Abstract
Background
Clinical guidelines have a role in medical education and in the standardization of patient care. However, it is not clear whether guidelines created by subspecialists reach relevant practicing physicians or influence patient care. In 2007 the Endocrine Society released “Guidelines on the Management of Thyroid Dysfunction During Pregnancy and Postpartum.” The objective of this study was to characterize the role of these guidelines in provider education and in subsequent patient care decisions.
Method
In 2009 three waves of mail surveys were distributed to 1601 Wisconsin health care providers with a history of providing obstetric care. Survey participants were members of the American College of Obstetricians and Gynecologists or the American Academy of Family Physicians. There were 881 returned surveys (55%) and 575 were eligible for the study (adjusted rate 52.5%).
Results
Although only 11.5% of providers read the Endocrine Society's guidelines, reading the guidelines was associated with increased likelihood of prepregnancy counseling on levothyroxine management (p < 0.0001), increased likelihood of screening for thyroid disease risk factors (p = 0.0007), and increased likelihood of empiric levothyroxine dose increase in pregnant patients (p = 0.0005). After controlling for provider sex, membership affiliation, practice setting, and number of years in practice, reading the guidelines was still an independent predictor of patient education prepregnancy (p < 0.01).
Conclusion
The Endocrine Society's “Guidelines on the Management of Thyroid Dysfunction During Pregnancy and Postpartum” reached a minority of providers involved in obstetrics, but exposure to the guidelines did impact patient care. A multidisciplinary approach to guideline creation would improve the dissemination and practical application of guidelines.
Introduction
Up to 4.6% of the U.S. population has either overt or subclinical hypothyroidism, with a greater incidence in women than in men (1). The impact of maternal hypothyroidism on pregnancy can be profound, including an associated increased risk of miscarriage, premature delivery, preeclampsia, low birth weight, C-section, fetal death, and decreased infant IQ (2–7). Although there is still controversy regarding the implications of subclinical hypothyroidism and hyperthyroidism on pregnancy outcome (8–10), there are clear data confirming that pregnancy does alter thyroid function (11), and women on thyroid hormone replacement prepregnancy do need dose adjustments during pregnancy (2,12–17).
In an effort to prevent uncontrolled maternal thyroid dysfunction, the Endocrine Society released “Guidelines on the Management of Thyroid Dysfunction During Pregnancy and Postpartum” in 2007. The participants involved in creating the guidelines included members of the Endocrine Society and cosponsoring organizations such as American Association of Clinical Endocrinologists, Asia and Oceania Thyroid Association, American Thyroid Association, European Thyroid Association, and Latin American Thyroid Association. A representative of the American College of Obstetricians and Gynecologists (ACOG) was part of the original guidelines committee, but ACOG subsequently declined to endorse the final document. Applicable published and peer-reviewed literature of the past 20 years was reviewed. The guidelines were then created and graded using the U.S. Preventive Service Task Force System of grading and where possible, Grades of Recommendation Assessment, Development and Evaluation (GRADE) (18).
In general, the development of clinical guidelines is a response to the reality that the time constraints of providing preventive and chronic care (19,20), combined with the breadth of medical information available, limit the ability of a general health care provider to read, analyze, and apply all published data on all relevant topics (21). Although guidelines run the risk of being too narrowly focused on a disease or even worse of being based on weak evidence (22), there is a need for guidelines due to significant practice variability and difficulty disseminating subspecialty information across multiple fields. Clinical guidelines can be powerful education tools with the capability of altering a provider's belief about what medical care a patient needs (23). However, when guidelines are created by a subspecialty organization with implications for practitioners across a variety of fields, two important questions arise: (i) Do the guidelines reach their target population? (ii) Is patient care influenced? If guidelines reach their target population without influencing patient care, the next relevant questions include the following: (i) Are the guidelines practically applicable? (ii) What obstacles limit implementation?
The following study was designed to determine the current management of thyroid hormone replacement in women of reproductive age and the role of the Endocrine Society's guidelines in influencing care. It was hypothesized that the guidelines would impact provider education and subsequent patient care but that the penetrance would not be 100%.
Materials and Methods
This study was created to evaluate the current management of thyroid hormone replacement in women of reproductive age and the role of the Endocrine Society's guidelines in influencing care. The University of Wisconsin Health Sciences' Institutional Review Board (IRB) approved this study for IRB exemption. The survey instrument consisted of a two-sided, one-page mail survey administered by the University of Wisconsin Survey Center in the spring of 2009. Based on the Dillman method (24), there were three waves of mailings sent to 914 Wisconsin members of the American Academy of Family Physicians (AAFP) and 687 members of the Wisconsin Chapter of ACOG.
Of the 1601 distributed surveys, there were 881 (55%) returned surveys, which included 278 that were ineligible because the providers did not provide care to pregnant patients in 2008, 2 refusals, 4 deceased providers, and 22 returned undeliverable. This left 575 eligible completed surveys, and after accounting for the rate of ineligibility the adjusted response rate was 52.5%.
After compiling data, all statistical analysis was performed with SAS 9.2 (SAS Inc., Cary, NC). Means were reported as mean ± standard error of the mean. Descriptive statistics were used to find frequency, and chi-square tests were used for categorical variables. Multivariable logistic regression was used to model dichotomous outcomes. Statistical significance was set to a probability value of p < 0.05.
Results
To determine if the subset of participants answering the survey were representative of the population surveyed, the distributions of membership type and urban versus rural practice were compared between those who completed the survey (n = 575) and the total survey population (n = 1601). For the purpose of this assessment, urban was defined as residence in five of the largest cities in Wisconsin: Milwaukee, Madison, Green Bay, Appleton-Oshkosh, and Racine. Rural was defined as residence in all other areas of Wisconsin. The percentage of urban medical providers completing the survey versus rural was exactly the same at 36% (184/514 and 391/1087, respectively). The percentage of AAFP members completing the survey versus ACOG members was nonsignificantly higher at 36% versus 35% (334/914 and 241/687, respectively).
There was a similar percentage of male and female providers completing the survey (n = 278 and 291, respectively). Three hundred and eighty-eight providers reported working in a private practice setting, while 180 reported working at an academic center. Of those providers completing the survey, 19% (108/575) were residents or fellows. The mean number of years in practice was 15 ± 0.39 and the mean number of pregnant patients seen in 2008 was 85 ± 6.91. Members of ACOG saw an average of 172 ± 14.89 pregnant patients a year, while members of AAFP saw an average of 24 ± 1.13 pregnant patients a year (Table 1).
Table 1.
Survey Participant Characteristics (n = 575)
| n | |
|---|---|
| Provider sex | |
| Male | 278/569 (48.9%) |
| Female | 291/569 (51.1%) |
| Practice setting | |
| Private practice | 388/568 (68.3%) |
| Academic center | 180/568 (31.7%) |
| Mean number of years in practice | 15 ± 0.39 |
| Residents or fellows | 108/575 (18.8%) |
| Mean number of patients seen in 2008 | 85 ± 6.91 |
| Mean number seen by ACOG | 172 ± 14.89 |
| Mean number seen by AAFP | 24 ± 1.13 |
ACOG, American College of Obstetricians and Gynecologists; AAFP, American Academy of Family Physicians.
Seventy-six percent of the providers (435/575) schedule their initial prenatal visits between 7 and 12 weeks of gestation. For patients on levothyroxine (LT4), 70% (403/575) of the providers address LT4 dose at first visit, while 19% (108/575) address the dose at time of positive home pregnancy test. After the initial adjustment in dose, most providers (484/575) use thyroid function tests as the basis for LT4 adjustments during the remainder of the pregnancy. Only 3.5% (20/575) refer all patients to an endocrinologist for management of thyroid hormone replacement during pregnancy, and only 9% (52/575) refer most of their patients to an endocrinologist. The likelihood of endocrinologist involvement for patients receiving LT4 as treatment of thyroid cancer is much higher as 25.4% (146/575) of providers refer all thyroid cancer patients and 15.7% (90/575) refer most thyroid cancer patients. Of note, nonresponse was high (21%) with this question and likely represents lack of exposure to patients with a history of thyroid cancer. Supportive of this conclusion, many of the nonresponders wrote in not applicable (Table 2).
Table 2.
Management of Pregnant Patients on Levothyroxine
| Week in gestation | <4 weeks | 4–6 weeks | 7–9 weeks | 10–12 weeks | >12 weeks | Missing |
| Patients first seen | 7/575 (1.2%) | 85/575 (14.8%) | 278/575 (48.3%) | 157/575 (27.3%) | 42/575 (7.3%) | 6/575 (1.0%) |
| When LT4 dose is addressed | Home pregnancy test positive | First antenatal visit | Follow-up visit | Other | Missing | |
| 108/575 (18.8%) | 403/575 (70.1%) | 13/575 (2.3%) | 45/575 (7.8%) | 6/575 (1.0%) | ||
| Method of initial LT4 dose adjustment | Empiric increase | Based on TFTs | Other | Missing | ||
| 55/575 (9.6%) | 447/575 (77.7%) | 59/575 (10.3%) | 14/575 (2.4%) | |||
| Further management of LT4 in pregnancy | Symptomatic | Based on TFTs | Other | Missing | ||
| 23/575 (4%) | 485/575 (84.3%) | 50/575 (8.7%) | 17/575 (3.0%) | |||
| Endo referral | None | A few | Some | Most | All | Missing |
| 217/575 (37.7%) | 170/575 (29.6%) | 86/575 (15%) | 52/575 (9%) | 20/575 (3.5%) | 30/575 (5.2%) | |
| Endo referral if Thyroid Cancer | 155/575 (27%) | 36/575 (6.3%) | 25/575 (4.3%) | 90/575 (15.7%) | 146/575 (25.4%) | 123/575 (21.4%) |
LT4, levothyroxine; TFT, thyroid function test.
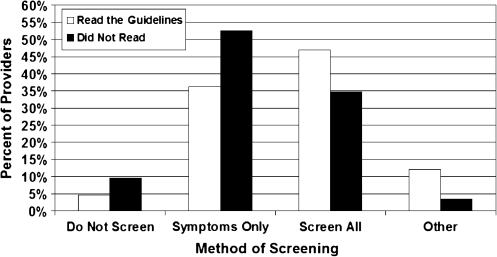
For patients not on LT4, only 36% (208/575) of providers routinely screen all patients for thyroid disease risk factors. Another 50% (287/575) of providers only question patients about risk factors for thyroid disease if they have symptoms of hypothyroidism or hyperthyroidism. The presence of risk factors for thyroid disease is used to determine the need for thyroid-stimulating hormone (TSH) testing by 66% (379/575) of providers, whereas 15% (88/575) check TSH on all pregnant patients regardless of risk factors (Table 3).
Table 3.
Screening for Thyroid Disorders in Pregnant Patients Not on Levothyroxine
| n | |
|---|---|
| Screen for risk factors for thyroid disease | |
| Do not screen | 50/575 (8.7%) |
| Screen if symptoms | 287/575 (49.9%) |
| Universal screening | 208/575 (36.2%) |
| Other | 26/575 (4.5%) |
| Missing data | 4/575 (0.7%) |
| Policy for routine TSH | |
| Do not check | 86/575 (15.0%) |
| Check if + risk factors | 379/575 (65.9%) |
| Check on all | 88/575 (15.3%) |
| Other | 19/575 (3.3%) |
| Missing data | 3/575 (0.5%) |
TSH, thyroid-stimulating hormone.
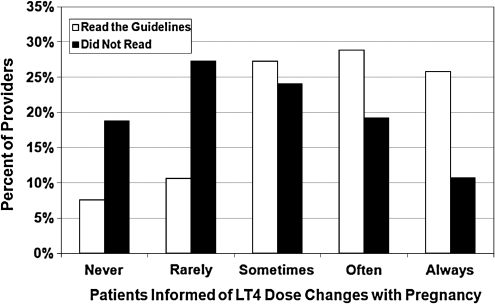
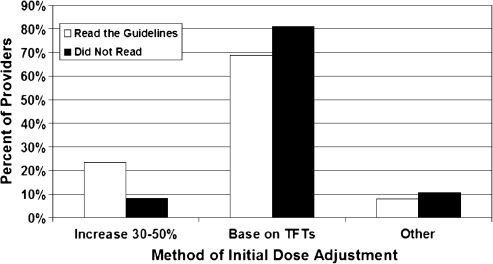
Although only 11.5% (66/575) of all providers read the 2007 Endocrine Society's “Guidelines on the Management of Thyroid Dysfunction During Pregnancy and Postpartum,” reading the guidelines was associated with increased likelihood of prepregnancy counseling on changes in thyroid hormone dose with pregnancy (p < 0.0001) and increased likelihood of screening for thyroid disease risk factors (p = 0.0007) (Figs. 1 and 2). Reading the guidelines was also associated with an increased likelihood of empiric dose increase in LT4 at time of confirmed pregnancy versus adjusting LT4 based on thyroid function tests (p = 0.0005) (Fig. 3). When residents and fellows were evaluated separately from the entire cohort, a similar percent read the guidelines (10%). In addition, there was no significant difference between trainees versus nontrainees in regard to likelihood of prepregnancy counseling (p = 0.22).
FIG. 1.
Multivariable Logistic Regression Analysis of Patient Education and Screening
FIG. 2.
Multivariable Logistic Regression Analysis of Patient Education and Screening
FIG. 3.
Multivariable Logistic Regression Analysis of Patient Education and Screening
After controlling for membership affiliation, provider sex, practice setting, and number of years in practice, reading the guidelines was still an independent predictor of prepregnancy counseling (p < 0.01). With multivariable logistic regression, there was a trend between reading the guidelines and screening for thyroid disease risk factors (p = 0.11) and working in a private practice setting and screening for risk factors (p = 0.08). No one variable was independently predictive of screening for thyroid disease risk factors (Table 4).
Table 4.
Multivariable Logistic Regression Analysis of Patient Education and Screening
| Independent variables | p-Value | OR (CI) |
|---|---|---|
| Often or always educating the patient prepregnancy | ||
| Reading the guidelines | <0.01a | 2.42 (1.33–4.39) |
| Provider sex | 0.90 | 1.03 (0.67–1.57) |
| Private practice setting | 0.52 | 0.85 (0.51–1.40) |
| No. of years in practice | 0.46 | 0.99 (0.97–1.02) |
| Member of ACOG | <0.01a | 2.87 (1.92–4.31) |
| Often or always screening for risk factors for thyroid disease | ||
| Reading the guidelines | 0.11 | 1.61 (0.90–2.86) |
| Provider sex | 0.18 | 0.76 (0.51–1.14) |
| Private practice setting | 0.09 | 1.55 (0.94–2.54) |
| No. of years in practice | 0.56 | 0.99 (0.97–1.02) |
| Member of ACOG | 0.18 | 1.30 (0.88–1.91) |
Significant at p < 0.05.
OR, odds ratio; CI, confidence interval.
Discussion
When evaluating the role of guidelines in clinical care, the Endocrine Society's “Guidelines on the Management of Thyroid Dysfunction During Pregnancy and Postpartum” were ideal guidelines to evaluate for several reasons: (i) hypothyroidism and pregnancy are common events, (ii) there are adverse outcomes related to untreated hypothyroidism during pregnancy, (iii) certain guideline recommendations were strongly advocated based on U.S. Preventive Service Task Force System and GRADE, and (iv) the guidelines were created by subspecialists but have large implications for medical providers across a variety of disciplines.
The first relevant question was, “Do these guidelines reach their target population?” Based on this study, the guidelines reach a minority of the target population. Obstetricians and family physicians provide the majority of antenatal care, but only 11.5% of these providers read the guidelines. Although the role of maternal thyroid function in pregnancy outcome has been well published over the past 10 years, a larger volume of work has been published in subspecialty journals than in high-impact general journals such as New England Journal of Medicine. Many of these subspecialty journals may not be accessible by primary care physicians. Although guidelines provide opportunity for subspecialty information to reach a broader population, the publication of these guidelines also occurred in a subspecialty journal, thus limiting the ability of generalists to access relevant information. Since this study shows that a small percent of these patients are ultimately referred to endocrinologists, publishing solely in an endocrine journal may not be adequate.
The second relevant question on the role of guidelines in provider education centers on the ability of guidelines to influence patient care. Although previous work has found a link between degree of subspecialization, number of years in practice, and provider knowledge on thyroid disease in pregnancy (25), no previous study has evaluated factors involved in providing patient education. Importantly, this study found that reading the Endocrine Society's guidelines was associated with increased prepregnancy counseling on changes in thyroid hormone replacement during pregnancy, even when controlling for other variables such as provider sex, practice setting, number of years in practice, and membership to ACOG versus AAFP. This prepregnancy counseling is critical since this empowers a patient to be her own advocate once conception occurs.
Despite improvements in care with exposure to the guidelines, there were other areas of care that were not influenced. If the guidelines do not influence care, the next important questions are the following: (i) Are the guidelines practically applicable? (ii) What obstacles limit implementation?
In this study, reading the guidelines did not impact the time of first prenatal visit. The initial antenatal visit is typically 8–10 weeks of gestation, with the majority of our responders seeing patients between 7 and 12 weeks of gestation. Based on good evidence, the Endocrine Society guidelines strongly recommend providers know that there is a high likelihood that the LT4 dose needs to be increased by 4–6 weeks of gestation and may require a 30%–50% increase (18). However, the initial antenatal visit is typically scheduled at 8–10 weeks of gestation for two reasons: first, many women do not know they are pregnant soon after conception; second, depending on method of detection, between 30% and 73% of conceptions end in miscarriage, most prior to 6 weeks of gestation (26–30). Thus, first antenatal visits are typically scheduled when the fetal heart rate can be assessed.
If the first antenatal visit is after the dose change should have occurred, it may be better to check thyroid function tests at time of positive home pregnancy test. However, only 19% of physicians surveyed addressed LT4 dose at time of positive home pregnancy test, while 70% addressed LT4 dose at the first prenatal visit. There were care providers who added commentary and reported addressing the dose at time of conception if medication history was known, but in many cases, the patient's history is unknown until the initial visit. This lag time between when a dose increase is needed and when it occurs is one explanation for 49% of women on LT4 having a TSH level outside goal range during the first trimester (14). Since the fetal thyroid does not develop until 13 weeks of gestation, and since the initial antenatal visit may occur after initial dose adjustment is warranted, a second option is to empirically increase the LT4 dose by 30% once conception is confirmed (13). Although the method of addressing the necessary dose change was not explicitly stated in the guidelines, reading the guidelines was associated with a significantly higher likelihood of empirically increasing the dose. This may be the most practical option given the lag time between change in dose requirement and initial visit. However, not all physicians will be comfortable with this empiric dose increase (14) since the effects of hyperthyroidism, a potential risk of empiric treatment, on pregnancy are debated (9,10). Despite hesitancy of some providers in accepting empiric dose increase, the 2007 Endocrine Society's guidelines suggest that in pregnant women, there are likely no adverse effects of subclinical hyperthyroidism, a more likely outcome of empiric dose increase than overt hyperthyroidism.
This brings to point the issue of the appropriate audience for guidelines. An audience of endocrinologists is clearly too narrow for the topic of thyroid hormone replacement in women of reproductive age. Given that most patients are not under obstetric care before 7–12 weeks of gestation, perhaps an audience of obstetric care providers is also too narrow. It is possible that the audience for these guidelines would be all medical providers who start women of reproductive age on LT4 as most of the patients will not see their obstetric provider until after an initial dose adjustment is warranted. Patient education at the time of initial LT4 prescription is necessary so that the patient can then inform the obstetric team at the time of known conception instead of at the scheduled antenatal visit. If the burden of responsibility falls on all LT4-prescribing providers and the patient, this warrants distribution of the guidelines to an even larger audience.
In regard to women not already on LT4, the current guidelines do not recommend checking a TSH on all pregnant patients but instead recommend TSH screening in patients with risk factors for thyroid disease (18). There are multiple risk factors for thyroid disease, including a family history of thyroid disease, personal history of thyroid surgery, history of head or neck radiation, goiter, type 1 diabetes or other autoimmune disorder, positive thyroid antibodies, symptoms or signs of thyroid dysfunction, iodine deficiency, history of previous thyroid disorder, infertility, miscarriage, or previous preterm delivery (18). In this study, only 36% of providers routinely screen pregnant patients for thyroid disease risk factors, whereas close to 50% selectively screen patients with symptoms of hypothyroidism or hyperthyroidism, and another 9% do not routinely screen any patients. Although it is not clear why only a little over one-third of providers routinely screen for thyroid disease risk factors in pregnant patients, given the lengthy list of risk factors and the variety of other patient care issues being addressed at an initial antenatal visit, time constraints may be a limiting factor. Although the likelihood of screening for these risk factors increased if the guidelines were read, on multivariable analysis this association was only a trend.
In our study, only 15% of care providers routinely check TSH on all pregnant patients independent of known risk factors. Previous studies found that 19% of mid-Atlantic ACOG members and 48% of Maine physicians routinely check TSH on all pregnant patients (31,32). Despite the Endocrine Society's guidelines reporting inadequate evidence for routine TSH testing in all patients, there are some practical issues that may support this option. First, a recent study found that targeting high-risk patients alone misses one-third of women with overt and subclinical hypothyroidism (33). Second, if there is an increased likelihood of adverse pregnancy outcome with hypothyroidism, routine TSH testing is cost efficient (34,35). Third, the list of relevant risk factors is lengthy, and in our study, only 36% of providers routinely screen for thyroid disease risk factors, thus increasing the likelihood of undiagnosed hypothyroidism during pregnancy. Given the potential risks of not checking TSH, the time constraints of clinical practice limiting screening for all thyroid disease risk factors (19,20), and the cost–benefit ratio (34,35), checking a TSH on all pregnant patients may be practical despite inconclusive long-term data.
Despite several important findings, there are limitations of this study. First, only members of Wisconsin ACOG and Wisconsin AAFP were surveyed. It is possible that this cohort is not representative of all ACOG and AAFP members. Although no previous survey study has evaluated the current management of thyroid hormone replacement in pregnancy, previous studies have looked at routine testing of TSH in pregnant patients not previously found to have hypothyroidism (31,32). TSH screening practices vary widely based on geographic region, and one may extrapolate that the management of thyroid hormone replacement may also vary by geographic region. A second limitation of this study is that practice patterns were based on self-report and thus subject to respondent recall. There was an attempt to decrease recall bias by eliminating subjects who did not see a pregnant patient in 2008 and by surveying subjects February–March of 2009, just 2–3 months after the specified time period to recall. Finally, although all survey studies are at risk for bias by nonresponse, the comparison between the completed survey subjects to the total surveyed population found that they were nearly identical in regard to membership affiliation and urban versus rural practice setting.
In summary, despite their limitations (21,22), clinical guidelines do serve a purpose: provider education and standardization of care. In this study, reading the Endocrine Society's “Guidelines on the Management of Thyroid Dysfunction During Pregnancy and Postpartum” was associated with positive changes in clinical practice. Yet, the guidelines reached a minority of obstetric care providers, likely secondary to their publication in a subspecialty journal. In many instances, clinical guidelines are only applicable to a subset of providers, and thus it is appropriate to limit dissemination to scientific journals within relevant societies. However, in the case of thyroid hormone replacement in women of reproductive age, the burden of awareness falls upon multiple care providers and the patient herself. Therefore, these guidelines may have been more effective if they were disseminated to a wider audience.
This study clearly shows that clinical guidelines can influence patient care. However, it also highlights how selective dissemination and lack of clear options for practical implementation can limit the true potential of guidelines. Endocrinologists previously advocated for a multidisciplinary approach to the creation of guidelines on pregnancy and thyroid function (36). This solution would improve both dissemination and practical applicability. Because of the potential impact on practice patterns and the variety of physicians involved in the care of women of reproductive age, creating and publishing applicable guidelines for this topic should include representatives of relevant physician groups, including experts in endocrinology, obstetrics, and health service research. The role of guidelines in physician education and ultimately in the standardization of care can be optimized with a multidisciplinary approach.
Footnotes
Abstract was presented as an oral presentation at the 2009 American Thyroid Association Meeting.
Acknowledgments
The author would like to thank the family physicians and obstetricians in the state of Wisconsin who took the time to respond to the survey. The author would like to acknowledge both Mary Beth Plane, Ph.D., Director of Research Services at the University of Wisconsin Department of Family Medicine, and Joel B. Henry, M.D., Chairman, Wisconsin Section of The American College of Obstetricians and Gynecologists, who previewed the survey instrument before administration. The author would like to acknowledge Mark Albanese, Ph.D., who offered advice on statistical analysis and Ron Koenig, M.D., Ph.D., who proof read the article. At the time of data collection Dr. Haymart was at the University of Wisconsin. The University of Wisconsin Paul P. Carbone Comprehensive Cancer Center funded the data collection and supported the research of Megan Haymart through CA K12087718.
Disclosure Statement
The author declares that there is no conflict of interest and nothing to disclose.
References
- 1.Hollowell JG. Staehling NW. Flanders WD. Hannon WH. Gunter EW. Spencer CA. Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 2.Idris I. Srinivasan R. Simm A. Page RC. Maternal hypothyroidism in early and late gestation: effects on neonatal and obstetric outcome. Clin Endocrinol (Oxf ) 2005;63:560–565. doi: 10.1111/j.1365-2265.2005.02382.x. [DOI] [PubMed] [Google Scholar]
- 3.Benhadi N. Wiersinga WM. Reitsma JB. Vrijkotte TG. Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009;160:985–991. doi: 10.1530/EJE-08-0953. [DOI] [PubMed] [Google Scholar]
- 4.Antolic B. Gersak K. Verdenik I. Novak-Antolic Z. Adverse effects of thyroid dysfunction on pregnancy and pregnancy outcome: epidemiologic study in Slovenia. J Matern Fetal Neonatal Med. 2006;19:651–654. doi: 10.1080/14767050600850332. [DOI] [PubMed] [Google Scholar]
- 5.Allan WC. Haddow JE. Palomaki GE. Williams JR. Mitchell ML. Hermos RJ. Faix JD. Klein RZ. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7:127–130. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 6.Stagnaro-Green A. Maternal thyroid disease and preterm delivery. J Clin Endocrinol Metab. 2009;94:21–25. doi: 10.1210/jc.2008-1288. [DOI] [PubMed] [Google Scholar]
- 7.Haddow JE. Palomaki GE. Allan WC. Williams JR. Knight GJ. Gagnon J. O'Heir CE. Mitchell ML. Hermos RJ. Waisbren SE. Faix JD. Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 8.Cleary-Goldman J. Malone FD. Lambert-Messerlian G. Sullivan L. Canick J. Porter TF. Luthy D. Gross S. Bianchi DW. D'Alton ME. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112:85–92. doi: 10.1097/AOG.0b013e3181788dd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anselmo J. Cao D. Karrison T. Weiss RE. Refetoff S. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292:691–695. doi: 10.1001/jama.292.6.691. [DOI] [PubMed] [Google Scholar]
- 10.Casey BM. Dashe JS. Wells CE. McIntire DD. Leveno KJ. Cunningham FG. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107:337–341. doi: 10.1097/01.AOG.0000197991.64246.9a. [DOI] [PubMed] [Google Scholar]
- 11.Glinoer D. de Nayer P. Bourdoux P. Lemone M. Robyn C. van Steirteghem A. Kinthaert J. Lejeune B. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 12.Mandel SJ. Larsen PR. Seely EW. Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96. doi: 10.1056/NEJM199007123230204. [DOI] [PubMed] [Google Scholar]
- 13.Alexander EK. Marqusee E. Lawrence J. Jarolim P. Fischer GA. Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241–249. doi: 10.1056/NEJMoa040079. [DOI] [PubMed] [Google Scholar]
- 14.Hallengren B. Lantz M. Andreasson B. Grennert L. Pregnant women on thyroxine substitution are often dysregulated in early pregnancy. Thyroid. 2009;19:391–394. doi: 10.1089/thy.2008.0206. [DOI] [PubMed] [Google Scholar]
- 15.Loh JA. Wartofsky L. Jonklaas J. Burman KD. The magnitude of increased levothyroxine requirements in hypothyroid pregnant women depends upon the etiology of the hypothyroidism. Thyroid. 2009;19:269–275. doi: 10.1089/thy.2008.0413. [DOI] [PubMed] [Google Scholar]
- 16.Kothari A. Girling J. Hypothyroidism in pregnancy: pre-pregnancy thyroid status influences gestational thyroxine requirements. BJOG. 2008;115:1704–1708. doi: 10.1111/j.1471-0528.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 17.Verga U. Bergamaschi S. Cortelazzi D. Ronzoni S. Marconi AM. Beck-Peccoz P. Adjustment of L-T4 substitutive therapy in pregnant women with subclinical, overt or post-ablative hypothyroidism. Clin Endocrinol (Oxf ) 2009;70:798–802. doi: 10.1111/j.1365-2265.2008.03398.x. [DOI] [PubMed] [Google Scholar]
- 18.Abalovich M. Amino N. Barbour LA. Cobin RH. De Groot LJ. Glinoer D. Mandel SJ. Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 19.Yarnall KS. Pollak KI. Ostbye T. Krause KM. Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostbye T. Yarnall KS. Krause KM. Pollak KI. Gradison M. Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sniderman AD. Furberg CD. Why guideline-making requires reform. JAMA. 2009;301:429–431. doi: 10.1001/jama.2009.15. [DOI] [PubMed] [Google Scholar]
- 22.Shaneyfelt TM. Centor RM. Reassessment of clinical practice guidelines: go gently into that good night. JAMA. 2009;301:868–869. doi: 10.1001/jama.2009.225. [DOI] [PubMed] [Google Scholar]
- 23.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 24.Dillman D. Mail and Telephone Surveys: The Total Design Method. John Wiley and Sons, Inc; New York: 1978. [Google Scholar]
- 25.Rinaldi MD. Stagnaro-Green AS. Thyroid disease and pregnancy: degrees of knowledge. Thyroid. 2007;17:747–753. doi: 10.1089/thy.2007.0080. [DOI] [PubMed] [Google Scholar]
- 26.Boklage CE. Survival probability of human conceptions from fertilization to term. Int J Fertil. 1990;35 75, 79–94. [PubMed] [Google Scholar]
- 27.Wilcox AJ. Weinberg CR. O'Connor JF. Baird DD. Schlatterer JP. Canfield RE. Armstrong EG. Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 28.Wang X. Chen C. Wang L. Chen D. Guang W. French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577–584. doi: 10.1016/s0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- 29.Zinaman MJ. Clegg ED. Brown CC. O'Connor J. Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]
- 30.Tong S. Kaur A. Walker SP. Bryant V. Onwude JL. Permezel M. Miscarriage risk for asymptomatic women after a normal first-trimester prenatal visit. Obstet Gynecol. 2008;111:710–714. doi: 10.1097/AOG.0b013e318163747c. [DOI] [PubMed] [Google Scholar]
- 31.Haddow JE. McClain MR. Palomaki GE. Kloza EM. Williams J. Screening for thyroid disorders during pregnancy: results of a survey in Maine. Am J Obstet Gynecol. 2006;194:471–474. doi: 10.1016/j.ajog.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 32.Stovall DW. Loveless MB. Walden NA. Karjane N. Cohen SA. Primary and preventive healthcare in obstetrics and gynecology: a study of practice patterns in the mid-atlantic region. J Womens Health (Larchmt) 2007;16:134–138. doi: 10.1089/jwh.2006.0066. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya B. Anthony S. Bilous M. Shields B. Drury J. Hutchison S. Bilous R. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203–207. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 34.Dosiou C. Sanders GD. Araki SS. Crapo LM. Screening pregnant women for autoimmune thyroid disease: a cost-effectiveness analysis. Eur J Endocrinol. 2008;158:841–851. doi: 10.1530/EJE-07-0882. [DOI] [PubMed] [Google Scholar]
- 35.Thung SF. Funai EF. Grobman WA 2009 The cost-effectiveness of universal screening in pregnancy for subclinical hypothyroidism. Am J Obstet Gynecol. 200 doi: 10.1016/j.ajog.2008.10.035. 267 e261–e267. [DOI] [PubMed] [Google Scholar]
- 36.Burman KD. Controversies surrounding pregnancy, maternal thyroid status, and fetal outcome. Thyroid. 2009;19:323–326. doi: 10.1089/thy.2009.1570. [DOI] [PubMed] [Google Scholar]