Abstract
Studies of prolonged separation from the attachment figure that were conducted with infant monkeys during the middle of the 20th century identified a passive behavioral response, termed “despair,” that appeared to model human depressive illness. Studies in guinea pigs, which exhibit filial attachment that resembles attachment in monkeys, have described a similar passive response to briefer periods of maternal separation. Recent evidence indicates that elements of the immune system mediate the passive behavioral response of guinea pigs. These findings accord well with current ideas that immune responses contribute to depressive illness, suggest new hypotheses about how maternal separation might promote depression, and give us a rodent model in which such hypotheses might be tested.
Keywords: maternal separation, depression, sickness, inflammation, stress
Research during the 1960s and 70s by Harry Harlow and others clearly established the depressive-like effects that social separation can have on the behavior of nonhuman primates (e.g., Suomi, Eisele, Grady, & Harlow, 1975). When first removed from the mother, infant monkeys exhibited active behaviors such as vocalizing and locomotor activity. Many hours later, some infants entered a second, passive phase (called “despair”) marked by inactivity, disinterest in the environment, a slouched posture, and the appearance of grief (Kaufman & Rosenblum, 1967). The monkey studies, while severe, were valuable for highlighting the consequences of comparably severe procedures to which institutionalized children of the day were routinely subjected: long periods of separation from the mother and other attachment figures. A subgroup of these children exhibited passivity, indifference to their surroundings, and grief that resembled the reaction of separated monkeys. The reaction of human infants was considered a form of depression (called anaclitic depression), and the reaction of infant monkeys became one of the first animal models of depressive illness. Over the years, evidence has continued to accumulate that maternal separation and other disruptions of attachment relationships increase risk for depression (Gillespie & Nemeroff, 2007). Yet the monkey model was never widely used in depression research, due in large part to practical considerations, including the availability of primates and the enormous time and expense that such studies require.
A RODENT MODEL OF ATTACHMENT-FIGURE SEPARATION
An alternative approach is to examine maternal separation in laboratory rodents. Among commonly studied rodents, guinea pigs show evidence of an attachment process that best approximates filial attachment in primate infants (Hennessy, 2003). Unlike laboratory rats and mice, guinea pigs are physically mature at birth and fully capable of wandering off from the mother. Because the mother shows little active maternal care and does not retrieve pups, it is the strong attraction that pups show for the mother that ensures the proximity of mother and young. Guinea pigs, therefore, seem the rodent of choice for studies aimed at understanding how absence of the attachment figure per se (as opposed to the absence of specific forms of maternal care-taking activities) affects biobehavioral processes leading to depression.
Separation from the mother can evoke behavioral and physiological responses in guinea pigs similar to those of nonhuman primates (Hennessy, 2003). Among these similarities is a two-stage, active/passive behavioral response, although the course of the response is greatly compressed in time in guinea pigs relative to monkeys. When placed alone into an unfamiliar cage, a guinea pig pup initially vocalizes at a high rate, but after an hour or so it quiets and begins showing a constellation of passive behaviors consisting of a crouched stance, closure of the eyes, and piloerection (i.e., bristling of the fur) over most the body (Fig. 1), which at least superficially resembles the passive despair-like response of separated monkeys. The response of guinea pig pups can be attributed to the absence of the mother rather than just exposure to novelty or being alone, because the presence of the pup’s mother resolves the passive response but the presence of an unfamiliar female does not.
Fig. 1.
A guinea pig pup exhibiting the crouching, eye-closure, and piloerection (bristling of the fur) characteristic of the passive stage of separation.
Although the passive response of guinea pig pups resembles that of nonhuman primate infants, the descriptor “despair” appears inappropriate because the passive response can be elicited by a seemingly innocuous procedure—that is, placing a physically mature, though preweaning, pup into a new cage for several hours. Moreover, the concept of despair seems to imply emotional/cognitive capabilities beyond the capacities of a rodent, such as the guinea pig. But if evolution of a similar two-stage, active/passive response to maternal separation in species as diverse as guinea pigs and Old World monkeys is more than coincidence, then perhaps a better understanding of the passive response of guinea pig pups would shed light on the basic underlying processes or function of the response in primate infants as well. Recent evidence from our laboratories suggests that the passive behavioral response in separated guinea pigs is mediated by elements of the immune system—elements that can be activated by exposure to stressors. To understand how the immune system might intervene between exposure to a stressor, such as maternal separation, and a depressive-like behavioral response, some background on the process of nonspecific, or innate, immunity is helpful.
IMMUNE INFLUENCES DURING SEPARATION
While the immune system is best recognized for highly specific properties (e.g., production of antibodies to particular pathogens), the immune system’s first response to invasion by bacteria or other pathogens is a nonspecific, inflammatory reaction that can occur both locally (e.g., at the site of an injury) and systemically. The reaction, which is designated the acute-phase response, or just sickness, is triggered by immune cells, such as macrophages, which release an array of cytokines—hormone-like, chemical messengers—that orchestrate the global reaction. Sickness shifts bodily resources to better defend against pathogens. Physiological components of sickness include changes in the types of proteins produced by the liver, elevation of circulating stress-hormone levels, and fever. There also are behavioral components of sickness including inactivity, sleepiness, seeking of warmth, and postures that conserve body heat. These behaviors, like the physiological components, appear to be adaptive for instance by promoting fever and conserving energy. The concept of sickness behaviors is readily appreciated by anyone experiencing a bout with the flu. Evidence that symptoms of sickness, including sickness behaviors, result from proinflammatory cytokines is provided by demonstrations that natural substances or drugs that disrupt the action of proinflammatory cytokines (e.g., nonsteroidal anti-inflammatory drugs such as indomethacin) also alleviate physiological and behavioral signs of sickness (Johnson, Curtis, Dantzer, & Kelley, 1993).
The potential connection between sickness and separation derives from findings that physiological and behavioral aspects of sickness sometimes are seen following exposure to stressors such as electric shock. The adaptive function of sickness behaviors following stressors is not entirely clear, though conservation of energy would seem to be important for organisms both during pathogen exposure and in threatening circumstances. From an evolutionary perspective, one could argue that periods of stress (e.g., pursuit by a predator) would often predict need for recuperation and/or physical injury, both of which would be aided by activation of immune-related pathways (Deak, 2007). In any event, stress-induced sickness behaviors appear to be caused by proinflammatory cytokines acting in the brain (e.g., hypothalamus, limbic system), because the behaviors can be reduced or fully reversed with administration of anti-inflammatory agents to the central nervous system (Maier & Watkins, 1998). In light of such findings, we proposed that passive behaviors of isolated guinea pig pups, and possibly of socially separated monkey infants, represent sickness behaviors induced by the stress of the maternal-separation procedure (Hennessy, Deak, & Schiml-Webb, 2001).
PROINFLAMMATORY PROCESSES DURING SEPARATION
The appearance of guinea pig pups during the passive stage clearly suggests a physically ill animal (Fig.1), and interestingly, early investigators noted that separated monkey and human infants also appeared ill (Kaufman & Rosenblum, 1967). But appearances alone are not convincing. If, in fact, the crouching, eye-closing, and piloerection seen in separated guinea pig pups are true components of sickness, one would also expect that (a) direct activation of the innate immune system would increase levels of the same three passive behaviors, (b) anti-inflammatory agents would reduce passive behaviors evoked by separation, and (c) physiological components of an acute-phase response would accompany the passive behaviors during separation. Evidence for each of these predictions now exists. To test the first prediction, pups were injected with lipopolysacchride, a substance commonly used to elicit an acute-phase response in laboratory animals. As predicted, lipopolysaccharide increased the passive behaviors in a dose-dependent manner (Hennessy et al., 2004).
Several anti-inflammatory agents have been used to test the second prediction. Alpha-melanocyte stimulating hormone (α-MSH) is a naturally occurring neurochemical and hormone with a variety of physiological and behavioral influences, including a wide ranging and robust anti-inflammatory effect. α-MSH, which does not readily enter the brain when injected peripherally, was administered directly into the ventricles of the brain (administration into the cavernous ventricles helps ensure widespread distribution through the brain). Pups spent less time exhibiting passive behavior during a 3-hour period of isolation when given α-MSH than they did when administered a control injection of saline solution (Schiml-Webb, Deak, Greenlee, Maken, & Hennessy, 2006). The same dose and administration procedures also diminished passive behavior produced by lipopolysaccharide, suggesting it was the anti-inflammatory property of α-MSH that reduced the passive behaviors during the isolation period (Hennessy, Schiml-Webb, et al., 2007).
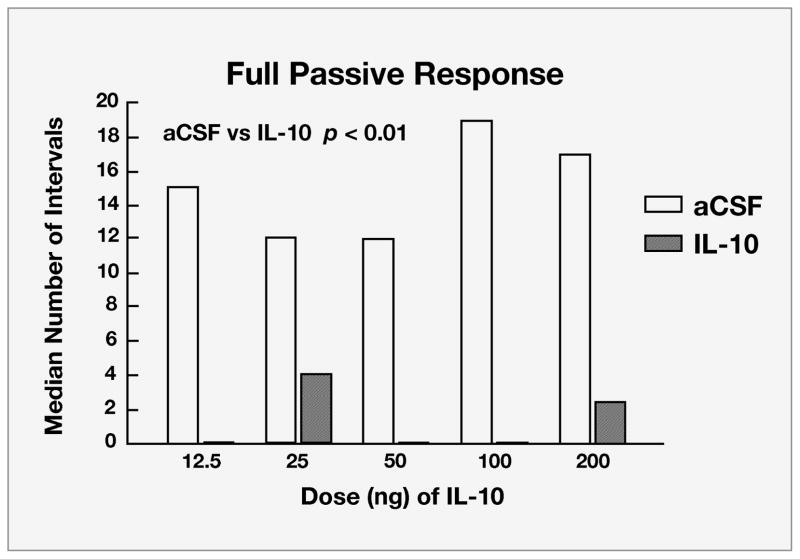
Prostaglandins mediate a variety of physiological functions including smooth muscle contraction, pain, and aspects of the acute-phase response, including inflammation and sickness behaviors. Injection of indomethacin, an inhibitor of prostaglandin synthesis, also was found to reduce the passive behaviors of maternally separated guinea pig pups (Hennessy, Schiml-Webb, et al., 2007). A recently completed study examined the effect of infusion of Interleukin-10 (IL-10) into the ventricles. IL-10 is a naturally occurring cytokine but one with potent anti-inflammatory, rather than proinflammatory, properties. IL-10 is released along with proinflammatory cytokines and appears to moderate or regulate proinflammatory activity. Guinea pig pups were administered five different doses of IL-10 prior to isolation (Perkeybile, Schiml-Webb, O’Brien, Deak, & Hennessy, 2009). All five doses reduced the time pups spent exhibiting the passive behaviors (Fig. 2). Together then, three very different anti-inflammatory agents have now been found to reduce passive behavior of maternally separated guinea pig pups.
Fig. 2.
Median number of 60-second intervals during which guinea pig pups separated from their mothers in an unfamiliar cage exhibited the full passive response (consisting of crouching, eye-closure, and piloerection) following administration of the anti-inflammatory cytokine Interleukin-10 (IL-10). Pups were tested following infusion into the ventricles of the brain of one of five doses of IL-10dissolved in artificial cerebrospinal fluid (aCSF) or of just the aCSF control substance alone.
Finally, the third prediction has been addressed by showing that an elevation of a proinflammatory cytokine in the spleen and increased core temperature—both physiological aspects of an acute-phase response—also occur during separation (Hennessy, Deak, Schiml-Webb, & Barnum, 2007; Hennessy, Deak, Schiml-Webb, Carlisle, & O’Brien, 2008). In all, these findings provide convergent evidence that passive behavioral responses of guinea pig pups during prolonged maternal separation are mediated, at least in part, by proinflammatory factors.
These findings raise two obvious questions: (a) To what degree do the findings in guinea pigs generalize to primates, and (b) does the involvement of proinflammatory factors in the behavioral response to separation have any relevance for our understanding of depressive illness? The first question remains to be tested, though preferably in a way that does not require resorting to the prolonged separation procedures of 40 years ago. One potential approach is suggested by findings in an Old World primate, the cynomolgous monkey. A percentage of adult females—usually socially subordinate females—exhibit a hunched “depressive” posture when housed under laboratory conditions (Shively et al., 2006). These monkeys also show reduced binding of serotonin receptors and increased stress-hormone activity, changes that parallel those seen during human depression. Therefore, it may be possible to examine the role of cytokines in the passive behavior of adult monkeys experiencing moderate social stress instead of infant monkeys exposed to prolonged maternal separation.
PROINFLAMMATORY PROCESSES IN DEPRESSION
As for the second question, the recent emergence of the so-called “cytokine” or “macrophage” hypothesis of depression, which contends that proinflammatory cytokines contribute to some forms of depression, was prompted by observations of increasing circulating cytokines in depressed individuals and was reinforced by depressive reactions in cancer patients following administration of cytokines for chemotherapy (Dantzer, 2006). Support from animal studies include findings that anhedonia (loss of interest in pleasurable activity)—a central feature of depression—also accompanies cytokine-induced sickness (De La Garza, 2005). Though the sickness response itself appears to be adaptive, depression may result from prolonged proinflammatory stimulation or proinflammatory stimulation in already-vulnerable individuals (Dantzer, 2006). The initially provocative notion that proinflammatory cytokines might be a mechanism underlying depression has now been integrated into a larger picture of the neurobiology of major depressive illness, in which cytokines are viewed as contributing to depression through their interaction with other putative mechanisms. Proinflammatory cytokines can reduce serotonin activity, increase stress-hormone secretion, and increase production of neurochemicals that are damaging to nerve cells, all events that in themselves appear related to the onset of depression (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Miura et al., 2008). Importantly in the present context, stress-induced cytokine release may be a crucial link between exposure to stressors and the onset of depression (Miura et al., 2008). Further, proinflammatory activity appears to underlie some outcomes of what is probably the most well-recognized animal model of stress-induced depression—that of learned helplessness (Chourbaji et al., 2006). Thus, examination of proinflammatory influences provides a novel perspective from which to view the long-established link between the psychosocial stressor of maternal separation and the development of depression.
FUTURE DIRECTIONS
At present, many gaps remain in our understanding of how cytokines are involved in the separation response of guinea pigs, much less of nonhuman or human primates. One thing we do not know is the mechanism by which perception of maternal absence in a novel environment activates cytokine activity. One possibility is that it occurs through release of corticotropin-releasing factor (CRF), a major stress-related neurochemical. CRF release is known to be sensitive to psychogenic stressors, and CRF can induce activation of proinflammatory processes. Injection of CRF elicits the passive responses in guinea pig pups, and these responses can be reduced with administration of α-MSH (Hennessy, Deak, et al., 2007). However, whether endogenous release—rather than injection—of CRF during separation affects proinflammatory processes, and does so in such a way as to promote passive responding, remains to be determined. Another point requiring clarification is the effect of antidepressant agents. If passive behaviors during separation model depression, antidepressants might be expected to reduce the passive responses and to do so through reduction of cytokine activity.
Finally, there are issues of individual differences and vulnerability. Because not all monkey or human infants respond during separation with passive, depressive-like behavior, the factors that might determine this differential vulnerability (e.g., differences in temperament or threshold for a stress-induced proinflammatory response) will require further study. Moreover, much of the current focus on the link between separation and depression is not on the immediate response to maternal separation (e.g., anaclitic depression) but, rather, on the vulnerability that such separations or related traumatic early-life experiences seem to confer for bouts of depression in adolescence and adulthood. Studies are needed to determine if proinflammatory factors underlie this lasting vulnerability. It is known, however, that early activation of proinflammatory processes can have lasting biobehavioral effects reminiscent of those produced by early stress (Hennessy, Deak, et al., 2007; Meaney et al., 1996), so examination of whether early-attachment-figure separation has proinflammatory effects on behavior that persist into later life appears warranted.
A recurring theme in the field of developmental psychobiology is that early manipulations of the social environment often change behavior and influence later development through unanticipated actions on basic physiologic processes (Hofer, 2006). Our results suggest that maternal separation in guinea pigs produces passive, depressive-like behavior through activation of proinflammatory processes. These findings, together with evidence that similar proinflammatory processes appear to contribute to human depression, afford a means by which a variation of an old animal model of depression can be used to examine new hypotheses about the way in which attachment-figure separation might lead to depressive illness.
Recommended Reading
Maier, S.F., & Watkins, L.R. (1998). (See References). This review provides an introduction for psychologists to sickness behavior and the induction of sickness behavior by stressors.
Dantzer, R., O’Connor, J.C., Freund, G.G., Johnson, R.W., & Kelley, K.W. (2008). (See References). This is an up-to-date review of research on the relationship of cytokines and depression.
Blum, D. (2002). Love at Goon Park: Harry Harlow and the Science of Affection. New York: Berkeley Books. This book for a general audience presents a very readable summary of the routine separation of children from attachment figures in the mid 1900s and of some of the monkey studies prompted by this practice.
Acknowledgments
This work was supported by Grant MH068228 from the National Institute of Mental Health.
References
- Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiology of Disease. 2006;23:587–594. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurological Clinics. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T. From classic aspects of the stress response to neuroinflammation and sickness: Implications for individuals and offspring of diverse species. International Journal of Comparative Psychology. 2007;20:96–110. [Google Scholar]
- De La Garza R. Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: Focus on anhedonia. Neuroscience and Biobehavioral Reviews. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Corticotropin-releasing factor and the psychobiology of early-life stress. Current Directions in Psychological Science. 2007;16:85–89. [Google Scholar]
- Hennessy MB. Enduring maternal influences in a precocial rodent. Developmental Psychobiology. 2003;42:225–236. doi: 10.1002/dev.10095. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA. Stress-induced sickness behaviors: An alternative hypothesis for responses during maternal separation. Developmental Psychobiology. 2001;39:76–83. doi: 10.1002/dev.1031. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Barnum CJ. Immune influences on behavior and endocrine activity in early-experience and maternal separation paradigms. In: Czerbska MT, editor. Psychoneuroendocrinology Research Trends. Hauppauge, NY: Nova Science Publishers; 2007. pp. 293–319. [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Carlisle C, O’Brien E. A second, daily maternal separation evokes a separation-specific rise in core temperature and sensitized behavioral response in guinea pig pups. Developmental Psychobiology. 2008;50:730. [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Wilson SE, Greenlee TM, McCall E. Responses of guinea pig pups during isolation in a novel environment may represent stress-induced sickness behaviors. Physiology and Behavior. 2004;81:5–13. doi: 10.1016/j.physbeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml-Webb PA, Miller EE, Maken DS, Bullinger KL, Deak T. Anti-inflammatory agents attenuate the passive responses of guinea pig pups: evidence for stress-induced sickness behavior during separation. Psychoneuroendocrinology. 2007;32:508–515. doi: 10.1016/j.psyneuen.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. Psychobiological roots of early attachment. Current Directions in Psychological Science. 2006;15:84–88. [Google Scholar]
- Johnson RW, Curtis SE, Dantzer R, Kelley KW. Central and peripheral prostaglandins are involved in sickness in birds. Physiology and Behavior. 1993;53:127–131. doi: 10.1016/0031-9384(93)90020-g. [DOI] [PubMed] [Google Scholar]
- Kaufman JC, Rosenblum LA. The reaction to separation in infant monkeys: Anaclitic depression and conservation withdrawal. Psychosomatic Medicine. 1967;29:748–675. doi: 10.1097/00006842-196711000-00010. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: Shift in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, Hennessy MB. Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinology. 2009;34:1101–1108. doi: 10.1016/j.psyneuen.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiml-Webb PA, Deak T, Greenlee T, Maken DS, Hennessy MB. Alpha melanocyte stimulating hormone reduces putative stress-induced sickness behaviors in isolated guinea pig pups. Behavioural Brain Research. 2006;168:326–330. doi: 10.1016/j.bbr.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Shively CA, Friedman DP, Gage D, Bounds MC, Brown-Proctor C, Blair JB, et al. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Archives of General Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Eisele CD, Grady SA, Harlow HF. Depressive behavior of adult monkeys following separation from family environment. Journal of Abnormal Psychology. 1975;84:576–578. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]