang and Yang, 2006). To test this, 1C12 cells were inoculated from the 7-day old algal plate stock to liquid medium and the motility (Fig. 4A) and cell density (Fig. 4B) were measured for 4 consecutive days (D1-D4). The rescued 1C12 transformants served as a control (WT*). As predicted, 1C12 cells generated full-length flagella within 1 hr after resuspension but cells were paralyzed (D0, Fig. 4A). Occasionally, several cells jiggled or moved locally. The motility level did not change significantly within the same day. The percentage of swimmers gradually increased in the next two days during early log phase as shown by the cell density plot (D1 and D2, Fig. 4B) and then declined as the culture approached or reached the stationary phase (D3 and D4). Although the absolute percentages and density plots varied in each preparation, all exhibited a similar bell-shape curve in motility changes. Similar to the observation of pf25 cells, replacing old medium with fresh medium reduced the deterioration level in the next day while old medium did not affect motility until the next day as well (Yang and Yang, 2006). In contrast, flagellated WT* cells always swam, regardless of culture age.
Fig. 4.
The changing motility phenotypes of 1C12 cells. (A) A representative histogram demonstrating changes in the percentage of swimmers during five consecutive days of the liquid culture of 1C12 (open bars) and rescued 1C12 control (WT*, grey bars). Freshly resuspended 1C12 cells from agar plates (Day 0) were paralyzed. Motility improved during the early log phase and then declined as the culture reached the stationary phase. (B) A representative plot of cell densities of WT* (triangle) and 1C12 cells (square). The density at the inoculation date was too low to determine. At the fourth day, the culture reached stationary phase. Each data point was the average from four measurements. (C) The trajectories of cells from log-phase cultures. Many WT* cells display helical trajectory. In contrast, the 1C12 swimmers change directions frequently without a discernable trajectory. Yet the distance that processive 1C12 swimmers transversed was not significantly different from that of WT* cells. (D) The effect of media on the reactivated cell models. Motile fractions of live cells and reactivated cell models with a buffer containing detergent and 0.5 mM ATP were determined for the log phase and stationary phase cultures. The reactivated motile cells were further classified into swimmers that transverse processively and spinners that rotated around a fixed spot. Spinners were more prominent in 1C12 cell model and in older cultures of WT*. Notably, despite complete paralysis in early stationary phase, a fraction of reactivated 1C12 cell models still can spin.
The motility of 1C12 cells was sensitive to temperature as well. The motility of pf26ts was determined by the temperature at which flagellar assembly occurred and shifting temperature afterwards did not affect motility (Huang et al., 1981). In contrast, the 1C12 swimmers with motile flagella generated at room temperature (~ 24°C) became completely paralyzed within 1 hr at 32°C (Table II). The motile fraction decreased at 28°C as well. Paralysis did not recover on the same day after the temperature was reduced to 24°C. Thus, although RSP6 is not essential for the assembly of spokeheads or motility, the RSP6-minus cells easily become paralyzed at higher temperature or in spent media.
Table 2.
Temperature-sensitive paralysis of 1C12 swimmers. As shown by the percentage of swimmers in the liquid culture, 1C12 swimmers, either from the log-phase or stationary-phase culture, became completely paralyzed within 1 hr after transferring to the water bath at 32°C. The motile fraction was greatly reduced at 28 °C as well. Each number was obtained from the average of six randomly chosen fields.
| Log phase |
Stationary phase |
|
|---|---|---|
| 24°C | 80% | 20% |
| 28°C | 40% | 5% |
| 32°C | 0% | 0% |
Motile RSP6 mutant cells cannot maintain a helical trajectory
As RSP6 swimmers at the permissive temperature and in the early log phase appeared almost like WT* control, video microscopy and image analyses were performed to detect subtle anomalies. Both WT rescue (WT*) and 1C12 were very light sensitive, turning toward the illumination source and thus sticking to the glass slide and cover slip. To prevent light-induced anomalies, a 625 nm-cutoff filter was inserted in the optic path to remove short wavelength stimulating light and the image was recorded by a light-sensitive CCD camera. As shown by the tracked motion, most WT* cells maintain helical trajectories fairly well under the filtered illumination (Fig. 4C, left panel). Contrarily, except the few cells that move locally, the 1C12 processive swimmers in the log phase actually changed direction frequently, failing to maintain helical trajectories (right panel). However, their velocity was only slightly slower (compare the distance of the tracks). The light sensitivity precluded the possibility of waveform analysis as high-speed video-microscopy requires bright light to compensate short exposure durations (Yang et al., 2008).
To further assess motility, in vitro reactivation of cell models was conducted. WT* and 1C12 cells from early log-phase (day 2, D2) and early stationary-phase (day 4, D4) culture were permeabilized with detergent and reactivated with 0.5 mM MgATP. For the day 2 culture, the percentages of motile reactivated cell model for mutant and WT* control prepared from the log-phase culture were similar, although the mutant group evidently contained more spinners (Fig. 4D). Interestingly, although 1C12 cells were all paralyzed at D4, 20% of the cell models were motile, albeit spinning only. The motile fraction of the reactivated WT* cell model from the log phase was slightly lower but the ratio of spinners increased significantly. Thus, RSP6-minus cell models have a higher propensity to spin while their axonemes in paralyzed cells retained sufficient motility to support spinning of cell models but not swimming of live cells. Furthermore, spent media also reduced the capacity of WT* axonemes albeit less prominently.
Drastic reduction of spokeheads in flagella from spent media cultures
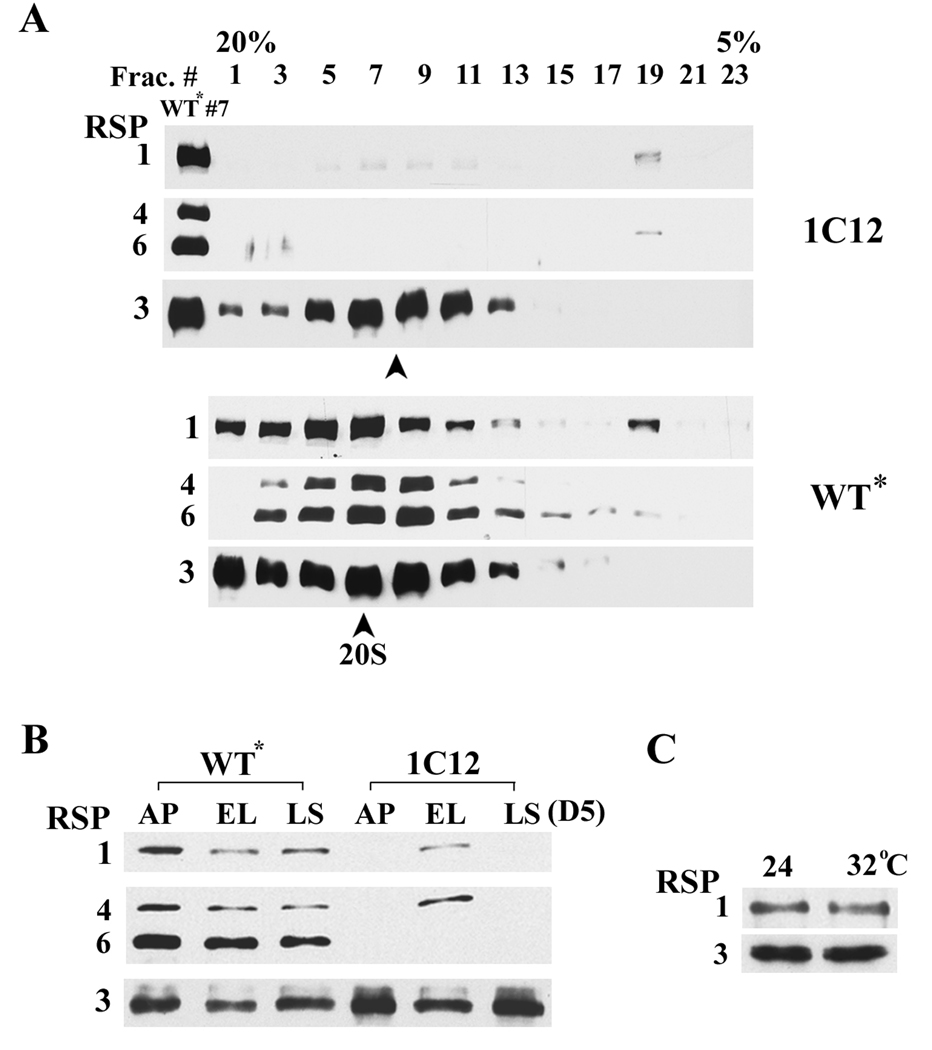
To further access the defect of RSP6-minus spokeheads, spokes were extracted from axonemes with chaotropic 0.6 M KI buffer and separated by velocity sedimentation through a 5–20% sucrose gradient. To prepare a large quantity of flagella for this experiment, the cells were harvested from 8-liter stationary phase culture after 6 days in liquid media. The cells were entirely paralyzed. The fractions were probed for RSP1, 4, 6 in the spokehead and RSP3 in the stalk as a control. As anticipated, all four RSPs in the rescued 1C12 (WT*) gradient sedimented as 20S particles with the peak in fraction #7 (Fig. 5A). Surprisingly, RSP4 and RSP1 were barely detectable in the gradient of the mutant (upper panel, compare with WT* #7 fraction). Furthermore, the major fraction of the residual RSP1 and RSP4 sedimented near the top of the gradient (fraction #19), indicative of dissociation. Contrarily, the stalk protein RSP3 was not reduced and sedimented as a slightly smaller particle (compare two arrowheads in the two gradients). Thus, RSP6-minus spokeheads in such preparations decreased drastically and were unstable.
Fig. 5.
Western blots showed drastic reduction of RS head domain in flagella of mutant cells grown in spent medium. (A) RS extracted from WT* axonemes, as shown by the bands of RSP1, 3, 4 and 6 sedimented around fraction #7 as 20S intact particles (arrowhead) in the sucrose gradient. Contrary to fraction #7, RSP1 and 4 from the 1C12 gradient were drastically reduced. Furthermore, a major fraction of these two proteins sedimented near the top of the gradient, indicative of dissociation. In contrast, stalk proteins in 1C12, represented by RSP3, were not reduced and sedimented as a slightly smaller particle (compare the two arrowheads). (B) The spokehead proteins were drastically reduced in the axonemes from 1C12 harvested from 7-day old agar plates (AP) and late stationary phase liquid culture (LS, Day 5). All cells were entirely paralyzed. Contrarily, these proteins from the early log phase (EL) with many swimmers were comparable with WT* samples that were not notably affected by the media conditions. (C) Temperature-induced paralysis was not caused by drastic dissociation of RS heads. 1C12 axonemes were prepared from the log-phase culture, either maintained at 24 °C or shifted to 32 °C for 1 hr.
We reasoned that the diminished spokehead content was an extended scenario of the reduced spokehead that was observed occasionally from the 4-day-old small liquid culture (Fig. 1) at the stationary phase and the deficiency revealed by western blots might be more prominent and reproducible if axonemes were prepared from older cultures. To test this, axonemes were prepared one day later, from the late stationary phase (Day 5) liquid culture, and from 7-day old agar plates (the stock that were usually used for inoculation of liquid cultures). The cells from both preparations were entirely paralyzed. The controls were the early log-phase 1C12 liquid culture with about 80% swimmers and WT* cultures. RSP3, a stalk protein, provided a loading control. The spoke contents in WT* axonemes prepared from the three types of cultures were not obviously affected. Contrarily, as predicted, the amount of head proteins RSP1 and RSP4 were barely detectable in mutant axonemes prepared either from agar plates (AP, Fig. 5B) or the late stationary phase culture (LS), but appeared normal in the early log phase culture (EL) with an abundance of swimmers. Thus the motility as well as the spokehead assembly of RSP6 mutants was much more sensitive to media conditions than was WT.
To test if the paralysis at the restrictive temperature was also due to dissociation of the spokehead, we compared axonemes prepared from duplicated early log-phase 1C12 cultures, one maintained at 24°C and the other placed at 32°C for 1 hr in which cells were entire paralyzed. Western blots showed that spokehead amounts, represented by RSP1, were similar (Fig. 5C), indicating that the temperature-induced paralysis was not due to a drastic dissociation of the RSP6-minus spokehead.
Discussion
This study uncovers an unexpected phenotype from a slight defect in the control system of 9+2 axonemes which has implications for the understanding of subtle axonemal defects in general.
The mercurial phenotypes of two new RSP6 mutants are different from the typical motility mutants that display steady-state deficiencies. It also differs from the first RSP6 mutant, pf26ts, whose flagella contains the mutated RSP6 and the other spokehead proteins and whose motility will not change once flagella have assembled. The flagella of the new RSP6 mutants most likely do not contain any RSP6 polypeptides as shown by the nature of their mutations, transformation rescue (Fig. 2) and western analyses of axonemes (Fig. 1). Yet the rest of the spokehead components could still assemble and the flagella are motile when cells are in the log phase and at room temperature. Hence, despite the high homology of RSP4 and RSP6, only RSP4 is essential for spokehead assembly and motility. In line with this conclusion, the duplicated RSP4/6 genes are not universal (Fig. 3). For organisms that have only a single RSP4/6 gene, the gene product may functions as a homodimer, since RSP4 and RSP6 extracted by concentrated KI buffer do not associate with RSP1 but remain co-sedimenting with each other, likely as a heterodimer (Kelekar et al., in press).
The 2nd RSP4/6 gene is used differently in different organisms. Although nonessential in the standard condition, RSP6-minus green algae easily become paralyzed, at slightly higher temperature or in spent media. Yet similar conditions occur frequently in the natural habitat. In this sense, the second paralogue is essential for the motility of these organisms. For human and mouse, both northern blots (Eriksson et al., 2001) and EST profile show that one of the two RSP4/6 genes, RSHL1, is only expressed in testis. Contrarily, the other gene encoding RSPH4A is expressed in all tissues with motile cilia and flagella and is a causative gene for primary cilia dyskinesia (Castleman et al., 2009). Thus RSPH4A, likely equivalent to RSP4, is essential for all motile cilia and flagella in mammals while RSHL1 may enhance fertility.
The most perplexing phenotypes of RSP6 mutants and the RSP11 mutant pf25 are mixed populations of cells with drastically different motility despite identical genetic background, and a 2-day delay of recovery. Although inhibition of PKA activity enhances the dynein-driven sliding velocity and reactivation (Hasegawa et al., 1987; Gaillard et al., 2006), paralyzed pf25 or pf26 cells will not become motile after treatment with the kinase inhibitor staurosporine, which is effective in vivo (e.g. Zhang and Snell, 1994). The possibilities of preferential selection of motile cells or second mutations have also been ruled out (Yang and Yang, 2006). Instead, the motility swing can be best explained by the varied assembly deficiencies detected by western blots (Fig.s 1 and 5B), although reductions of spokehead proteins are noticeable after most mutant cells become immotile. We reason that the lag of obvious assembly defects is because of the stringent requirement of RS for oscillatory beating and the limited sensitivity of western analyses.
Although the lack of spokehead, RS or CP results in paralyzed flagella, mere reduction of RS is sufficient to cause paralysis of two RS mutants, pf5 and pf27 (Huang et al., 1981; Yang and Yang, 2006). On the other hand, defective spoke HSP40 (RSP16) results in sporadic stalling, twitching flagella. Interestingly, spoke HSP40 and the other spokehead proteins decrease in RSP6-minus axonemes (Fig. 1A), suggesting that this U-shaped chaperone stabilizes the bulbous head/stalk junction by directly interacting with RSP2 on one end (Yang et al., 2008) and spokehead molecules on the other (this study). Similar motility phenotypes were observed in various CP mutants (Randall et al., 1964; Witman et al., 1978; Dutcher et al., 1984; Yokoyama et al., 2004). In addition, the planar waveform of reactivated sea-urchin axonemes became three dimensional after incubation with anti-RSP4/6 antibody (Gingras et al., 1998). These phenotypes support the model that bend propagation of a planar waveform, typical of the 9+2 axoneme, is founded on a mechanical feedback transduced between CP, RS and 9 outer doublets that inherently beat in three dimensions (Yang et al., 2008). The failure of motile RSP6 mutant cells in maintaining helical trajectories further underscores the importance of a precise feedback system in maintaining rhythmic beating and thus a helical trajectory.
We envision that the RSP4/6 heterodimer promotes effective assembly of spokeheads that guide the tilt-and-return of RS against CP (Warner and Satir, et al., 1974) along the length of flagella to propagate bend seamlessly in two dimensions and thus support helical trajectory. RSP6-minus RS, or lacking a few spokeheads causes glitches of this repeated movement leading to asynchrony and subsequent beat re-initiation and changes in trajectory directions, while further deficiencies result in frequent asynchrony, sporadic stalling and hence local moving cells. Those whose spoke deficiencies exceed a threshold level become immotile. Yet the reduction is not significant enough to be reliably revealed in protein gels (Huang et al., 1981) or western blots that are influenced by protein loads and antigen-antibody affinities unless the axonemes are prepared from the cultures older than usually used, in which spokeheads decreased drastically.
We postulate that the different facets of the changing phenotypes are due to the decreased availability of nutrients and the reduced assembly efficiency of defective complexes. Without RSP6, the other spokehead components assemble at a reduced rate. The inefficiency is negligible in healthy cells but becomes evident when nutrients are restricted, in spent media or on agar plates in which nutrients diffuse passively. Under the deprived conditions, a myriad of cellular reactions is critically reduced and yet the assembly of all components into WT RS remains coupled, albeit slower or less precise. However, the assembly of spokeheads without RSP6 becomes even slower than that of spokestalks, leading to the delivery of a mixed population of spokes, with or without the spokehead content, into flagella. Consequently, the motility does not improve until next day when new flagella generate after cell division and flagellar components are replenished. One day in rich media is insufficient for starved cells to recover fully and thus the percentage of swimmers does not reach the peak level until 2 days after resuspension (Fig, 4A). Furthermore, as vegetative algal cells usually multiply four fold each day during the log phase and then the rate tapers toward the stationary phase (Harris, 2009), the cell density, the available nutrients and the nutritional state of every cell may vary after logarithmic growths.
The media-dependent assembly deficiency likely is not unique to RSP6 or RS mutants. Although western blots are not adequate to reveal changes in the axonemal content of WT (Fig. 5B) or pf25 (Yang and Yang, 2006), reactivation profiles did differ. The motile fraction prepared from older WT culture (D4) is only slightly lower than that from earlier culture (D2) but the fraction of spinners is significantly higher (Fig. 4B). As reactivated cell models that lack RSP6 and some spokeheads have propensity to spin, the increased reactivated spinners from older WT culture (Fig. 4D) or after prolonged reactivation (Kamiya and Witman, 1984) may reflect subtle axonemal defects and the inherent difference of two flagella. Likewise, structural defects may underlie the ineffectiveness of the kinase inhibitor to enhance reactivation of damaged WT axonemes (Hasegawa et al., 1987) and RSP6-minus cell models. By the same token, the subtle axonemal defects in conjunction with the deficiency in two RSPs may be sufficient to paralyze old pf25 cells. It is quite interesting that immotile mutant cells can be reactivated to spin, consistent with the fact that sufficient RS are still present. Apparently, some factors in vivo hinder the residual motility of defective axoneme. Ample ATP in vitro or removal of the hydrated flagellar glycoproteins that increase the stroke load (Nakamura et al., 1996) come into mind.
As environmental factors can influence assembly of the defective axonemal complex of Chlamydomonas mutants, it is critical to investigate if similar scenarios occur to subtypes of the polygenic primary cilia dyskinesia (Afzelius, 2004).
Supplementary Material
Acknowledgements
We are grateful to Jessica Feldman and Dr. W. F. Marshall (University of California, San Francisco) and Dr. G. B. Witman (University of Massachusetts) for generously sharing additional RSP6 mutants and to Dr. Chun Yang for technical assistance and BAC DNA preparation. This work is supported by NIH Grant, GM068101 (to P. Yang) and GM044228 (to D.R. Mitchell).
References
- Afzelius BA. Cilia-related diseases. J Pathol. 2004;204(4):470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton. 1987;8(1):68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- Castleman VH, Romio L, Chodhari R, Hirst RA, de Castro SC, Parker KA, Ybot-Gonzalez P, Emes RD, Wilson SW, Wallis C, et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet. 2009;84(2):197–209. doi: 10.1016/j.ajhg.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry AM, Rosenbaum JL. Flagellar radial spoke: a model molecular genetic system for studying organelle assembly. Cell Motil Cytoskeleton. 1993;24(4):224–232. doi: 10.1002/cm.970240403. [DOI] [PubMed] [Google Scholar]
- Curry AM, Williams BD, Rosenbaum JL. Sequence analysis reveals homology between two proteins of the flagellar radial spoke. Mol Cell Biol. 1992;12(9):3967–3977. doi: 10.1128/mcb.12.9.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK, Huang B, Luck DJ. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J Cell Biol. 1984;98(1):229–236. doi: 10.1083/jcb.98.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Ansved T, Anvret M, Carey N. A mammalian radial spokehead-like gene, RSHL1, at the myotonic dystrophy-1 locus. Biochem Biophys Res Commun. 2001;281(4):835–841. doi: 10.1006/bbrc.2001.4465. [DOI] [PubMed] [Google Scholar]
- Frey E, Brokaw CJ, Omoto CK. Reactivation at low ATP distinguishes among classes of paralyzed flagella mutants. Cell Motil Cytoskeleton. 1997;38(1):91–99. doi: 10.1002/(SICI)1097-0169(1997)38:1<91::AID-CM8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gaillard AR, Fox LA, Rhea JM, Craige B, Sale WS. Disruption of the A-kinase anchoring domain in flagellar radial spoke protein 3 results in unregulated axonemal cAMP-dependent protein kinase activity and abnormal flagellar motility. Mol Biol Cell. 2006;17(6):2626–2635. doi: 10.1091/mbc.E06-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras D, White D, Garin J, Cosson J, Huitorel P, Zingg H, Cibert C, Gagnon C. Molecular cloning and characterization of a radial spoke head protein of sea urchin sperm axonemes: involvement of the protein in the regulation of sperm motility. Mol Biol Cell. 1998;9(2):513–522. doi: 10.1091/mbc.9.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HH. Chlamydomonas SourceBook. 2nd Ed. Volume 1. Oxford, UK: Elsevier Inc.; 2009. Chaper 8. [Google Scholar]
- Hasegawa E, Hayashi H, Asakura S, Kamiya R. Stimulation of in vitro motility of Chlamydomonas axonemes by inhibition of cAMP-dependent phosphorylation. Cell Motil Cytoskeleton. 1987;8(4):302–311. doi: 10.1002/cm.970080403. [DOI] [PubMed] [Google Scholar]
- Hayashibe K, Shingyoji C, Kamiya R. Induction of temporary beating in paralyzed flagella of Chlamydomonas mutants by application of external force. Cell Motil Cytoskeleton. 1997;(3):232–239. doi: 10.1002/(SICI)1097-0169(1997)37:3<232::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Huang B, Piperno G, Luck DJ. Paralyzed flagella mutants of Chlamydomonas reinhardtii. Defective for axonemal doublet microtubule arms. J Biol Chem. 1979;254(8):3091–3099. [PubMed] [Google Scholar]
- Huang B, Piperno G, Ramanis Z, Luck DJ. Radial spokes of Chlamydomonas flagella: genetic analysis of assembly and function. J Cell Biol. 1981;88(1):80–88. doi: 10.1083/jcb.88.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell. 1982;28(1):115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Kamiya R. Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants. Int Rev Cytol. 2002;219:115–155. doi: 10.1016/s0074-7696(02)19012-7. [DOI] [PubMed] [Google Scholar]
- Kamiya R, Witman GB. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984;98(1):97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar P, Wei M, Yang P. Isolation and analysis of radial spoke proteins. In: King SM, Pazour GJ, editors. Methods in Cell Biology. San Diego: Elsevier; 2009. In press. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Pedersen LB, Feely M, Rosenbaum JL, Mitchell DR. ATP production in Chlamydomonas reinhardtii flagella by glycolytic enzymes. Mol Biol Cell. 2005;16(10):4509–4518. doi: 10.1091/mbc.E05-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Sale WS. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol. 1999;144(2):293–304. doi: 10.1083/jcb.144.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-King RS, Gorbatyuk O, Takebe S, King SM. Flagellar radial spokes contain a Ca2+-stimulated nucleoside diphosphate kinase. Mol Biol Cell. 2004;15(8):3891–3902. doi: 10.1091/mbc.E04-04-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Huang B, Luck DJ. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977;74(4):1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Huang B, Ramanis Z, Luck DJ. Radial spokes of Chlamydomonas flagella: polypeptide composition and phosphorylation of stalk components. J Cell Biol. 1981;88(1):73–79. doi: 10.1083/jcb.88.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151(5):F37–F42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall J, Warr JR, Hopkins JM, McVittie A. A Single-Gene Mutation of Chlamydomonas Reinhardii Affecting Motility: a Genetic and Electron Microscope Study. Nature. 1964;203:912–914. doi: 10.1038/203912a0. [DOI] [PubMed] [Google Scholar]
- Satouh Y, Padma P, Toda T, Satoh N, Ide H, Inaba K. Molecular characterization of radial spoke subcomplex containing radial spoke protein 3 and heat shock protein 40 in sperm flagella of the ascidian Ciona intestinalis. Mol Biol Cell. 2005;16(2):626–636. doi: 10.1091/mbc.E04-09-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol Biol Cell. 2002;13(9):3303–3313. doi: 10.1091/mbc.E02-04-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF, Sale WS. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science. 1992;257(5076):1557–1559. doi: 10.1126/science.1387971. [DOI] [PubMed] [Google Scholar]
- Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskeleton. 2004;57(1):8–17. doi: 10.1002/cm.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FD, Satir P. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule sliding. J Cell Biol. 1974;63(1):35–63. doi: 10.1083/jcb.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Hendrickson T, Sale WS. Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation. Cell Motil Cytoskeleton. 2007;64(8):569–579. doi: 10.1002/cm.20211. [DOI] [PubMed] [Google Scholar]
- Witman GB, Plummer J, Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978;76(3):729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Kamimura S, Kamiya R. Nanometer scale vibration in mutant axonemes of Chlamydomonas. Cell Motil Cytoskeleton. 1994;29(2):177–185. doi: 10.1002/cm.970290209. [DOI] [PubMed] [Google Scholar]
- Yang C, Owen HA, Yang P. Dimeric heat shock protein 40 binds radial spokes for generating coupled power strokes and recovery strokes of 9 + 2 flagella. J Cell Biol. 2008;180(2):403–415. doi: 10.1083/jcb.200705069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yang P. The flagellar motility of Chlamydomonas pf25 mutant lacking an AKAP-binding protein is overtly sensitive to medium conditions. Mol Biol Cell. 2006;17(1):227–238. doi: 10.1091/mbc.E05-07-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Diener DR, Rosenbaum JL, Sale WS. Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J Cell Biol. 2001;153(6):1315–1326. doi: 10.1083/jcb.153.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Diener DR, Yang C, Kohno T, Pazour GJ, Dienes JM, Agrin NS, King SM, Sale WS, Kamiya R, et al. Radial spoke proteins of Chlamydomonas flagella. J Cell Sci. 2006;119(Pt 6):1165–1174. doi: 10.1242/jcs.02811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama R, O'Toole E, Ghosh S, Mitchell DR. Regulation of flagellar dynein activity by a central pair kinesin. Proc Natl Acad Sci U S A. 2004;101(50):17398–17403. doi: 10.1073/pnas.0406817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mitchell DR. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci. 2004;117(Pt 18):4179–4188. doi: 10.1242/jcs.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Snell WJ. Flagellar adhesion-dependent regulation of Chlamydomonas adenylyl cyclase in vitro: a possible role for protein kinases in sexual signaling. J Cell Biol. 1994;125(3):617–624. doi: 10.1083/jcb.125.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.