Abstract
Shp2/Ptpn11 tyrosine phosphatase is a general regulator of the RTK pathways. By genetic ablation, we demonstrate that Shp2 is required for lacrimal gland budding, lens cell proliferation, survival and differentiation. Shp2 deletion disrupted ERK signaling and cell cycle regulation, which could be partially compensated by activated Kras signaling, confirming that Ras signaling was the main downstream target of Shp2 in lens and lacrimal gland development. We also showed that Sprouty2, a general suppressor of Ras signaling, was regulated by Shp2 positively at the transcriptional level and negatively at the post-translational level. Only in the absence of Sprouty2 could activated Kras signaling robustly rescue the lens proliferation and lacrimal-gland-budding defects in the Shp2 mutants. We propose that the dynamic regulation of Sprouty by Shp2 might be important not only for modulating Ras signaling in lens and lacrimal gland development, but also for RTK signaling in general.
Keywords: FGF, Ras, Shp2, Sprouty, Lacrimal gland
INTRODUCTION
Despite their morphological differences, both the lens and the lacrimal gland of the vertebrate eye originate from the surface head ectoderm during embryonic development. The presumptive lens ectoderm is first induced by the underlying optic vesicle to become a single layer of thickening cells, the lens placode, which invaginates at mouse embryonic day 10.5 (E10.5) to form a lens vesicle. The posterior lens epithelial cells next elongate into the primary lens fibers to fill up the lens vesicle cavity at E12.5. Further lens growth is driven by the anterior lens epithelial cells that proliferate and migrate inward at the equatorial region of the lens, eventually differentiating into orderly arrays of the secondary lens fibers. A few days after lens induction, at E12.5, the lacrimal gland anlage first appears as a thickening of conjunctival epithelium at the temporal side of the eye. This ectodermal placode also invaginates to form the lacrimal bud, invading the surrounding neural-crest-derived periocular mesenchyme. Eventually, the elongating lacrimal gland undergoes branching morphogenesis to form dense glandular structures.
Development of the lens and lacrimal gland of the eye also share the requirement for RTK pathways, especially FGF signaling. Although several RTK signaling factors, including PDGF, IGF, EGF and HGF, can promote lens cell proliferation in vitro, only FGFs can induce lens cell differentiation in mammals (Choi et al., 2004; Hyatt and Beebe, 1993; Kok et al., 2002; Liu et al., 1996; McAvoy and Chamberlain, 1989; Reddan and Wilson-Dziedzic, 1983; Wormstone et al., 2000). In transgenic mice, misexpression of the secreted forms of Fgf1, 3, 4, 7, 8 or 9 led to premature differentiation of lens epithelial cells into fiber cells, whereas dominant-negative Fgfr1 suppressed lens epithelial cell proliferation (Faber et al., 2001; Lovicu and Overbeek, 1998; Robinson et al., 1998; Robinson et al., 1995). These studies were further corroborated by genetic depletion of Fgfr1/2/3 after lens induction, which resulted in an empty lens vesicle, completely devoid of lens fiber cells (Zhao et al., 2008). Similarly, Fgf10 has been shown to induce ectopic lacrimal gland in explant cultures and transgenic animals, whereas ablation of Fgf10 or Fgfr2 abolished mouse lacrimal gland development (Govindarajan et al., 2000; Makarenkova et al., 2000; Pan et al., 2008). Consistent with this, we have shown recently that defective biosynthesis of heparan sulfate, a crucial co-receptor of FGFR, also resulted in loss of lens and lacrimal gland (Pan et al., 2008; Pan et al., 2006). These results firmly established the essential role of FGF signaling in lens and lacrimal gland development.
How is FGF signaling transduced in these two organs? Previous studies have shown that loss of FGF signaling in both lens and lacrimal gland disrupted ERK signaling, but the functional significance and regulatory mechanism of the FGF-ERK pathway remain to be explored. In this paper, we address these questions by studying Shp2 (Ptpn11 — Mouse Genome Informatics), a protein tyrosine phosphatase that controls RTK signaling through a yet unknown mechanism (Feng, 1999; Neel et al., 2003). Biochemical studies have indicated that Shp2 can be recruited to FGFR by direct binding to the scaffold protein Frs2α, and together they provide docking sites for the adaptor protein Grb2 (Kouhara et al., 1997; Ong et al., 2000). Grb2 then activates Ras-ERK signaling via the nucleotide exchange factor Sos or stimulates PI3K signaling by its association with Gab1 protein (Hadari et al., 2001). Furthermore, Shp2 has been proposed to suppress the negative Ras regulators, Sprouty (Spry — Mouse Genome Informatics) and RasGAP (Rasa1 — Mouse Genome Informatics), or to activate Src family kinases, thus indirectly promoting Ras-ERK signaling (Cunnick et al., 2002; Hanafusa et al., 2004; Zhang et al., 2004b). By genetic analysis, we now show that Shp2 ablation results in ERK signaling failure and developmental defects in lacrimal gland and lens, which can be partially reversed by constitutively activated Kras signaling. However, loss of Shp2 function also exposes Sprouty2 (Spry2 — Mouse Genome Informatics) to excessive phosphorylation, which leads to downregulation of the overall Ras-ERK signaling. Only by both deleting Sprouty2 and activating Kras can we restore robust lens proliferation and lacrimal gland budding. These results demonstrate that Shp2 controls Sprouty2 to regulate Ras-ERK signaling in lens and lacrimal gland development.
MATERIALS AND METHODS
Mice
Shp2flox mice have been previously described (Zhang et al., 2004a). Drs Ruth Ashery-Padan (Tel Aviv University, Tel Aviv, Israel) and Richard Lang (Children's Hospital Research Foundation, Cincinnati, OH) kindly provided the Le-Cre mice, which exhibit normal lens and lacrimal gland development as heterozygotes (see Fig. S1 in the supplementary material) (Ashery-Padan et al., 2000; Pan et al., 2008). LSL-KrasG12D mice were obtained from the Mouse Models of Human Cancers Consortium (MMHCC) Repository at the National Cancer Institute (Tuveson et al., 2004). The genotyping primers used for detecting the loss of the LSL cassette in the Le-Cre;Shp2flox/flox;LSL-KrasG12D lens were: LSL (forward: 5′-CTAGCCACCATGGCTTGAGT-3′; reverse: 5′-GCTCCAACCACCACAAGTTT-3′) and Kras (forward: 5′-GTCGACAAGCTCATGCGGGTG-3′; reverse: 5′-CCTTTACAAGCGCACGCAGACTGTAGA-3′). Sprouty2flox mice came from the Mutant Mouse Regional Resource Centers (MMRRC) (Shim et al., 2005) and were crossed with a germ cell active Cre line EIIa-Cre from the Jackson Laboratory (Bar Harbor, Maine) to generate the systemic Sprouty2KO mice (Xu et al., 2001). All experiments were performed in accordance with institutional guidelines.
Histology
After overnight fixation in 4% paraformaldehyde (PFA), the embryos were dehydrated progressively through 30, 50, 70, 90 and 100% ethanol, cleared in xylene and embedded in paraffin. The paraffin sample blocks were oriented on a Leica 2125 microtome and sectioned at 10 μm. These sections were rehydrated, stained with Hematoxylin and Eosin, and mounted with coverslips, before digital pictures of the sections were taken on a Leica DM500 compound microscope. For the quantification of the lens size and lacrimal gland length, the maximum area of the lens and the longest distance from the tip of the lacrimal gland to the deepest rim of the conjunctival epithelium were measured using the ImageJ program (National Institute of Health, Bethesda, MD), and the statistical significance was calculated by one-way ANOVA analysis.
Immunohistochemistry
BrdU, TUNEL staining and immunohistochemistry were performed as previously described (Pan et al., 2008; Pan et al., 2006). The antibodies used were anti-Shp2 (Sc-280, Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-ERK1/2 (#9101), anti-Cyclin D1 (#2926) and D3 (#2936) (all from Cell Signaling Technology, Beverly, MA), anti-phospho-Histone H3 (#06-570, Upstate, Temecula, CA), anti-E-cadherin (U3254, Sigma, St Louis, Missouri), anti-BrdU (G3G4, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), anti-Ki67 (#550609, BD Pharmingen San Diego, CA), anti-Prox1 (PRB-238C) and anti-Pax6 (PRB-278P) (both from Covance, Berkeley, CA). Anti-α, β and γ crystallins were kindly provided by Sam Zigler (National Eye Institute, Bethesda, MD). The cell proliferation and apoptosis were measured as the ratio of BrdU, phospho-Histone H3, Ki67 or TUNEL-positive cells versus DAPI-positive cells, and analyzed by one-way ANOVA analysis.
RNA in situ hybridization
RNA in situ hybridizations were carried out as previously described (Pan et al., 2008). The following probes were used: Erm, Pea3 and Er81 (all from Dr Bridget Hogan, Duke University Medical Center, Durham, NC), Sprouty2 (from Gail Martin, University of California at San Francisco, San Francisco, CA). The 288 bp Shp2 probe spanning exon 4 of Shp2 was cloned from a mouse Shp2 cDNA kindly provided by Dr Rebecca Chan (Indiana University School of Medicine, Indianapolis, IN). At least three embryos of each genotype were analyzed for each probe.
Western blot and immunoprecipitation
The lenses were collected at E16.5 and homogenized in RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS) with protease and phosphatase inhibitors (Pierce, Rockford, IL). After heat denaturation, equal amounts of lens protein lysates, as previously determined by BCA protein assay (Pierce, Rockford, IL), were separated on 12% SDS-polyacrylamide gels and transferred to PVDF membranes (Millipore, Billerica, MA). The blots were blocked in 5% non-fat dry milk for 30 minutes and incubated overnight with anti-phospho-ERK1/2 (#9101), anti-ERK1/2 (#4695), anti-phospho-AKT (#3787) anti-AKT (#9272), (1:1000, all from Cell Signaling Technology, Beverly, MA) or anti-β-actin (A5441) (1:5000, Sigma) at 4°C. After incubation with the HRP-conjugated secondary antibody for 2 hours, the protein signals were revealed by enhanced chemiluminescence (Pierce, Rockford, IL) and exposed on X-ray films. For two-color quantitative western analysis, the blot was probed simultaneously with the mouse anti-phospho-ERK1/2 (sc-7383, Santa Cruz Biotechnology) and the rabbit anti-ERK1/2 (#4695, Cell Signaling Technology), followed by incubation with infrared-conjugated secondary antibodies. The membrane was scanned in an Odyssey SA scanner (LICOR Biosciences, Lincoln, NE), and band intensities were quantified using the Odyssey software.
For immunoprecipitation, lenses dissected from newborn pups were snap frozen in liquid nitrogen and stored at −80°C until use. Lens samples of 600 μg (about 20 pairs) were lysed in 300 μl of non-denaturing buffer (20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 1% NP40, 2 mM EDTA) containing protease and phosphatase inhibitors (Pierce, Rockford, IL) and incubated with 2 μl normal goat serum for 1 hour, followed by 5 μl A/G protein agarose for 30 minutes to remove non-specific proteins. After centrifugation, the supernatant was incubated sequentially with 2 μg anti-Sprouty2 or control IgG antibodies and 20 μl A/G protein agarose overnight at 4°C, before being washed three times in 1 ml of lysis buffer. The pellet was collected by centrifugation and subjected to western blot as described above using the following antibodies: anti-Shp2 (Sc-280, Santa Cruz Biotechnology), anti-phosphotyrosine (clone 4G10, Upstate) and anti-Sprouty2 (S1444, Sigma).
RESULTS
Shp2 ablation disrupts lens and lacrimal gland development
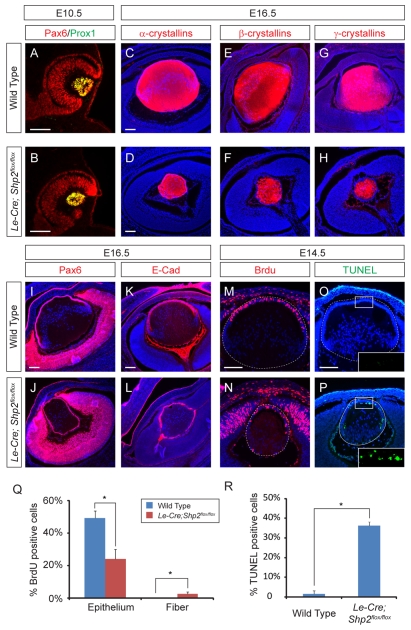
The Shp2 conditional knockout was generated by crossing the Shp2flox allele with the Le-Cre transgene, which is expressed as early as E9.5 in the surface ectoderm before it gives rise to the lens and the lacrimal gland. At E12.5, however, the Le-Cre;Shp2flox/flox mutant embryos were still indistinguishable from the wild-type controls, and histological analysis showed that the primary lens fiber cells had elongated anteriorly to fill up the lens vesicle (Fig. 1A,B). The lens phenotype became visible after E14.5, when the Le-Cre;Shp2flox/flox mutants exhibited reduced lens size and abnormal posterior shift of the lens epithelial cells (Fig. 1C-F, arrowheads). More severe defects, as we have shown previously, were observed in the development of lacrimal gland, which had clearly budded from the conjunctival epithelium in the wild-type embryos at E15.5, but never formed in the Shp2 mutants (Fig. 1G,H, arrows) (Pan et al., 2008). At postnatal day 0 (P0), the anterior lens epithelial cells, which normally migrate to the equatorial regions of the lens to differentiate, now completely surrounded the small Shp2 mutant lens, and the nuclei of the lens fiber cells inside the lens were also grossly disorganized amid numerous vacuoles (Fig. 1I,J, arrowheads). These results show that Shp2 is required for lens and lacrimal gland development.
Fig. 1.
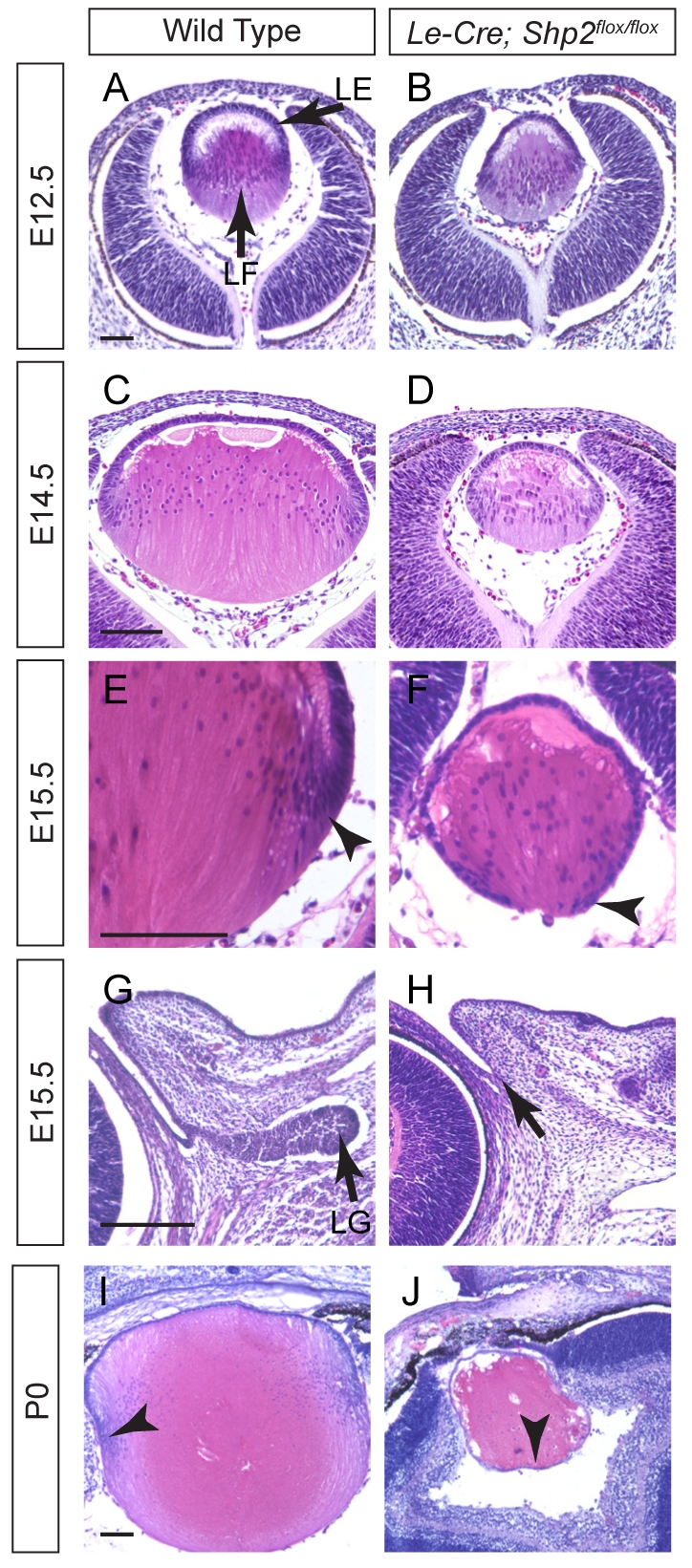
Shp2 is required for lens and lacrimal gland development. (A-D) The Le-Cre;Shp2flox/flox mutant lens exhibited normal primary lens fiber cell elongation at E12.5 but reduced lens size at E14.5. (E-H) The anterior lens epithelial cells shifted posteriorly in the E15.5 Le-Cre;Shp2flox/flox mutant lens (arrowheads), and no lacrimal gland was detected (arrows). (I,J) At P0, the Le-Cre;Shp2flox/flox mutant lens was completely surrounded by epithelial cells (arrowheads), indicating a failure of lens epithelial cell differentiation. Scale bars: 100 μm. LE, lens epithelium; LF, lens fiber; LG, lacrimal gland.
We next explored the molecular defects in the Shp2 mutant lens. The early lens vesicle development proceeded normally at E10.5, as shown by the strong expression of the lens determination proteins, Pax6 and Prox1 (Fig. 2A,B). Similarly, the lens differentiation markers α-, β- and γ-crystallins were also abundantly expressed at E16.5, suggesting that the loss of Shp2 did not abrogate the primary lens fiber cell differentiation (Fig. 2C-H). By contrast, Pax6 and E-cadherin expression, which at E16.5 labeled the lens epithelium, now extended to the posterior rim of the lens (Fig. 2I-L). This is consistent with the histological analysis above that showed failure of the lens epithelial cells to migrate inside the lens to differentiate into secondary lens fibers. As shown by the BrdU incorporation assay and TUNEL staining, the Shp2 mutant lens epithelium also exhibited reduced cell proliferation and extensive apoptosis, which explained the significant reduction in lens size (Fig. 2M-R). Taken together, these data support the idea that Shp2 function is essential for lens epithelial cell proliferation, survival and differentiation.
Fig. 2.
Defective lens epithelial cell development in the Shp2 mutant lens. (A-H) Similar to wild type controls, the Le-Cre;Shp2flox/flox mutant lens expressed Pax6 and Prox1 at E10.5, and α-, β- and γ-crystallins at E16.5. (I-L) The Pax6 and E-Cadherin positive lens epithelium encircled the Le-Cre;Shp2flox/flox mutant lens, indicative of the failure of lens epithelial cells to differentiate into secondary lens fibers. (M-P) Cell proliferation and survival defects in the Le-Cre;Shp2flox/flox mutant lens as shown by reduced BrdU incorporation and increased TUNEL staining. (Q) The ratio of BrdU-positive cells versus DAPI-positive cells decreased in the epithelial but increased in the fiber compartments of the E14.5 Le-Cre;Shp2flox/flox lens (n=3) compared with the wild type (n=4) (*P<0.001). The dashed lines in M and N encircled the lens cells that were counted. (R) The percentage of TUNEL-positive cells increased in the E14.5 Le-Cre;Shp2flox/flox mutant lens (n=5) compared with the wild type (n=3) (*P<0.0001). All lens cells within the dashed circle in O and P were counted. Scale bars: 100 μm.
Shp2 regulates ERK signaling
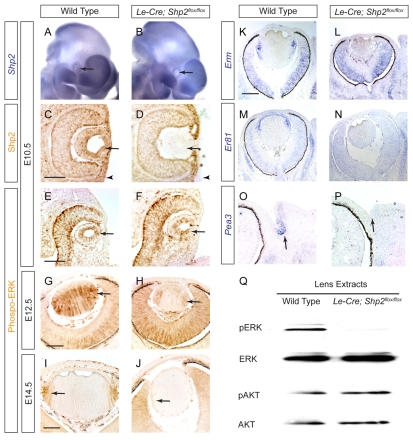
Considering that the Le-Cre transgene in the Le-Cre;Shp2flox/flox animals was active at E9.5, it is surprising that no lens abnormality was detected before E12.5. This prompted us to examine the timing of Shp2 ablation in the Le-Cre;Shp2flox/flox embryos. Using a probe specific to exon 4 of Shp2, which is flanked by LoxP sites in the Shp2flox allele, we showed by RNA in situ hybridization that this crucial segment of Shp2 transcript was indeed lost in the mutant lens at E10.5 (Fig. 3A,B). Similarly, Shp2 protein was undetectable by immunohistochemistry in the E10.5 mutant lens vesicle and in the surface ectoderm, which was the precursor to the future lacrimal gland (Fig. 3C,D, arrows and arrowheads). We have previously shown that Shp2 is required for ERK signaling in lacrimal gland budding (Pan et al., 2008). However, using a phospho-specific antibody that recognizes the active form of ERK, we did not detect any reduction of phospho-ERK staining in the E10.5 lens (Fig. 3E,F). Phospho-ERK staining in the Shp2 mutant lens was eventually lost after E12.5, which coincided with the onset of the lens phenotype (Fig. 3G-J). This suggests that Shp2 ablation in the Le-Cre;Shp2flox/flox mutants did not impact ERK signaling until after the lens induction phase.
Fig. 3.
Shp2 ablation disrupted ERK but not AKT signaling. (A-D) At E10.5 in the Shp2 mutant, both Shp2 mRNA and protein were lost in the lens vesicle (arrows) and the adjacent ectoderm destined to become lacrimal gland (arrowheads). (E-J) Phospho-ERK expression in the Shp2 mutant lens was preserved at E10.5, but abolished after E12.5 (arrows). (K-P) Shp2 deletion abolished the expression of the RTK responsive genes, Erm, Er81 and Pea3 in lens and lacrimal gland development. (Q) Western blot confirmed that phospho-ERK (pERK) but not phospho-AKT (pAKT) was lost in the E16.5 Shp2 mutant lens. Scale bars: 100 μm.
We next assayed the known RTK downstream response genes, Erm (Etv5 — Mouse Genome Informatics), Er81 (Etv1 — Mouse Genome Informatics) and Pea3 (Etv4 — Mouse Genome Informatics), the expression of which is regulated by FGF and GDNF signaling in many developing tissues (Haase et al., 2002; Munchberg and Steinbeisser, 1999; Raible and Brand, 2001; Roehl and Nusslein-Volhard, 2001). At E14.5, the expression of Erm and Er81, which are normally present in the anterior lens epithelium and the lens equator, respectively, were abolished by Shp2 mutation (Fig. 3K-N). Similarly, Erm and Pea3 expression, which mark the budding lacrimal gland, were also completely lost in the Shp2 mutants (Fig. 3O,P, arrows; Fig. 4I-J′, arrowheads). Finally, we collected the lens from E16.5 wild-type and Shp2 mutant embryos and performed western blot experiments. Although ERK, AKT and phospho-AKT were present at comparable levels, phospho-ERK was lost in the Shp2 mutant lens (Fig. 3Q). Therefore, we conclude that Shp2 controls ERK but not AKT signaling in lens development.
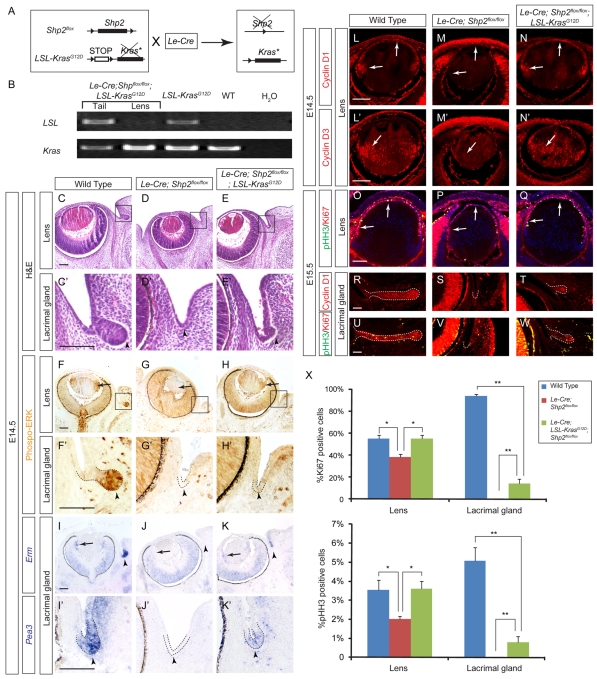
Fig. 4.
Kras activation partially compensated for the loss of Shp2. (A) The LSL-KrasG12D allele can be activated when a LoxP sites-flanked transcriptional STOP cassette (LSL) is removed by the Le-Cre driver, which also inactivates the Shp2flox allele in lens and lacrimal gland. This results in activated Kras signaling in the Shp2 mutant background. (B) Allele-specific genotyping revealed the loss of the LSL allele in the Le-Cre;Shp2flox/flox;LSL-KrasG12D mutant lens but not in the tail, demonstrating the efficiency and specificity of the Le-Cre transgene in cleaving the STOP cassette. As a control, the Kras allele was unaffected. (C-E′) At E14.5, Kras activation led to increased lens size and slight bulging of lacrimal gland ectoderm (arrowheads). (F-H′) At E14.5, KrasG12D mutation induced significant phospho-ERK expression in the lens (arrows), and to a lesser extent, in the lacrimal gland (arrowheads). (I-K′) RTK signaling response genes Erm and Pea3 were weakly but clearly induced in the lacrimal gland primordium by Kras activation (arrows). (L-Q) The KrasG12D mutation also reversed the loss of Cyclin D1 and D3 expression in the Shp2 mutant lens, leading to increased phospho-Histone H3 (pHH3) and Ki67 expressions (arrows). (R-W) At E15.5, Cyclin D1, pHH3 and Ki67 expressions were observed in the small lacrimal gland bud in the Le-Cre;Shp2flox/flox;LSL-KrasG12D mutants (highlighted by dashed lines). (X) Cell proliferation in the E15.5 lens and lacrimal gland as measured by the percentage of the Ki67- and pHH3-positive cells in the wild type (n=8), the Le-Cre;Shp2flox/flox (n=6) and the Le-Cre;Shp2flox/flox;LSL-KrasG12D (n=8) mutants (*P<0.001, **P<0.0001). Scale bars: 100 μm.
Activated Kras attenuated the Shp2 lens and lacrimal gland phenotype
To investigate the molecular mechanism of Shp2 in ERK signaling, we next asked whether constitutively activated Ras signaling, which is upstream to ERK, could rescue the Shp2 mutant phenotype in vivo. This was accomplished by crossing Le-Cre;Shp2flox/flox with the LSL-KrasG12D allele, which contains a floxed transcriptional STOP cassette (LSL) in front of a constitutively active G12D mutation in the Kras gene (Tuveson et al., 2004). Cre-mediated recombination driven by the Le-Cre transgene could thus simultaneously disable Shp2 and activate Kras signaling by removing the STOP cassette, which had prevented the expression of KrasG12D (Fig. 4A). Furthermore, as a genetic knock-in, the LSL-KrasG12D allele was under the same transcriptional regulation as the endogenous Kras locus, which ensures the expression of KrasG12D at the normal physiological level. Indeed, allele-specific genotyping showed that the LSL cassette was lost in the lens but not the tail of the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants, whereas the Kras allele was unaffected (Fig. 4B). We further showed that the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants exhibited a statistically significant increase in lens size (Fig. 4C-E,6W). Importantly, whereas the Le-Cre;Shp2flox/flox embryos never exhibited any lacrimal gland bud, the Le-Cre;Shp2flox/flox;LSL-KrasG12D mutants now showed a slight bulging at the fornix of conjunctival epithelium at E14.5 and a more obvious extrusion at E15.5, although the lacrimal gland budding was still not comparable to the wild type when quantified (Fig. 4C′-E′, arrowheads, R-W, dashed lines; Fig. 6W). Therefore, at least morphologically, activated Kras signaling partially compensated for the loss of Shp2 in lens and lacrimal gland development.
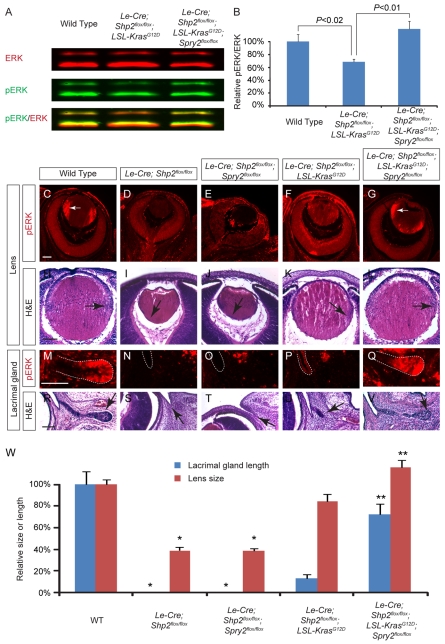
Fig. 6.
Sprouty2 ablation synergized with Kras activation to rescue the Shp2 mutant phenotype. (A,B) Western blot analysis with infrared fluorescence showed that the phospho-ERK (pERK) level in the Le-Cre;Shp2flox/flox;LSL-KrasG12D mutant lens was lower than that in wild type or the Le-Cre;Shp2flox/flox;LSL-KrasG12D;Spry2flox/flox mutant. The relative pERK/ERK ratios were averaged from three independent sets of samples. (C-G) The lens phospho-ERK expression was lost in the Le-Cre;Shp2flox/flox and the Le-Cre;Shp2flox/flox;Spry2flox/flox mutants, upregulated in the Le-Cre;Shp2flox/flox;LSL-KrasG12D mutants, and fully recovered in the Le-Cre;Shp2flox/flox;LSL-KrasG12D;Spry2flox/flox mutants (arrows). (H-L) Sprouty2 deletion had no effect in the Le-Cre;Shp2flox/flox mutant background, but when combined with the KrasG12D mutation, it helped to restore the Shp2 lens growth and the normal lens epithelium cell migration pattern (arrows). (M-V) Similarly, the lacrimal gland phospho-ERK staining and outgrowth (arrows) was partially rescued by Kras activation alone and fully restored after additional Sprouty2 deletion. (W) Quantification of the lacrimal gland length and lens sizes at E15.5. [*P<0.001 for the Le-Cre;Shp2flox/flox (n=5) or the Le-Cre;Shp2flox/flox;Spry2flox/flox mutants (n=7) compared with wild type (n=5) or the Le-Cre;Shp2flox/flox;LSL-KrasG12D mutants (n=4); ** P<0.01 for the Le-Cre;Shp2flox/flox;LSL-KrasG12D;Spry2flox/flox mutants (n=4) compared with the Le-Cre;Shp2flox/flox;LSL-KrasG12D mutants (n=5)]. Scale bars: 100 μm.
We next examined the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants in molecular detail. Consistent with the role of Kras in ERK signaling, phospho-ERK staining was now clearly observed in the double mutant lens and became weakly detectable at the conjunctival fornix (Fig. 4F-H, arrows; 4F′-H′, arrowheads). The relatively low level of ERK activation was further supported by the expression of Erm and Pea3, which was also weakly induced in the Le-Cre;Shp2flox/flox;LSL-KrasG12D lacrimal gland primordium (Fig. 4I-K′, arrowheads). This would explain the very modest initiation of lacrimal gland budding in the double mutants compared with that of the wild types. By immunostaining, we showed that Shp2 ablation downregulated the expression of the cell cycle regulators Cyclin D1 and Cyclin D3 in the Le-Cre;Shp2flox/flox single mutant lens, as expected from the cell proliferation defects observed earlier. In the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants, however, both Cyclin D1 and Cyclin D3 expression was elevated (Fig. 4L-N′, arrows), which also led to increased levels of the general cell cycle markers Ki67 and M-phase-specific marker phospho-Histone H3 in the lens (Fig. 4O-Q,X). Cyclin D1, Ki67 and phospho-Histone H3 expressions were also increased in the Le-Cre;Shp2flox/flox;LSL-KrasG12D lacrimal gland, although the percentage of Ki67 or phospho-Histone H3 positive cells was still significantly lower than that of the wild-type lacrimal gland (Fig. 4R-W, dashed lines). Direct activation of Kras could thus partially restore ERK signaling and cell proliferation in the Shp2 lens and lacrimal gland.
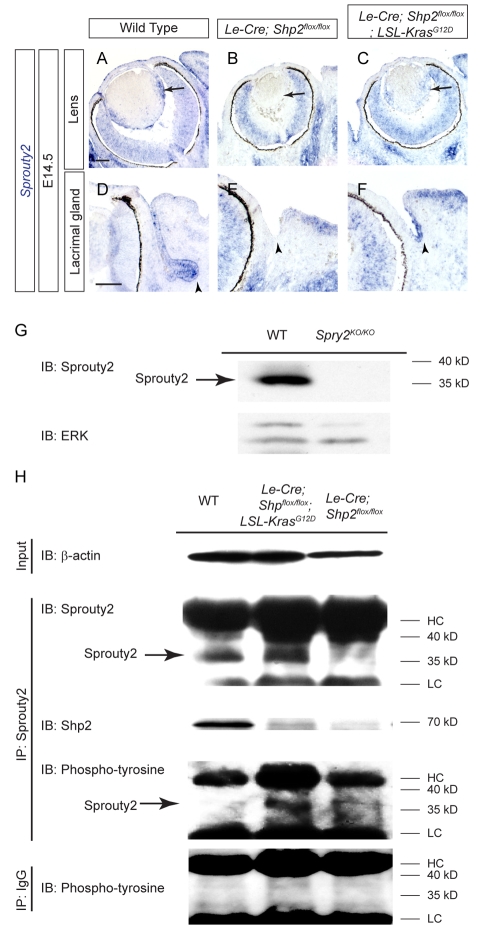
Shp2 controls Sprouty2 tyrosine phosphorylation in the lens
The incomplete rescue in the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants suggested that there existed additional Shp2 signaling defects not compensated by the direct Kras activation. Although the direct targets of Shp2 tyrosine phosphatase are still uncertain, biochemical studies have shown that Shp2 can dephosphorylate Sprouty family proteins, which are general suppressors of RTK signaling (Hanafusa et al., 2004; Jarvis et al., 2006; Tefft et al., 2005). This will negatively regulate the Sprouty activities and eventually lead to increased ERK signaling. Interestingly, we noticed that Sprouty2 was expressed in the wild-type but not in the Shp2 mutant lens and lacrimal gland primordium, demonstrating that Shp2 signaling also positively regulated Sprouty2 expression (Fig. 5A,B,D,E, arrows and arrowheads). Introduction of the LSL-KrasG12D allele also induced Sprouty2 expression in both lens and lacrimal gland (Fig. 5C,F, arrow and arrowhead), but this did not necessarily indicate that Sprouty2 protein was appropriately phosphorylated in the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants. We therefore immunoprecipitated Sprouty2 protein from the lens extracts collected from newborn animals. The antibody we used is highly specific to Sprouty2 protein, as demonstrated in the western blot of the systemic Sprouty2 null (Spry2KO/KO) lysate (Fig. 5G). As expected from the Sprouty2 mRNA expression above, western blot analysis showed that Sprouty2 protein was present in both wild type and the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants but not in the Le-Cre;Shp2flox/flox single mutant (Fig. 5H). Importantly, Shp2 protein also co-immunoprecipitated with Sprouty2 in the wild-type lens extracts, consistent with the previous reports of direct protein-protein interaction between Shp2 and Sprouty (Fig. 5H) (Hanafusa et al., 2004; Jarvis et al., 2006; Tefft et al., 2005). Finally, we showed by phosphotyrosine immunoblotting that Sprouty2 was hyperphosphorylated in the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants (Fig. 5H). These results indicate that loss of Shp2 phosphatase activity leads to unchecked tyrosine phosphorylation in Sprouty2 protein.
Fig. 5.
Shp2 controls Sprouty2 expression and tyrosine phosphorylation. (A-F) Sprouty2 mRNA expression (arrows, arrowheads) was lost in the Shp2 mutants but induced by the addition of the KrasG12D allele. (G) The specificity of the Sprouty2 antibody was demonstrated in the western blot (IB) of the Spry2KO/KO mutant lens. The same blot was also probed with the ERK antibody as a loading control. (H) Sprouty2 was immunoprecipitated (IP) from equal amounts of lens lysate from the newborn animals as shown by the β-actin western blots (Input). The western blots further confirmed the loss of Sprouty2 in the Le-Cre;Shp2flox/flox mutants, the association of Shp2 with Sprouty2 in the wild-type controls and increased tyrosine phosphorylation of Sprouty2 in Le-Cre;Shp2flox/flox;LSL-KrasG12D compound mutants. As a control, no tyrosine phosphorylated Sprouty2 were detected in a non-specific IgG immunoprecipitate. Scale bars: 100 μm. HC, immunoglobulin heavy chain (50 kD); LC, immunoglobulin light chain (25 kD).
Combined Sprouty2 ablation and Kras activation rescued Shp2 deletion in lens and lacrimal gland development
Sprouty2 phosphorylation is known to augment its inhibitory activity, which would dampen the ERK activation in the Le-Cre;Shp2flox/flox;LSL-KrasG12D double mutants. We thus hypothesized that the removal of Sprouty2 would relieve this excessive inhibitory loop, allowing full activation of Kras-ERK signaling to rescue the Shp2 mutant phenotype. Indeed, quantitative western blot analysis showed that, although ERK phosphorylation in the Le-Cre;Shp2flox/flox;LSL-KrasG12D lens remained significantly lower than that in the wild-type controls, additional ablation of Sprouty2 further elevated the phospho-ERK level (Fig. 6A,B). This was also demonstrated by the direct immunodetection of phospho-ERK on lens sections, which was weak and diffuse in the Le-Cre;Shp2flox/flox;LSL-KrasG12D mutant, but strongly activated in the equatorial regions of the wild type and the Le-Cre;Shp2flox/flox;LSL-KrasG12D;Spry2flox/flox triple mutants (Fig. 6C-G, arrows). Similarly, although phospho-ERK staining was only modestly induced in the Le-Cre;Shp2flox/flox;LSL-KrasG12D conjunctival epithelium, it appeared to reach the same intensity level as the wild type in the Le-Cre;Shp2flox/flox;LSL-KrasG12D;Spry2flox/flox triple mutant lacrimal gland bud (Fig. 6M-Q). Therefore, in both lens and lacrimal gland, combined activation of Kras and ablation of Sprouty2 fully restored ERK signaling in the Shp2 mutants.
We have also confirmed that the Sprouty2 systemic knockout (Spry2KO/KO) animals are viable and fertile, without any obvious ocular defects (Shim et al., 2005). Similarly, the conditional Sprouty2 knockout (Le-Cre; Spry2flox/flox) had no effect by itself or in the context of the Shp2 single mutant during lens and lacrimal gland development (Fig. 6I,J,S,T,W; see Fig. S1 in the supplementary material). However, when combined with the KrasG12D allele, Sprouty2 ablation restored the lens to the wild-type size in the Le-Cre;Shp2flox/flox;LSL-KrasG12D;Spry2flox/flox triple mutants, and completely reversed the abnormal posterior shift of the lens epithelium cells (Fig. 6H-L,W, arrows). Of note, although the lens cell proliferation and differentiation defects were rescued, there still exist extensive TUNEL-positive cells in the Le-Cre;Shp2flox/flox;LSL-KrasG12D;Spry2flox/flox triple mutants (data not shown). Nevertheless, although the KrasG12D allele could only induce at most a minuscule outgrowth of lacrimal gland in the context of Shp2 mutation, the combination of Sprouty2 ablation and Kras activation in the Le-Cre;Shp2flox/flox;LSL-KrasG12D;Spry2flox/flox triple mutants was able to promote lacrimal gland growth to approximately that of the E15.5 wild-type controls (Fig. 6R-W). Therefore, inhibition of Sprouty2 and activation of Kras signaling both contributed to the genetic rescue of the Shp2 phenotype in lens and lacrimal gland development.
DISCUSSION
In summary, we have shown that Shp2 tyrosine phosphatase is a key factor in regulating the growth, survival and differentiation of lens epithelial cells and lacrimal gland epithelial budding. Shp2 ablation disrupted ERK signaling, its downstream response gene expressions, and cell cycle regulator activities. Although the lens and lacrimal gland phenotype can be partially rescued by activated Kras signaling, the absence of Shp2 led to uncontrolled tyrosine phosphorylation of Sprouty2 protein, which ultimately prevented the full activation of ERK pathway. Only the removal of Sprouty2 allowed Kras activation to fully restore lens growth and lacrimal gland budding. These results suggest that Sprouty2 suppression is an integral part of Shp2 function in modulating Ras-ERK signaling.
Previous studies have uncovered many potential substrates of Shp2, but how these factors are integrated into a coherent signaling pathway and whether such biochemical interactions are functionally relevant in vivo remain unclear. It is especially perplexing that a protein phosphatase such as Shp2 can positively regulate a kinase pathway such as Ras-MAPK (Dance et al., 2008). It has been proposed that Shp2 dephosphorylates Sprouty to suppress its negative regulation of Ras activity, thus indirectly promoting Ras-MAPK signaling. This is supported by studies in Drosophila in which removing one copy of the Sprouty allele suppressed the photoreceptor defect caused by the inactivation of the Shp2 homolog Corkscrew. However, it should be noted that this genetic interaction was based on the expression of a dominant-negative Shp2 homolog Corkscrew, which was unlikely to fully suppress the endogenous Corkscrew function. Using a complete loss-of-function allele of Shp2, we have now shown that at least in lens and lacrimal gland development, Sprouty2 expression was abrogated by Shp2 ablation. If Sprouty2 was never expressed in the Shp2 null background, further deletion of the Sprouty2 allele would certainly not elicit any effect. Indeed, we showed that Sprouty2 genetic ablation did not ameliorate the Shp2 knockout ocular phenotype, and ERK signaling remained downregulated. Therefore, the loss of Sprouty does not preclude the need for Shp2 in activating Ras signaling, suggesting that there exist Sprouty-independent function(s) of Shp2 in RTK signaling.
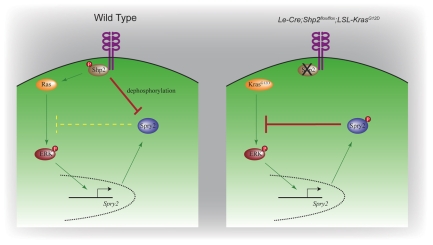
Our results thus support the idea that Shp2 induces Sprouty2 expression indirectly via Ras signaling, while directly downregulating Sprouty2 activity by controlling its phosphorylation state (Fig. 7). It might appear paradoxical that Shp2 evolves to be both a positive and a negative regulator of Sprouty2; however, it should be noted that Sprouty2 inhibition via direct Shp2 dephosphorylation is likely to be much faster than Sprouty2 induction via Ras signaling, because the latter requires new protein synthesis. The kinetic difference between these two pathways could potentially sharpen the dynamic response of Ras signaling to the change of extracellular stimulus, thus tightly controlling the progenitor cells to rapidly enter/exit the proliferation and differentiation states. This biphasic regulation of Sprouty by Shp2 is also consistent with the previous observations that Shp2 appears to employ distinct mechanisms to control both the immediate-early and late signaling responses to growth factors (Bennett et al., 1996). Finally, our model might further explain why Kras activation alone achieved much more robust rescue of the lens than the lacrimal gland in the Shp2 mutant background. Here we need to take into account the fact that the lacrimal gland tip cells must sustain intense RTK signaling to lead the continuous outgrowth of the lacrimal gland, whereas the lens epithelial cells only transiently experience a more moderate RTK activation during their differentiation, as evident by the differential intensity of phospho-ERK and Erm staining in these two tissues (Fig. 4F,I). This would lead to continuously high Sprouty2 expression, and as a consequence, a greater need for Shp2 to restrict its inhibitory effect in the lacrimal gland. It is tempting to speculate that, in the presence of unconstrained Sprouty2 inhibition, Kras activation might be sufficient to induce RTK downstream signaling to the moderate threshold required for lens development, but not enough to maintain the peak level required for the lacrimal gland budding. Numerous cell culture studies have shown that, in the absence of Shp2, most RTK signaling can still induce ERK activation, but its intensity and duration is much diminished (Feng, 1999; Neel et al., 2003). Our comparative analysis of the lens and the lacrimal gland not only show that this intriguing biochemical observation is applicable in vivo, but also suggests that at least one of its underlying mechanisms might be the dynamic regulation of Sprouty by Shp2.
Fig. 7.
Model of Shp2-Ras-Sprouty2 signaling in lens and lacrimal gland development. In wild-type cells, Shp2 activates Ras-ERK signaling to induce Spry2 transcription but also suppresses Sprouty2 protein activity by tyrosine dephosphorylation, thus weakening the intensity of the Sprouty2-Ras negative feedback loop. In the Le-Cre;Shp2flox/flox;LSL-KrasG12D cells, the activated KrasG12D mutant was still able to induce Spry2 transcription, but the Sprouty2 protein was hyperphosphorylated in the absence of Shp2 phosphatase, resulting in stronger suppression of Ras signaling.
How important, then, is the Shp2-Ras-ERK pathway in mediating RTK signaling, including FGF signaling, in lens and lacrimal gland? In lacrimal gland development, either Shp2 or Fgfr2 ablation abolished lacrimal gland budding. In lens development, however, the Shp2 phenotype is surprisingly milder than that of the reported FGFR signaling defects. Using a Cre driver that acted even later than Le-Cre, Zhao and colleagues showed that deletion of the Fgfr1/2/3 completely abrogated lens fiber cell elongation, resulting in a hollow lens structure without any γ-crystallin expression (Zhao et al., 2008). Moreover, Gotoh and colleagues showed in a systemic knockout model that mutations in the key FGFR adaptor protein Frs2α to disrupt its interaction with Shp2 resulted in lens induction failure (Gotoh et al., 2004). These phenotypes are in striking contrast to our Le-Cre;Shp2flox/flox mutant lens, which exhibited cell proliferation and apoptosis defects but maintained α, β and γ-crystallin expressions. The phenotypic discrepancy between the Shp2 and the Frs2α mutants is almost certainly due to the timing of knockouts, as we have shown that the Le-Cre mediated Shp2 knockout did not appreciably affect ERK signaling at E10.5. Although we cannot rule out the possibility that undetectable levels of Shp2 protein persisted after the lens induction phase to support further lens development, it should be noted that the Shp2 and Fgfr1/2/3 mutants both appeared to reduce phospho-ERK expression to a similar extent in the E12.5 lens, but only the Fgfr1/2/3 mutant exhibited primary lens fiber cell elongation and differentiation defects. This raises an important question about whether Shp2-Ras-ERK pathway can sufficiently account for all of the RTK signaling effects in the lens. Considering that RTK signaling is known to promote other downstream pathways, such as PI3K-AKT and PLCγ signaling, further studies are needed to investigate whether these pathways might cooperate with Shp2-Ras-ERK signaling to control lens development.
Supplementary Material
Acknowledgements
The authors thank Drs Ruth Ashley-Padan, Rebecca Chan, Bridget Hogan, Richard Lang, Gail Martin and Sam Zigler for mice and reagents, and members of the Zhang lab for discussions. The work was supported by NIH grants DK073945 to GSF, EY017061 and EY018868 to X.Z. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.042820/-/DC1
References
- Ashery-Padan R., Marquardt T., Zhou X., Gruss P. (2000). Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14, 2701-2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. M., Hausdorff S. F., O'Reilly A. M., Freeman R. M., Neel B. G. (1996). Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol. Cell. Biol. 16, 1189-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Park S. Y., Joo C. K. (2004). Hepatocyte growth factor induces proliferation of lens epithelial cells through activation of ERK1/2 and JNK/SAPK. Invest. Ophthalmol. Vis. Sci. 45, 2696-2704 [DOI] [PubMed] [Google Scholar]
- Cunnick J. M., Meng S., Ren Y., Desponts C., Wang H. G., Djeu J. Y., Wu J. (2002). Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J. Biol. Chem. 277, 9498-9504 [DOI] [PubMed] [Google Scholar]
- Dance M., Montagner A., Salles J. P., Yart A., Raynal P. (2008). The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell. Signal. 20, 453-459 [DOI] [PubMed] [Google Scholar]
- Faber S. C., Dimanlig P., Makarenkova H. P., Shirke S., Ko K., Lang R. A. (2001). Fgf receptor signaling plays a role in lens induction. Development 128, 4425-4438 [DOI] [PubMed] [Google Scholar]
- Feng G. S. (1999). Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res. 253, 47-54 [DOI] [PubMed] [Google Scholar]
- Gotoh N., Ito M., Yamamoto S., Yoshino I., Song N., Wang Y., Lax I., Schlessinger J., Shibuya M., Lang R. A. (2004). Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc. Natl. Acad. Sci. USA 101, 17144-17149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V., Ito M., Makarenkova H. P., Lang R. A., Overbeek P. A. (2000). Endogenous and ectopic gland induction by FGF-10. Dev. Biol. 225, 188-200 [DOI] [PubMed] [Google Scholar]
- Haase G., Dessaud E., Garces A., de Bovis B., Birling M., Filippi P., Schmalbruch H., Arber S., deLapeyriere O. (2002). GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. neuron 35, 893-905 [DOI] [PubMed] [Google Scholar]
- Hadari Y. R., Gotoh N., Kouhara H., Lax I., Schlessinger J. (2001). Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. USA 98, 8578-8583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Torii S., Yasunaga T., Matsumoto K., Nishida E. (2004). Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J. Biol. Chem. 279, 22992-22995 [DOI] [PubMed] [Google Scholar]
- Hyatt G. A., Beebe D. C. (1993). Regulation of lens cell growth and polarity by an embryo-specific growth factor and by inhibitors of lens cell proliferation and differentiation. Development 117, 701-709 [DOI] [PubMed] [Google Scholar]
- Jarvis L. A., Toering S. J., Simon M. A., Krasnow M. A., Smith-Bolton R. K. (2006). Sprouty proteins are in vivo targets of Corkscrew/SHP-2 tyrosine phosphatases. Development 133, 1133-1142 [DOI] [PubMed] [Google Scholar]
- Kok A., Lovicu F. J., Chamberlain C. G., McAvoy J. W. (2002). Influence of platelet-derived growth factor on lens epithelial cell proliferation and differentiation. Growth Factors 20, 27-34 [DOI] [PubMed] [Google Scholar]
- Kouhara H., Hadari Y. R., Spivak-Kroizman T., Schilling J., Bar-Sagi D., Lax I., Schlessinger J. (1997). A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89, 693-702 [DOI] [PubMed] [Google Scholar]
- Liu J., Chamberlain C. G., McAvoy J. W. (1996). IGF enhancement of FGF-induced fibre differentiation and DNA synthesis in lens explants. Exp. Eye Res. 63, 621-629 [DOI] [PubMed] [Google Scholar]
- Lovicu F. J., Overbeek P. A. (1998). Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development 125, 3365-3377 [DOI] [PubMed] [Google Scholar]
- Makarenkova H. P., Ito M., Govindarajan V., Faber S. C., Sun L., McMahon G., Overbeek P. A., Lang R. A. (2000). FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development 127, 2563-2572 [DOI] [PubMed] [Google Scholar]
- McAvoy J. W., Chamberlain C. G. (1989). Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development 107, 221-228 [DOI] [PubMed] [Google Scholar]
- Munchberg S. R., Steinbeisser H. (1999). The Xenopus Ets transcription factor XER81 is a target of the FGF signaling pathway. Mech. Dev. 80, 53-65 [DOI] [PubMed] [Google Scholar]
- Neel B. G., Gu H., Pao L. (2003). The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28, 284-293 [DOI] [PubMed] [Google Scholar]
- Ong S. H., Guy G. R., Hadari Y. R., Laks S., Gotoh N., Schlessinger J., Lax I. (2000). FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 20, 979-989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Woodbury A., Esko J. D., Grobe K., Zhang X. (2006). Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 133, 4933-4944 [DOI] [PubMed] [Google Scholar]
- Pan Y., Carbe C., Powers A., Zhang E. E., Esko J. D., Grobe K., Feng G. S., Zhang X. (2008). Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development 135, 301-310 [DOI] [PubMed] [Google Scholar]
- Raible F., Brand M. (2001). Tight transcriptional control of the ETS domain factors Erm and Pea3 by Fgf signaling during early zebrafish development. Mech. Dev. 107, 105-117 [DOI] [PubMed] [Google Scholar]
- Reddan J. R., Wilson-Dziedzic D. (1983). Insulin growth factor and epidermal growth factor trigger mitosis in lenses cultured in a serum-free medium. Invest. Ophthalmol. Vis. Sci. 24, 409-416 [PubMed] [Google Scholar]
- Robinson M. L., Overbeek P. A., Verran D. J., Grizzle W. E., Stockard C. R., Friesel R., Maciag T., Thompson J. A. (1995). Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development 121, 505-514 [DOI] [PubMed] [Google Scholar]
- Robinson M. L., Ohtaka-Maruyama C., Chan C. C., Jamieson S., Dickson C., Overbeek P. A., Chepelinsky A. B. (1998). Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev. Biol. 198, 13-31 [DOI] [PubMed] [Google Scholar]
- Roehl H., Nusslein-Volhard C. (2001). Zebrafish pea3 and erm are general targets of FGF8 signaling. Curr. Biol. 11, 503-507 [DOI] [PubMed] [Google Scholar]
- Shim K., Minowada G., Coling D. E., Martin G. R. (2005). Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev. Cell 8, 553-564 [DOI] [PubMed] [Google Scholar]
- Tefft D., De Langhe S. P., Del Moral P. M., Sala F., Shi W., Bellusci S., Warburton D. (2005). A novel function for the protein tyrosine phosphatase Shp2 during lung branching morphogenesis. Dev. Biol. 282, 422-431 [DOI] [PubMed] [Google Scholar]
- Tuveson D. A., Shaw A. T., Willis N. A., Silver D. P., Jackson E. L., Chang S., Mercer K. L., Grochow R., Hock H., Crowley D., et al. (2004). Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375-387 [DOI] [PubMed] [Google Scholar]
- Wormstone I. M., Tamiya S., Marcantonio J. M., Reddan J. R. (2000). Hepatocyte growth factor function and c-Met expression in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 41, 4216-4222 [PubMed] [Google Scholar]
- Xu X., Li C., Garrett-Beal L., Larson D., Wynshaw-Boris A., Deng C. X. (2001). Direct removal in the mouse of a floxed neo gene from a three-loxP conditional knockout allele by two novel approaches. Genesis 30, 1-6 [DOI] [PubMed] [Google Scholar]
- Zhang E. E., Chapeau E., Hagihara K., Feng G. S. (2004a). Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc. Natl. Acad. Sci. USA 101, 16064-16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Q., Yang W., Kontaridis M. I., Bivona T. G., Wen G., Araki T., Luo J., Thompson J. A., Schraven B. L., Philips M. R., et al. (2004b). Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13, 341-355 [DOI] [PubMed] [Google Scholar]
- Zhao H., Yang T., Madakashira B. P., Thiels C. A., Bechtle C. A., Garcia C. M., Zhang H., Yu K., Ornitz D. M., Beebe D. C., et al. (2008). Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.