Abstract
CD8+ lymphocytes are critical to the control and elimination of viral pathogens. Impaired CD8+ responses are well recognized in lentiviral infections; however, the mechanisms underlying CD8+ impairment remain elusive. Using the feline immunodeficiency virus (FIV) model for human AIDS, we reported previously that CD4+CD25+ Treg cells in both the acute and long-term, asymptomatic phase of infection are constitutively activated and suppress CD4+CD25– T cell responses. In the current study, we have demonstrated that CD4+CD25+ Treg cells suppress CD8+ responses to immune stimulation during both the acute and chronic, asymptomatic phase of FIV infection and that the mechanism of suppression may be mediated by membrane-associated TGF-β (mTGF-β) on CD4+CD25+ lymphocytes. Depletion of CD4+CD25+ lymphocytes from lymph node suspensions significantly enhanced production of IFN-γ during the acute phase of infection and coculture of CD8+ lymphocytes with CD4+CD25+ lymphocytes resulted in suppression of CD8+ IFN-γ during both the acute and chronic stages of infection. FACS analysis indicated that there was TGF-βRII upregulation on CD8+ cells from FIV+ cats during the acute and chronic stage of infection. In addition, there was upregulation of mTGF-β on the CD4+CD25+ subset in chronically infected cats. In support of activation of the TGF-β signaling pathway, Western blotting showed Smad 2 phosphorylation in CD8+ targets following CD4+CD25+/CD8+ coculture. These results demonstrate the suppressive effect CD4+CD25+ Treg cells have on the CD8+ immune response during the acute and chronic stages of FIV infection and suggest that the mechanism of suppression may be mediated by mTGF-β.
Introduction
In feline immunodeficiency virus (FIV) and human immunodeficiency virus (HIV) infections, progressive immune dysfunction is evident during the course of infection.1,2 Early immune dysfunction is characterized by reduced capacity to respond to FIV/HIV antigens, by poor response to recall antigens, and by the diminished capacity to mount a primary cell-mediated immune response to new infections.3,4 Later in the course of disease, immune dysfunction is characterized by a loss of mitogenic responses, a reduction in CD4+ T cell counts, and the development of opportunistic infections signaling the onset of AIDS.1,2,5
Although AIDS lentivirus infections are typically associated with an early CD8+ lymphocytosis, recent data indicate that the quality of the CD8+ response, not the magnitude of the response, appears to be most critical in controlling virus replication.6,7 Experiments utilizing AIDS lentivirus animal models have demonstrated that CD8+ depletion leads to increased virus production in vitro and to increased viremia and more severe disease in vivo.8,9 These combined results suggest that the CD8+ response plays a major role in the control of AIDS lentivirus replication.6–9 More importantly, these results indicate that even in the face of a robust CD8+ response there appear to be both viral and host factors that allow the virus to escape elimination and establish and maintain a chronic infection.
In acute and chronic viral infections, there are immunoregulatory mechanisms in place to control inflammatory responses that might prove detrimental to the host.1,10,11 A key component of the virus–host interaction is CD4+CD25+ T regulatory cells (Tregs) that temper the inflammatory response, but may also promote development of chronic infections. In a variety of different viral infections such as herpes simplex virus (HSV), hepatitis C virus (HCV), and Friend virus, CD4+CD25+ Tregs appear to play a pivotal role in immunopathogenesis.12–15 Suvas et al.12 demonstrated that CD4+CD25+ depletion, prior to HSV infection, enhanced CD8+ responses to immunodominant peptides 3- to 4-fold. Boettler et al.15 demonstrated that Tregs inhibited CD8+ interferon (IFN)-γ production and proliferation in response to HCV antigen. Furthermore, they reported that chronic HCV infection led to in vivo CD4+CD25+ expansion and impaired CD8+ responses to other viral antigens such as influenza. The common theme of these models is that depletion of CD4+CD25+ Tregs leads to enhanced CD4+ and CD8+ effector responses, both against viral antigens and against unrelated recall antigens, and this heightened effector activity may lead to virus clearance. Conversely, depletion of CD4+CD25+ Tregs may also result in increased immunopathology associated with unchecked inflammation. For example, in a murine HSV keratitis model, Suvas and colleagues reported that CD4+CD25+ depletion prior to infection led to more severe corneal lesions associated with a vigorous T effector response.11
The CD8+ immune response plays an important role in both the acute and chronic phases of lentiviral infection and the ability to escape elimination during the acute CD8+ response is one of the keys to establishing a chronic infection.16,17 Understandably, much attention has been focused on the mechanisms lentiviruses use to escape the initial CD8+ response and persistently evade elimination during the chronic phase of infection. Based on evidence from other models of virus infection, it is likely that Tregs play an important role in both the acute and chronic stages of HIV infection. Kinter et al.18 reported that CD4+CD25+ T cells in the majority of healthy, chronically infected HIV+ patients significantly suppressed proliferation and cytokine production by CD4+ and CD8+ T cells stimulated with HIV peptides in vitro. Interestingly, the level of Treg suppressor function correlated inversely with plasma viremia. More recently, Kinter and others19 demonstrated that Tregs from HIV-infected individuals suppressed CD8+ antigen-specific HIV gag responses, expression of IFN-γ and tumor necrosis factor-α (TNF-α), and antiviral chemokine secretion.
Using the FIV model we have previously shown that FIV infection phenotypically and functionally activates CD4+CD25+ Tregs during both the acute and chronic stages of infection and these cells are capable of inhibiting CD4+ effector responses.20,21 Activated feline Tregs from FIV+ cats upregulate CTLA4, B7.1 (CD80), and B7.2 (CD86), and suppress CD4+ proliferation and interleukin (IL)-2 production.20,21 Furthermore, we have demonstrated preferential in vitro and in vivo replication of FIV in the CD4+CD25+ subset of CD4+ T cells. We believe that due to FIV infection of CD4+CD25+ Tregs early during the course of infection as well as during the chronic stage of infection, Tregs become activated and capable of suppressing CD4+CD25– T helper responses.21,22
The mechanism(s) of CD4+CD25+ Treg-mediated suppression remains elusive. Different mechanisms of contact-mediated suppression have been proposed such as granzyme-dependent, CTLA-4-dependent, and tumor growth factor (TGF)-β-dependent suppression.23,24 Grossman and others25 reported that after CD3/CD46 stimulation, CD4+CD25+FOXP3+ lymphocytes isolated from human blood showed increased granularity caused by an accumulation of cytoplasmic granzyme A. In coculture experiments these stimulated Tregs were able to kill autologous CD4+, CD8+, and CD14+ target cells in a granzyme A–perforin-dependent fashion. However, granzyme-mediated killing of activated CD4+ and CD8+ targets is probably more relevant to autoimmune and transplantation models, because it appears that in many infectious disease models, effector cells are anergized and not destroyed following Treg interactions.24,25
Tregs constitutively express CTLA-4 and some studies have suggested that CD80/CD86 ligation by CTLA-4 accounts for at least partial T effector suppression.23,26,27 However, Katoaka et al.28 demonstrated that CTLA-4 was not required for in vitro Treg-mediated suppression. Therefore, it may be that the CTLA-4/CD80/86 pathway represents an alternative suppressive pathway in models of chronic viral infection such as FIV.29,30
Several laboratories, including ours, have demonstrated surface bound TGF-β (mTGF-β) on activated Tregs.31–33 However, the role of TGF-β in Treg-mediated suppression is controversial. Nakamura et al.31 demonstrated that activated Tregs expressed mTGF-β and that their contact-dependent suppressor function was abrogated by treatment with anti-TGF-β neutralizing antibody. Others have demonstrated that suppressor function of both mouse and human CD4+CD25+ thymocytes is at least partially inhibited by neutralization of TGF-β.32,34 In contrast, Piccirillo et al.35 reported that anti-TGF-β had no effect on in vitro Treg cell-mediated suppression. Additionally, studies have shown that Tregs from TGF-β-deficient mice were capable of suppression as well as those from normal mice.35,36
We previously reported that activated CD4+CD25+ Tregs express mTGF-β and that these cells mediate suppressor function by engagement of TGF-βRII on the surface of activated CD4+ target cells.37 Importantly, the TGF-β pathway is also essential to controlling CD8+ lymphocyte responses. Mice expressing a dominant negative TGF-βRII (dnTGF-βRII) specific to their T cells develop a profound CD8+ hyperproliferation resulting in massive expansion of their peripheral lymphoid tissue by 2–9 months of age.38 Interestingly, CD8+ T cell development in the thymus was normal, suggesting that the homeostatic control occurs in the periphery. In addition, introduction of a dnTGF-βRII into antigen-specific CD8+ cells rendered them resistant to Treg cell-mediated suppression.39 Further findings in this model demonstrated that TGF-β inhibits CD8+ antitumor effector function, although suppressed CD8+ lymphocytes maintain an activated phenotype.40 Similarly, in an autoimmune diabetes model, delayed progression of diabetes was associated with an increased percentage of mTGF-β-positive, CD4+CD25+ lymphocytes within the peripheral nodes and pancreas.41
It is evident from this discussion that the role of the TGF-β/TGF-βRII signaling pathway in Treg cell-mediated suppression likely depends on the model system used. Using the FIV model, we have demonstrated transient upregulation of TGF-β on the surface of CD4+CD25+ cells during acute FIV infection and we have shown that blocking TGF-β signaling blocks the Treg-mediated conversion of CD4+CD25– target cells to suppressor cells.21,37 The experiments that follow support the hypothesis that Treg-mediated CD8+ suppression likely occurs via the interaction between Tregs displaying mTGF-β and TGF-βRII on target CD8+ cell surfaces.
Materials and Methods
Cats
Specific pathogen-free (SPF) cats were obtained from Liberty Research, Inc. (Waverly, NY) at 6 months of age, and housed in the Laboratory Animal Resource Facility at the College of Veterinary Medicine, North Carolina State University. FIV-infected cats were housed separately from uninfected control cats. Protocols were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Infection with FIV
The NCSU1 isolate of FIV was originally obtained from a naturally infected cat at the North Carolina State University College of Veterinary Medicine and has been described in detail elsewhere.42 Virus inoculum was grown as a single tissue culture passage in an IL-2-dependent feline CD4+ cell line (FCD4-E cells) as previously described.43 The cats were inoculated intravenously with 1 × 105 TCID50 of cell-free virus culture and control cats were sham inoculated with equal volumes of sterile FCD4-E cell culture medium. Most of the cats used for the FIV acute phase infection studies were then utilized for the FIV chronic infection studies, and at the time of chronic phase experiments had been infected with FIV for approximately 1 year.
Sample collection
Blood was collected via jugular venipuncture into EDTA vacutainer tubes from FIV-infected and control cats at the indicated time points postinoculation. Two milliliters was retained for a complete blood cell count and lymphocyte subset analysis by multicolored flow cytometry. Plasma was separated from the remaining blood and frozen for analysis of viral load by reverse transcriptase polymerase chain reaction (RT-PCR). During acute phase studies cats were grouped such that popliteal lymph nodes (LNs) were biopsied at days 14, 21, 28, 35, 42, 56, and 84 from two to four FIV+ cats and four sham-inoculated cats were used as uninfected controls. LN biopsies were performed as previously described.21,44 Briefly, cats were anesthetized with intravenous ketamine and valium and anesthesia was maintained with isofluorane gas. Popliteal lymph nodes were excised through a small incision in the caudal aspect of the stifle and the incision was sutured with monofilament sutures. LNs were processed into a single cell suspension as previously described and used for phenotypic analysis by flow cytometry and for purification of lymphocyte subsets (CD8+, CD4+CD25+ cells). Two LNs were collected from each cat during the course of the acute phase study. For experiments during the chronic stage of infection, lymphocytes were harvested either by LN excision or following euthanasia.
Antibodies
Murine monoclonal antifeline CD4 [monoclonal antibody (mAb) 30A], CD8 (mAb 3.357), and CD25 (mAb 9F23) were produced in our laboratory.45 The antifeline CD25 (mAb 9F23) was originally provided by K. Ohno (University of Tokyo). The antibodies were conjugated to fluorescein isothiocyanate (FITC) (anti-CD8, anti-CD25), phycoerythrin (PE) (anti-CD4, anti-CD8), or biotin (anti-CD8) (developed with Streptavidin/PerCP). Antihuman TGF-β (mAb 240, R&D Systems), previously shown to bind feline TGF-β, was conjugated to antigen-presenting cells (APC).37 Goat anti-TGF-β receptor II (AF 241 NA, R&D systems) was conjugated to PE. Mouse anti-phospho-SMAD2 (138D4) was purchased from Cell Signaling Technologies. The isotype control antibody for the TGF-β–APC was irrelevant mouse IgG1 purchased from Southern Biotec. The isotype control for the TGF-β receptor II–PE was irrelevant polyclonal goat antibody purchased from R&D systems and conjugated to PE.
Lymphocyte subset analysis
The phenotype of lymphocytes from blood and LNs was determined by multicolored flow cytometric analysis. Peripheral blood mononuclear cells (PBMCs) were prepared using an established whole blood lysis protocol.3 Membrane TGF-β expression was determined using APC-conjugated antihuman TGF-β in combination with anti-CD4-PE and anti-CD25-FITC. TGF-βRII expression was determined using PE-conjugated anti-TGF-βRII in combination with anti-CD8-FITC. For flow cytometric analysis, lymphocytes were gated based on forward vs. side scatter, and approximately 20,000 gated events were acquired and stored list-mode fashion for analysis using CellQuest software. Absolute numbers of lymphocytes were calculated using the percentage from FACS analysis, multiplied by the total lymphocyte count from either peripheral blood or LNs.
T regulatory cell depletion assay
LN cells were stained with anti-CD4-PE and anti-CD25-FITC and CD4+CD25+ dual positive cells were depleted by flow cytometric cell sorting (MoFlo, Beckman-Colter). The resulting CD4+CD25+-depleted cells were cultured in the absence or presence of concanavalin A (Con A) (5 μg/ml) for 18 h then analyzed for production of IFN-γ by ELISpot. Unfractionated (nondepleted) LN cells were also passed through the cell sorter to ensure similar processing conditions then cultured in the presence or absence of Con A and analyzed for IFN-γ production. The effect of depleting CD4+CD25+ cells on LN IFN-γ production was determined by dividing the number of spot-forming cells (SFCs) from depleted cultures by the number of SFCs from unfractionated cultures.
T regulatory cell/CD8+ suppression assay
LN cells were stained with anti-CD4-PE, anti-CD25-FITC, and anti-CD8-biotin, developed with Streptavidin-PerCP and sorted into CD4+CD25+ and CD8+ populations. The sorted populations were greater than 95% pure. The CD8+ cells were incubated for 18 h in the presence or absence of Con A (5 μg/ml). Following incubation, 3 × 105 CD8+ cells per well were added to an IFN-γ ELISpot plate, and CD4+CD25+ cells added at a ratio of 0.5:1 (CD4+CD25+ to CD8+). The plates were analyzed for the presence of IFN-γ-producing cells as described below.
IFN-γ ELISpot
Following incubation without or with Con A, cells were plated into precoated (monoclonal antifeline IFN-γ, R&D systems) 96-well ELISpot plates at a concentration of 3 × 105 cells/well. The plates were incubated for 24 h, stained with detection antibody, and developed per the manufacturer's instructions. Once dry, each well was counted with an automated ELISpot reader for quantification of SFCs per number of cells plated in each well. Controls included recombinant feline IFN-γ (positive control), media only wells (to assess background staining), and wells treated without detection antibody (for nonspecific staining).
Plasma and cell-associated viremia
Evidence of infection was assessed on each plasma sample using a commercially available snap test ELISA (IDEXX laboratories) to detect antibodies against FIV. In addition, quantitative real-time PCR was used to determine viral gag-mRNA loads in each plasma sample. Briefly, 1 ml of plasma was used to extract viral RNA using the Qiagen QIAamp Ultrasense Virus Isolation kits. Then 10 μl of the isolated viral RNA was reverse transcribed in a separate reaction using the Promega Reverse Transcription System with random primers. This reaction was followed by a real-time PCR step using specific primers for FIV-gag mRNA, universal Taqman PCR Mastermix (Applied Biosystems), and the FIV-specific probe in the relative concentrations specified by the manufacturer. Primers sequences were as follows: (491f-GATTAGGAGGTGAGGAAGTTCAGCT), (617r-CTTTCATCCAATATTTCT TTATCTGCA), and (probe FIVNC555P-FAM/CATGGCCACATTAATAATGGCCGCA/36-TAMSp).
The reactions were run in duplicate in 96-well plates and incubated at 50°C for 2 min, 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min, before returning to 25°C. A standard curve was run in each reaction using serial dilutions of previously sequenced and quantified FIV-gag-mRNA. The standard curve was used to determine absolute viral mRNA copy numbers per milliliter of plasma. Sorted populations of 0.5–3 × 106 CD4+CD25+ and CD4+CD25– T cells from blood and LNs were maintained in RNA later and evaluated for relative quantities of FIV-gag mRNA using the ΔΔCt method.46 Equal amounts of RNA were used in each test. The calibrator sample consisted of a mixture of RNA samples obtained from all groups of cells. Cells from two to eight cats were evaluated individually at each time point.21
Reverse transcription real-time PCR for IFN-γ mRNA
CD8+ lymphocytes (2–4 × 106) from FIV– and FIV+ were untreated, Con A stimulated, or stimulated with Con A followed by either TGF-β (10 ng/ml) for 120 min or coculture with CD4+CD25+ cells for 120 min (CD4+CD25+ to CD8+ ratio = 1:1). RNA from cell cultures was isolated using the Qiagen RNeasy plus Mini Kit and reverse transcription was performed using the Promega Reverse Transcription System, following the manufacturer's instructions for both. This reaction was followed by a real-time PCR step using the universal Taqman PCR Mastermix (Applied Biosystems) and the Qiagen Quantitect Sybr Green PCR Kit (probe) using feline-specific IFN-γ primers. Primer sequences were as follows: (141f-TGGTGGGTCGCTTTTCGTAG) and (225r-GAAGGAGACAATTTGGCTTTGAA). The reactions were run in duplicate in 96-well plates. The fold induction was calculated by using the ΔΔCt value, where fold induction = 2−(ΔΔCt), as described by Winer et al.46 PBMCs from an FIV-negative cat and GAPDH as the internal control were used as the calibration sample value in the ΔΔCt equation.
Smad 2 Western blots
FACS purified CD8+ and CD4+CD25+ lymphocytes were serum starved for 18 h. Then 4 × 106 CD8+ cells were incubated with rhTGF-β (10 ng/ml, R&D Systems) or cocultured with CD4+CD25+ cells at a 1:1 ratio for 2 h. The CD8+ cells were then lysed with NP-40 and separated by SDS-PAGE. For the cocultured groups, CD4+CD25+ lymphocytes were depleted from CD8+ cultures using anti-PE microbeads and MACS separation columns (>90% CD8+ purity), both purchased from Miltenyi Biotec. The blots were analyzed using rabbit monoclonal anti-P-SMAD 2 (138D4, Cell Signaling Technology), followed by HRP-conjugated goat antirabbit IgG and detected by chemiluminescence. The blots were then stripped and reprobed with antiactin and HRP-conjugated goat antimouse antibody. For each treatment group, actin and P-SMAD 2 were evaluated by photodensitometry and normalized, with unstimulated CD8+ controls assigned a value of 1.
Statistical analysis
Statistical analysis was performed using a Student's t test to compare FIV– and FIV+ groups and/or treatment groups as indicated in each of the figures. p-values less than 0.1 (Fig. 3) or 0.05 (Figs. 4–7 and 9) were considered significant.
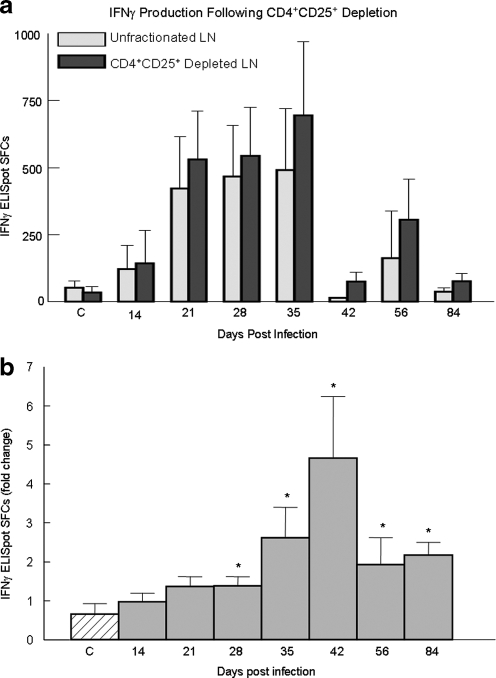
FIG. 3.
IFN-γ production from FIV+ cats following CD4+CD25+ Treg depletion is enhanced at all time points after 21 days postinfection. Popliteal lymph nodes were surgically excised at the indicated time point and the lymphocytes were sorted into two populations: unfractionated LN cells and CD4+CD25+-depleted LN cells. Cells were stimulated for 18 h with Con A then assayed for IFN-γ by ELISpot. The control group (C) was composed of sham-inoculated cats and the test group was represented by FIV+ cats sampled at the indicated time point pi. (a) The bars represent the absolute number of IFN-γ SFCs (mean ± SEM) as measured by ELISpot from unfractionated LNs (gray bars) and CD4+CD25+-depleted LNs (dark bars). (b) To compare control cats to FIV+ cats and to account for individual variation, the fold change following CD4+CD25+ depletion was calculated using the data shown in (a). The bars represent the mean ± SEM of fold increase in IFN-γ SFCs as measured by ELISpot, calculated by the following: SFCs from CD4+CD25+ depleted LN culture÷SFCs from unfractionated LN culture. The fold change following CD4+CD25+ depletion from each FIV+ group (gray) was compared to the control group (diagonal line, p < 0.1, asterisks). Control cats n = 4, FIV+ cats n = 2–5 for each time point. (Note: all numbers greater than 1 reflect an increase in IFN-γ SFCs following CD4+CD25+ depletion.)
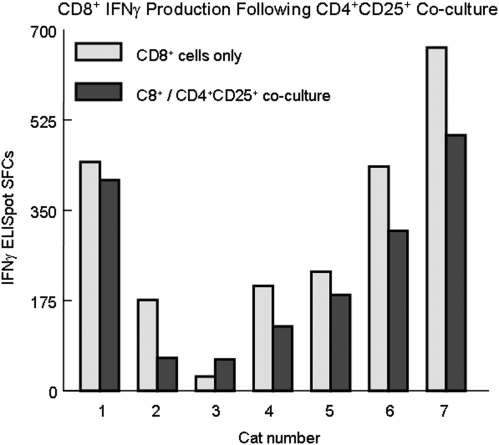
FIG. 4.
CD4+CD25+-mediated suppression of CD8+ IFN-γ production in acute FIV infection occurs after 21 days pi. FACS-purified CD8+ cells from FIV+ cats were stimulated with Con A for 18 h then cocultured with autologous CD4+CD25+ cells at a ratio of 0.5:1 (CD4+CD25+ to CD8+) and IFN-γ production was determined by ELISpot. The gray shaded bars represent CD8+ cells alone and the black bars represent CD8+/CD4+CD25+ cocultures with each pair of bars representing an individual cat. There is significant CD4+CD25+-mediated CD8+ suppression in six of seven cats at days 28–56 pi (p < 0.05). CD8+ suppression was not evident during the first 21 days of infection (not shown).
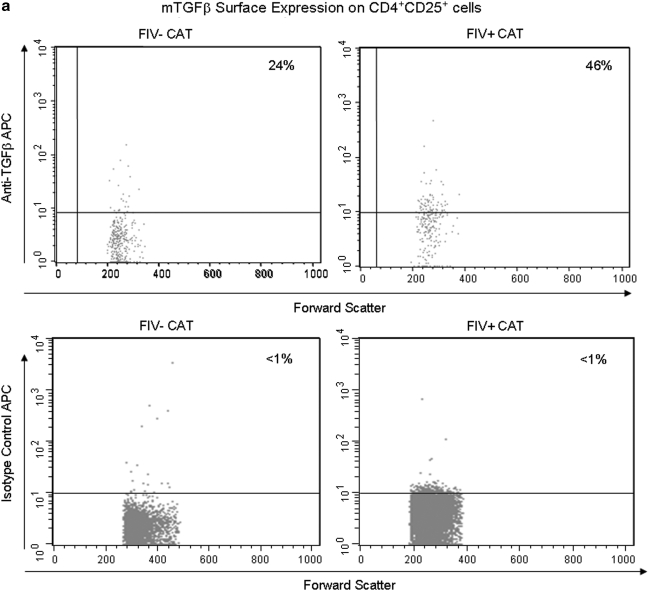
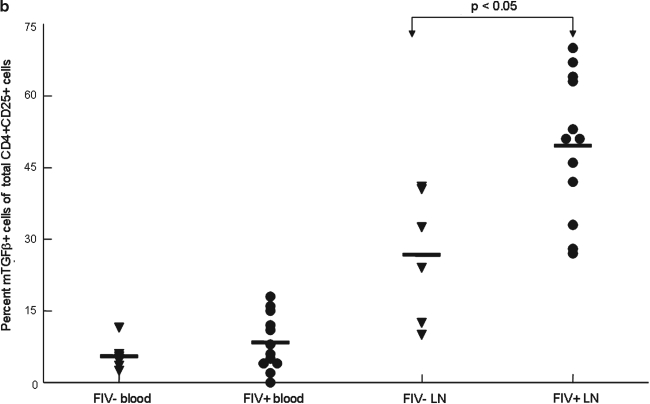
FIG. 7.
LN CD4+CD25+ lymphocytes exhibit increased expression of mTGF-β during chronic FIV infection. Peripheral blood and lymph node cells were stained with anti-CD4, anti-CD25, and anti-mTGF-β and analyzed by FACS. CD4+CD25+ cells were gated and then analyzed for percent mTGF-β+ cells. (a) Dot plots (upper row) demonstrating the percentage of mTGF-β-positive cells within the CD4+CD25+ population from the LNs of an uninfected and chronically infected FIV+ cat. The upper right quadrant represents the percent mTGF-β+ cells (24% and 46%, respectively). The lower row of dot plots represents irrelevant isotype control antibodies for anti-mTGF-β surface staining. (b) The graph depicts the mean percent mTGF-β+ of the CD4+CD25+ population (horizontal line) and dots represent individual cats. There was no difference between the FIV– (6%, n = 7) and FIV+ (8%, n = 15) blood. However, the mean mTGF-β+ was considerably higher in the FIV+ LNs (50%, n = 15) when compared to the FIV– LNs (27%, n = 6).
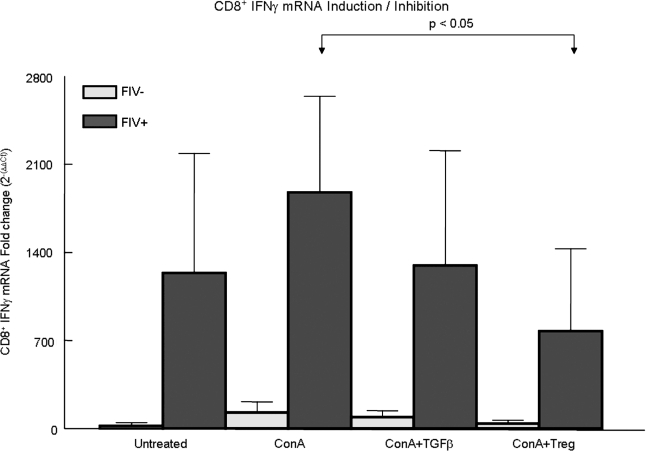
FIG. 9.
CD8+ IFN-γ mRNA is reduced in FIV+ cats by TGF-β treatment and following coculture with CD4+CD25+ Tregs. In all, 2–4 × 106 purified CD8+ lymphocytes from FIV– (gray bars) or FIV+ (dark bars) cats were serum starved overnight and were either untreated, treated with TGF-β, or cocultured with CD4+CD25+ Tregs, and RNA from CD8+ cells was then harvested for determination of IFN-γ mRNA by RT-PCR. For the CD4+CD25+/CD8+ cocultures, CD4+CD25+ cells were depleted by magnetic bead separation prior to RNA collection. The CD8+ cells were either untreated, Con A (5 μg/ml) stimulated for 30 min followed by TGF-β (10 ng/ml) for 120 min, or treated with Con A for 30 min followed by coculture with CD4+CD25+ cells (1:1 ratio) for 120 min. In FIV+ cats, there was a significant reduction in IFN-γ mRNA following coculture with CD4+CD25+ Tregs (p < 0.05, asterisks). Each bar represents the mean ± SEM from five experiments.
Results
CD8+ lymphocyte dynamics and plasma viremia during acute FIV infection
The 28 SPF cats inoculated with FIV-NCSU1 followed an infection course similar to that described previously for this isolate.21,43 All cats were antibody positive for FIV gag proteins (ELISA) by 5 weeks postinfection (pi). Blood and lymph node CD4+ cells as well as plasma were positive for FIV RNA by RT-PCR as early as 2 weeks postinfection (data not shown). Typical of FIV infection, there was an early T cell lymphopenia at day 14 pi in both the blood and lymph node followed by a gradual rebound in CD8+ cells (Fig. 1). At approximately week 5, all FIV-infected cats exhibited peripheral lymphadenopathy as has been previously described for acute FIV infection.5,47 Plasma viremia peaked at day 14 pi coinciding with the CD8+ nadir and then decreased as the CD8+ cell count increased (Fig. 2). FIV replication in the CD4+CD25+ subset paralleled the plasma viremia with FIV gag mRNA levels peaking at 14 days pi (Fig. 2).
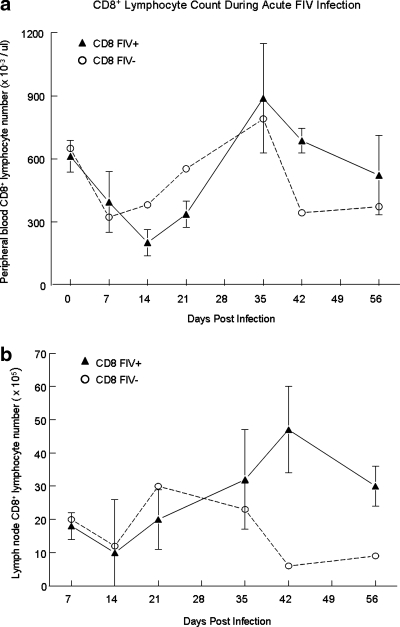
FIG. 1.
Peripheral blood and LN CD8+ lymphocyte counts during the acute phase of FIV infection. Peripheral blood (a) and lymph node (b) lymphocytes were collected at the time points post-FIV infection (shaded triangles) or sham infection (open circles) and stained with anti-CD8 and analyzed by flow cytometry. The CD8+ nadir for FIV+ cats occurs at day 14 in both the peripheral blood and LNs. A manual lymphocyte count multiplied by the percentage of CD8+ cells obtained via FACS analysis yielded the absolute count for the CD8+ population. Each time point represents the mean cell count (FIV+ cats n = 4, FIV– cats n = 2).
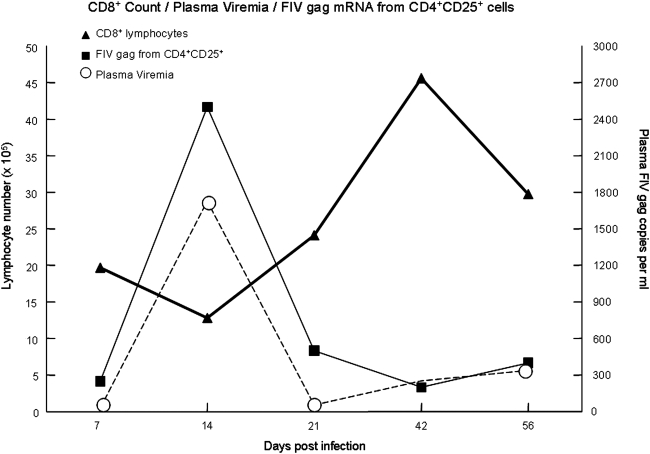
FIG. 2.
Lymph node CD8+ cell count with plasma viremia and FIV gag mRNA from CD4+CD25+ lymphocytes superimposed. Quantification of plasma viremia by real-time PCR for FIV-gag mRNA is depicted by the dashed line (circle), with the scale in copies per milliliter of plasma on the right-hand side (n = 8). Relative quantities of FIV gag mRNA from purified CD4+CD25+ lymphocytes were determined by the ΔΔCt method. The fold change in FIV gag mRNA (square, relative scale not shown, n = 8) in CD4+CD25+ lymphocytes coincides with both the peak in plasma viremia and the CD8+ nadir. As a point of reference, the CD8+ lymphocyte count (triangle) as shown in Fig. 1 is duplicated here.
CD4+CD25+ lymphocytes inhibit CD8+ IFN-γ production during acute FIV infection
As shown in Fig. 2, acute FIV infection is associated with a rebound in CD8+ cell numbers, following the CD8+ nadir. However, despite this rapid rebound, there is persistent, low-level plasma viremia. We recently reported that CD4+CD25+ Tregs are activated and suppress CD4+ T helper cells as early as 3 days post-FIV infection; we then asked whether Tregs also suppressed the CD8+ T cell response during acute FIV infection.21 CD8+ IFN-γ production as measured by ELISpot has been correlated with CD8+ CTL function and we used this method as an indicator of CD8+ antiviral potential.48 Lymphocytes from popliteal LNs were sorted into unfractionated LN cells and CD4+CD25+-depleted LN cells and were assayed for IFN-γ SFCs. Figure 3a depicts the absolute number of SFCs (mean ± SEM) for unfractionated and CD4+CD25+-depleted LNs. To account for the amount of individual variation between cats, the number of SFCs from CD4+CD25+-depleted LNs was divided by the number of SFCs from unfractionated LNs to reflect the fold increase following CD4+CD25+ depletion. As shown in Fig. 3b, the number of IFN-γ SFCs following CD4+CD25+ depletion was enhanced at all time points after 21 post-FIV infection. Once the cats transitioned toward the chronic phase of infection (days 56 and 84 pi), a sustained 2-fold increase in IFN-γ SFCs was evident following CD4+CD25+ depletion. The results indicate that CD4+CD25+ depletion from LN suspensions during acute FIV infection enhanced IFN-γ production after 21 days pi.
To characterize the effect of CD4+CD25+ Tregs specifically on CD8+ IFN-γ production, we performed a direct Treg suppression assay using a subset of the FIV+ cats depicted in Fig. 3. Lymphocytes were sorted into CD8+and CD4+CD25+ populations as described in the Materials and Methods section. After sorting, CD4+CD25+ lymphocytes were cocultured with CD8+ lymphocytes at a ratio of 0.5:1 (CD4+CD25+ to CD8+ targets) and assayed for IFN-γ SFCs. Consistent with the observations made in Fig. 3, during the first 21 days of infection, there was no evidence of CD4+CD25+-mediated suppression of CD8+ IFN-γ production (data not shown). However, as shown in Fig. 4, from days 28 to 56 pi, there is significant CD4+CD25+-mediated suppression of CD8+ IFN-γ SFCs, as measured by ELISpot.
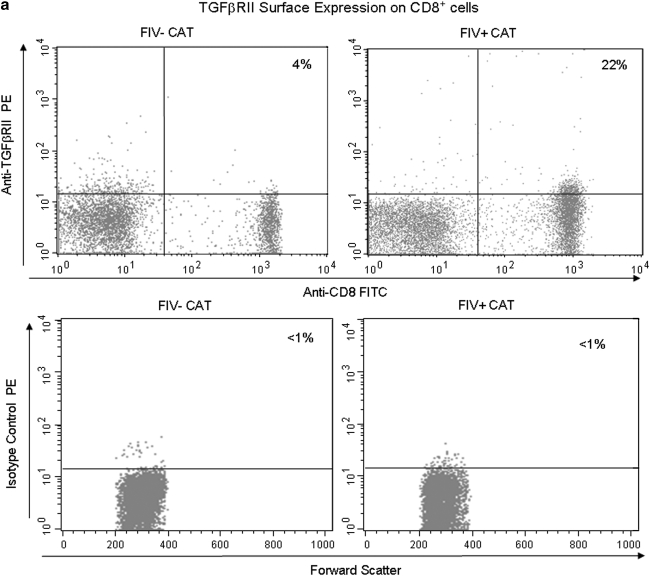
CD8+ lymphocytes exhibit increased TGF-βRII expression during acute FIV infection
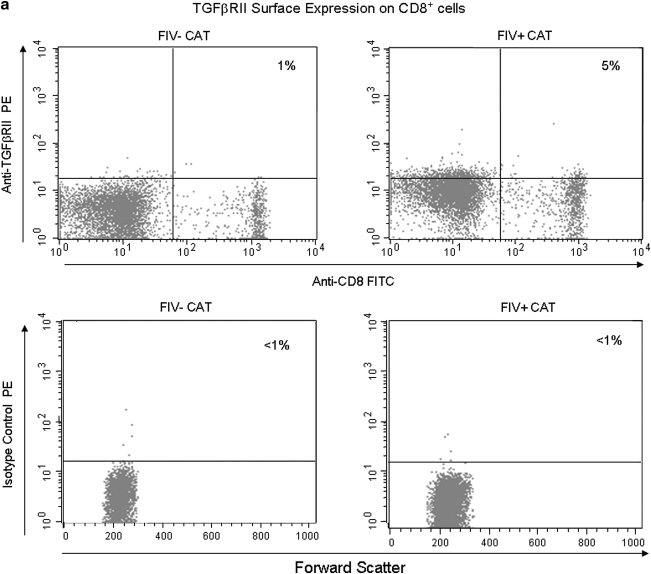
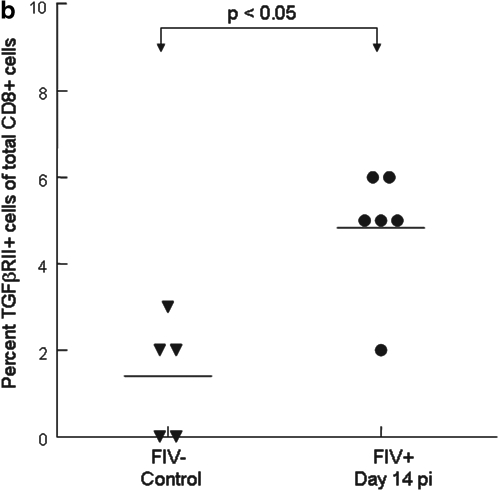
We reported previously that mTGF-β is transiently upregulated on CD4+CD25+ Treg cells from acutely FIV-infected cats, therefore lymphocytes targeted for T regulatory suppression should express TGF-βRII.21 As described in the introduction, TGF-β is important in controlling CD8+ responses. To assess expression of TGF-βRII on CD8+ cells during the acute stage of infection, peripheral blood CD8+ lymphocytes were examined by FACS for the percentage of TGF-βRII-positive cells. A representative dot plot from an FIV– control and an FIV-infected cat at day 14 pi is shown in Fig. 5a. As depicted in Fig. 5b, the mean percentage of TGF-βRII+CD8+ lymphocytes was approximately three times greater in FIV+ cats (5%) at day 14 pi, when compared to age-matched FIV– control cats (<2%). The mean percentage of TGF-βRII+CD8+ lymphocytes in FIV+ cats was also increased at days 42 and 98 pi (data not shown).
FIG. 5.
CD8+ lymphocytes exhibit increased expression of TGF-βRII in FIV+ cats when compared to FIV– control cats during acute FIV infection. Peripheral blood was stained with anti-CD8 and anti-TGF-βRII then analyzed by FACS. (a) Dot plots (upper row) demonstrating the percentage of TGF-βRII+ cells within the CD8+ population from the blood of an uninfected control cat and an FIV+ cat 14 days pi (1% and 5%, respectively). The lower row of dot plots represents irrelevant isotype control antibodies for anti-TGF-βRII surface staining. (b) The graph depicts the mean percent TGF-βRII+ cells within the CD8+ population (horizontal line) and dots represent individual cats. The mean percent TGF-βRII+ from the blood of FIV+ (5%, n = 6) cats 14 days pi is higher than that of FIV– (1%, n = 5) controls.
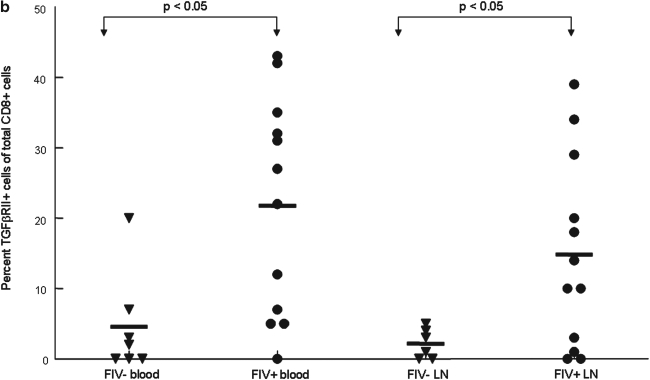
CD8+ lymphocytes upregulate TGF-βRII expression during the course of FIV infection
As demonstrated above, during acute FIV infection, TGF-βRII expression is increased in peripheral blood CD8+ cells. We then examined both peripheral blood and LN CD8+ lymphocytes from FIV chronically infected cats at 1 year pi. A representative dot plot from an FIV– control and FIV-infected cat at 1 year pi is shown in Fig. 6a. Figure 6b shows that in chronically infected cats the mean percentage of TGF-βRII+CD8+ lymphocytes in both PBMCs and LNs was more than five times greater in FIV+ cats when compared to age-matched FIV– control cats. More importantly, the mean expression of TGF-βRII on CD8+ lymphocytes was five times greater in FIV+ cats 1 year pi (22%) when compared to 14 days pi (5%). The results from Figs. 5 and 6 suggest TGF-βRII is progressively upregulated on CD8+ lymphocytes during the course of FIV infection.
FIG. 6.
CD8+ lymphocytes exhibit increased expression of TGF-βRII in FIV+ cats when compared to FIV– control cats during chronic FIV infection. Peripheral blood and lymph node cells were stained with anti-CD8 and anti-TGF-βRII and then analyzed by FACS. (a) Dot plots (upper row) demonstrating the percentage of TGF-βRII+ cells within the CD8+ population from the blood of an uninfected control cat and an FIV+ cat 1 year pi (4% and 22%, respectively). The lower row of dot plots represents irrelevant isotype control antibodies for anti-TGF-βRII surface staining. (b) The graph depicts the mean percent TGF-βRII+ cells within the CD8+ population (horizontal line) and dots represent individual cats. The mean percent TGF-βRII+ was higher in FIV+ cats in both the blood FIV+ (22%, n = 15), FIV– (5%, n = 7) and LN FIV+ (15%, n = 15), FIV– (2%, n = 6).
CD4+CD25+ lymphocytes exhibit increased expression of mTGF-β in chronically infected FIV+ cats
We have shown that mTGF-β is transiently upregulated on CD4+CD25+ Tregs from acutely FIV-infected cats and recently we have demonstrated that CD4+CD25+ Tregs convert CD4+CD25– T helper cells to T regulatory-like cells via a TGF-β-dependent mechanism.21,37 Data suggest this may also be the mechanism of T regulatory-mediated suppression.31,37,49 A representative dot plot from an FIV– control and FIV-infected cat at 1 year pi is shown in Fig. 7a. As shown in Fig. 7b, during the chronic stage of infection, there was a 2-fold increase in the percentage of lymph node mTGF-β+CD4+CD25+ lymphocytes from FIV+ cats (56%) compared to FIV– cats (27%). The mean percentage of mTGF-β+CD4+CD25+ cells from peripheral blood of FIV+ cats was somewhat higher than FIV– cats, but did not reach significance (p = 0.24).
CD4+CD25+ lymphocytes from chronically infected FIV+ cats activate the TGF-β signaling pathway and inhibit IFN-γ production in CD8+ target cells
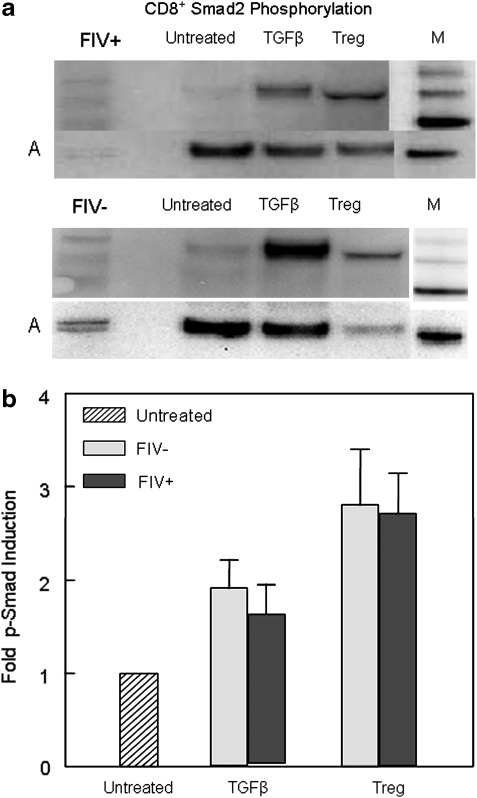
The data presented above suggest that mTGF-β may be a mechanism of T regulatory-mediated CD8+ suppression during FIV lentiviral infection. We asked if treatment of CD8+ cells with soluble TGF-β or coculture with CD4+CD25+ Tregs induced intracellular Smad 2 phosphorylation, a marker specific to the TGF-β signaling pathway.50 Synchronization of Smad 2 phosphorylation for visualization by Western blotting requires coordinated effector cell to target cell interaction. Therefore, we selected a CD4+CD25+ effector to CD8+ target ratio of 1:1. Figure 8 shows that after a 2-h coculture with CD4+CD25+ lymphocytes, CD8+ lymphocytes exhibit Smad 2 phosphorylation exceeding that of TGF-β-treated CD8+ cells.
FIG. 8.
Smad 2 phosphorylation is induced in CD8+ lymphocytes by TGF-β and following CD4+CD25+/CD8+ cocultures. In all, 4 × 106 FACS-purified CD8+ lymphocytes were serum starved overnight and were either untreated, treated with TGF-β (10 ng/ml) for 120 min, or cocultured with 4 × 106 FACS-purified CD4+CD25+ Tregs for 120 min at a ratio of 1:1, then lysed, and the amount of p-Smad 2 was determined by Western blotting. In the case of the CD4+CD25+/CD8+ cocultures, CD4+CD25+ cells were depleted by magnetic bead separation prior to lysis. (a) The upper and lower Western blots are from an FIV+ and FIV– cat, respectively. Only a trace amount of Smad 2 phosphorylation is visible in untreated CD8+ cells (Lane 3). Treatment of CD8+ lymphocytes with soluble TGF-β results in Smad 2 phosphorylation (Lane 4). Following coculture with CD4+CD25+ Tregs, CD8+ targets exhibit robust Smad 2 phosphorylation (Lane 5). The lower row represents the actin (A) control and Lane 6 represents the kDa marker (M, phosphorylated Smad 2 is found between 50 and 60 kDa). (b) Smad 2 phosphorylation in CD8+ targets following coculture with CD4+CD25+ cells exceeds that of TGF-β-treated targets. The bar graph represents the mean of Smad 2 phosphorylation from eight FIV+ cats and two FIV– control cats that were treated as described above. Untreated groups were given a value of 1, and the fold Smad 2 phosphorylation was then calculated for each treatment group.
To further characterize the role of TGF-β in Treg cell-mediated suppression of CD8+ cells, lymphocytes from chronically infected FIV+ cats and FIV– control cats were sorted into CD8+ and CD4+CD25+ populations. Following treatment of CD8+ cells with TGF-β or following CD4+CD25+ coculture, IFN-γ mRNA from CD8+ lymphocytes was assessed by real-time RT-PCR. Figure 9 shows a modest reduction in CD8+ IFN-γ mRNA following treatment with soluble TGF-β for 120 min. More importantly, in FIV+ cats there was marked suppression in CD8+ IFN-γ mRNA following a 120-min CD8+/CD4+CD25+ coculture. These results indicate that CD4+CD25+ Tregs from chronically infected FIV cats exhibit potent CD8+ immunosuppressive capability. The induction of Smad 2 phosphorylation combined with IFN-γ mRNA suppression in CD8+ target cells suggests that CD4+CD25+ Tregs utilize the TGF-β/TGF-βRII signaling pathway to inhibit CD8+ lymphocyte responses.
Discussion
During acute lentivirus infection, a CD8+ T cell lymphocytosis is noted several weeks pi.8,9,51–54 Investigations of acute HIV infection have shown that the initial control of plasma viremia parallels HIV-specific CTL activity.54,55 Using the FIV lentivirus model, Bucci et al.9 reported that cats with poor CD8+ anti-FIV activity did not have a subsequent reduction in cell-associated viremia. Conversely, cats with robust CD8+ anti-FIV activity demonstrated a progressive reduction in cell-associated virus, with several cats appearing to eliminate the virus entirely. In the experiments shown here, we have not observed any cats that appeared to clear their acute FIV infection. In a second study, Bucci et al.53 reported that CD8+ lymphocytes from FIV+ cats displayed an activated phenotype and that in vitro depletion of CD8+ lymphocytes led to increased FIV replication in PBMC cultures from FIV chronically infected cats. Similarly, Schmitz and others56 showed that in vivo depletion of CD8+ lymphocytes during chronic SIV infection led to increased viremia, which resolved with the reappearance of virus-specific CD8+ lymphocytes.
More recent investigations have examined the interaction between CD4+CD25+ Tregs and CD8+ targets in chronic HIV infection. Kinter et al.18 reported that CD4+CD25+ T cells in the majority of healthy, chronically infected HIV+ patients significantly suppressed proliferation and cytokine production by CD4+ and CD8+ T cells stimulated with HIV peptides in vitro. More recently, Kinter and others19 demonstrated that Tregs from HIV-infected individuals suppressed CD8+ antigen-specific HIV gag responses, expression of IFN-γ and TNF-α, and antiviral chemokine secretion. Eggena et al.57 similarly reported that in vitro depletion of Tregs from HIV+ PBMCs increased gag-specific CD8+ responses, as measured by IFN-γ ELISpot.
Using the FIV model, the aim of these experiments was to explore the events of acute lentiviral infection, specifically the interaction between CD4+CD25+ Tregs and CD8+ lymphocytes, and whether the observations noted during early FIV infection were also evident during the chronic, asymptomatic stage of infection. Figure 1 demonstrates CD8+ lymphocyte dynamics during the course of acute FIV infection. It has previously been reported that cats experience transient lymphadenopathy during the acute phase of infection, and this was noted in the FIV-infected cats in our experiment at about week 5 pi, corresponding to the increased lymphocyte count in both blood and LNs. At days 14 and 21, prior to the generalized lymphadenopathy, popliteal LNs were small and friable in every cat that had popliteal LN excision and in several cats the LNs were so shrunken, they could not be located for excision. These observations correspond to the lymphocyte count in both the blood and peripheral LNs and it is likely that acute viremia causes extensive lympholysis. In support of these observations, similar findings were also noted during acute SIV infection of rhesus macaques.58
We recently reported that the peak in CD4+CD25+ FIVgag mRNA also occurs at week 2 postinfection.21 The fold change in CD4+CD25+ FIV gag mRNA is shown in Fig. 2 in relation to the CD8+ cell count and plasma viremia. Although cause and effect are difficult to establish, we believe these observations may represent a series of key events involved in early CD8+ immunosuppression, which include lympholysis associated with acute viremia, concurrent CD4+CD25+ regulatory T cell activation, and a peak in FIV infection of CD4+CD25+ Tregs.21,59 This may be the time when activated CD4+CD25+ Tregs come into contact with the first wave of activated, virus-specific CD8+ cells and we are currently investigating these very early time points in detail.
The effect of CD4+CD25+ cell depletion on CD8+ responses during acute viral infection has been examined using the murine HSV model. Investigation has shown that CD4+CD25+ depletion, prior to HSV infection, enhances CD8+ responses to immunodominant peptides 3- to 4-fold and enhances in vivo CD8+ cytotoxic activity, including proliferation, granzyme B, and IFN-γ production.12,60 Although Treg cell activation appears to be antigen specific, once activated Tregs initiate both antigen-specific and antigen-nonspecific lymphocyte suppression.3,4 We therefore sought to demonstrate that ex vivo depletion of CD4+CD25+ regulatory cells, during the acute phase of FIV infection, enhanced CD8+ T cell mitogenic responses. During acute FIV infection, there is a moderate degree of variation in IFN-γ SFCs between individual cats, which is reflected in Fig. 3a. In an effort to account for this variation, we utilized a simple division formula to assess the effects of CD4+CD25+ depletion for each cat (CD4+CD25+ depleted LN SFCs ÷ unfractionated LN SFCs) to reflect the fold increase in IFN-γ production. As depicted in Fig. 3, depletion of CD4+CD25+ lymphocytes from LN suspensions during acute FIV+ infection boosted IFN-γ production in mitogen-stimulated lymphocytes starting after day 21 pi. More importantly, Fig. 4 demonstrates that CD4+CD25+ coculture with CD8+ targets suppressed CD8+ IFN-γ production after 21 days pi. We recently reported that acute FIV infection leads to increased intranuclear FOXP3 expression in CD4+CD25+ lymphocytes as early as day 21 pi.21 A short lag time between the increase in Treg cell FOXP3 expression and CD8+ suppression would be expected. These data suggest that the overall CD8+ response during acute FIV is diminished by CD4+CD25+ Tregs, and that the magnitude of CD8+ inhibition follows the course of FOXP3 upregulation in the CD4+CD25+ subset.
The exact mechanism of Treg-mediated suppression is not completely understood and it is likely that Tregs are capable of suppression by different means and that the mechanisms of suppression vary between the experimental models used. In 2001, Nakamura and colleagues31 reported that CD4+CD25+-mediated suppression of CD4+CD25– proliferation was inhibited by the presence of anti-TGF-β antibody. More importantly, they showed that CD4+CD25+ regulatory T cells stimulated with anti-CD3 and IL-2 expressed high levels of membrane TGF-β, as assessed by FACS and western blot. In a follow-up study, Nakamura et al.49 reported that treatment of CD4+CD25+ T cells with recombinant latency-associated peptide of TGF-β (rLAP) also inhibited CD4+CD25+-mediated suppression. They also described experiments in which CD4+CD25+ cells from TGF-β-deficient mice were capable of suppressing CD4+CD25– proliferation in vitro, but did not protect recipient mice from the development of colitis in an in vivo SCID transfer model.
In concert with the findings from Nakamura et al., we have reported that CD4+CD25+ Tregs from FIV-infected cats exhibit increased expression of mTGF-β.21,61 We have recently reported that mTGF-β is transiently upregulated on lymph node CD4+CD25+ lymphocytes during acute FIV infection.21 Similar to the findings from acute FIV infection, Fig. 7 shows a significantly increased percentage of mTGF-β+CD4+CD25+ lymphocytes within the lymph node of FIV+ cats, approximately 1 year postinfection. An increased percentage of mTGF-β+ CD4+CD25+ lymphocytes was not evident in the blood during acute or chronic FIV infection. Taken together these observations suggest that mTGF-β is upregulated on lymph node CD4+CD25+ T regulatory cells during the progression from acute to chronic FIV infection.
The inhibitory action of TGF-β is important in regulating CD8+ immune responses. For example, mice expressing a dnTGF-βRII specific to their T cells develop a profound CD8+ hyperproliferation and adoptive transfer experiments utilizing dnTGF-βRII CD8+ lymphocytes have demonstrated the development of different autoimmune diseases.38,41,62,63 In a human melanoma vaccine study, TGF-β was shown to inhibit CD8+ effector function in vitro, whereas the suppressed CD8+ lymphocytes maintained an activated phenotype.40 In an infectious disease model, blocking TGF-β restored in vitro CD8+ T cell IFN-γ production, and more importantly restored protective immunity against Plasmodium yoelli in vivo.64
There is little information regarding the role of TGF-β in controlling CD8+ immune responses during lentiviral infections, but there is ample evidence for CD8+ immune dysfunction during the course of AIDS lentiviral infections.1,2,5,65,66 In considering possible mechanisms for CD8+ dysfunction, we hypothesized that FIV infection may cause increased TGF-βRII expression on CD8+ lymphocytes, thereby making them much more sensitive to TGF-β inhibition. Figures 5 and 6 demonstrate that CD8+ lymphocytes from FIV+ cats have increased surface expression of TGF-βRII when compared to FIV– cats and these findings suggest that there is progressive upregulation of TGF-βRII during the course of FIV infection. Similar to the human melanoma vaccine study referenced above, activation of the TGF-β pathway in CD8+ lymphocytes may help to explain one of the apparent paradoxes of chronic lentiviral infections, in that CD8+ T cells display an activated phenotype but exhibit reduced effector function.1,53
In CD8+ lymphocytes, TGF-β ligation of TGF-βRII leads to TGF-βRI/TGF-βRII dimerization followed by Smad 2/3 phosphorylation for intracellular propagation of the TGF-β signal. The phosphorylated Smad complex translocates to the nucleus and binds transcription factors, which promote cell cycle arrest and inhibit cytolytic gene products, leading to CD8+ anergy and reduction in CD8+ function.67,68 Studies of CD8+ antitumor activity have emphasized the importance of the TGF-β signaling pathway as a mechanism for tumor escape. Using the murine EL-4 thymoma model, Tomas and Massague67 showed in a series of experiments that TGF-β inhibits the expression of CD8+ IFN-γ through Smad-ATF1-mediated binding of the IFN-γ promoter.
In accordance with these findings, Fig. 8b shows that coculture of CD8+ lymphocytes with CD4+CD25+ Tregs induces Smad 2 phosphorylation exceeding that of soluble TGF-β. Utilization of an mTGF-β-dependent mechanism relies on effector and target cell contact and in feline CD8+ lymphocytes Smad 2 phosphorylation occurs within 2–4 h of TGF-βRII ligation.31 Therefore, a CD4+CD25+ to CD8+ ratio of 1:1 was selected to maximize effector to target cell contact and to coordinate Smad 2 phosphorylation. Vahlenkamp et al.20 have previously reported that Tregs from FIV+ cats inhibit IL-2 production in CD4+CD25– targets at relatively low CD4+CD25+ to CD4+CD25– ratios. However, at effector to target ratios of 1:1, they showed that Tregs from FIV-negative cats also suppressed IL-2 production in CD4+ targets. Figure 7b shows that approximately 24% of the CD4+CD25+ cells from the FIV– control cats are also mTGF-β+. Given the high CD4+CD25+ to CD8+ ratio and the results of Fig. 7b, it is not surprising that Fig. 8b demonstrates that CD4+CD25+ cells from FIV– cats can also induce Smad 2 phosphorylation. However, despite the induction of Smad 2 phosphorylation in FIV– cats, Fig. 9 shows that only activated Tregs from FIV+ but not FIV– control cats are capable of suppressing IFN-γ production in stimulated CD8+ target cells. Figure 9 also demonstrates that coculture of CD8+ cells with activated CD4+CD25+ Tregs from FIV+ cats results in more potent inhibition of CD8+ IFN-γ production than treatment with soluble TGF-β.
In conclusion, during the course of FIV infection, mTGF-β is upregulated on CD4+CD25+ Tregs, with these cells being found in LNs during the acute and chronic stage of infection. CD8+ lymphocytes exhibit an increased percentage of TGF-βRII in both the blood and LN when compared to FIV– cats. It is likely that once in the LN CD8+ lymphocytes interact with CD4+CD25+ lymphocytes in a TGF-β-dependent fashion. In support of this, TGF-β-dependent Smad 2 phosphorylation in CD8+ lymphocytes was evident following CD4+CD25+ coculture. We have also shown that CD8+ IFN-γ production is inhibited following CD4+CD25+ coculture in FIV+ cats but not in FIV– controls. Taken together, these results indicate that in FIV lentiviral infection, CD4+CD25+ Tregs suppress CD8+ responses during both the acute and chronic stages of infection and that CD4+CD25+ Tregs utilize a membrane TGF-β-dependent mechanism to suppress IFN-γ in CD8+ lymphocytes.
Acknowledgments
This work was funded in part by National Institute of Health Grants AI080288 (M.B.T.), AI058691 (W.A.T.), 1K08A1073102 (A.M.M.), and 1K08AI074445 (J.E.F.). The authors would like to thank Deb Anderson, Janet Dow, and Lawana Hartsell for their excellent technical assistance and Dr. M. Correa for assistance with the statistical analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tompkins MB. Tompkins WA. Lentivirus-induced immune dysregulation. Vet Immunol Immunopathol. 2008;123:45. doi: 10.1016/j.vetimm.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazenberg MD. Otto SA. van Benthem BH. Roos MT, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 3.Davidson MG. Rottman J. English RV. Lappin MR, et al. Feline immunodeficiency virus predisposes cats to acute generalized toxoplasmosis. Am J Pathol. 1993:143–1486. [PMC free article] [PubMed] [Google Scholar]
- 4.Dean GA. Bernales JA. Pedersen NC. Effect of feline immunodeficiency virus on cytokine response to Listeria monocytogenes in vivo. Vet Immunol Immunopathol. 1998;65:125. doi: 10.1016/s0165-2427(98)00148-2. [DOI] [PubMed] [Google Scholar]
- 5.Burkhard MJ. Dean GA. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr HIV Res. 2003;1:15. doi: 10.2174/1570162033352101. [DOI] [PubMed] [Google Scholar]
- 6.Lopez M. Soriano V. Lozano S. Ballesteros C, et al. No major differences in the functional profile of HIV Gag and Nef-specific CD8 + responses between long-term nonprogressors and typical progressors. AIDS Res Hum Retroviruses. 2008;24:1185. doi: 10.1089/aid.2008.0006. [DOI] [PubMed] [Google Scholar]
- 7.Jagannathan P. Osborne CM. Royce C. Manion MM, et al. Comparisons of CD8 + T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J Virol. 2009;83:2728. doi: 10.1128/JVI.02128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz JE. Kuroda MJ. Santra S. Sasseville VG, et al. Control of viremia in simian immunodeficiency virus infection by CD8 + lymphocytes. Science. 1999;283:857. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 9.Bucci J. English R. Jordan H. Childers T, et al. Mucosally transmitted feline immunodeficiency virus induces a CD8 + antiviral response that correlates with reduction of cell-associated virus. J Infect Dis. 1998;177:18. doi: 10.1086/513822. [DOI] [PubMed] [Google Scholar]
- 10.Tompkins WA. Mexas AM. Fogle JE. In Regulatory T Cells and Clinical Application. Springer; New York: 2008. CD4+CD25+ regulatory T cells in viral infections. [Google Scholar]
- 11.Suvas S. Azkur AK. Kim BS. Kumaraguru U, et al. CD4 + CD25 + regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 12.Suvas S. Kumaraguru U. Pack CD. Lee S, et al. CD4 + CD25 + T cells regulate virus-specific primary and memory CD8 + T cell responses. J Exp Med. 2003;198:889. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumforth KR. Birgersdotter A. Reynolds GM. Wei W, et al. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin's lymphoma cells mediates up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173:195. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson SJ. Messer RJ. Carmody AB. Hasenkrug KJ. In vitro suppression of CD8 + T cell function by Friend virus-induced regulatory T cells. J Immunol. 2006;176:3342. doi: 10.4049/jimmunol.176.6.3342. [DOI] [PubMed] [Google Scholar]
- 15.Boettler T. Spangenberg HC. Neumann-Haefelin C. Panther E, et al. T cells with a CD4 + CD25 + regulatory phenotype suppress in vitro proliferation of virus-specific CD8 + T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benito JM. Lopez M. Soriano V. The role of CD8 + T-cell response in HIV infection. AIDS Rev. 2004;6:79. [PubMed] [Google Scholar]
- 17.Collins KL. How HIV evades CTL recognition. Curr HIV Res. 2003;1:31. doi: 10.2174/1570162033352138. [DOI] [PubMed] [Google Scholar]
- 18.Kinter AL. Hennessey M. Bell A. Kern S, et al. CD25 + CD4 + regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4 + and CD8 + HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinter AL. Horak R. Sion M. Riggin L, et al. CD25 + regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8 + T cells in vitro. AIDS Res Hum Retroviruses. 2007;23:438. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]
- 20.Vahlenkamp T. Tompkins M. Tompkins W. Feline immunodeficiency virus (FIV) infection phenotypically and functionally activates immunosuppressive CD4 + CD25 + T regulatory (Treg) cells. J Immunol. 2004;172:4752. doi: 10.4049/jimmunol.172.8.4752. [DOI] [PubMed] [Google Scholar]
- 21.Mexas AM. Fogle JE. Tompkins WA. Tompkins MB. CD4+CD25+ Regulatory T cells are infected and activated during acute FIV infection. Vet Immunol Immunopathol. 2008;126:263. doi: 10.1016/j.vetimm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi A. Vahlenkamp TW. Garg H. Tompkins WA, et al. Preferential replication of FIV in activated CD4(+)CD25(+) T cells independent of cellular proliferation. Virology. 2004;321:307. doi: 10.1016/j.virol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 23.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 24.Gondek DC. Lu LF. Quezada SA. Sakaguchi S, et al. Cutting edge: Contact-mediated suppression by CD4 + CD25 + regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 25.Grossman WJ, et al. Human T regulatory cells can use the perforin pathway to cause autologoous target cell death. Immunity. 2004;21:589. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Read S. Greenwald R. Izcue A. Robinson N, et al. Blockade of CTLA-4 on CD4 + CD25 + regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedline RH. Brown DS. Nguyen H. Kornfeld H, et al. CD4 + regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataoka H. Takahashi S. Takase K. Yamasaki S, et al. CD25(+)CD4(+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int Immunol. 2005;17:421. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 29.Taylor PA. Noelle RJ. Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paust S. McCarty N. Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101:10398. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura K. Kitani A. Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annunziato F. Cosmi L. Liotta F. Lazzeri E, et al. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196:379. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oida T. Zhang X. Goto M. Hachimura S, et al. CD4 + CD25- T cells that express latency-associated peptide on the surface suppress CD4 + CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 34.Gil-Guerrero L, et al. In vitro and in vivo down-regulation of regulatory T cell activity with a peptide inhibitor of TGF-B1. J Immunol. 2008;181:126. doi: 10.4049/jimmunol.181.1.126. [DOI] [PubMed] [Google Scholar]
- 35.Piccirillo CA. Letterio JJ. Thornton AM. McHugh RS, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kullberg MC. Hay V. Cheever AW. Mamura M, et al. TGF-beta1 production by CD4 + CD25 + regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol. 2005;35:2886. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 37.Petty CS. Tompkins MB. Tompkins WA. Transforming growth factor-beta/transforming growth factor-betaRII signaling may regulate CD4 + CD25 + T-regulatory cell homeostasis and suppressor function in feline AIDS lentivirus infection. J Acquir Immune Defic Syndr. 2008;47:148. doi: 10.1097/QAI.0b013e318160df70. [DOI] [PubMed] [Google Scholar]
- 38.Lucas PJ. Kim SJ. Melby SJ. Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor. J Exp Med. 2000;191:1187. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen ML. Pittet MJ. Gorelik L. Flavell RA, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmadzadeh M. Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green EA. Gorelik L. McGregor CM. Tran EH, et al. CD4 + CD25 + T regulatory cells control anti-islet CD8 + T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.English RV. Johnson CM. Gebhard DH. Tompkins MB. In vivo lymphocyte tropism of feline immunodeficiency virus. J Virol. 1993;67:5175. doi: 10.1128/jvi.67.9.5175-5186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.English RV. Nelson P. Johnson CM. Nasisse M, et al. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J Infect Dis. 1994;170:543. doi: 10.1093/infdis/170.3.543. [DOI] [PubMed] [Google Scholar]
- 44.Smithberg SR. Fogle JE. Mexas AM. Reckling SK, et al. In vivo depletion of CD4 + CD25 + regulatory T cells in cats. J Immunol Methods. 2008;329:81. doi: 10.1016/j.jim.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tompkins MB. Gebhard DH. Bingham HR. Hamilton MJ, et al. Characterization of monoclonal antibodies to feline T lymphocytes and their use in the analysis of lymphocyte tissue distribution in the cat. Vet Immunol Immunopathol. 1990;26:305. doi: 10.1016/0165-2427(90)90115-9. [DOI] [PubMed] [Google Scholar]
- 46.Winer J. Jung CK. Shackel I. Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 47.Bendinelli M. Pistello M. Lombardi S. Poli A, et al. Feline immunodeficiency virus: An interesting model for AIDS studies and an important cat pathogen. Clin Microbiol Rev. 1995;8:87. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horton H. Russell N. Moore E. Frank I, et al. Correlation between interferon- gamma secretion and cytotoxicity, in virus-specific memory T cells. J Infect Dis. 2004;190:1692. doi: 10.1086/424490. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura K. Kitani A. Fuss I. Pedersen A, et al. TGF-beta 1 plays an important role in the mechanism of CD4 + CD25 + regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 50.Feng XH. Derynck R. Specificity and versatility in TGF-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 51.Rowland-Jones SL. Nixon DF. Aldhous MC. Gotch F, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 52.De Maria A, et al. Occurrence of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T cell activity in apparently uninfected childeren born to HIV-1-infected mothers. J Infect Dis. 1994;170:1296. doi: 10.1093/infdis/170.5.1296. [DOI] [PubMed] [Google Scholar]
- 53.Bucci J. Gebhard D. Childers T. English R, et al. The CD8 + phenotype mediating antiviral activity in FIV-infected cats is characterized by reduced surface expression of the CD8 beta chain. J Infect Dis. 1998;178:968. doi: 10.1086/515699. [DOI] [PubMed] [Google Scholar]
- 54.Borrow P. Lewicki H. Hahn BH. Shaw GM, et al. Virus-specific CD8 + cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koup R. Safrit J. Cao Y. Andrews C, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitz JE. Simon MA. Kuroda MJ. Lifton MA, et al. A nonhuman primate model for the selective elimination of CD8 + lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol. 1999;154:1923. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eggena MP. Barugahare B. Jones N. Okello M, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 58.Cumont MC. Diop O. Vaslin B. Elbim C, et al. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Virol. 2008;82:1175. doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joshi A. Garg H. Tompkins MB. Tompkins WA. Preferential feline immunodeficiency virus (FIV) infection of CD4 + CD25 + T-regulatory cells correlates both with surface expression of CXCR4 and activation of FIV long terminal repeat binding cellular transcriptional factors. J Virol. 2005;79:4965. doi: 10.1128/JVI.79.8.4965-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez MA. Puttur FK. Wang YM. Howden W, et al. T regulatory cells contribute to the attenuated primary CD8 + and CD4 + T cell responses to herpes simplex virus type 2 in neonatal mice. J Immunol. 2008;180:1556. doi: 10.4049/jimmunol.180.3.1556. [DOI] [PubMed] [Google Scholar]
- 61.Emani S. In Immunology. North Carolina State University; Raleigh, NC: 2006. Molecular characterization of T regulatory cells in FIV infection. [Google Scholar]
- 62.Yang GX. Lian ZX. Chuang YH. Moritoki Y, et al. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leveen P. Carlsen M. Makowska A. Oddsson S, et al. TGF-beta type II receptor-deficient thymocytes develop normally but demonstrate increased CD8 + proliferation in vivo. Blood. 2005;106:4234. doi: 10.1182/blood-2005-05-1871. [DOI] [PubMed] [Google Scholar]
- 64.Ocana-Morgner C. Wong KA. Lega F. Dotor J, et al. Role of TGF-beta and PGE2 in T cell responses during Plasmodium yoelii infection. Eur J Immunol. 2007;37:1562. doi: 10.1002/eji.200737068. [DOI] [PubMed] [Google Scholar]
- 65.Scott-Algara D. Buseyne F. Porrot F. Corre B, et al. Not all tetramer binding CD8 + T cells can produce cytokines and chemokines involved in the effector functions of virus-specific CD8 + T lymphocytes in HIV-1 infected children. J Clin Immunol. 2005;25:57. doi: 10.1007/s10875-005-0358-3. [DOI] [PubMed] [Google Scholar]
- 66.Arrode G. Finke JS. Zebroski H. Siegal FP, et al. CD8 + T cells from most HIV-1-infected patients, even when challenged with mature dendritic cells, lack functional recall memory to HIV gag but not other viruses. Eur J Immunol. 2005;35:159. doi: 10.1002/eji.200425744. [DOI] [PubMed] [Google Scholar]
- 67.Thomas DA. Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Moustakas A. Pardali K. Gaal A. Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]