Perchlorate anion is a known environmental contaminant in the United States, that is associated with its use in aerospace industry,1 fireworks and automobile airbag.2 In most cases, it is present as an inorganic salt and highly soluble in water. Because of its poor affinity to the mineral surfaces and organic materials, perchlorate anion can readily move to aqueous medium resulting in the contamination of ground water.3 Perchlorate is also known to disrupt normal thyroid functions competing with iodide, when consumed in excess than the recommended limit. Based on the recommendations of the National Academy of Sciences, the US Environmental Protection Agency, recently set a new limit of perchlorate to 15 µgL−1 that is 9.5 µgL−1 lower than that was previously set.4 Therefore, there is a need to develop new systems that can be used effectively in the remediation of this target anion.
While there has been a significant progress in binding of a variety of inorganic anions during the last two decades,5 however, reports on the perchlorate structures with macrocyclic hosts are quite limited.6–8 Given that the low charge to radius ratio of the perchlorate as predicted from the single charge shared with the four oxygens, this anion interacts weakly with synthetic hosts.9 The first structural evidence of cryptand based perchlorate complex was reported by Nelson in 1995, showing a complete encapsulation in a furan based azacryptand,6 followed by an another report published in 2000, that was in a m-xylyl analogue.7 Later, the cleft binding of perchlorate was observed in the pyridine based analogue, in which perchlorates are bound between the arms of the cryptand.8 On the other hand, two cyclophane based perchlorate structures are known from the work of Steed et al. that are with 2, 6, 10-triaza[11]metacyclophane, and 2,6,9,13-tetraaza[14] metacyclophane.10
Although, azamacrocycles do not possess sterically controlled preorganized cavity, but they mimic many biological polyamines, e.g. putrescine, spermidine and spermine, and are known to interact nucleotides strongly and selectively by electrostatic interactions between the positively charged ammonium groups of receptors and anionic phosphates.11 Indeed, since late 70’s azamacrocycles are the most widely investigated hosts for binding anionic guests in both solution and solid states.12 In many cases, the monocyclic based receptors enjoy flexibility to interact anions from both sides of the cycle leading to the formation of ditopic complexes, e.g. halide complexes with [18]N613 and metacyclophanes.10 Hexaazamacrocycles that are readily obtainable from Schiff base condensation reaction reported by Martell and coworkers,14 are capable in binding a wide range of anions including halides, nitrate and phosphate, and even in complexing biological anions like carboxylates and nucleotides.15 Surprisingly, with the exception of one example,16 there is no structural report of perchlorate complex with a Schiff base deriven macrocycle. Herein, we present the crystallographic reports of two ditopic perchlorate complexes with the hexaazamacrocycles, H6[L1]6+ and H4[L2]4+, and the results of 1H NMR binding studies in aqueous solution.
Both LI17 and L218 were reported previously in the literature, and prepared from the cyclization of bis(2-aminoethyl)amine with 2,5-thiophenedicarboxaldehyde and teraphthalaldehyde, respectively, followed by the reduction with sodium borohydride. Perchlorate salts were obtained from the reaction of the corresponding neutral ligands with HClO4 in water. Crystals suitable for X-ray analysis were obtained from the slow evaporation of the salts dissolved in water.‡
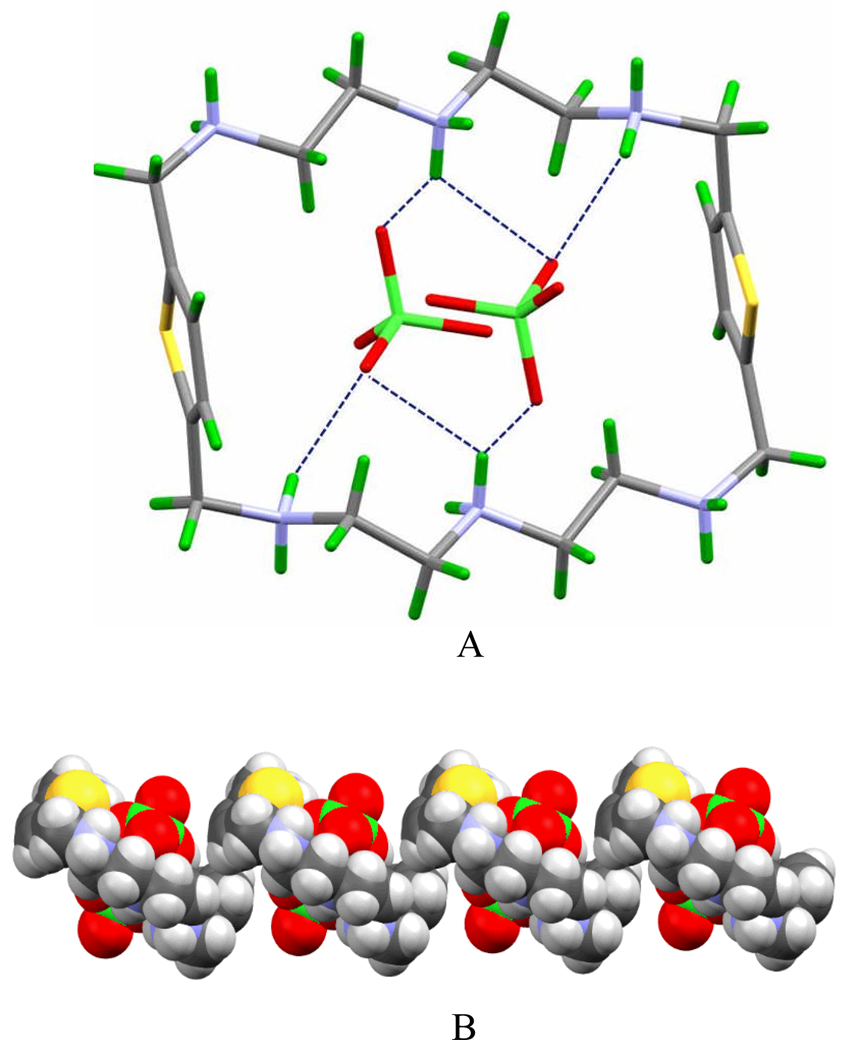
Structural analysis indicates that both ligands act as hosts for two perchlorate guests that are held via several hydrogen bonds to protonated nitrogen centers. The perchlorate complex of L1 lies in its triclinic system possessing an inversion center. As shown in Fig. 1A, the ligand L1 crystallizes in its full protonated form hosting two perchlorate species with a total of six hydrogen bonds (N…O = 2.808 − 2.960 Å). In the complex, both protons on the central nitrogens pointing toward the cavity, become available for hydrogen bonding from both sides. Each anion is linked to both central NH2+ as well as to one secondary NH2+ linking the aromatic unit. The involvement of both central NH2+ in binding the anion, results into a shortening of N…N distance, 6.296 Å. The protons on the two remaining symmetry related secondary NH2+ are directed away from the cavity and involved in hydrogen bonding with external perchlorates. The aromatic units are antiparallel to each other separating with a distance of 8.898 Å. Extending the structure along the b axis, results a helix type structure, in which perchlorates are bound in helical pockets (viewed along the c axis) formed by the macrocycles (Fig. 1B).
Fig. 1.
Crystal structure of H6L1(ClO4)2]4+: (A) side view showing two perchlorates bonded to the macrocycle. (B) One dimensional packing to the b direction viewed along the c axis.
In the perchlorate complex of L2, the ligand is tetraprotonated, and crystallized with four perchlorate anions and one water. The central nitrogens remain unprotonated. Fig. 2A shows the X-ray structure of the complex in which two perchlorates sitting above the plane of the macrocycle, are bonded to all of secondary NH2+ via N…O hydrogen bonds ranging in the donor–acceptor distances of 2.94 to 3.06 Å.
Fig. 2.
Crystal structure of H4L2(ClO4)2]2+: (A) side view showing two perchlorates and one water bonded to the macrocycle. (B) One dimensional packing to the a direction viewed along the c axis.
This structure differs from that observed for the perchlorate complex of L1, in which the macrocycle interacts with two anions from both sides. The remaining two anions, however, are out side the cavity and associated with the macrocycle via single hydrogen bonding interaction with the amine protons. The central N…N distance is 10.56 Å, that is much longer than the corresponding distance in H6L1(ClO4)2]4+. Interestingly, one water molecule is found in the cavity lying on the N…N axis, and closely located to one terminal nitrogen. This internal water is coordinated asymmetrically through hydrogen bonds with two amine proton, and with one perchlorate anion (Ow…O = 2.846 Å). The second perchlorate anion is located at a longer distance from the water (Ow…O, 3.48 Å) indicating the lack of hydrogen bond between the two species. The distance between two aromatic centroids is 6.674 Å and both rings are facing to one oxygen of the later anion with the distances of Arcentroids…O of 3.901 and 3.912 Å. As shown in two dimensional packing (Fig. 2B), perchlorates bridge the neighbouring macrocycles resulting in a channel that is filled by the anionic species.
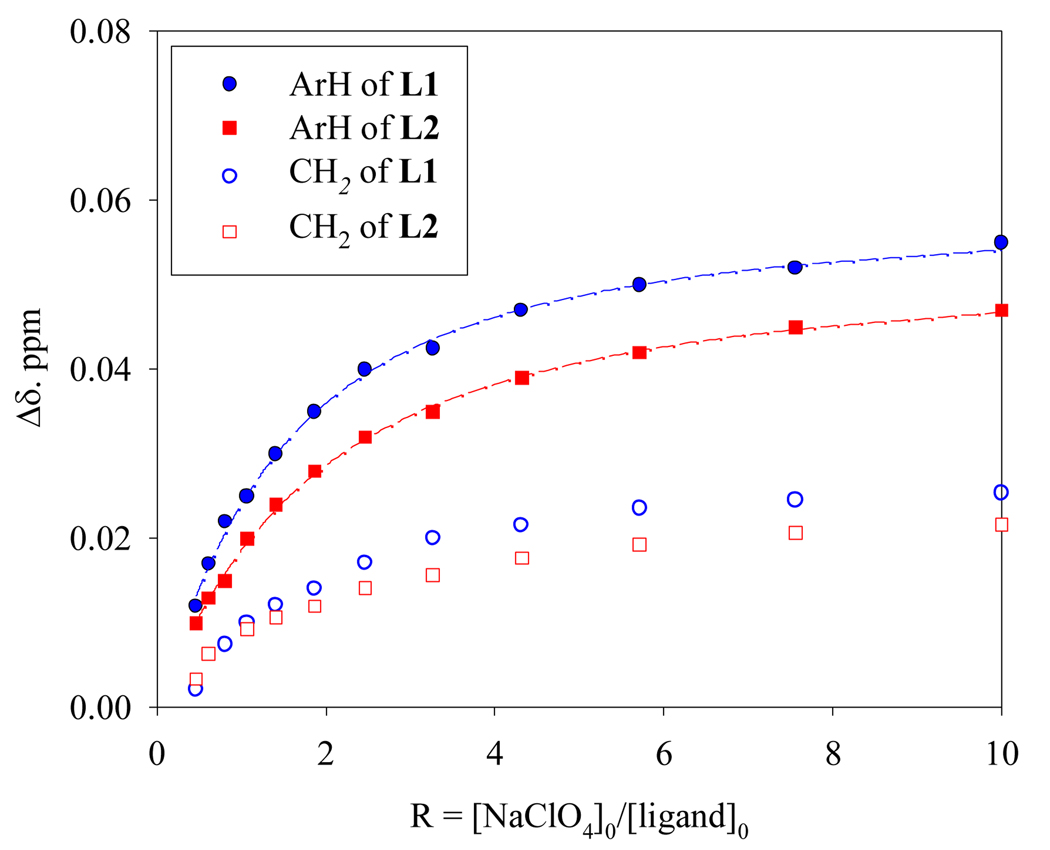
In order to get the solution binding properties, 1H NMR titrations studies were performed at pH 4.019 in D2O using the tosylate salts of ligands (2 mM). The addition of NaClO4 solution (20 mM) resulted in a downfield shift (ämax ~ 0.05 ppm) for the aliphatic protons close to aromatic units in both ligands. Other protons did not shift significantly. Analysis of the NMR titration data gave the best fit for a 1:1 binding model (Fig. 3), yielding association constants (log K), 2.52 and 2.42 for L1 and L2, respectively. The 1:1 binding stoichiometry was further supported by the Job plots performed in D2O at the same pH, showing a maximum at a 0.5 mole fraction for both ligands (see supplementary information). The observed stoichiometry is, however, inconsistent with that in the solid state, but not unprecedented, as reported earlier in several cases.13,20 The above binding constants are not strong, as expected in water - a competitive polar solvent. The anionic guest also competes with surrounding tosylates that are likely associated with macrocyclic cations.21 Attempts to study in non-polar solvent like CDCl3 or CD3CN were hampered due to the poor solubility of the ligand salts.
Fig. 3.
1H NMR titration curves for perchlorate binding with the tosylate salts of L1 (blue) and L2 (red) in D2O at pH = 4.0.
In summery, we presented two rare examples of perchlorate structures with hexaazamacrocycles. The anions are coordinated by the hydrogen bonding interactions with L1 and L2 in hexa- and tetra- protonated forms, respectively, resulting into ditopic complexes in both cases. In L2, all four protonated sites are utilized in binding anions, however, in L1 two protonated nitrogen remain unbonded. While an inert perchlorate interacts weakly with synthetic hosts, these findings will enhance the level of our understanding for the rational design of the selective anion receptors, for this and other tetrahedral anionic species.
Supplementary Material
Acknowledgments
The project described was supported by Grant Number G12RR013459 from the National Center for Research Resources. This material is based upon work supported by the National Science Foundation under C H E-0821357. Purchase of the diffractometer was made possible by grant No. LEQSF (1999-2000)-ENH-TR-13, administered by the Louisiana Board of Regents.
Footnotes
Electronic supplementary information (ESI) available: The details of the synthetic procedure, NMR binding studies, Job plots and crystallographic details. CCDC reference numbers 739057 and 739058. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b918152k
Crystal data: For [H6L1(ClO4)2](ClO4)4·4H2O: C20H48N6S2Cl6O28, M =1097.46, crystal size 0.27 × 0.19 × 0.10 mm3, Triclinic, P1, a = = 8.3060 (6), b = 10.5205 (9), c = 12.4149 (10) Å, α = 103.619 (5)°, β = 90.201 (5)°, γ = 94.123 (5)°, V = 1051.39 (15)Å3, Z = 1, dcalc = 1.733 g cm−3, T = 90 K, F(000) = 568, µ(Cu-Kα) = 5.59 mm−1, 3690 independent reflections (10176 measured), Rint = 0.030, R1( obsd data) = 0.032, wR2(all data) = 0.087. For [H4L2(ClO4)2(H2O)](ClO4)2: C24H44N6Cl4O17, M = 830.45, crystal size 0.25 × 0.10 × 0.08 mm3, Orthorhombic, P212121, a = 11.9131 (10), b = 14.5341 (15), c = 20.786 (2) (10) Å, V = 3599.0 (6) Å3, Z = 4, dcalc = 1.533 g cm−3, T = 90 K, F(000) = 1736, µ(Mo-Kα) = 0.41 mm−1, 7932 independent reflections (61377measured), Rint = 0.097, R1(obsd data) = 0.051, wR2(all data) = 0.105. Flack parameter = 0.28 (5).
Notes and references
- 1.USEPA. Perchlorate environmental contamination: Toxicological review and risk characterization. External review draft. NCEA-1-0503. Washington, DC: 2002. [Google Scholar]
- 2.Mendiratta SK, Doston RL, Brokker RT. Encyclopedia of chemical Tech. New york: John Wiley and Sons; 1996. pp. 18–57. [Google Scholar]
- 3.Macmillan DK, Dalton WR, Bednar AJ, Waisner SA, Arora PN. Chmeosphere. 2007;67:344. doi: 10.1016/j.chemosphere.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 4.USEPA. “Interim Drinking Water Health Advisory For Perchlorate” EPA 822-R-08-025. Washington, DC: 2008. (December 2008) [Google Scholar]
- 5.(a) Bianchi A, García-España E, Bowman-James K. Supramolecular Chemistry of Anions. New York: Wiley-VCH; 1997. [Google Scholar]; (b) Schmidtchen FP, Berger M. Chem. Rev. 1997;97:1609. doi: 10.1021/cr9603845. [DOI] [PubMed] [Google Scholar]; (c) Gale PA, editor. Coord. Chem. Rev. 2003. 35 years of Synthetic Anion Receptor Chemistry; p. 240. [Google Scholar]; (d) Gale PA. Acc. Chem. Res. 2006;39:465. doi: 10.1021/ar040237q. [DOI] [PubMed] [Google Scholar]; (e) Gale PA. Chem. Commun. 2005:3761. doi: 10.1039/b504596g. [DOI] [PubMed] [Google Scholar]; (f) Bowman- James K. Acc. Chem. Res. 2005;38:671. doi: 10.1021/ar040071t. [DOI] [PubMed] [Google Scholar]; (g) Sessler JL, Gale PA, Cho WS. In: Anion Receptor Chemistry, (Monographs in Supramolecular Chemistry) Stoddart JF, editor. Cambridge: Royal Society of Chemistry; 2006. [Google Scholar]; (h) Hossain MA. Curr. Org. Chem. Vol. 12. 2008. p. 1231. [Google Scholar]
- 6.Morgan G, McKee V, Nelson J. J. Chem. Soc., Chem. Commun. 1995:1649. [Google Scholar]
- 7.Hynes MJ, Maubert B, McKee V, Town RM, Nelson J. J. Chem. Soc., Dalton Trans. 2000:2853. [Google Scholar]
- 8.McKee V, Morgan GG. Acta Cryst. 2003;C59:150. [Google Scholar]
- 9.Arranz P, Bencini A, Bianchi A, Diaz P, García-España E, Giorgi C, Luis SV, Querol M, Valtancoli B. J. Chem. Soc., Perkin Trans. 2001;2:1765. [Google Scholar]
- 10.Illioudis CA, Steed JW. Organ. Biomol. Chem. 2005;3:2935. doi: 10.1039/b506828b. [DOI] [PubMed] [Google Scholar]
- 11.Nagarajan S, Ganem B. J. Org. Chem. 1987;52:5044. [Google Scholar]
- 12.(a) Llinares JM, Powell D, Bowman-James K. Coord. Chem. Rev. 2003;240:57. [Google Scholar]; (b) García-España E, Díaz P, Llinares JM, Bianchi A. Coord. Chem. Rev. 2006;250:2952. [Google Scholar]
- 13.Warden AC, Warren M, Hearn MTW, Spiccia L. New J. Chem. 2004;28:1160. [Google Scholar]
- 14.Chen D, Martell AE. Tetrahedron. 1991;47:6895. [Google Scholar]
- 15.(a) Motekaitis RJ, Martell AE, Dietrich B, Lehn JM. Inorg. Chem. 1984;23:1588. [Google Scholar]; (b) Beer PD, Wheeler JW, Moore C. In: Supramolecular Chemistry. Balzani V, De Cola L, editors. Dordrecht: Kluwer Academic Publishers; 1992. p. 105. [Google Scholar]; (c) Katz HE. In: Inclusion Compounds. Atwood JL, Davies JED, MacNichol DD, editors. Oxford: Oxford University Press; 1991. p. 391. [Google Scholar]; (d) Seel C, Galán A, de Mendoza J. Top. Curr. Chem. 1995;175:101. [Google Scholar]; (e) Papoyan G, Gu K, Wiórkiewicz-Kuczera J, Kuczera K, Bowman-James K. J. Am. Chem. Soc. 1996;118:1354. [Google Scholar]; (f) Wiórkiewicz-Kuczera J, Kuczera K, Bazzicalupi C, Bencini A, Valtancoli B, Bianchi A, Bowman-James K. New J. Chem. 1999;23:1007. [Google Scholar]; (g) Clifford T, Danby A, Llinares JM, Mason S, Alcock NW, Powell D, Aguilar JA, Garcia-Espana E, Bowman-James K. Inorg.Chem. 2001;40:4710. doi: 10.1021/ic010135l. [DOI] [PubMed] [Google Scholar]
- 16.Bazzicalupi C, Bencini A, Bianchi A, Fusi V, Giorgi C, Paoletti P, Stefani1 A, Valtancoli B. J. Chem. Soc., Perkin Trans. 1995;2:275. [Google Scholar]
- 17.Dancey KF, Fenton ED, Moss S, Jones G, Price R. Synthetic Communications. 1986;16:795. [Google Scholar]
- 18.Gao J, Reibenspies J, Martell AE. Inorg. Chim. Acta. 2002;335:125. [Google Scholar]
- 19.The logarithm of the protonation constant for [H6L2]/[H5L2][H] was reported to 3.04, and the ligand was seen to be predominantly tetrateprotonated at pH =4, see, Anda C, Martínez M-A, Llobet A. Supramol. Chem. 2005;17:257.
- 20.Hossain MA, Morehouse P, Powell PD, Bowman-James K. Inorg. Chem. 2005;44:2143. doi: 10.1021/ic048937e. [DOI] [PubMed] [Google Scholar]
- 21.Saeed MA, Thompson JJ, Fronczek FR, Hossain MA. Acta Cryst. 2009;E65 doi: 10.1107/S1600536809035648. o2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.