Abstract
Background
N∊-(carboxymethyl)-lysine (CML) is an advanced glycation end product (AGE), the accumulation of which has been implicated in the etiology of diabetes complications. Skeletal muscle in diabetes demonstrates altered function, and increased accumulation of CML has been found in several fast-twitch muscles of diabetic animals. Objective: This study aims to explore the accumulation of CML in soleus (a slow muscle) in diabetic animals, with and without insulin therapy.
Methods
Twenty-one rats were randomly divided into control and diabetes groups (DNI: diabetes without insulin; DI: diabetes with insulin; C: control). Diabetes was induced by intravenous administration of streptozotocin. At the end of the 12-week experimental period the soleus muscle was excised and snap frozen in liquid nitrogen. Muscle cross-sections were immunolabeled for CML. The number of CML-labeled muscle fibers was quantified; fibers were also evaluated for fiber types and cross-sectional areas.
Results
The percentage of myofibers immunolabeling for CML was highest in the DNI group (13.8 ± 2.5%), lower in the DI group (5.4 ± 1.1%) and lowest in the C group (2.1 ± 0.6%). Statistical analysis revealed that AGE accumulation was significantly greater in the DNI group than in both C and DI groups (p = 0.0002). There was no significant difference between C and DI groups. In the DNI animals, AGE-positive myofibers showed a higher percentage of fast fiber types than did the AGE-negative fibers (49.5 ± 6.9 vs. 13.7 ± 1.5%, p = 0.002). No differences existed in cross-sectional areas between AGE-positive and AGE-negative fibers within any group.
Conclusion
The greatest accumulation of AGE was in the soleus of the DNI group, and was significantly less in the DI group. These findings may be linked to disordered glucose metabolism, increased oxidative stress and/or fiber type transformation in these muscles.
Key Words: Advanced glycation end products, Diabetes mellitus, Immunohistochemistry, Skeletal muscle, N∊-(carboxymethyl)-lysine, Insulin
Introduction
Diabetes mellitus is a condition of disordered glucose metabolism characterized by hyperglycemia. It is associated with complications such as accelerated cardiovascular disease, nephropathy, retinopathy and neuropathy [1,2]. Insulin is a requisite treatment for type 1 diabetes and is also used in treatment of type 2 diabetes. It is a hormone that facilitates uptake of glucose by skeletal muscle (insulin-stimulated glucose uptake) [3,4], and also has important functions in protein metabolism, as both an anabolic hormone and an anticatabolic factor [5,6,7].
Skeletal muscle plays a pivotal role in the management of diabetes mellitus in that it is the primary organ system that metabolizes glucose at rest and during exercise [3,8]. The response of skeletal muscle to glucose and insulin is not uniform across all muscles. There are documented differences in the insulin sensitivity and glucose uptake of a muscle depending on its predominant fiber type [9,10]. In experimental diabetes, muscles with a predominantly slow-twitch fiber profile exhibit greater insulin sensitivity and greater glucose uptake than muscles with a predominantly fast-twitch fiber profile. Diabetes has several effects on skeletal muscle as well. Untreated diabetes is associated with skeletal muscle wasting [11] and altered skeletal muscle function [12,13,14].
Accumulation of advanced glycation end products (AGE) has been associated with diabetes complications [15,16,17,18]. AGE are compounds formed in nonenzymatic reactions between reducing sugars and proteins with long half-lives. Glycation can also occur between reducing sugars and lipids and/or DNA. The exact relationship of AGE accumulation to the diabetes complications is not yet clear. AGE formation may alter three-dimensional protein structure, and thereby alter protein function. AGEs have also been identified as markers of oxidative stress [17,19,20]. N∊-(carboxymethyl)-lysine (CML) is a non-cross-linking AGE that is present in vivo [15,21,22] and has been studied in animal models [23,24].
In experimental diabetes, AGE accumulation has been documented in rat skeletal muscle. Alt et al. [23] determined that gastrocnemius muscle in diabetic rats showed increased amounts of CML accumulation in total muscle and in myofibrillar proteins. Snow et al. [24] used immunohistochemistry to identify patterns of AGE accumulation in plantaris myofibers of rats with untreated diabetes. They showed that CML accumulated at sites in the cell interior as well as at the fiber periphery. Much remains to be elucidated regarding characteristics of AGE accumulation and its effects in skeletal muscle in diabetes mellitus.
It was the primary aim of this study to investigate the effect of insulin on AGE accumulation in soleus muscle of diabetic rats. Secondary aims were (1) to evaluate the degree of atrophy in AGE-labeled fibers, as well as their fiber types, and (2) to compare AGE accumulation in soleus, as predominantly slow muscle, with plantaris, a predominantly fast muscle. The data for AGE accumulation in plantaris of diabetic rats has previously been published [24]. It was hypothesized that insulin administration would be associated with decreased CML accumulation in skeletal muscle, and that soleus, a predominantly slow muscle, would exhibit less CML accumulation than plantaris, a predominantly fast muscle.
Methods
Animal Protocol
A total of 21 male Sprague Dawley rats (Charles River, Wilmington, Mass., USA), weighing 200–250 g, were randomly assigned to 1 of 3 groups (7 animals per group): control (C), diabetes without insulin (DNI) and diabetes with insulin (DI).
Diabetes was induced by intravenous injection (50–60 mg/kg in saline solution) of streptozotocin (STZ; Sigma, St. Louis, Mo., USA), a pancreatic β cell toxin.
Control animals were given intravenous injections of normal saline. Animals were included in the study if they showed serum glucoses of ≥250 mg/dl on at least 3 daily measurements taken beginning on the 5th day after STZ administration. After the animals were assigned to study groups, serum glucoses in the diabetic animals were determined 3 times per week by glucose oxidase method (Glucose Analyzer II; Beckman Instruments, Palo Alto, Calif., USA). Serum glucoses of the control animals were determined at the beginning and at the end of the 12-week study period.
Subcutaneous injections of neutral protamine Hagedorn insulin (Novolin, recombinant DNA origin; Novo Nordisk Pharmaceuticals Inc., Princeton, N.J., USA) were administered to 1 group of diabetic animals (DI, n = 7), with dose determined by sliding scale. Insulin was given for serum glucose of >200 mg/dl, and usually was given daily. The insulin was injected in the evenings, at the beginning of the rats’ active phase. Blood was drawn for serum glucose determination in the mornings, to approximate the time of expected peak insulin effect. Mean insulin dose was 2.6 ± 0.3 units. The insulin was intended to prevent life-threatening metabolic complications, but not to provide tight glucose control, due to concern about hypoglycemia. Insulin was administered over the entire 12-week experimental period. Control and DNI groups were not given insulin.
All rats were housed in standard cages and standard environmental conditions in the same room. They were given standard rat chow and water ad libitum. Their physical exercise level was limited to spontaneous cage-based activity. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
At the end of the 12-week experimental period, animals were deeply anesthetized by injection (50–60 mg/kg, intraperitoneal) of sodium pentobarbital (Fisher Scientific, Fair Lawn, N.J., USA). Soleus muscle was excised, placed on corks in embedding medium and snap frozen in isopentane over liquid nitrogen. Muscle specimens were stored at −80°C until cross-sections were cut.
Tissue Staining and Analysis
Cross-sections from mid-belly of soleus muscles were cut on a cryostat (Leica Microsystems, Nussloch, Germany) at −20°C, with thickness of 10 μm. Sections were placed on slides (Superfrost; Fisher Scientific, Pittsburgh, Pa., USA) and immunolabeled for CML. The immunolabeling was performed using 6D12, a monoclonal IgG antibody from mouse (TransGenic Inc., Kumamoto, Japan). Per manufacturer's insert, this antibody specifically labels CML and also recognizes N∊-(carboxyethyl)-lysine [25]. Findings will be noted using the terms AGE or CML, but with the understanding that N∊-(carboxyethyl)-lysine could be represented by these findings as well. Primary antibody dilution was 1:300 (vol/vol). Negative controls were performed by completing the immunolabeling procedure, but with elimination of the primary antibody incubation step. The preparation and CML immunolabeling of the soleus muscle were performed in identical manner to that of the plantaris, which has been previously described [24].
General fiber typing of the soleus muscle was performed by immunolabeling cross-sections for myosin heavy chain (MHC) isoforms. Mouse monoclonal IgG antibodies were used against slow and fast MHC (Novocastra, Newcastle upon Tyne, UK). Dilutions used were: slow MHC 1:20 (vol/vol) and fast MHC 1:10 (vol/vol). Negative controls were included in this analysis with omission of the primary antibody incubation step. The MHC immunolabeling of the soleus muscle was performed by the same method as that used to immunolabel the plantaris [24].
Using a microscope, digital images were obtained of muscle cross-sections immunolabeled for AGE and for MHC isoforms, using a digital imaging program (Diagnostic Instruments, Sterling Heights, Mich., USA); magnification was ×200.
For CML labeling, the entire cross-section was evaluated. The mean number of fibers evaluated for AGE immunolabeling was 1,451 per animal. MHC isoform characteristics were determined for a subset of cells (mean 323 cells per animal) in randomly selected regions of the cross-sections. Fiber types were assigned based on the MHC labeling combinations expressed [26]. AGE characteristics of those cells were then matched to the MHC profile. AGE immunolabeling results were expressed as percent of AGE-labeled cells of the total number of cells counted per cross-section. Assessment for AGE labeling was performed by professional students who were blinded to the experimental groups. Interrater reliability was good (mean κ value 0.75). A myofiber was designated as AGE positive if labeling was clearly present intracellularly (intracellular stippling pattern), or clearly accumulated along the cell periphery (peripheral labeling pattern). A myofiber was designated AGE negative if no labeling was present or if it was uncertain if labeling was present. Cross-sectional areas were determined for the cells evaluated for MHC isoforms using a computer program (Scion Imaging for Windows; Scion, Frederick, Md., USA).
Statistical Analysis
Descriptive statistics included means and standard errors of the means (SEM). Differences between groups were determined with the use of one-way ANOVA. If significant differences were present with the ANOVA, Tukey-Kramer post hoc test was performed. Pearson correlation coefficient determination and regression analysis were also performed for further evaluation of the effect of insulin versus CML accumulation in the diabetes groups. Significance level for all analyses was set at ≤0.05. Analyses were performed with use of the NCSS statistics program (Kaysville, Utah, USA).
Results
Animal Characteristics
Animal weights, serum glucoses and muscle weights are shown in table 1. Mean serum glucose in the diabetic groups was significantly greater than in the controls. However, the glucose values between the diabetic groups did not differ significantly. Initial (prediabetes induction) body weights did not differ between the 3 groups. However, at the end of the 12-week experimental period, body weights and soleus muscle weights were significantly smaller in the DNI group than in the other groups. The muscle weight/body weight ratio did not differ between the 3 groups.
Table 1.
Animal characteristics
| Group | Initial body weight, g (before STZ) | Final body weight, g | Soleus weight, g | Muscle weight/body weight1 ratio | Initial serum glucose, mg/dl (beforeSTZ) | Final serum glucose, mg/dl | Mean insulin dose, units |
|---|---|---|---|---|---|---|---|
| C (n = 7) | 255.9 ± 16.1 | 552.7 ± 30.8 | 0.2163 ± 0.0136 | 3.93 ± 0.156 | 134.7 ± 4.1 | 125.6 ± 5.1 | 0 |
| DI (n = 7) | 258.5 ± 15.5 | 511 ± 33.3 | 0.2234 ± 0.0089 | 4.43 ± 0.021 | 133.4 ± 7.3 | 311.6 ± 24.2+ | 2.6 ± 0.3 |
| DNI (n = 7) | 227.7 ± 14.5 | 372.5 ± 19.8∗ | 0.1650 ± 0.0141∗∗ | 4.40 ± 0.024 | 110.7 ± 5.4 | 338.7 ± 16.6+ | 0 |
Animal characteristics for DNI animals were previously reported in Snow et al. [24].
Significantly less than C and DI, p = 0.0008;
significantly less than C and DI, p = 0.007;
significantly greater than C. No significant difference between initial body weights, p = 0.3.
Values are given as ×10−4.
AGE Accumulation
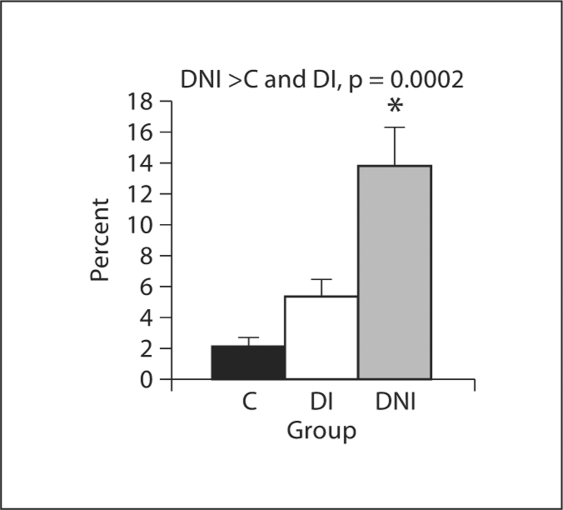
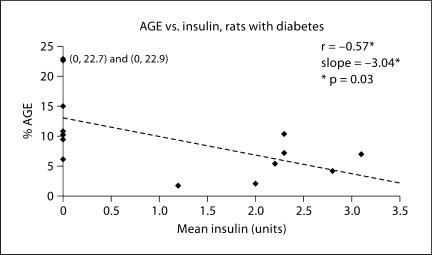
Percent AGE accumulation in soleus is shown in figure 1 and illustrated in figure 2. Correlation between AGE accumulation and insulin dose is presented in figure 3. AGE accumulation was increased in the DNI group. No significant difference was found between CML accumulation in the C and DI groups in the one-way ANOVA. Additionally, there was a significant negative correlation between insulin dose and CML accumulation in the soleus.
Fig. 1.
Percent AGE (CML) accumulation in soleus. * AGE accumulation is significantly greater in the DNI group than in control and DI groups, p = 0.0002.
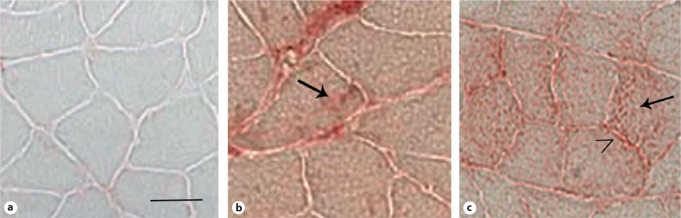
Fig. 2.
AGE accumulation in soleus of diabetic and control rats. a Control. b Diabetes with insulin. c Diabetes without insulin. Arrows indicate intracellular AGE immunolabeling; caret denotes AGE immunolabeling at myofiber periphery. Scale bar = 50 μm.
Fig. 3.
Correlation between AGE accumulation and insulin dose in soleus of diabetic rats. * There is a significant negative correlation between AGE accumulation and insulin dose, p = 0.03; the slope of the line is also significant at p = 0.03.
For reference, the previously published AGE accumulation data for the plantaris is summarized as follows [24]: C = 4.3 ± 2.2%, DNI = 20.5 ± 3.4%. Only control and DNI groups were evaluated in that study [24]. Thus, the addition of the insulin treatment is a new parameter in the current study.
Other Characteristics of AGE-Positive Myofibers in Soleus
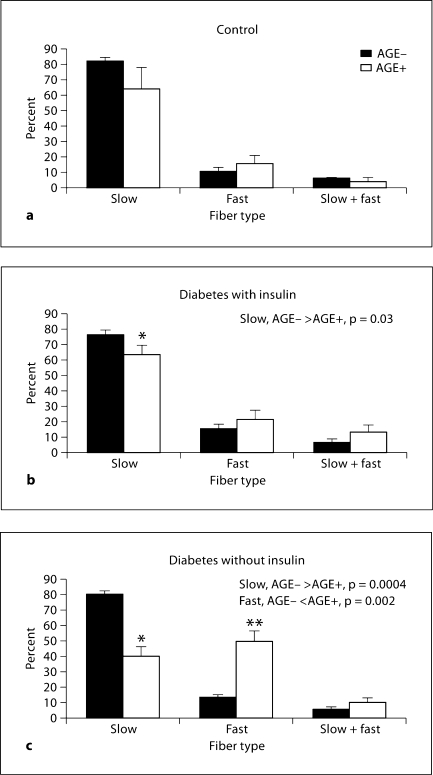
Figure 4 identifies fiber type characteristics of AGE-positive myofibers compared with AGE-negative myofibers in soleus muscle.
Fig. 4.
AGE accumulation by fiber types in soleus. a Control (C); no statistical differences in fibers between AGE-negative and AGE-positive fiber types. b Diabetes with insulin (DI); * percentage of slow fibers in AGE-positive cells is significantly less than in AGE-negative cells (p = 0.03). c Diabetes without insulin (DNI); * percentage of slow fibers in AGE-positive cells is significantly less than in AGE-negative fibers (p = 0.0004); ** percentage of fast fibers in AGE-positive cells is significantly greater than in AGEnegative cells (p = 0.002).
This data indicates that the percentage of AGE-positive fibers expressing the slow MHC is lower than the percentage of AGE-negative fibers expressing slow MHC in both DI and DNI groups. Additionally, the distribution of AGE-positive fibers expressing the fast MHC is increased compared to the AGE-negative fibers in the DNI group. This pattern of fiber type distribution mirrors the fiber type distributions identified in the soleus overall in DI and DNI rats, and thus reflects the fiber type transformation seen in these groups [26].
Cross-sectional areas of AGE-positive and AGE-negative fibers are presented in table 2. There were no significant differences between AGE-positive and AGE-negative cross-sectional areas within each group. Previous evaluation of overall fiber cross-sectional areas [26] revealed atrophy in the fibers from the DNI group, which is also reflected in the data above.
Table 2.
Cross-sectional areas, AGE-positive versus AGE-negative soleus myofibers
| Group | AGE positive | AGE negative | p value |
|---|---|---|---|
| C (n = 6) | 3,901 ± 328 | 3,751 ± 257 | 0.7 |
| DI (n = 7) | 3,682 ± 217 | 3,912 ± 167 | 0.4 |
| DNI (n = 7) | 2,669 ± 178 | 3,179 ± 250 | 0.1 |
Values are given as μm2. No statistically significant differences between cross-sectional areas of AGE-positive and AGE-negative myofibers within any of the groups.
Discussion
In this study, the effect of insulin administration was evaluated with regard to AGE accumulation in soleus muscle of diabetic rats. Characteristics of the soleus muscle, such as fiber area and fiber type, were also evaluated in the context of AGE accumulation in these myofibers. Lastly, AGE accumulation in soleus was compared to that in plantaris in order to evaluate the influence of predominant muscle fiber type on AGE accumulation.
Animal Characteristics
Results are consistent with a prior report [26], showing lower body weight and soleus muscle weight in the DNI group than in the other groups, consistent with the characteristic skeletal muscle atrophy and catabolic state associated with untreated diabetes. There was no statistically significant difference in serum glucose levels in the DI compared to the DNI group. However, the administration of the small doses of insulin was associated with normalized body and skeletal muscle weights, consistent with the known anticatabolic and anabolic effects of insulin [5,6,7].
Effect of Insulin on AGE Accumulation
In addition to the evidence of the effects of insulin on muscle and body weight, the results of the current study show that treatment with small doses of insulin is associated with lower AGE accumulation in the soleus muscle of diabetic rats compared to the untreated condition. This result occurred despite the fact that serum glucose levels were not different between the DI and the DNI groups, and both were higher than in controls. The mechanisms for this result remain to be elucidated. However, several characteristics of insulin's effect on glucose metabolism and on intramuscular oxidative stress may play a part.
Insulin and Glucose Metabolism – The Polyol Pathway in Skeletal Muscle
Insulin facilitates glucose uptake by skeletal muscle and allows metabolism of the glucose through the oxidative, glycolytic and glycogen synthesis pathways [7]. However, in conditions of hypoinsulinemia with hyperglycemia, glucose flux also increases through the polyol pathway [27]. In the polyol pathway, glucose is reduced to sorbitol, which in turn is oxidized to form fructose [27]. Increased metabolism of glucose via the polyol pathway has been implicated in the etiology of diabetic complications [27]. Cotter et al. [28] reported not only increased intracellular concentrations of glucose, but also increased concentrations of sorbitol and fructose in tibialis anterior of diabetic rats not given insulin. Fructose is a reducing sugar that has been implicated in AGE formation [15,17]. By directing glucose metabolism away from the polyol pathway, administration of insulin may alter the concentrations of other potential AGE-forming sugars in the myofiber (such as fructose) so that there is less substrate available for AGE formation [15,17].
Insulin and Oxidative Stress in Skeletal Muscle
AGE accumulation has been described as a marker of oxidative stress [17,19,20]. Furthermore, skeletal muscle atrophy associated with untreated diabetes mellitus has been attributed to increased oxidative stress in the muscle. Mastrocola et al. [11] have identified increases in reactive oxygen species, hydroxynonenal, and superoxide dismutase in gastrocnemius muscles of rats with STZ-induced diabetes and associated muscle wasting. Body weight and gastrocnemius muscle weight returned to normal with antioxidant administration. The authors thus concluded that the muscle atrophy in the STZ model of diabetes was at least in part due to oxidative stress. With administration of insulin, muscle weights and body weights of STZ diabetic rats increase to essentially normal levels, presumably due to the anabolic and anticatabolic effects of insulin on protein homeostasis [5,6,7]. In light of that conclusion, if the atrophy of untreated diabetes is associated with increased oxidative stress, resolution of the atrophy with insulin administration may be linked to decreased oxidative stress in muscle. The lower AGE accumulation observed in skeletal muscle with insulin treatment may be an indicator of this lower level of oxidative stress.
Other Considerations
Another observation from this study is that a low dose of insulin decreased AGE accumulation despite continued hyperglycemia. It is therefore possible that the dose of insulin necessary to influence AGE accumulation is not as great as that needed for tight glucose control. Alternatively, as suggested by Wallberg-Henriksson et al. [29], chronically diabetic animals may have a blunted response to exogenous insulin, with a subsequent possible limitation to an insulin-associated decrease in AGE formation. Further study is needed to gain more understanding of these possibilities.
Effect of Insulin as Influenced by Predominant Muscle Fiber Type
It has been determined that skeletal muscle response to insulin is associated with skeletal muscle fiber type. In rodent models, muscles with predominantly oxidative fiber type (slow twitch, type I) have been found to exhibit greater insulin binding and greater glucose uptake than muscles with a predominantly glycolytic fiber profile (fast twitch, type IIb) [9,10,30,31]. Intermediate fibers (type 2a) seem to be more closely associated with the oxidative fibers in their responses to insulin sensitivity [30]. Concentrations of GLUT 4 have also been found to be higher in predominantly oxidative muscles [32]. Processes in the insulin signaling pathway such as phosphorylation of insulin receptor, insulin receptor substrates 1 and 2, and Akt serine follow this pattern as well [33]. Additionally, decreases in GLUT 4 content [29,31] and glucose uptake [31] have been documented in STZ diabetes, and improvements in GLUT 4 protein content occurs with administration of insulin. These improvements were more pronounced in oxidative-dominant muscle than in glycolytic-dominant muscle [32,34].
If AGE accumulation is influenced by insulin, as the current results suggest, it would be expected that the soleus muscle would show less AGE accumulation than faster muscles (that is, plantaris or EDL), since it would be more sensitive to the effects of exogenously administered insulin.
AGE Accumulation Differences between Soleus and Plantaris
Snow et al. [24] have previously described AGE accumulation in plantaris of diabetic rats not given insulin compared to controls. Plantaris is a hind limb muscle with a predominantly fast fiber type [35]. As noted above, soleus has a predominantly slow fiber type [35]. In comparison with the AGE accumulation presented here for soleus, the plantaris values showed somewhat greater AGE accumulation in both C and DNI groups. This difference did not reach levels of statistical significance, however (C: p = 0.14; DNI: p = 0.07; two-sample t test between plantaris and soleus within each group). Taken together, the findings in these 2 studies are indeterminate regarding an association between AGE accumulation and a muscle's predominant fiber type. However, more study is necessary to further clarify this potential relationship.
Finally, one other finding that could contribute to the AGE accumulation pattern seen in this study is related to soleus fiber type transformation in diabetes. Snow et al. [26] determined that the percentage of fast myosin heavy chain-expressing myofibers was increased in soleus of rats in the DNI group compared to DI and C groups. The MHC fiber types of the AGE-positive myofibers in the present study follow the same pattern. Therefore, given the fiber type transformation to a faster profile in the DNI soleus, there possibly could be decreased insulin sensitivity in the muscles of this group, which may contribute to increased AGE accumulation.
Conclusion
This study evaluated accumulation of CML, an AGE, in the soleus muscles of diabetic rats, with and without insulin therapy. The primary finding is that AGE accumulation is decreased in association with administration of exogenous insulin, even when the insulin dose is not great enough to normalize serum glucose. AGE accumulation is also greater in fast fibers in the noninsulin diabetic animals, which may reflect patterns of decreased insulin sensitivity in muscle with a more glycolytic fiber type profile. The exact nature of the association of insulin and AGE accumulation remains to be elucidated in future studies.
Acknowledgements
The authors wish to acknowledge Janice Shoeman, Catherine Waisanen, Kristi Youdas, Kristen Doherty, Rachel Eggenberger, Julie Eibensteiner, Eric Lindquist, Vicki Lucas, Matt Marcellus, Michelle McFaddin, Adam Meierbachtol and Abby Metz. This work was funded in part by NIH HD049459 (L.M.S.).
References
- 1.Boel E, Selner J, Flodgaard H, Jensen T. Diabetic late complications: Will aldose reductase inhibitors or inhibitors of advanced glycosylation endproduct formation hold promise? J Diabetes Complications. 1995;9:104–129. doi: 10.1016/1056-8727(94)00025-j. [DOI] [PubMed] [Google Scholar]
- 2.Sheetz M, King G. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 3.Ryder JW, Chibalin AV, Zierath JR. Intracellular mechanisms underlying increases in glucose uptake in response to insulin or exercise in skeletal muscle. Acta Physiol Scand. 2001;171:249–257. doi: 10.1046/j.1365-201x.2001.00827.x. [DOI] [PubMed] [Google Scholar]
- 4.Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol. 2005;99:330–337. doi: 10.1152/japplphysiol.00175.2005. [DOI] [PubMed] [Google Scholar]
- 5.Charlton M, Balogopal P, Nair K. Skeletal muscle myosin heavy chain synthesis in type 1 diabetes. Diabetes. *;1997:1336–1340. doi: 10.2337/diab.46.8.1336. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Chinkes D, Wolf S, Wolfe R. Insulin but not growth hormone stimulates protein anabolism in skin wound and muscle. Am J Physiol. 1999;276:E712. doi: 10.1152/ajpendo.1999.276.4.E712. [DOI] [PubMed] [Google Scholar]
- 7.Hadley ME, Levine JE: Pancreatic Hormones and Metabolic Regulation; Endocrinology. Upper Saddle River, Pearson Prentice Hall Pearson Education Inc., 2007, pp 237–263.
- 8.Song X, Ryder JW, Kawano Y, Chibalin AV, Krook A, Zierath JR. Muscle fiber type specificity in insulin signal transduction. Am J Physiol. 1999;277:R1690. doi: 10.1152/ajpregu.1999.277.6.R1690. [DOI] [PubMed] [Google Scholar]
- 9.Bonen A, Tan MH, Watson-Wright WM. Insulin binding and glucose uptake differences in rodent skeletal muscles. Diabetes. 1981;30:702–704. doi: 10.2337/diab.30.8.702. [DOI] [PubMed] [Google Scholar]
- 10.Henriksen EJ, Boury RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990;259:E593. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- 11.Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, Aragno M, Costelli P. Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med. 2008;44:584–593. doi: 10.1016/j.freeradbiomed.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 12.Andersen H, Poulsen P, Mogensen C, Jakobsen J. Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes. 1996;45:440–445. doi: 10.2337/diab.45.4.440. [DOI] [PubMed] [Google Scholar]
- 13.Andersen H. Muscular endurance in long-term IDDM patients. Diabetes Care. 1998;21:604–609. doi: 10.2337/diacare.21.4.604. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson GM, O'Callaghan AO, Stephenson DG. Single-fiber study of contractile and biochemical properties of skeletal muscles in streptozotocin-induced diabetic rats. Diabetes. 1994;43:622–628. doi: 10.2337/diab.43.5.622. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed N. Advanced glycation endproducts – role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Forbes JM, Soulis T, Thallas V, Panagiotopoulos S, Long DM, Vasan S, Wagle D, Jerums G, Cooper M. Renoprotectvie effects of a novel inhibitor of advanced glycation. Diabetologia. 2001;44:108–114. doi: 10.1007/s001250051587. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Barden A, Mori T, Bellin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, Horii Y, Nishino T, Shiiki H, Sakaguchi Y, Kagoshima T, Dohi K, Makita Z, Vlassara H, Bucala R. Immunohistochemical localization of advanced glycosylation endproducts in coronary atheroma and cardiac tissue in diabetes mellitus. Am J Pathol. 1993;143:1649–1656. [PMC free article] [PubMed] [Google Scholar]
- 19.Fu XM, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, N∊-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 20.Nagai R, Ikeda K, Higashi T, et al. Hydroxyl radical mediates N∊-(carboxymethyl)lysine formation from amadori product. Biochem Biophys Res Commun. 1997;234:167–172. doi: 10.1006/bbrc.1997.6608. [DOI] [PubMed] [Google Scholar]
- 21.Basta G, Schmidt A, DeCaterina R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K, Higashi T, Sano H, Jinnouchi Y, Yoshida M, Arake T, Ueda S, Horiuchi S. N∊-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the maillard reaction. Biochemistry. 1996;35:8075–8083. doi: 10.1021/bi9530550. [DOI] [PubMed] [Google Scholar]
- 23.Alt N, Carson J, Alderson N, Wang Y, Nagai R, Henle T, Thorpe S, Baynes J. Chemical modification of muscle protein in diabetes. Arch Biochem Biophys. 2004;425:200–206. doi: 10.1016/j.abb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Snow LM, Lynner C, Nielsen E, Neu H, Thompson LV. Advanced glycation end product in diabetic rat skeletal muscle in vivo. Pathobiology. 2006;73:244–251. doi: 10.1159/000098210. [DOI] [PubMed] [Google Scholar]
- 25.Koito W, Araki T, Horiuchi S, Nagai R. Conventional antibody against N∊-(carboxymethyl)lysine (CML) shows cross-reaction to N∊-(carboxyethyl)lysine (CEL): immunochemical quantification of CML with a specific antibody. J Biochem. 2004;136:831–837. doi: 10.1093/jb/mvh193. [DOI] [PubMed] [Google Scholar]
- 26.Snow LM, Sanchez O, McLoon L, Serfass R, Thompson LV. Myosin heavy chain isoform immunolabelling in diabetic rats with peripheral neuropathy. Acta Histochem. 2005;107:221–229. doi: 10.1016/j.acthis.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Brownlee M. The pathobiology of diabetic complications – a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 28.Cotter ME, Cameron NE, Robertson S, Ewing I. Polyol pathway-related skeletal muscle contractile and morphological abnormalities in diabetic rats. Exp Physiol. 1993;78:139–155. doi: 10.1113/expphysiol.1993.sp003675. [DOI] [PubMed] [Google Scholar]
- 29.Wallberg-Henriksson H, Holloszy JO. Activation of glucose transport in diabetic muscle: Responses to contraction and insulin. Am J Physiol. 1985;249:C233. doi: 10.1152/ajpcell.1985.249.3.C233. [DOI] [PubMed] [Google Scholar]
- 30.Megeney LA, Neufer PD, Dohm GL, Tan MH, Blewett CA, Elder GCB, Bonen A. Effects of muscle activity and fiber composition on glucose transport and glut-4. Am J Physiol. 1993;264:E583. doi: 10.1152/ajpendo.1993.264.4.E583. [DOI] [PubMed] [Google Scholar]
- 31.Koerker DJ, Sweet IR, Baskin DG. Insulin binding to individual rat skeletal muscles. Am J Physiol. 1990;259:E517. doi: 10.1152/ajpendo.1990.259.4.E517. [DOI] [PubMed] [Google Scholar]
- 32.Hardin DS, Dominguez JH, Garvey WT. Muscle group-specific regulation of glut 4 glucose transporters in control, diabetic, and insulin-treated diabetic rats. Metabolism. 1993;42:1310–1315. doi: 10.1016/0026-0495(93)90130-g. [DOI] [PubMed] [Google Scholar]
- 33.Song XM, Ryder JW, Kawano Y, Chibalin AV, Krook A, Zierath JR. Muscle fiber type specificity in insulin signal transduction. Am J Physiol. 1999;277:R1690. doi: 10.1152/ajpregu.1999.277.6.R1690. [DOI] [PubMed] [Google Scholar]
- 34.Richardson JM, Balon TW, Treadway JL, Pessin JE. Differential regulation of glucose transporter activity and expression in red and white skeletal muscle. J Biol Chem. 1991;266:12690–12694. [PubMed] [Google Scholar]
- 35.Armstrong R, Phelps R. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]