Abstract
Basement membrane matrix proteins are known to up-regulate granulocyte–macrophage colony-stimulating factor (GM-CSF) signaling in neutrophils and mononuclear phagocytes, but the mechanisms involved are poorly understood. We used the intracellular portion of the α subunit of the GM-CSF receptor (αGMR) to search for interacting proteins and identified the 67-kDa laminin receptor (LR), a nonintegrin matrix protein receptor expressed in several types of host defense cells and certain tumors, as a binding partner. LR was found to interact with the β subunit of the GMR (βGMR) as well. Whereas GM-CSF functions by engaging the αGMR and βGMR into receptor complexes, LR inhibited GM-CSF-induced receptor complex formation. Laminin and fibronectin binding to LR was found to prevent the binding of βGMR to LR and relieved the LR inhibition of GMR. These findings provide a mechanistic basis for enhancing host defense cell responsiveness to GM-CSF at transendothelial migration sites while suppressing it in circulation.
Neutrophils and mononuclear phagocytes circulate in the blood and migrate from blood vessels into tissues to perform their host defense function under instructions from signaling molecules. Transendothelial migrating cells must integrate extracellular matrix and cytokine signals to be activated at the appropriate site for host defense with minimum collateral damage to normal tissues. Laminin (LM), fibronectin (FN), and collagen are subendothelial basement membrane matrix proteins that regulate cell responsiveness to cytokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-5 although the molecular mechanisms involved are poorly understood (1, 2). Metastasizing tumor cells also migrate through blood vessels and some aspects of their migration are similar to that of host defense cells (3). An understanding of the molecular mechanisms coordinating cytokine action to adhesion molecules therefore may help in illuminating host defense and tumor metastasis.
Human GM-CSF regulates the growth, differentiation, and maturation of myeloid precursor cells and enhances the function of mature neutrophils, eosinophils and mononuclear phagocytes (4). GM-CSF binds to its cognate receptor which is composed of a ligand-binding low-affinity α subunit GM-CSF receptor (αGMR) and a β subunit (βGMR) that together form the high affinity receptor responsible for most GM-CSF activated signaling (5, 6). The GM-CSF, IL-3 and IL-5 receptors have distinct α subunits but share a common β subunit, βGMR (7, 8). Signaling through the GM-CSF, IL-3, and IL-5 receptors is relatively well understood with two main pathways defined: Janus kinase (Jak)–signal transducer and activator of transcription (Stat) activation and ras/raf-mitogen-activated protein (MAP) kinase (MAPK) activation (9, 10). The function of αGMR in conjunction with βGMR is complex and αGMR appears to have a role independent of βGMR. We have shown that αGMR signals for increased glucose transport through activation of phosphatidylinositol 3-kinase (11). Although the cytoplasmic domain of αGMR is short (54 aa), it is essential for MAPK and Jak2–Stat5 signaling as well as cell survival induced by GM-CSF (12, 13).
The 67-kDa LM receptor (LR, also known as LRP, LBP, and 67 LR) is a nonintegrin membrane protein with the N terminus inside the cell (14). LR cDNA encodes 295 aa (37 kDa), and it is not fully understood how cells make the 67-kDa LR, although it might involve dimerization and acylation (15, 16). In addition to LM, the known ligands for LR are FN and type IV collagen (17, 18). LR appears to have a function in host defense with the expression in cells including T lymphocytes, mast cells, and monocytic acute myeloid leukemia cells (19–21). The interaction of cancer cells with LM is implicated in tumor metastasis, and the LR is involved in this process (22–24).
While searching for proteins interacting with the cytoplasmic domain of the human αGMR, we discovered that the LR binds to this region of the receptor. Here we show how the LR regulates the GMR system and how certain matrix proteins modulate GMR function.
Materials and Methods
Cell Lines, Neutrophils, and Culture Methods. The 293T cells were maintained in high-glucose DMEM supplemented with 10% bovine calf serum, 1% sodium pyruvate, 1% l-glutamine, and antibiotics. U937 cells were maintained in RPMI medium 1640 supplemented with 10% FBS. Human neutrophils were purified from fresh whole blood of healthy donors by centrifugation through Polymorphprep (Axis-Shield, Oslo) following the manufacturer's specifications. Erythrocytes were removed by hypotonic lysis. The purified neutrophils were resuspended in RPMI medium 1640 for MAPK and Stat5 phosphorylation analysis.
Expression of the Intracellular Cytoplasmic Domain (ICD) of αGMR (αGMR-ICD). The cDNA for αGMR-ICD was subcloned into the NdeI and SapI sites of the pTYB1 vector of the T7 select protein expression system (New England Biolabs). The 5′ primer is GCT TGCATACATATGAAAAGGTTCCTTAGGATACAG, and the 3′ primer is GCGTGTCGCTCTTCCGCAGGTAATTTCC TTCACGGTCAA. The plasmid encoding a fusion protein of the chitin-binding domain (CBD) and αGMR-ICD was electroporated into Escherichia coli strain ER2566, and the expression was induced with 0.3 mM isopropyl β-d-thiogalactoside for 3 h at room temperature. Bacteria were sonicated in lysis buffer (modified PBS with 135 mM K+, 5 mM Na+, 0.1% Triton X-100, and protease and phosphatase inhibitor cocktails from Sigma). The fusion protein αGMR-ICD/CBD was captured on chitin beads, and the beads were washed with the lysis buffer at 4°C. The αGMR Ab C18 (Santa Cruz Biotechnology) was used for Western blotting of the fusion protein.
Binding of U937 Proteins to αGMR-ICD/CBD and Protein Identification. U937 cells (1 × 108) were sonicated in 10 ml of lysis buffer (modified PBS), and the lysate was cleared by centrifugation for 30 min at 20,000 × g. Chitin beads (100 μl) loaded with αGMR-ICD/CBD were incubated in the U937 lysate for 16 h at 4°C and the beads washed with the lysis buffer. The αGMR-ICD was then eluted with 1 ml of lysis buffer containing 100 mM DTT for 1 h. Native chitin beads were used to capture nonspecific binding proteins in the eluted solution, and the beads were then washed. The nonspecific control beads were analyzed in SDS/PAGE alongside total binding proteins for identifying specific binding proteins. Gel-resolved proteins were digested with trypsin and partially fractionated; the resulting peptide mixtures were then analyzed by using matrix-assisted laser-desorption ionization/reflection time-of-flight MS (Reflex III, Bruker, Billerica, MA) as described (25) and by using an electrospray ionization triple quadrupole MS/MS instrument (API300, ABI/MDS Sciex, Thornhill, Canada) modified with an ultra-fine ionization source (26). Selected precursor or fragment ion masses from the matrix-assisted laser-desorption ionization/time-of-flight MS or NanoES-MS/MS spectra were taken to search the Homo sapiens segment of a protein nonredundant database as described (27). MS/MS spectra also were inspected for y′′ ion series to compare to the computer-generated fragment ion series of the predicted tryptic peptides.
Analysis of MAPK and Stat5 Phosphorylation. U937 cells were incubated for 16 h in serum-free medium, washed with PBS, and resuspended in the same medium at 5 × 106 cells per ml. These cells were treated with different concentrations of LM for 30 min followed by treatment with GM-CSF (R & D Systems) for 10 min at 37°C. Cells were then washed twice with PBS at 4°C and sonicated in the phosphorylation-analysis buffer (20 mM Tris, pH 7.4/1 mM EGTA/1 mM sodium orthovanadate/1 mM NaF/25 μg/ml soy trypsin inhibitor/25 μg/ml aprotinin/25 μg/ml leupeptin/1 mM PMSF). After sonication, cell debris was removed by centrifugation at 10,000 rpm for 10 min. Proteins were separated by SDS/PAGE and transferred to nitrocellulose (Bio-Rad). MAPK was detected with anti-MAPK Ab (Upstate Biotechnology, Lake Placid, NY) and anti-phospho-MAPK Ab (New England Biolabs). Stat5 was detected with anti-Stat5 (Santa Cruz Biotechnology), and the phosphorylated Stat5 was detected with anti-phospho-Stat5 (New England Biolabs). LM (human placenta or Engelbreth-Holm-Swarm murine sarcoma) and FN (human plasma or cellular from human foreskin fibroblasts) are from Sigma. Digitalized images from the developed films were quantified for individual band intensities with nih image software.
Tumor Necrosis Factor (TNF)-α Production Assay. Human neutrophils were freshly purified and incubated with GM-CSF and LM or FN for 16 h in RPMI medium 1640 supplemented with 10% FBS and antibiotics. Cells were spun down, and TNF-α in the medium was measured with a high sensitivity human TNF-α immunoassay kit (R & D Systems). The assay was done in triplicate, and the standard deviations are shown in Figure 3D.
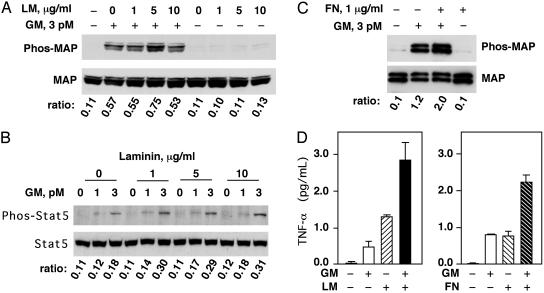
Fig. 3.
LM and FN enhance GM-CSF function in U937 cells and neutrophils. (A) U937 cells were serum-starved and stimulated with 3 pM GM-CSF (GM) for 10 min. LM (from murine sarcoma) at the indicated concentrations (0, 1, 5, and 10 μg/ml) was added 30 min before GM-CSF treatment. Phosphorylated MAPK (Upper) was analyzed by Western blotting, and the total protein level of MAPK (Lower) was shown as a control. Phosphorylated MAP to total MAP band intensity ratios are indicated. (B) U937 cells were serum-starved and stimulated with 1 pM or 3 pM GM-CSF (GM) for 10 min. LM (from murine sarcoma) at indicated concentrations was added 30 min before GM-CSF treatment. Phosphorylated Stat5 (Upper) was analyzed by Western blotting, and the total protein level of Stat5 (Lower) is shown as a control. Phosphorylated Stat5 to total Stat5 ratios are indicated. (C) Freshly purified human neutrophils were treated with 1 μg/ml FN (from human plasma) for 30 min before 10-min treatment with 3 pM GM-CSF. Phosphorylated MAPK (Upper) was analyzed by Western blotting, and the total protein level of MAPK (Lower) is shown as a control. Phosphorylated MAP to total MAP ratios are indicated. (D) Human neutrophils were treated with GM-CSF (10 pM), LM (from human placenta, 5 μg/ml), or a combination of both for 16 h. TNF-α in the medium was measured by ELISA (Left). Human neutrophils were treated with GM-CSF (5 pM), FN (from human foreskin fibroblasts, 5 μg/ml), or a combination of both for 16 h, and TNF-α production was measured by ELISA (Right).
Immunoprecipitation of Proteins Expressed in 293T Cells. Plasmids for human αGMR (in pMX), βGMR (in pMX), and LR (expression-ready in pcDNA3.1 from Invitrogen, containing V5 and His tags at the C terminus for detection) were transiently transfected (≈5 μg of each plasmid) into 293T cells in 10-cm tissue culture plates by calcium phosphate precipitation (28). After transfection (48 h), cells were sonicated in lysis buffer (modified PBS) and the lysates were cleared by centrifugation. αGMR and βGMR were immunoprecipitated with αGMR Ab S20 and βGMR Ab S16 (Santa Cruz Biotechnology). Lysates were incubated with 1 μgoftheAbsfor3h followed by 2 h with protein A beads (Sigma) capturing at 4°C. αGMR was detected in Western blot with its Ab C18 and βGMR with its Ab C20 (Santa Cruz Biotechnology). LR was detected by horseradish peroxidase (HRP)-conjugated anti-V5 Ab (Invitrogen).
The 8×GAS (8 IFN-γ-Activated Site Elements)–Luciferase Reporter Assay. The 293T cells were transfected in 10-cm tissue culture plate for 24 h. The cells were harvested and seeded into 96-well plate for 24 h. Then, 5 μg/ml human placenta LM or plasma FN was added for 30 min and GM-CSF (300 pM) was added to the cells. After 4 h the medium was aspirated and cells lysed with brief sonication, and then luciferase activity was measured (Promega).
Results
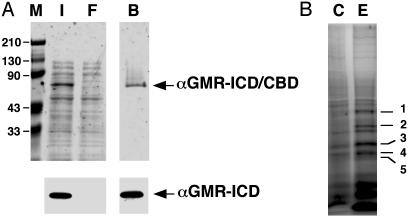
The LR Binds to the Cytoplasmic Domain of Human αGMR. To find proteins that might bind to the αGMR-ICD, we constructed a recombinant fusion protein composed of the CBD and αGMR-ICD. The cDNA for αGMR-ICD was subcloned into pTYB1 and the fusion protein expressed in E. coli and affinity-purified with chitin beads. The recombinant protein was at least 90% pure as analyzed by silver staining, and the protein was confirmed with αGMR Ab C18 (Fig. 1A). The fusion protein αGMR-ICD/CBD bound to chitin beads was cleaved by DTT, releasing αGMR-ICD and leaving CBD bound to the beads. We searched for proteins in U937 lysates that bound to αGMR-ICD on the chitin bead-bound αGMR-ICD/CBD protein. U937 was selected because these cells express high levels of αGMR. The DTT cleaved the fusion protein and αGMR-ICD with the binding proteins were eluted from the chitin beads that were then separated with SDS/PAGE. Five discrete proteins bands appeared to be specific because they did not bind to chitin beads (Fig. 1B). The identities of αGMR-ICD bound proteins were determined by a combination of peptide mass fingerprinting by using matrix-assisted laser desorption–ionization/ time-of-flight MS and mass spectrometric sequencing by using NanoES triple-quadrupole MS/MS (27). The identified proteins were: band 1, bacterial hsp70; band 2, nucleolin; band 3, nucleolin and LR; band 4, bacterial hsp70, α-actin and LR; and band 5, human SET. We considered the LR and SET likely to be true binding proteins.
Fig. 1.
Proteins from U937 bind to the ICD of αGMR. (A) Silver staining of αGMR-ICD/CBD expressed in bacteria. Lane M contains protein molecular weight markers. Lane I contains bacteria total protein lysate input. Lane F is chitin beads flow-through fraction, and lane B contains proteins bound to chitin beads (Upper). The identity of the αGMR-ICD was confirmed by Western blotting by using anti-αGMR Ab C18 (Lower). (B) The chitin beads with the fusion protein αGMR-ICD/CBD were incubated with a total cell extract from U937 cells. After washing and DTT elution, αGMR-ICD and its binding proteins (lane E) were analyzed by SDS/PAGE and silver staining. Control binding proteins (lane C) were those DTT-eluted proteins that bind to native chitin beads. Five proteins bands (bands 1–5) appear to be specific binding proteins to αGMR-ICD, and they were sequenced by MS.
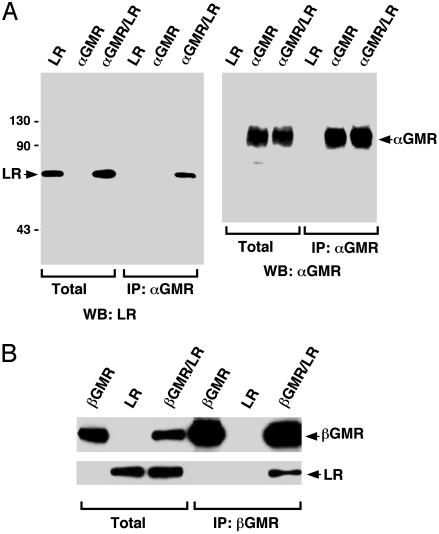
The LR Binds to αGMR and βGMR. To confirm that αGMR interacts with the LR, the plasmids encoding αGMR and LR were transiently transfected into 293T cells (28), and expression of the LR and αGMR were assayed by immunoblotting (Fig. 2A). The anti-V5 tag Ab detected the 67-kDa LR but not the 37-kDa precursor. The αGMR Ab S20 did not immunoprecipitate LR when it was expressed alone but did so when coexpressed with αGMR (Fig. 2A). These results confirm the association of the LR with αGMR. The human SET protein was similarly expressed and did not coimmunoprecipitate with αGMR (data not shown). Because LR binds FN and the βGMR has FN domains, we investigated whether LR could also associate with βGMR (29). The 293T cells were transfected with βGMR, LR, or both. Whereas βGMR Ab S16 did not immunoprecipitate LR when expressed alone, the Ab immunoprecipitated LR when coexpressed with βGMR (Fig. 2B). We generated a rabbit polyclonal Ab against a peptide from the N terminus sequence of LR. This Ab detected LR with a molecular mass of 67 kDa in monocytic U937 cells and neutrophilic HL-60 cells by Western blotting, and 293T cells did not express detectable LR (data not shown). Our results indicate that the LR is a membrane molecule that can physically interact with both αGMR and βGMR.
Fig. 2.
LR binds to both αGMR and βGMR. (A) The 293T cells were transfected with LR (5 μg) or αGMR (5 μg) or cotransfected with LR (5 μg) and αGMR (5 μg). Total protein lysates and αGMR Ab S20 immunoprecipitated proteins were analyzed by SDS/PAGE followed by Western blotting analysis (WB) by using LR Ab anti-V5-HRP (Left) and αGMR Ab C18 (Right). (B) The 293T cells were transfected with βGMR (5 μg) or LR (5 μg) or cotransfected with βGMR (5 μg) and LR (5 μg). Total protein lysates and βGMR Ab S16-immunoprecipitated (IP) proteins were analyzed by SDS/PAGE followed by Western blotting by using LR Ab anti-V5-HRP (Lower) and βGMR Ab C20 (Upper).
LM and FN Enhance GM-CSF Signaling in U937 Cells and Human Neutrophils. The interaction between the LR and αGMR as well as βGMR prompted us to investigate the functional relationship between matrix proteins and GM-CSF signaling. GM-CSF induces phosphorylation of MAPK (Erk1 and Erk2) and the Stat5 (9, 10). We investigated the effect of LM on GM-CSF signaling in the monocytic cell line U937. GM-CSF (3 pM) induced MAPK phosphorylation and LM alone had no effect; however, LM (5 μg/ml) substantially enhanced GM-CSF induced MAPK phosphorylation (Fig. 3A). GM-CSF (1 pM and 3 pM) also stimulated Stat5 phosphorylation and LM enhanced this GM-CSF response (Fig. 3B). In fresh human neutrophils, GM-CSF (3 pM) induced MAPK phosphorylation and FN (1 μg/ml) enhanced the GM-CSF response (Fig. 3C). TNF-α is an important cytokine in host defense and inflammation and GM-CSF stimulates neutrophils to produce TNF-α (30). We measured TNF-α production by human neutrophils and found that LM and FN enhanced GM-CSF-induced TNF-α production (Fig. 3D). LM or FN alone stimulated measurable TNF-α production (Fig. 3D), reflecting the complex stimulatory effects of the matrix proteins on cytokine production (31). These results indicate that the matrix proteins LM and FN can enhance GM-CSF signaling.
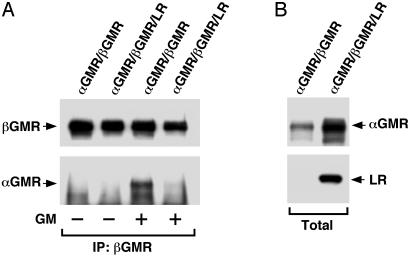
The LR Inhibits GMR Complex Formation. Because αGMR, βGMR, and LR reside in the cell membrane, it is possible that modulation might occur at the receptor level accounting for the functional relationship between the matrix proteins and GM-CSF signaling. The GMR subunits αGMR and βGMR can associate in a “preformed” receptor complex and the ligand GM-CSF induces further complex formation (32). We investigated the effect of LR on GMR complex formation in 293T cells transfected with αGMR and βGMR or αGMR, βGMR, and LR. Cells expressing the receptor proteins were lysed and lysates cleared by centrifugation, and βGMR Ab S16 was used to immunoprecipitate βGMR and proteins bound to βGMR. The proteins captured by protein A were separated by SDS/PAGE and Western blots developed with anti-αGMR Ab. In the absence of GM-CSF, the coimmunoprecipitated αGMR is from preformed αGMR/βGMR complexes whereas the increment of coimmunoprecipitated αGMR in lysates with ligand corresponds to ligand-induced receptor complexes. In the lysate containing αGMR and βGMR, there were preformed αGMR/βGMR complexes and ligand-induced receptor complexes as shown in Fig. 4A. In the lysate containing αGMR, βGMR, and LR, however, the level of preformed receptor complex as well as ligand-induced receptor complex was substantially lower (Fig. 4A), presumably because of the presence of LR. The total expression levels of αGMR and LR are shown in Fig. 4B. The experiments with cell lysates showed that the effect of LR on the GMR is a molecular phenomenon independent of other signaling activities. Experiments with GM-CSF treatment of cells instead of in lysates showed similar inhibition by LR (data not shown). These results indicate that the LR inhibits GMR complex formation.
Fig. 4.
LR inhibits GMR complex formation. (A) The 293T cells were transfected with αGMR plus βGMR (2.5 μg each) or 2.5 μg of αGMR plus 2.5 μg of βGMR plus 5 μg of LR. After transfection (48 h), cells were harvested and lysed; the lysates were then cleared by centrifugation. The lysates were treated with 0.5 nM GM-CSF for 12 h at 4°C. Immunoprecipitation (IP) was done with βGMR Ab S16. In Western blot, αGMR was detected by C18 Ab and the immunoprecipitated βGMR was detected by Ab C20. (B) Total protein levels of αGMR and LR in lysates used for the immunoprecipitation experiment (A) were detected by C18 Ab and anti-V5-HRP, respectively.
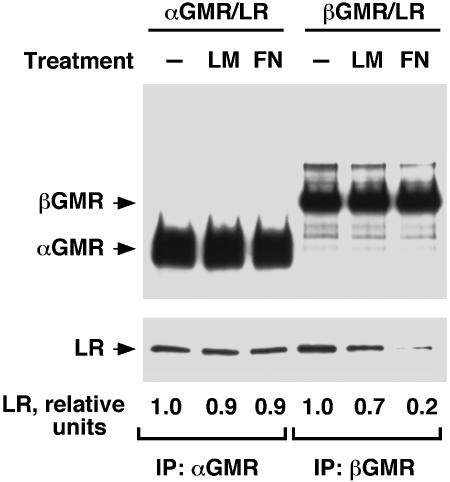
LM and FN Inhibit the Binding of βGMR to LR. Because the LR interacts with both αGMR and βGMR, we investigated how those interactions might change in response to the LR ligands, LM and FN. For αGMR and LR, there is no specific information for predicting how the interaction would change upon LM or FN treatment; however, it is possible that LR binds βGMR through its FN domains and that FN might compete with βGMR for LR binding. Therefore FN might inhibit the βGMR/LR interaction. Cells (293T) were transfected with αGMR and LR or βGMR and LR, treated with LM or FN, and the αGMR/LR or βGMR/LR complexes immunoprecipitated with the respective GMR Abs. The associated LR was analyzed by immunoblotting (Fig. 5 Lower). The αGMR/LR complex appeared to be unaffected by LM or FN treatment whereas the βGMR/LR complex was inhibited by LM and FN (Fig. 5).
Fig. 5.
LM and FN disrupt LR/βGMR complex. The 293T cells were transfected with αGMR plus LR (4 μg each) or βGMR plus LR (4 μg each). After transfection (60 h), cells were harvested and treated with LM (mixture of 5 μg/ml human placenta LM and 5 μg/ml murine sarcoma LM) or FN (mixture of 5 μg/ml plasma FN and 5 μg/ml cellular FN) for6hat room temperature with rocking. Cells were centrifuged and lysed, and LM or FN was added to the lysates to maintain the original concentrations. Immunoprecipitation of cleared lysates was done with αGMR Ab S20 and βGMR Ab S16 at 4°C for 16 h. In Western blotting, αGMR and βGMR were detected with Abs C18 and C20, respectively (Upper). LR was detected with anti-V5-HRP Ab (Lower). Relative ratios of LR to αGMR or βGMR band intensities are indicated.
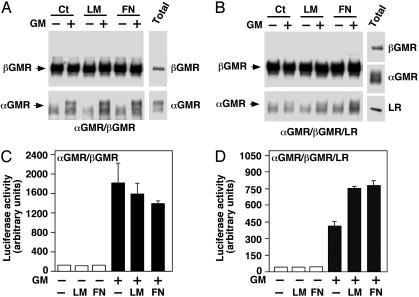
LM and FN Relieve the Inhibition of LR on the GMR. The LR binds to matrix proteins including LM, FN, and type IV collagen. We investigated whether these matrix proteins modulate GMR signaling through LR. In 293T cells transfected with αGMR and βGMR, GM-CSF-induced receptor complex formation and the matrix proteins LM and FN had no effect as shown by βGMR Ab coimmunoprecipitated αGMR (Fig. 6A Lower). In cells transfected with αGMR, βGMR and LR, GM-CSF was unable to induce receptor complex formation due to the presence of LR, however, the addition of LM or FN restored the ligand-induced GMR complex (Fig. 6B). To ascertain the physiological consequences of LM and FN effects on the GMR, we measured GMR signaling quantitatively with the 8×GAS-luciferase reporter construct that responds to Stat5 activation (33, 34). In 293T cells transfected with αGMR, βGMR, and 8×GAS-luciferase, GM-CSF activated the reporter and LM and FN had no effect on reporter activity (Fig. 6C). In cells transfected with αGMR, βGMR, 8×GAS-luciferase and LR, however, both LM and FN enhanced GM-CSF function in activating the luciferase reporter gene (Fig. 6D). Type IV collagen had a measurable effect but less than LM or FN in increasing GMR complex formation or 8×GAS-luciferase activity (data not shown). These results indicate that although the LR inhibits the GMR system, the matrix proteins can relieve this inhibition (Fig. 7).
Fig. 6.
LM and FN relieve the LR inhibition on GMR system. (A) The 293T cells were transfected with αGMR (2.5 μg) and βGMR (2.5 μg). Cells were treated with LM (mixture of 2 μg/ml human placenta and 2 μg/ml human cellular) or FN (human plasma, 5 μg/ml) for 10 min followed by 0.5 nM GM-CSF treatment for 1 h at room temperature. Cells were centrifuged and lysed with no further addition of matrix proteins or GM-CSF. Proteins were immunoprecipitated by βGMR Ab S16 and βGMR (Upper) and αGMR associated with βGMR (Lower) were detected with Western blotting. αGMR and βGMR in the total protein lysate are shown (Right). (B) The 293T cells were transfected with αGMR (2.5 μg), βGMR (2.5 μg), and LR (5 μg). Cells were treated as in A. Proteins immunoprecipitated by βGMR Ab S16 and βGMR (Upper) and αGMR associated with βGMR (Lower) were detected with Western blotting. αGMR, βGMR, and LR were detected in the total protein lysate (Right). (C) The 293T cells were transfected with αGMR plus βGMR plus pcDNA3.1/GS empty vector plus 8×GAS-luciferase (2 μg each). Luciferase activity was measured after different treatments as indicated. Human placenta LM and human plasma FN were used at 5 μg/ml, and GM-CSF (GM) was used at 300 pM. (D) The 293T cells were transfected with αGMR plus βGMR plus LR plus 8×GAS-luciferase (2 μg each). Luciferase activity was measured after different treatments as indicated. Human placenta LM and human plasma FN were used at 5 μg/ml, and GM-CSF (GM) concentration was 300 pM.
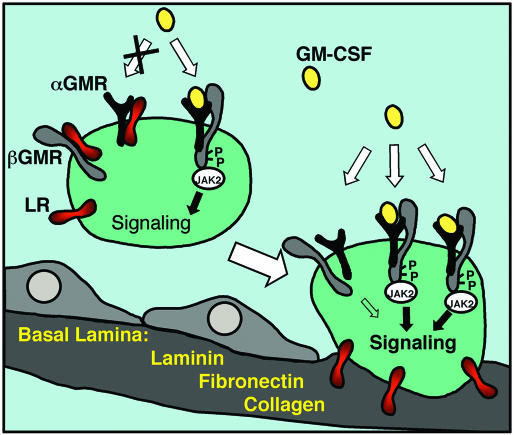
Fig. 7.
Schematic model of the LR modulation of GMR system. In circulating host defense cells that are not attached to the basement membrane, the GMR complex formation is inhibited by the LR through its interaction with αGMR and βGMR. The inhibition results in low responsiveness to the GM-CSF cytokine. In cells attached to the basement membrane, the matrix proteins LM, FN, and collagen bind to the LR and the inhibition of the LR on the GMR system is relieved. The basement membrane attachment results in increased GM-CSF signaling. The mechanism implicates that only cells undergoing transendothelial migration are activated and those nonattaching cells do not get activated even though they might see similar concentration of GM-CSF in the infected local milieu. The exact stoichiometry of the functional GMR complex is not reflected.
Discussion
Host defense cells are regulated by interacting signals from extracellular matrix proteins, cytokines, and chemotactic peptides. GM-CSF and TNF-α prime the neutrophils for an enhanced respiratory burst triggered by molecules such as the bacterial peptide fMLP (35, 36). Although GM-CSF or TNF-α alone does not induce a respiratory burst, adherence to matrix proteins primes human neutrophils for the respiratory burst in response to TNF-α and GM-CSF (1, 37). The hierarchical regulation of host defense cells by matrix proteins, inflammatory cytokines, chemokines, and chemotaxins precisely limits host defense actions to appropriate sites. Host defense cell migration through endothelial basement membrane matrix proteins appears to initiate the activation process; however, the molecular mechanisms involved in matrix protein regulation of host defense cells remain unclear.
The short cytoplasmic domain of the GMR α subunit has attracted considerable attention because it is critical for GMR signaling (12, 13, 38, 39). In searching for proteins interacting with the αGMR ICD, we found GM-CSF receptor α-subunit-associated protein, whose function has yet to be determined (40) and phosphatidylinositol 3-kinase, which is involved in GM-CSF signaling for increased glucose uptake (11). Here we used an alternative approach for finding αGMR-interacting proteins by expressing the αGMR ICD as a CBD fusion protein and using it in binding experiments with U937 cell extract on chitin beads. U937 cells were selected because they express relatively high levels of αGMR, and we reasoned they might therefore offer a better chance of finding interacting proteins. MS identified the LR in two separate protein bands, probably reflecting the presence of 37- and 67-kDa forms (16). The other αGMR-binding protein identified by MS was human SET (41). SET seemed to be nonspecific, because it did not coimmunoprecipitate with αGMR (data not shown).
Although GM-CSF is a cytokine involved in host defense, LR may play a host defense role as well. LR expression in T cells is increased in a subset of activated memory T lymphocytes (19) and human T cells produce neuropeptides GnRH-II and GnRH-I, which increase LR gene expression (42). The murine mast cell line PT18 and mouse bone marrow-derived mast cells express LR (20). In acute myeloid leukemia, 67-kDa LR expression is prominent and high LR-expressing cells often exhibit monocytic or myelomonocytic properties (21). Cultured colon cancer cells bound to LM but not to FN and the binding was inhibited by Abs to integrins but not by LR mAb (43). These observations support a signaling role for LR.
It was unexpected to find that LR interacted with βGMR in addition to αGMR in coimmunoprecipitation studies. LR may interact with the βGMR extracellular domain as the LR extracellular domain interacts with FN and βGMR has FN domains extracellularly. The ability of FN and LM to compete with βGMR for LR binding supports the notion that the βGMR and LR extracellular domains are involved in the interaction. We found LR inhibits both preformed (ligand independent) αGMR/βGMR complex formation and GM-CSF induced complex formation. The function of the βGMR/LR interaction seems straightforward, suggesting that LR interaction with βGMR prevents it from participating in GM-CSF signaling. The LR ligands, LM or FN compete with βGMR for binding to LR and therefore βGMR is freed from LR and available for GM-CSF signaling.
The interaction between αGMR and LR was found by using the αGMR ICD, implying that both ICDs are involved in the interaction. The function of the αGMR/LR interaction is less obvious than that of βGMR/LR interaction because theoretically only one point of interception is required to achieving inhibition of a linear signaling pathway. A possible explanation is that the inhibition is more efficient when LR also sequesters the αGMR ICD, which is required for cooperating with βGMR in GM-CSF signaling and has additional functions independent of βGMR (12, 13, 38, 44). The αGMR/LR interaction was not disrupted by LM or FN, as was βGMR/LR interaction. It is possible that the αGMR/LR interaction involves primarily the ICDs and βGMR/LR interaction mainly involves extracellular domains, implying distinct mechanisms of modulation. It is also possible that the αGMR/LR interaction is simply less important than βGMR/LR interaction in modulating αGMR/βGMR complex formation, as βGMR freed from LR could use αGMR that is or is not bound to LR for signaling.
A model of LR regulation GM-CSF signaling is summarized in Fig. 7. For host defense cells in the circulation, LR sequesters αGMR and βGMR and inhibits GMR complex formation; therefore cell responsiveness to GM-CSF is low. For host defense cells contacting basement membrane, LR is bound by matrix proteins LM, FN, or collagen and the inhibition on GMR is relieved. Therefore host defense cells migrating out of the circulation may have higher responsiveness for GM-CSF that facilitates host defense actions.
LR inhibition of GM-CSF signaling at the receptor level appears very efficient and specific as compared to mitigating extracellular ligands or suppressing downstream cascade components. The type of receptor–receptor interaction described here for LR and GMR has not been reported previously with respect to cytokine signaling. A conceptually similar form of interaction has been shown for the hepatocyte growth factor receptor Met, sequestering Fas in the membrane and preventing Fas ligand binding and subsequent induction of apoptosis (45). Our present findings define a mechanism for modulating GM-CSF responses based on receptor–receptor inhibitory interaction and release of inhibition by basement membrane proteins. The results also expand our understanding of microenvironmental effects of cytokine responsiveness especially in bone marrow where matrix proteins play important roles in cell growth and differentiation.
Acknowledgments
We thank Dr. Steven J. Ackerman for providing the 8×GAS-luciferase reporter gene construct originally made by Dr. Christopher Glass. This work was supported by grants from the National Institutes of Health (CA 30388 to D.W.G. and CA 08748) and the Lebensfeld Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GM-CSF, granulocyte–macrophage colony-stimulating factor; LM, laminin; LR, LM receptor; GMR, GM-CSF receptor; FN, fibronectin; MAP, mitogen-activated protein; MAPK, MAP kinase; CBD, chitin-binding domain; TNF, tumor necrosis factor; HRP, horseradish peroxidase; ICD, intracellular domain; 8×GAS, 8 IFN-γ-activated site elements; Stat, signal transducer and activator of transcription.
See Commentary on page 13737.
References
- 1.Nathan, C. F. (1989) Blood 73, 301–306. [PubMed] [Google Scholar]
- 2.Tourkin, A., Anderson, T., LeRoy, E. C. & Hoffman, S. (1993) Cell Adhes. Commun. 1, 161–176. [DOI] [PubMed] [Google Scholar]
- 3.Young, M. R., Lozano, Y., Djordjevic, A., Devata, S., Matthews, J., Young, M. E. & Wright, M. A. (1993) Int. J. Cancer 53, 667–671. [DOI] [PubMed] [Google Scholar]
- 4.Gasson, J. C. (1991) Blood 77, 1131–1145. [PubMed] [Google Scholar]
- 5.Gearing, D. P., King, J. A., Gough, N. M. & Nicola, N. A. (1989) EMBO J. 8, 3667–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashida, K., Kitamura, T., Gorman, D. M., Arai, K., Yokota, T. & Miyajima, A. (1990) Proc. Natl. Acad. Sci. USA 87, 9655–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavernier, J., Devos, R., Cornelis, S., Tuypens, T., Van der Heyden, J., Fiers, W. & Plaetinck, G. (1991) Cell 66, 1175–1184. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura, T., Sato, N., Arai, K. & Miyajima, A. (1991) Cell 66, 1165–1174. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki, K., Hino, M., Hato, F., Tatsumi, N. & Kitagawa, S. (1999) Blood 93, 341–349. [PubMed] [Google Scholar]
- 10.Quelle, F. W., Sato, N., Witthuhn, B. A., Inhorn, R. C., Eder, M., Miyajima, A., Griffin, J. D. & Ihle, J. N. (1994) Mol. Cell. Biol. 14, 4335–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhar-Mascareno, M., Chen, J., Zhang, R. H., Carcamo, J. M. & Golde, D. W. (2003) J. Biol. Chem. 278, 11107–11114. [DOI] [PubMed] [Google Scholar]
- 12.Matsuguchi, T., Zhao, Y., Lilly, M. B. & Kraft, A. S. (1997) J. Biol. Chem. 272, 17450–17459. [DOI] [PubMed] [Google Scholar]
- 13.Doyle, S. E. & Gasson, J. C. (1998) Blood 92, 867–876. [PubMed] [Google Scholar]
- 14.Castronovo, V., Taraboletti, G. & Sobel, M. E. (1991) J. Biol. Chem. 266, 20440–20446. [PubMed] [Google Scholar]
- 15.Landowski, T. H., Dratz, E. A. & Starkey, J. R. (1995) Biochemistry 34, 11276–11287. [DOI] [PubMed] [Google Scholar]
- 16.Castronovo, V., Claysmith, A. P., Barker, K. T., Cioce, V., Krutzsch, H. C. & Sobel, M. E. (1991) Biochem. Biophys. Res. Commun. 177, 177–183. [DOI] [PubMed] [Google Scholar]
- 17.Narasimhan, S., Armstrong, M. Y., Rhee, K., Edman, J. C., Richards, F. F. & Spicer, E. (1994) Proc. Natl. Acad. Sci. USA 91, 7440–7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwabuchi, K., Nagaoka, I., Someya, A. & Yamashita, T. (1996) Blood 87, 365–372. [PubMed] [Google Scholar]
- 19.Canfield, S. M. & Khakoo, A. Y. (1999) J. Immunol. 163, 3430–3440. [PubMed] [Google Scholar]
- 20.Thompson, H. L., Burbelo, P. D., Segui-Real, B., Yamada, Y. & Metcalfe, D. D. (1989) J. Immunol. 143, 2323–2327. [PubMed] [Google Scholar]
- 21.Montuori, N., Selleri, C., Risitano, A. M., Raiola, A. M., Ragno, P., Del Vecchio, L., Rotoli, B. & Rossi, G. (1999) Clin. Cancer Res. 5, 1465–1472. [PubMed] [Google Scholar]
- 22.Martignone, S., Menard, S., Bufalino, R., Cascinelli, N., Pellegrini, R., Tagliabue, E., Andreola, S., Rilke, F. & Colnaghi, M. I. (1993) J. Natl. Cancer Inst. 85, 398–402. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto, Y., Robey, F. A., Graf, J., Sasaki, M., Kleinman, H. K., Yamada, Y. & Martin, G. R. (1987) Science 238, 1132–1134. [DOI] [PubMed] [Google Scholar]
- 24.Barsky, S. H., Rao, C. N., Williams, J. E. & Liotta, L. A. (1984) J. Clin. Invest. 74, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdjument-Bromage, H., Lui, M., Lacomis, L., Grewal, A., Annan, R. S., McNulty, D. E., Carr, S. A. & Tempst, P. (1998) J. Chromatogr. A 826, 167–181. [DOI] [PubMed] [Google Scholar]
- 26.Geromanos, S., Freckleton, G. & Tempst, P. (2000) Anal. Chem. 72, 777–790. [DOI] [PubMed] [Google Scholar]
- 27.Sebastiaan Winkler, G., Lacomis, L., Philip, J., Erdjument-Bromage, H., Svejstrup, J. Q. & Tempst, P. (2002) Methods 26, 260–269. [DOI] [PubMed] [Google Scholar]
- 28.Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. (1993) Proc. Natl. Acad. Sci. USA 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustin, S. E., Church, A. P., Ford, S. C., Mann, D. A., Carr, P. D., Ollis, D. L. & Young, I. G. (2001) Eur. J. Biochem. 268, 2905–2911. [DOI] [PubMed] [Google Scholar]
- 30.Jablonska, E., Kiluk, M., Markiewicz, W. & Jablonski, J. (2002) Melanoma Res. 12, 123–128. [DOI] [PubMed] [Google Scholar]
- 31.Chana, R. S., Martin, J., Rahman, E. U. & Wheeler, D. C. (2003) Kidney Int. 63, 889–898. [DOI] [PubMed] [Google Scholar]
- 32.Woodcock, J. M., McClure, B. J., Stomski, F. C., Elliott, M. J., Bagley, C. J. & Lopez, A. F. (1997) Blood 90, 3005–3017. [PubMed] [Google Scholar]
- 33.Horvai, A. E., Xu, L., Korzus, E., Brard, G., Kalafus, D., Mullen, T. M., Rose, D. W., Rosenfeld, M. G. & Glass, C. K. (1997) Proc. Natl. Acad. Sci. USA 94, 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du, J., Alsayed, Y. M., Xin, F., Ackerman, S. J. & Platanias, L. C. (2000) J. Biol. Chem. 275, 33167–33175. [DOI] [PubMed] [Google Scholar]
- 35.Berkow, R. L., Wang, D., Larrick, J. W., Dodson, R. W. & Howard, T. H. (1987) J. Immunol. 139, 3783–3791. [PubMed] [Google Scholar]
- 36.Weisbart, R. H., Golde, D. W. & Gasson, J. C. (1986) J. Immunol. 137, 3584–3587. [PubMed] [Google Scholar]
- 37.Nathan, C. F. (1987) J. Clin. Invest. 80, 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polotskaya, A., Zhao, Y., Lilly, M. B. & Kraft, A. S. (1994) J. Biol. Chem. 269, 14607–14613. [PubMed] [Google Scholar]
- 39.Lilly, M. B., Zemskova, M., Frankel, A. E., Salo, J. & Kraft, A. S. (2001) Blood 97, 1662–1670. [DOI] [PubMed] [Google Scholar]
- 40.Tu, J., Karasavvas, N., Heaney, M. L., Vera, J. C. & Golde, D. W. (2000) Blood 96, 794–799. [PubMed] [Google Scholar]
- 41.Li, M., Makkinje, A. & Damuni, Z. (1996) J. Biol. Chem. 271, 11059–11062. [DOI] [PubMed] [Google Scholar]
- 42.Chen, A., Ganor, Y., Rahimipour, S., Ben-Aroya, N., Koch, Y. & Levite, M. (2002) Nat. Med. 8, 1421–1426. [DOI] [PubMed] [Google Scholar]
- 43.Kitayama, J., Nagawa, H., Tsuno, N., Osada, T., Hatano, K., Sunami, E., Saito, H. & Muto, T. (1999) Br. J. Cancer 80, 1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding, D. X., Rivas, C. I., Heaney, M. L., Raines, M. A., Vera, J. C. & Golde, D. W. (1994) Proc. Natl. Acad. Sci. USA 91, 2537–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, X., DeFrances, M. C., Dai, Y., Pediaditakis, P., Johnson, C., Bell, A., Michalopoulos, G. K. & Zarnegar, R. (2002) Mol. Cell 9, 411–421. [DOI] [PubMed] [Google Scholar]