In the interval since a trial of orthotopic liver transplantation was resumed at the University of Colorado, an attempt has been made to treat 14 patients. The most important conclusion that has emerged from this experience is that survival for at least 13 months can be obtained in humans. However, the present communication will focus attention upon several special problems of orthotopic liver transplantation including the high incidence of vascular thrombosis that has been encountered, the necessity for retransplantation in some cases if life is to continue, some miscellaneous technical complications, the development of metastases in patients whose original disease was hepatoma, and the occurrence and character of indolent late rejection.
Mortality
Five of the 14 patients are still alive 7, 6, 5, 3½, and 1½ months postoperatively. The deaths occurred ½, 1, 4, 35, 60, 105, 133, 186, and 400 days after transplantation. The causes of failure will be returned to below.
Vascular Complications
Regional De-arterialization
In previous publications1,2 selective thrombosis of the right hepatic artery was described. In 4 of the first 6 cases in the series, this accident occurred from 2 to 104 days after operation and led to regional hepatic gangrene, septicemia, and immediate or delayed death. A fifth patient amongst the first 6 also had this complication, but recovered only to die more than a year later from other causes. In the latter case, it was found at autopsy that the left hepatic artery had also thrombosed, probably at a later time. Nevertheless, the dearterialized liver had provided good function for many months.
The explanation for lobar arterial thrombosis is probably a complex one. However, there is some evidence2 that distortion of the right hepatic artery, which may result from failure to fix the homograft in its normal anatomic position, can be a contributing factor. Segmental liver infarction has not been seen in the subsequent cases in which the ligaments of the liver were reattached to the companion structures in the recipient.
Acute Total De-arterialization
This calamity has cost the lives of 2 pediatric patients. One was a 12 month old 5.7 kg child with biliary atresia who received an orthotopic homograft that had been minimally damaged by ischemia. The donor was 21 months old and weighed 10.5 kg. The new liver was barely accomodated in the hepatic fossa. After revascularization the homograft immediately elaborated large quantities of bile. However, within a half hour, the arterial anastomosis clotted, necessitating its excision and reconstruction. She was returned to the intensive care unit awake and with a systolic blood pressure of 150 mmHg. Five hours later the blood pressure suddenly fell to 80 mmHg and she became somnolent.
Reclotting of the hepatic artery was suspected but the serum transaminases then and at 6 to 12 hour intervals were only slightly elevated for the next 24 hours (Figure 1). The SGOT then abruptly rose to more than 10,000 units. A liver scan showed almost no isotope concentration in the area of the liver. The child died 2½ days later. Hemolysis which began on the second postoperative day became so overwhelming that the hematocrit fell to zero during the last day of life (Figure 1); concomitantly there was rapidly deepening jaundice. At autopsy the liver had a thrombosed artery, an intact portal vein and massive but incomplete necrosis.
Fig. 1.
Course of a child whose homograft hepatic artery thrombosed a few hours postoperatively. Diagnostic serum enzyme changes did not develop until 24 hours after the vascular accident. Note the evidence of hemolysis.
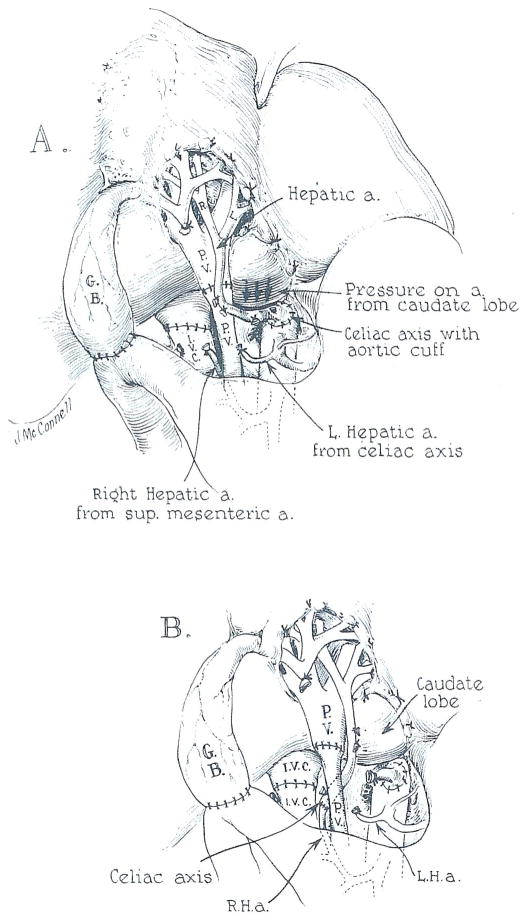
In another child, aged 8 years, a double arterial supply to the diseased liver was found at the time of hepatectomy, the larger vessel originating from the superior mesenteric artery and supplying the right lobe. It was thought that the caliber of the mesenteric branch was too small to permit adequate perfusion of the homograft hepatic artery. Consequently, the celiac axis of the transplanted liver was attached to the side of the recipient upper abdominal aorta (Figure 2A). The revascularized homograft became pink and secreted bile. There was some difficulty in closing the incision since the liver, which was taken from a 27.7 kg 10 year old donor, was too large for the 15.3 kg recipient.
Fig. 2. Anomalous hepatic arterial supply encountered in 2 patients. In each, the larger of 2 hepatic arteries originated from the superior mesenteric artery.
(A) Attempted vascular reconstruction. Note the compression of the homograft arterial blood supply by the caudate lobe of the oversized homograft. This was not detected in one case and resulted in early death.
(B) In the second patient, the graft hepatic artery was eventually anastomosed to the superior mesenteric branch. However, the child died of portal vein thrombosis to which the size disparity of the portal vessels probably contributed.
Two hours after returning to the recovery room, the patient developed refractory shock and lapsed into coma; she died 5 hours later. At autopsy, the liver was cyanotic and enormously swollen. The vascular anastomoses were patent. However, the arterial pedicle was under considerable tension and was displaced inferiorly by a protruding caudate lobe. Apparently, the unsatisfactory disposition of the vessel had been aggravated by closure of the abdominal incision (Figure 2A).
Portal Vein Thrombosis
The cause of failure in the foregoing case was not fully appreciated until exactly the same arterial anomaly was encountered in the next patient in the series, in which the other condition of an oversized organ was also present. The recipient was a 26 month old child with biliary atresia who was given the liver of a 5 year old donor. As in the previous case, the homograft celiac axis was anastomosed to the recipient aorta. It was demonstrated that when the abdominal incision was approximated, the hepatic artery was pinched off (Figure 2A). Consequently, the patient was given 3 mg/kg intravenous heparin and the homograft hepatic artery was transected and anastomosed to the recipient mesenteric branch (Figure 2B). Neutralizing protamine sulfate was not given. The homograft appeared to have a good arterial supply, although the gallbladder did not bleed as vigorously as usual.
After operation, the child woke promptly. Within 8 hours, the serum bilirubin had fallen from 16 to 0.8 mg%. Twenty hours after completion of the transplantation she developed acute abdominal distention and massive gastrointestinal bleeding. Death followed 90 minutes later. At autopsy the arterial anastomosis was patent, but the portal vein was completely occluded with fresh thrombus. In this case, the recipient portal vein had been much smaller than that of the homograft (Figure 2B).
Adrenal Infarction
In removing the recipient liver and the enclosed segment of vena cava, the right adrenal vein must be sacrificed. The adrenal gland underwent venous infarction in a 24 year old woman, whose indication for the orthotopic procedure was hepatoma; she did not have portal hypertension before transplantation nor any indication of abnormal venous collaterals. An emergency right adrenalectomy was performed. She recovered but died 5 weeks later of Pseudomonas pneumonitis. In one of our earlier cases, not included in this report, right adrenal necrosis also occurred but without secondary hemorrhage; the complication was discovered at autopsy one week postoperatively.
Size Discrepancy
Ideally, liver homografts should be obtained from donors of approximately the same size and age as the recipients. However, the extent to which disparities are acceptable is an important practical question which has not yet been fully answered. In the last 14 cases there have been 6 in which the donor and recipient sizes were equivalent, one in which the donor was considerably smaller (body weight ratio 2:3), and 7 in which the donor weighed much more than the recipient (ratios 1.5–2.3:1). All of the patients in the first 2 categories lived for at least one month after operation. Three of the 7 in the third group had early death from the hepatic arterial or portal occlusions cited in the preceding section. The significance of these findings was obscured by the fact that anomalies of the recipient hepatic arteries complicated the operations in 2 of the 3 technically unsuccessful cases. Nevertheless, the way in which the excessive size of the homografts contributed to at least 2 of the failures was emphasized earlier.
It is also clear, however, that the use of oversized homografts is not incompatible with success. One of the patients who is alive after 6 months is a 39 kg girl who received the liver of an 82 kg man. Two infants who weighed 11 and 14 kg at the time of operation are alive with the livers of 25 and 32 kg donors.
Coagulation Abnormalities
It is unlikely that either the lobar or the main arterial or portal thromboses mentioned above had an entirely mechanical basis. It has been recognized for several years3,4 that there are unique risks of both bleeding and clotting after liver transplantation, that the danger of a hemorrhagic diathesis is greatest during the intraoperative period, and that this hazard may often be succeeded by that of hypercoagulability particularly if a minimally damaged organ is used which is capable of rapid correction of deficient clotting factors. Thrombosis of part or all of the homograft arterial supply has also been seen in centers other than our own.5,6
The trepidation with which intraoperative anticoagulation with heparin might be prophylactically undertaken is understandable, especially when recipient hepatectomy has been made difficult and bloody by pre-existing portal hypertension. Nevertheless, this step should probably be considered in selected cases in which homograft procurement and storage have been carried out under optimum conditions.
Late Rejection
In all the cases in this series, azathioprine, prednisone, and horse antilymphocyte globulin (ALG) were begun just before or at the time of transplantation. The first 2 agents were continued indefinitely in variable doses. The ALG was ultimately discontinued in all but one of the long surviving patients either because the end of an arbitrary 4 month period of administration had been reached or because there was evidence of increasing sensitization to the foreign protein. There were no instances of irreversible acute rejection during the interval of triple drug therapy. However, an indolent but moderately severe deterioration of liver function characteristically appeared after the discontinuance of ALG. We have had 6 patients who have been observed after stopping ALG. In 5, jaundice recurred within one to 8 weeks, necessitating drastic revisions in therapy.
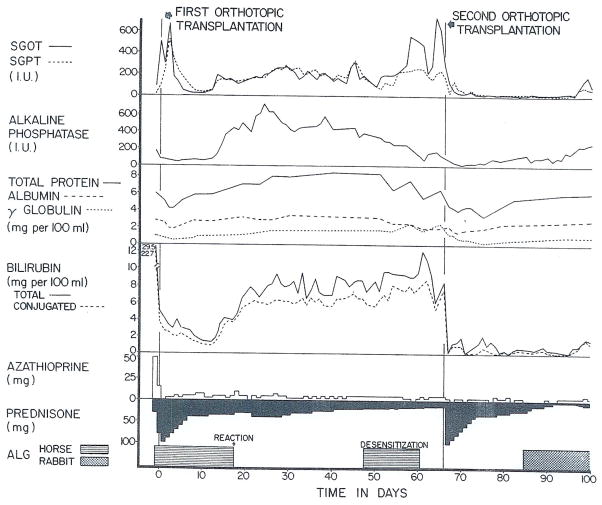
Examples are shown in Figures 3 and 4. The first recipient, a 2 year old child with biliary atresia, had no overt rejection for the first 6 months, but then became jaundiced within 8 weeks after the cessation of ALG injections (Figure 3). The prednisone dose was increased without effect. A course of rabbit ALG was subsequently started with a gradual but incomplete return of the functional abnormalities toward normal. The other patient (Figure 4) had equine ALG discontinued 19 days after operation for biliary atresia because of severe local reactions. Progressive rejection promptly developed, eventually necessitating retransplantation. Rabbit ALG was used on the second occasion. The experience with these cases, as well as that after cadaveric renal transplantation, has convinced us of the necessity to devise improved protocols for ALG administration. Several possible alternative approaches are discussed elsewhere in the proceedings of this meeting.7
Fig. 3.
The course of a patient with biliary atresia after orthotopic liver transplantation. Jaundice recurred within 2 months of discontinuing treatment with horse ALG. Treatment with rabbit ALG was ultimately instituted.
Fig. 4.
An example of orthotopic retransplantation of the liver. Uncontrolled rejection of the first homograft occurred shortly after horse ALG had been stopped because of severe reactions at the injection sites. Desensitization to horse protein was attempted. A second liver was eventually transplanted.
Metastatic Disease
Four patients in the series had hepatomas. One died of pneumonitis after 5 weeks and had no residual tumor. The child who survived for more than 13 months, eventually died from widespread metastases within the abdomen, thorax, cranial vault, and soft tissues of the extremities. Another patient, a 43 year old man, has x-ray findings suggestive of small metastases in the right lung, 5 months postoperatively. There is no evidence of recurrent tumor in a 16 year old girl after 6 months observation.
Retransplantation
The patient was 23 months old at the time of the first operation on May 26, 1968. The donor, aged 3½ years, had total conformity with the recipient of the 9 HLA groups then being measured by Dr. Paul Terasaki of Los Angeles. Initial hepatic function was excellent until the horse ALG was stopped prematurely because of intense swelling and erythema at the injection sites. Rejection began 3 days later and could not be reversed (Figure 4). The child became progressively more ill with high fevers, persistent tachycardia of 160–220/minute, and generalized edema.
On July 31, 1968 the first homograft was removed and replaced with the liver of a 25 kg, 7 year old donor; the histocompatibility match was less favorable than with the original transplantation in that there were mismatches in the Terasaki groups 6 and 7. The excised rejecting homograft was enormous (weight 880 grams) and had many superficially located infarcts. Histologically there were thromboses in some of the peripheral arterial and portal venous radicles as well as moderately heavy mononuclear cell infiltration which was concentrated mostly in the portal tracts.
Liver chemistries were promptly restored to normal (Figure 4). The postoperative course was very stormy, primarily because of the disproportionate size of the transplant which made both breathing and alimentation difficult; the slight additional abdominal distention caused by eating precipitated respiratory crises. In spite of these early problems and a subsequent diffuse pneumonitis of unknown etiology, the child survived and is in good condition with good liver function 6 weeks later. He is being treated with very small doses of azathioprine, 0.5 mg/kg per day prednisone, and rabbit ALG.
The 5 patients who were surviving at the time of the meeting are still alive 2½ months later.
Acknowledgments
Supported by United States Public Health Service grants AM-06344, HE-07735, AM-07772, AI-04152, FR-00051, FR-00069, FO5-TW-1154, AM-12148, and AI-AM-08898.
References
- 1.Starzl TE, Groth CG, Brettschneider L, Moon JB, Fulginiti VA, Cotton EK, Porter KA. Extended survival in 3 cases of orthotopic homotransplantation of the human liver. Surgery. 1968;63:549. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation before and after hepatectomy or transplantation in dogs and man. Arch Surg. 1966;92:71. doi: 10.1001/archsurg.1966.01320190073016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groth CG, Pechet L, Starzl TE. Coagulation during and after orthotopic transplantation of the human liver. Arch Surg. 1968 doi: 10.1001/archsurg.1969.01340070049006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonkalsrud EW. Personal communication. Feb, 1968.
- 6.Birtch A. Personal communication. Aug 26, 1968.
- 7.Starzl TE, Brettschneider L, Penn I, Schmidt RW, Bell P, Kashiwagi N, Townsend CM, Putnam CW. A trial with heterologous antilymphocyte globulin in man; Transplantation Proceedings; New York: Grune and Stratton Inc; 1968. [PMC free article] [PubMed] [Google Scholar]