Abstract
Objective
Guanfacine has been shown to reduce hyperactive behaviors in children with attention-deficit/hyperactivity disorder (ADHD) and possibly in children with pervasive developmental disorder (PDD) and hyperactivity. The aim of this exploratory study was to examine whether gene variants encoding the multidrug resistance protein (MDR1 or ABCB1) , a drug transporter at the blood–brain barrier, are associated with variability in the efficacy of guanfacine in children with PDD and hyperactivity.
Methods
Children with PDD who participated in an 8-week open-label trial of guanfacine were genotyped for the C3435T single-nucleotide polymorphism (SNP) variant of the MDR1 gene, a variant reported to alter function of the transporter. The decrease from baseline to 8 weeks in parent-rated Aberrant Behavior Checklist (ABC) hyperactivity and Swanson, Nolan, and Pelham (SNAP) scores were analyzed by MDR1 genotype. Response was compared between subjects homozygous for the minor allele T of the C34535T MDR1 variant (T/T) versus other genotypes (C/T and C/C).
Results
Disruptive behavior decreased during guanfacine treatment as assessed by several end points in the 25 enrolled children (23 boys and 2 girls). Genotype data were available from 22 children. Subjects with either C/T or C/C (n = 16) genotypes showed a three-fold greater improvement than T/T MDR1 C3435T genotype (n = 6) (mean decrease of 15.1 ± 12.6, or 50.7% from baseline, versus 4.5 ± 5.1, or 15.6% from baseline) in parent-rated ABC Hyperactivity scores over 8 weeks (p = 0.03). Parent-rated ADHD SNAP scores also differed by genotype (p = 0.05).
Conclusions
Gene variants in MDR1 may influence guanfacine response on hyperactive-impulsive behaviors via altered membrane transport. If replicated in larger samples, additional studies would be important to clarify the mechanisms underlying this effect and to determine its clinical significance.
Introduction
An emerging body of research data suggests that guanfacine, an α2A adrenergic agonist, is efficacious in reducing disruptive behaviors seen in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) and other groups of children with high levels of ADHD symptoms (Scahill et al. 2001; Newcorn et al. 2003; Pliszka et al. 2006; Biederman et al. 2008a). Hyperactivity and disruptive behaviors in individuals with pervasive developmental disorders (PDDs) are common targets for intervention, with as many as 40% of children with PDDs shown to display impairing levels of such symptoms (Lecavalier et al. 2006). Pharmacotherapeutic approaches have been considered useful in comprehensive treatment programs for PDD, and, among many options, α agonists have gained popularity in community practice (Rush and Francis 2000). Preliminary evidence supporting such use has emerged from a recently completed open-label prospective trial (Scahill et al. 2006) and from prior reports (Jaselskis et al. 1992).
This open trial conducted by the Research Units on Pediatric Psychopharmacology (RUPP) Autism Network showed solid reductions in parent- and teacher reported hyperactive-impulsive behaviors in children unresponsive to previous trials of a stimulant. Symptom decreases from baseline were 25–36% by 8 weeks, depending on the source of ratings, and 49% of subjects were rated by study clinicians as clinically significantly improved on global measures (Scahill et al. 2006).
Despite the apparent promise of guanfacine for the treatment of hyperactive-impulsive behaviors in this population, prominent variability in treatment efficacy has been noted. In the Scahill et al. report (2006), the improvement from baseline in Swanson, Nolan, and Pelham (SNAP) total ADHD symptom scores ranged from −1 to 41 points (mean 12.8). Similarly, in a large chart review study of children with PDD and other disorders receiving guanfacine, the rate of positive response ranged from 13% to 39% (average 23%), depending on the patient population (Posey et al. 2004). In a sample of ADHD children with co-occurring tic disorders, guanfacine was superior to placebo and reduced ADHD symptoms by 37% from baseline, but end-point ADHD ratings also showed large interindividual variability (Scahill et al. 2001). Another small placebo-controlled trial in children with tic disorders also showed evidence of interindividual variability in response (Cummings et al. 2003)
Pharmacogenetic studies attempt to reveal sources of such interindividual variation in drug response, often by examining genetic variation in drug targets, drug transporters, or regulatory enzymes as predictors of treatment response or adverse events. The family of drug transporters has been the focus of many recent studies in other areas of medicine; studies of drug transporters in psychopharmacology are relatively sparse. P-glycoprotein, also known as multidrug resistance protein (MDR1) or adenosine triphosphate (ATP)-binding cassette B1 (ABCB1), is a transporter across the blood–brain barrier of over 70 structurally varied types of drugs (Schinkel and Jonker 2003; Marzolini et al. 2004). MDR1 has been well characterized in terms of commonly occurring genetic variants and their relationship to MDR1 expression (Hoffmeyer et al. 2000; Wang et al. 2007), including a common synonymous single-nucleotide polymorphism (SNP), C3435T, which reportedly alters substrate specificity (Kimchi-Sarfaty 2007). The purpose of the present study was to perform an exploratory examination of the possible influence of MDR1 C3435T genotypes on the response of hyperactivity to guanfacine in the treatment of children with PDD. Outcome and safety data from this prospective open label trial have been published previously (Scahill et al. 2006).
Methods
This was a multisite, 8-week, prospective, open-label trial of guanfacine in children with PDD and high levels of hyperactivity and distractibility conducted by the RUPP Autism Network, which was funded by the National Institute of Mental Health (NIMH), and was approved by local site institutional review boards (IRBs) and a NIMH Data Safety Monitoring Board (DSMB). Eligible subjects were boys and girls between the ages of 5 and 14 years; Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) diagnosis of PDD (PDD–not otherwise specified [NOS], Asperger's disorder or autistic disorder) based on a DSM-IV clinical diagnosis and corroborated by the Autism Diagnostic Interview–Revised (Lord et al. 1997), accompanied by clinically significant symptoms of ADHD (i.e., impulsiveness and hyperactivity) as evidenced by a score of at least Moderate (≥4) on the Clinical Global Impressions–Severity (CGI-S) score for ADHD symptoms. Subjects also had to have an average score of 1.7 or greater on the parent-rated or teacher-rated Hyperactive-Impulsive items of the SNAP-IV (Swanson et al. 2001; http://adhd.net). Subjects also were required to show an inadequate response or intolerance to methylphenidate (MPH). Other entry criteria required a mental age of at least 18 months by psychometric testing.
Following a detailed screening that included medical, psychiatric, and developmental assessments, eligible subjects were seen weekly for the first 4 weeks to evaluate response and tolerability and then every other week until week 8. Visits included: Vital signs, height and weight, and a systematic review of adverse events. Outcome ratings were collected at baseline, and weeks 2, 4, 6, and 8 of treatment.
Measures
Outcome measures were the parent-rated Hyperactivity subscale of the Aberrant Behavior Checklist (ABC) and the Clinical Global Impressions–Improvement scale (CGI-I). The Hyperactivity subscale of the ABC contains 16-items that reflect hyperactivity and impulsive behavior (Aman et al. 1985). It was designed to measure change in treatment studies and has been normed in developmentally disabled populations (Marshburn et al. 1992; Brown et al. 2002). Higher scores reflect greater symptom severity. Other important outcomes included the teacher-rated Hyperactivity subscale of the ABC and the parent- and teacher-rated SNAP-IV. The SNAP-IV is an 18-item scale based on the DSM-IV symptoms of ADHD (Swanson et al. 2001) and has been employed in many clinical trials in typically developing children with ADHD (The MTA Cooperative Group 1999), but less commonly in children with PDD.
Medication
The medication regimen was determined by body weight. Children weighing below 25 kg started with 0.25 mg at bedtime, and increased to 0.25 mg twice a day (b.i.d.) on day 4. Thereafter, dosage increases were made in 0.25-mg increments, approximately every fourth day as tolerated, to a maximum of 3.5 mg per day, given on a three times a day (t.i.d.) schedule (e.g., 8 am, 2 pm, and 8 pm). For children weighing ≥25 kg, the guanfacine dose schedule was similar, but starting with 0.5 mg at night and increases in 0.5-mg increments. The maximum dose for these children was 5.0 mg per day on a t.i.d. schedule. There were no planned dose increases after week 5. Medication decreases to manage adverse effects were permitted at any time.
Genomic DNA was extracted from blood samples using a commercially available protocol (Qiagen). MDR1 was genotyped for the C3435T SNP polymorphism using the polymerase chain reaction (PCR) according to a published protocol (Hoffmeyer et al. 2000). A genotype by Intent-to-Treat design was used to analyze the data. Last observation carried forward (LOCF) was used to complete missing data from week 2 onward. Subjects without week 2 outcome data were not included in the analysis. The primary analysis of the effect of genotype was performed using PROC GLM in SAS 9.1.3. An analysis of covariance (ANCOVA) model was used to predict drop in the ABC–Hyperactivity subscale or total SNAP (Inattentive plus Hyperactive-Impulsive items) scores from week 0 to week 8 using the MDR1 genotype as the independent variable while controlling for baseline ABC or SNAP scores.
Results
Twenty-seven subjects met eligibility criteria at baseline; the parents of 25 subjects provided permission to participate (mean age = 9.03 ± 3.14 years). The sample included 92% boys (N = 23); 72% (n = 18) were Caucasian, 24% (n = 6) were African American, and 4% (n = 1) was Hispanic. Five subjects withdrew prior to completion due to lack of efficacy (n = 2) or emotional lability (n = 3). Blood draws for genotyping were successful for 22 subjects, of which 18 completed the 8-week protocol. There were no significant differences in baseline ADHD ratings between those subjects who were not genotyped and those who provided DNA.
Overall parent ratings showed declines in hyperactive-impulsive symptoms during the 8 weeks (see Table 1). Parent-rated Hyperactivity on the ABC–Hyperactivity scale declined 58% from 29.5 ± 8.5 at baseline to 17.3 ± 9.4 at end point. On the parent-rated SNAP, mean scores declined 56% from 37.0 ± 7.8 at baseline to 21.8 ± 9.5 at end point.
Table 1.
ABC and SNAP Scores at Baseline and 8 Weeks (End Point)
| Subscale | MDR1genotype | Baseline (SD) | Endpoint (SD) | Change score | % Change | ANOVA F-test (controlling for baseline score) | p value |
|---|---|---|---|---|---|---|---|
| ABC Hyperactivity | C/C or T/C* | 29.8 (8.9) | 14.7 (8.7) | 15.1 | 50.7 | 6.63 | 0.03 |
| T/T** | 28.8 (8.2) | 24.3 (7.8) | 4.5 | 15.6 | |||
| SNAP Total | C/C or T/C* | 37.6 (9) | 19.6 (9.9) | 18 | 47.9 | 4.45 | 0.05 |
| T/T** | 35.5 (3.5) | 27.7 (5) | 7.8 | 22.0 | |||
| SNAP Inattention | C/C or T/C* | 20.5 (4.7) | 11.6 (6.7) | 8.9 | 43.4 | 3.18 | 0.09 |
| T/T** | 19.2 (4) | 15.3 (2) | 3.9 | 20.3 | |||
| SNAP Hyperactivity | C/C or T/C* | 15.9 (5.2) | 8.1 (4.2) | 7.8 | 49.1 | 3.66 | 0.08 |
| T/T** | 15.3 (4.8) | 12.3 (5) | 3 | 19.6 |
n = 16.
n = 6.
Abbreviations: SD = Standard deviation; ANOVA = analysis of variance; ABC = Aberrant Behavior Checklist; SNAP = Swanson, Nolan, and Pelham.
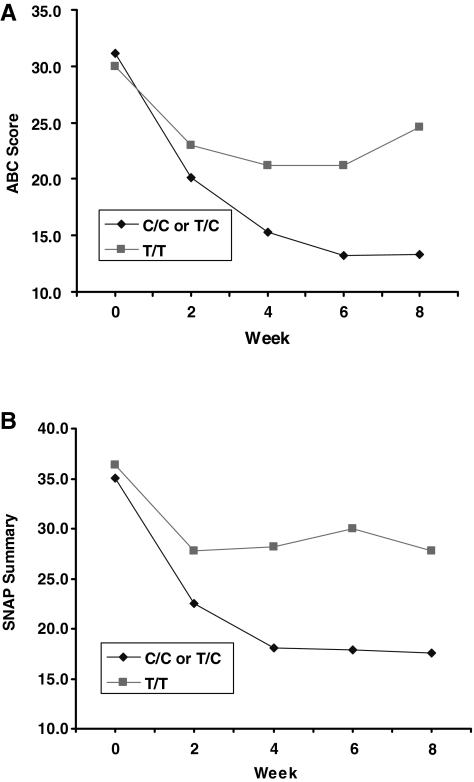
Because of prior data showing most pronounced effects of gene variants on gene expression for the T/T homozygotic condition (Kimchi-Sarfaty et al. 2007), we contrasted the change across baseline to 8 weeks using all outcome points for hyperactive-impulsive symptoms for two groups, the C/C and C/T versus the T/T subjects. Allele frequencies were 55% for the C and 45% for the T allele, respectively; the allele frequencies observed were comparable to other reports (Maeda and Sugiyama 2008). Genotypes were grouped as C/C or T/C (n = 16) versus T/T (n = 6). MDR1 genotype frequencies were in Hardy–Weinberg equilibrium; χ2 = 2.72, p = 0.10. Genotype groups did not differ in study completion rates, age, race, gender, final dose, or SNAP or ABC–Hyperactivity baseline symptom ratings (see Table 2), but the T/T genotype showed a significantly smaller drop in the SNAP and ABC–Hyperactivity summary score versus the C/C and C/T subjects (mean decrease of 22.5% and 16.2% versus 47.9% and 50.0%;),; SNAP summary and ABC–Hyperactivity, respectively) (Fig. 1). The amount of variation in drop of ABC–Hyperactivity and SNAP summary scores over 8 weeks that can be attributed to the MDR1 genotype was 53% and 40%, respectively. Effect sizes for guanfacine on SNAP total scores calculated from pre- versus posttreatment differences divided by average standard deviations (SDs) for the C/C and C/T group was 1.73 versus 0.54 for the T/T subjects. Similarly, effect sizes for guanfacine on ABC parent-rated Hyperactivity were 1.48 versus 0.31 for C/C and C/T versus T/T groups, respectively.
Table 2.
Clinical Characteristics By Genotype Groups
| C/C | C/T | T/T | P value | |
|---|---|---|---|---|
| Age | 8.3 ± 1.1 | 8.1 ± 1.2 | 9.0 ± 1.3 | 0.89 |
| Race | 6 W, 3 B | 5 W, 1 B, 1 H | 4 W, 2 B | 0.59 |
| Sex | 9 M | 7 M | 5 M, 1 F | 0.24 |
| Final guanfacine dose | 1.58 ± 0.34 | 1.75 ± 0.38 | 1.71 ± 0.41 | 0.94 |
Abbreviations: W = white; B = black; H = Hispanic; M = male; F = female.
FIG. 1.
Clinical Characteristics By Genotype Groups
Discussion
This prospective, open-label trial in children with PDD accompanied by hyperactivity and impulsive behavior showed that, despite highly significant decreases of 41% (total SNAP scores) and 42% (ABC–Hyperactivity) in parent ratings of ADHD symptoms during guanfacine administration, exploratory analyses of MDR1 gene variants showed prominent differences in response by genotype. The magnitude of the difference between the two genotype groups was greater than 1 SD in the final week-8 ratings, indicative of a large influence on treatment response. This is one of few observations of the influence of MDR1 on psychotropic treatment response.
MDR1 is a member of the ABC family of membrane-bound drug transporter proteins that are present at the blood–brain barrier. Besides MDR1 (ABCB1), the group includes multidrug resistance (-associated) protein 1 (MRP1, ABCC1), MRP2 (ABCC2), MRP3 (ABCC3), MRP4 (ABCC4), MRP5 (ABCC5), and the breast cancer resistance protein (BRCP, ABCG2) (Wang et al. 2007). The substrates and inhibitors of each of these transporters vary widely, but psychotropic drugs have been found to bind to many of these proteins. In the case of the best-studied member of the family, MDR1, 29 SNPs have been identified. The MDR1 variant we chose to examine has previously been shown to influence gene expression (Hoffmeyer et al. 2000) and substrate specificity (Kimchi-Sarfaty et al. 2007). Indeed, one report described a reduction of nearly 50% in MDR1 protein concentration between the C/C versus the T/T genotype; however, not all reports have observed effects on expression (Sakaeda 2005). The effects of such reductions in MDR1 can be complex. Reduced MDR1 has been associated with increased brain concentrations of drugs known to be transported by MDR1; however, reduced MDR1 can also reduce absorption and increase renal excretion of some substrates, defying straightforward attempts to explain differences in clinical effects by genotype.
Our report should be viewed as exploratory, given the small sample composed only of individuals previously shown to be unresponsive to or intolerant to MPH, with a restricted age range, predominance of males and Caucasian subjects, a lack of treatment blinding, and short observation period. Data from much larger clinical trial samples are needed to confirm or refute this observation and to better estimate the magnitude of the difference, if any, associated with the MDR1 genotype. Another limitation is the absence at present of definitive in vitro data confirming that guanfacine is a major substrate for MDR1 transport. If confirmed that guanfacine transport is influenced by MDR1, it would also be critical to examine in a controlled system the precise impact of MDR1 gene variants on guanfacine absorption, distribution, blood–brain levels, and excretion. Our ability to examine MDR1 genotype effects on adverse events is also limited in this pilot study. However, a large clinical trial sample could examine whether more commonly observed adverse events, such as sedation or mood liability, adverse events that we observed often mandated dose reduction or predicted intolerance, may also be associated with the MDR1 genotype. Such observations hold obvious potential clinical importance if applicable at the level of the individual patient.
Clinically, additional trials are needed to determine the benefits and safety of the α agonists as common treatments for pediatric neuropsychiatric disorders, especially in individuals with PDD with ADHD symptoms. More data are needed on the durability of benefits, as some reports suggest waning effects over time (Jaselskis et al. 1992), effects of sedation and fatigue on adherence (Biederman et al. 2008b), and to confirm the apparent acceptable cardiovascular safety profile from larger, recent reports (Biederman et al. 2008a; Daviss et al. 2008). As clinical support for guanfacine is growing, given the modest benefits of stimulants in subgroups of children such as PDD (Aman et al. 2003; Research Units on Pediatric Psychopharmacology [RUPP] Autism Network 2005), it is important to attempt to identify possible predictors of treatment benefit. Accumulated findings from initial guanfacine trials in children for the management of ADHD behaviors have varied, perhaps due to differences in clinical populations sampled. Although our data support MDR1 gene variants as significant moderators to guanfacine response, there yet may be other important variants in other genes that further contribute to variability in guanfacine response. Examination of other relevant target genes thought to play a role in guanfacine distribution, neurochemical effect, or metabolism, including α2A (ADRA2A), other noradrenergic system genes, CYP450 genes, and others could well reveal more robust genetic influences on clinical response to guanfacine.
In conclusion, the results of this study suggest that gene variants of MDR1 may serve as a potent moderator of the benefits of guanfacine as a treatment for hyperactivity in children with PDD who do not show improvement with MPH. Besides encouraging efforts for replication, the results should also stimulate a greater awareness in child psychopharmacology of the potential importance of the family of drug transporters as key components in drug distribution and clinical effect.
Footnotes
This research was supported by contracts from the National Institute of Mental Health (N01MH70010 to Dr. McCracken; N01MH70001 to Dr. McDougle; N01MH70009 to Dr. Scahill; N01MH80011 to Dr. Aman); National Institute of Mental Health (NIMH) grant MH01805 to Dr. McCracken, and General Clinical Research Center grants from the National Institutes of Health (M01 RR00750, to Indiana University; M01 RR06022, to Yale University; M01 RR00034, to Ohio State University; and M01 RR00052, to Johns Hopkins University); and the Korczak Foundation to Dr. Scahill. We also acknowledge the efforts of Mark Davies, M.P.H., Shirley Chuang, Ph.D., James Robinson, M.E.D., and Don McMahon, M.S., for statistical consultation and data management.
Statistical analyses were performed by Fiona Whelan, M.S.
Disclosures
Dr. McCracken has affiliations with Shire, Eli Lilly, Bristol Myers Squibb, Wyeth, Pfizer, and Aspect. Dr. Scahill has affiliations with Janssen Pharmaceutica, Neuropharm, Supernus, and Bristol-Myers Squibb. Dr. Aman has affiliations with Janssen Pharmaceutica, Eli Lilly, Forest Labs, and Abbott. Dr. McDougle has affiliations with AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen Pharmaceutica, PediaMed Pharmaceuticals, and Pfizer, Inc. Dr. Arnold has affiliations with Lilly, McNeil, Novartis, and Shire. Dr. Posey has affiliations with Forest and Lilly. Drs. Tierney and Vitiello and Ms. Shiraga, Ms. Whelan, and Ms. Ritz have no conflicts of interest or financial ties to disclose.
References
- Aman MG. Singh NN. Stewart AW. Field CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Deficiency. 1985;89:485–491. [PubMed] [Google Scholar]
- Aman MG. Buican B. Arnold LE. Methylphenidate treatment in children with borderline IQ and mental retardation: Analysis of three aggregated studies. J Child Adoles Psychopharmacol. 2003;13:27–38. doi: 10.1089/104454603321666171. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. (DSM-IV). [Google Scholar]
- Biederman J. Melmed RD. Patel A. McBurnett K. Konow J. Lyne A. Scherer N. SPD503 Study Group: A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008a;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- Biederman J. Melmed RD. Patel A. McBurnett K. Donahue J. Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr. 2008b;13:1047–1055. doi: 10.1017/s1092852900017107. [DOI] [PubMed] [Google Scholar]
- Brown EC. Aman MG. Havercamp SM. Factor analysis and norms for parent ratings on the Aberrant Behavior Checklist—Community for young people in special education. Res Develop Disabil. 2002;23:45–60. doi: 10.1016/s0891-4222(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Cummings DD. Singer HS. Krieger M. Miller TL. Mahone EM. Neuropsychiatric effects of guanfacine in children with mild Tourette syndrome: A pilot study. Clin Neuropharmacol. 2002;25:325–332. doi: 10.1097/00002826-200211000-00009. [DOI] [PubMed] [Google Scholar]
- Daviss WB. Patel NC. Robb AS. McDermott MP. Bukstein OG. Pelham WE. Palumbo D. Harris P. Sallee FR The CAT STUDY TEAM. Clonidine for attention-deficit/hyperactivity disorder: ECG changes, adverse events analysis. J Am Acad Child Adolesc Psychiatry. 2008;47(2):189–198. doi: 10.1097/chi.0b013e31815d9ae4. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S. Burk O. von Richter O. Arnold HP. Brockmöller J. Johne A. Cascorbi I. Gerloff T. Roots I. Eichelbaum M. Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaselskis CA. Cook EH., Jr Fletcher KE. Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autism. J Clin Psychopharmacol. 1992;12:322–327. [PubMed] [Google Scholar]
- Kimchi-Sarfaty C. Oh JM. Kim I-W. Sauna ZE. Calcagno AM. Ambudkar SV. Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- Lecavalier L. Behavior and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. J Autism Develop Disord. 2006;36:1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Lord C. Pickles A. McLennan J. Rutter M. Bregman J. Folstein S. Fombonne E. Leboyer M. Minshew N. Diagnosing autism: Analyses of data from the Autism Diagnostic Interview. J Autism Develop Disord. 1997;27:501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- Maeda K. Sugiyama Y. Impact of genetic polymorphisms of transporters on the pharmacokinetic, pharmacodynamic, and toxicological properties of anionic drugs. Drug Metab Pharmacokinet. 2008;23:223–235. doi: 10.2133/dmpk.23.223. [DOI] [PubMed] [Google Scholar]
- Marshburn EC. Aman MG. Factor validity and norms for the Aberrant Behavior Checklist in a community sample of children with mental retardation. J Autism Develop Disord. 1992;22:357–373. doi: 10.1007/BF01048240. [DOI] [PubMed] [Google Scholar]
- Marzolini C. Paus E. Buclin T. Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): Recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Newcorn JH. Schulz KP. Halperin JM. Charney DS. Leckman JF. Adrenergic agonists: Clonidine, guanfacine. In: Martin A, editor; Scahill L, editor; Pediatric Psychopharmacology: Principles and Practice. New York: Oxford; 2003. pp. 264–273. [Google Scholar]
- Posey DJ. Puntney JI. Sasher TM. Kem DL. McDougle CJ. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: A retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol. 2004;14:233–241. doi: 10.1089/1044546041649084. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology (RUPP) Autism Network: Methylphenidate in children with PDD and hyperactivity Arch Gen Psychiatry 621266–1274.2005. 16275814 [Google Scholar]
- Rush AJ. Francis A. Expert Consensus Guideline Series: Treatment of psychiatric and behavioral problems in mental retardation. (Special issue.) Am J Mental Retardat. 2000;105:159–228. [PubMed] [Google Scholar]
- Sakaeda T. MDR1 genotype-related pharmacokinetics: Fact or fiction? Drug Metab Pharmacokinet. 2005;20:391–414. doi: 10.2133/dmpk.20.391. [DOI] [PubMed] [Google Scholar]
- Scahill L. Chappell PB. Kim YS. Schultz RT. Katsovich L. Shepherd E. Arnsten AFT. Cohen DJ. Leckman JF. Guanfacine in the treatment of children with tic disorders and ADHD: A placebo-controlled study. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Scahill L. Aman MG. McDougle CJ. McCracken JT. Tierney E. Dziura J. Arnold LE. Posey D. Young C. Shah B. Ghuman J. Ritz L. Vitiello B. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16:589–598. doi: 10.1089/cap.2006.16.589. [DOI] [PubMed] [Google Scholar]
- Schinkel AH. Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv Drug Deliv Rev. 2003;21(55):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Kraemer HC. Hinshaw SP. Arnold LE. Conners CK. Abikoff HB. Clevenger W. Davies M. Elliott G. Greenhill LL. Hechtman L. Hoza B. Jensen PS. March JS. Newcorn JH. Owens L. Pelham WE. Schiller E. Severe J. Simpson S. Vitiello B. Wells CK. Wigal T. Wu M. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- The MTA Cooperative Group. A 14-Month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Wang JS. Newport DJ. Stowe ZN. Donovan JL. Pennell PB. DeVane CL. The emerging importance of transporter proteins in the psychopharmacological treatment of the pregnant patient. Drug Metab Rev. 2007;39:723–746. doi: 10.1080/03602530701690390. [DOI] [PubMed] [Google Scholar]