Abstract
Characterizing intraregional differences in current pediatric HIV care and treatment in Asia can guide the development of clinical practice guidelines and improve the understanding of local resource availability. The Therapeutics Research, Education, and AIDS Training in Asia (TREAT Asia) Pediatric Program is a collaboration of clinics and referral hospitals studying pediatric HIV outcomes in the region. A Web-based survey to characterize clinical management practices and monitoring resources was developed and distributed to 20 sites in January 2008. Seventeen (85%) sites from 6 countries responded through April 2008; 14 (82%) were hospital-based and 16 (94%) were public facilities. Of 4050 HIV-infected children under care, 3606 (89%) were on antiretroviral treatment; 80% were on their first mono-, dual-, or triple-drug regimen and 74% were on nevirapine- or efavirenz-based regimens. Fifteen (88%) sites had consistent access to polymerase chain reaction (PCR) testing for infant diagnosis. All sites had access to CD4 testing, with 13 (76%) routinely monitoring patients every 3–6 months; 7 (41%) sites monitored viral load at 6- to 12-month intervals. Although there is some variation in clinical practices, high levels of treatment and monitoring resources were available at these sites. The availability of PCR for early infant diagnosis positions them to implement recent WHO recommendations to treat HIV-infected children younger than 1 year of age. This information will be used to develop future research and programs to support children with HIV in Asia.
Introduction
In 2008, UNAIDS estimated that there were 140,000 children less than 15 years of age living with HIV in South and Southeast Asia.1 The region includes 20 low- to upper middle-income countries, in varying stages of their pediatric HIV epidemics. The relative social stability, economic development, and availability of health care providers make prevention and control of pediatric HIV in Asia a realistic goal. Many of these countries report initiating antiretroviral treatment (ART) in an increasing number of HIV-infected patients over the past few years. However, only a few of these countries have reported greater than 25% national ART coverage for either adults or children meeting treatment criteria or for antiretrovirals to prevent mother-to-child transmission (PMTCT) of HIV.1,2 Moreover, few countries in Asia have national pediatric surveillance data or participate in monitoring programs that follow HIV-exposed infants from birth through childhood.
More detailed regional surveillance data and understanding of clinical practices would help guide research and policies to better serve the needs of children and adolescents living with HIV and their families. The Therapeutics Research, Education, and AIDS Training in Asia (TREAT Asia) network was established by amfAR, The Foundation for AIDS Research, in 2001 to promote safe and effective HIV/AIDS treatment throughout Asia and the Pacific.3 The TREAT Asia Pediatric Program was later created in 2005 to provide the first platform from which pediatric HIV clinical providers and researchers in Asia could conduct regional-level observational research. Pediatric sites were recruited from the major clinical and research centers in developing countries, including Cambodia, China, India, Indonesia, Malaysia, Thailand, and Vietnam (Appendix). In recognition of the diversity of experience across the network, a detailed site survey was conducted to assess clinical resources, laboratory testing practices, and approaches to ART management.
Methods
In 2008, the TREAT Asia Pediatric Program involved 20 sites, including 15 clinical centers, 2 clinical research programs, 2 non-governmental organizations providing support to orphans with HIV, and 1 national program. Most are tertiary-care referral centers. The group is governed by a steering committee composed of principle investigators from each site, and representatives from a data management center (National Centre in HIV Epidemiology and Clinical Research [NCHECR], University of New South Wales, Australia) and a program management team (TREAT Asia).
An internal working group developed the survey instrument. It included 79 questions, which were divided into 4 sections: site description (31 questions), PMTCT (10 questions), clinical care and ART (16 questions), and laboratory testing (22 questions). The first antiretroviral regimen was defined as first antiretroviral exposure of any combination of drugs, and could include mono- or dual-therapy. The survey was available online or as an electronic soft-copy for sites with limited internet access. The survey was pilot tested in January 2008 before a final version was distributed. Each site's data were current as of the date they completed the survey. Institutional Review Board approval was not obtained, because this was considered an operational survey and did not involve accessing individual-level patient data. All sites provided aggregated information on the patients under their care at the time of survey submission. Survey data were exported into Microsoft Excel (Microsoft, Redmond, WA), and then into Stata 9.0 (StataCorp, College Station, TX) for further analysis at NCHECR.
Results
Clinical centers
Seventeen (85%) sites from 6 countries responded from January through April 2008, including Cambodia (1 site), India (1 site), Indonesia (1 site), Malaysia (4 sites), Thailand (6 sites), and Vietnam (4 sites). Of the 17 participating sites, 14 (82%) were located in an urban area, 16 (94%) were public or governmental facilities, and 7 (41%) were university-affiliated, hospital-based clinics. Among the 14 (82%) sites that were hospital-based, the median number of pediatric beds was 155 (interquartile range [IQR] 120, 311). Sites began providing HIV-specific care for children between 1990–2004, and ART between 1994–2006. The median number of outpatient clinic visits and admissions for HIV-related medical care in 2007 was 115 (IQR 55, 167) and 5 (IQR 1, 8) per month, respectively.
A total of 4050 HIV-infected children were under care. Of these, children were less than 5 years (n = 920; 23%), 5–9 years (n = 1579; 39%), 10–14 years (n = 1218; 30%), and 15–18 years (n = 331; 8%) of age. Each site followed a median of 209 (IQR 106, 264) patients, with a median of 26 (IQR 18, 86) less than 5 years, 85 (IQR 45, 106) 5–9 years, 29 (IQR 10, 113) 10–14 years, and 7 (IQR 1, 21) 15–18 years of age. Almost all sites (16 sites; 94%) provided Bacillus Calmette-Guérin vaccination after birth and before HIV diagnosis. Six (35%) sites had formalized HIV disclosure protocols.
Availability of PMTCT
Sites reported that for HIV-positive pregnant women who did not meet criteria for ART, the most commonly used maternal regimen in their local PMTCT programs was zidovudine (AZT) plus single dose-nevirapine (NVP) starting at 28 weeks gestation (7 sites; 41%). Sites reported directly providing the following interventions at no cost to patients: infant antiretroviral prophylaxis (13 sites; 76%), formula powder for replacement feeding (14 sites; 82%), and infant diagnostic testing (11 sites; 65%). Most of the sites used polymerase chain reaction (PCR) as a first diagnostic test, whether or not testing was freely provided (DNA PCR 13 sites, 76%; RNA PCR 2 sites, 12%), and usually performed the first test within 2 months of life (Table 1). All sites used enzyme-linked immunosorbent assay (ELISA) to diagnose HIV-exposed infants over 18 months of age.
Table 1.
PMTCT Management in HIV-Exposed Infants
| Variable | Number of sites (%) |
|---|---|
| PMTCT program components provided for free by sites | |
| Infant antiretrovirals | 13 (76) |
| Infant formula | 14 (82) |
| HIV-diagnostic testing | 11 (65) |
| Infant antiretroviral regimen | |
| AZT only | 6 (35) |
| AZT plus single-dose NVP | 9 (53) |
| Unknown | 2 (12) |
| Duration of postnatal infant antiretrovirals | |
| 1 week | 5 (29) |
| 4 weeks | 2 (12) |
| 6 weeks | 7 (41) |
| Unknown | 3 (18) |
| Type of infant feeding promoted by site | |
| Formula feeding only | 15 (88) |
| Maternal choice | 2 (12) |
| Diagnostic test for infants <9 months of age | |
| DNA PCR | 15 (88) |
| RNA PCR | 2 (12) |
| Diagnostic test for infants between 9–18 months of age | |
| DNA PCR | 10 (59) |
| RNA PCR | 2 (12) |
| ELISA | 5 (29) |
PMTCT, prevent mother-to-child transmission; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay.
ART
Fifteen sites from 4 countries reported that their countries had their own national pediatric HIV care and treatment guidelines. The most preferred first-line ART regimen for children less than 3 years was AZT, lamivudine (3TC), and NVP (11 sites; 65%); efavirenz (EFV)-based ART was often preferred in children older than 3 years (10 sites; 59%). At the time of data collection, 3 (18%) sites were treating all infants younger than 12 months, with other sites following immunologic and clinical criteria to determine treatment eligibility. Sites were following different guidelines for initiating treatment in HIV-infected children based on national guidelines (4 sites; 24%), World Health Organization (WHO) 2003 (2 sites: 12%), WHO 2006 (7 sites; 41%), or Centers for Disease Control and Prevention (CDC) 2006 (4 sites; 24%) guidelines. Free ART was provided by 94% of sites and 65% were using fixed-dose combination (FDC) tablets in their regimens.
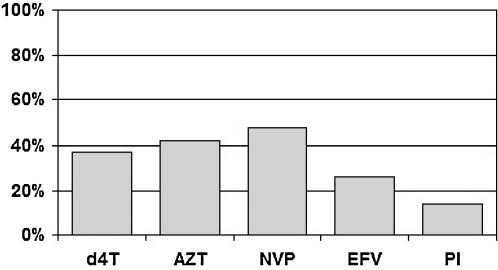
A total of 3606 (89%) of all patients had commenced ART throughout the participating sites; 2882 (80%) of these children were on their first antiretroviral regimen. There was a variety of ART regimens in use with 37% containing stavudine (d4T), and 48% containing NVP; 14% contained protease-inhibitors (Fig. 1).
FIG. 1.
Level of Access to Diagnostic and Monitoring tests
Monitoring testing
Sites usually had consistent access to CD4, DNA PCR, and RNA PCR testing, and 47% had limited or no access to resistance genotyping (Table 2). CD4 was monitored every 3–6 months at 76% of sites, and 59% monitored viral load at least every 12 months (Table 3). None of the sites performed genotyping before commencing ART. When available, genotyping was done to assess treatment failure and/or for research purposes.
Table 2.
Level of Access to Diagnostic and Monitoring tests
| |
Number of sites (%) |
||
|---|---|---|---|
| Test | All the time | Some of the time | Rarely or do not have access |
| Diagnostic test for HIV infection in children | |||
| DNA PCR | 13 (76) | 2 (12) | 2 (12) |
| ELISA method | 15 (88) | 2 (12) | 0 (0) |
| Western blot | 5 (29) | 7 (41) | 5 (29) |
| Laboratory test for monitoring antiretroviral therapy | |||
| CD4 number | 17 (100) | 0 (0) | 0 (0) |
| CD4 percentage | 15 (88) | 1 (6) | 1 (6) |
| RNA PCR | 14 (82) | 2 (12) | 1 (6) |
| HIV genotype | 8 (47) | 1 (6) | 8 (47) |
PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay.
Table 3.
Utilization of Monitoring Tests
| Variable | Number of sites (%) |
|---|---|
| Frequency of CD4 testing | |
| Baseline only | 1 (6) |
| Every 3–6 months | 13 (76) |
| Every 6–12 months | 3 (18) |
| Funding for CD4 testing | |
| Site or funder | 15 (88) |
| Both site and patient | 2 (12) |
| Frequency of viral load testing | |
| Every 3–6 months | 3 (18) |
| Every 6–12 months | 7 (41) |
| Every 12–24 months | 2 (12) |
| Assess treatment failure only | 2 (12) |
| Not routinely done | 3 (18) |
| Funding for viral load testing | |
| Site or funder | 15 (88) |
| Patient | 2 (12) |
| Indication for resistance genotyping | |
| Assessment of treatment failure only | 9 (53) |
| For research and assessment of treatment failure | 2 (12) |
| For research only | 1 (6) |
| Not done | 5 (29) |
| Funding for resistance genotyping | |
| Site or funder | 11 (65) |
| Both site and patient | 1 (6) |
Discussion
Experience with and resources for managing the pediatric HIV epidemic have lagged behind those for the adult epidemic. Problems encountered in expansion of pediatric HIV/AIDS treatment have included limited access to appropriate diagnostic testing for infants and young children, and the narrow range of available pediatric formulations or FDCs.2,4 Only the KIDS-ART-LINC group has previously published an assessment of regional-level ART accessibility and availability of resources for children in developing countries.5 The objective of this survey was to learn how clinical practices varied and what resources were available in TREAT Asia Pediatric Program sites. Although the sites participating in this survey were predominantly in urban settings and often university-affiliated, hospital-based clinics, these sites are commonly national referral centers that provide advice and direction for pediatric HIV care throughout the region. Consequently, the survey characterizes resources reflecting the highest levels of HIV care and treatment for children in many of these Asian countries.
Although sites were generally not involved with prenatal maternal PMTCT interventions, most provided infant antiretrovirals and diagnostic testing with PCR. Almost all sites responding to the survey promoted formula feeding to avoid breastfeeding-associated transmission. This is in contrast to multiple studies in sub-Saharan Africa that have demonstrated improved survival with exclusive breastfeeding.6–8 A number of Asian countries (e.g., Thailand, Vietnam, Malaysia) have made commitments to support formula provision, and acceptability among HIV-positive mothers is high.9–11 Although at the time of the survey few sites reported starting treatment for all HIV-infected infants, the availability of early infant diagnostic testing increases their ability to follow WHO recommendations, released after the survey was completed, to initiate ART in all HIV-infected infants less than 12 months of age whenever feasible.12
Even by early 2008, 89% of all children under care were already on ART. This is higher than the 73% on ART in the KIDS-ART-LINC cohort in 2007, but similar to the 91% of vertically infected children on ART in the NICHD International Site Development Initiative (NISDI) regional cohort in Latin American and the Caribbean in 2008.5,13 Reports of second-line ART use in sub-Saharan Africa have also been much lower than the 20% of children on their second or salvage regimen in the TREAT Asia cohort.13–15 This may be related to the early availability of antiretrovirals in some of the surveyed countries that led to the use of less durable mono- or dual-nucleoside reverse transcriptase inhibitor regimens.14,16,17 The increasing numbers of children in Asia developing treatment failure will require broader access to genotyping and pediatric protease inhibitors to help construct more complex ART regimens.17,18 In order to ensure that children with HIV can live into adulthood, public health programs need to plan for the procurement and delivery of pediatric second-line and salvage regimens.
Access to diagnostic and monitoring HIV tests was almost universal at the sites surveyed and largely supported through government funding. This allowed sites to more closely monitor children to determine when to start and when to switch regimens. The optimal intervals for CD4 and viral load testing remain unknown, but more frequent monitoring (i.e., at least every 6 months) could be beneficial for treatment failure monitoring. The median viral loads and age of children at the time of failure in two recent Thai studies were 5.3 log and 4.1 years in Bangkok, and 4.2 log and 7.6 years in northern Thailand, respectively.19,20 Delayed ART switching can lead to greater accumulation of resistance mutations,21 making access to regular viral load testing for early identification of virologic failure a priority for ensuring long-term durability of pediatric ART.
The survey was limited by the level of detail that could be collected, particularly with regards to the ART histories of children in the cohort. The level of clinical resources and laboratory capacity seen in this cohort also may not be generalizable to other clinical centers within each country represented. However, HIV care is centralized in some countries in the region because of the smaller numbers of children needing care and having fewer clinicians with pediatric HIV expertise. Participating sites also play an important leadership role in driving and developing the pediatric HIV care agenda in Asia.
This site survey provides the first assessment of the state of resource provision and pediatric HIV management practices at a number of tertiary-level pediatric sites providing HIV care in Asia. The results emphasize the importance of identifying optimal second-line regimens, and expanding pediatric antiretroviral and FDC options. Moreover, that 38% of children in this survey were 10–18 years old underscores the importance of preparing for managing social and treatment issues in adolescents. Future research through this regional cohort will explore ART outcomes and optimal monitoring strategies.
Appendix: The TREAT Asia Pediatric HIV Program, 2008
| B.V. Huy and N.V. Lam, National Hospital of Pediatrics, Hanoi, Vietnam. |
| D.A. Cooper, M.G. Law, J. Amin, and A. Kariminia, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia.a |
| F.J. Zhang and N. Han, Beijing Ditan Hospital, Beijing, China. |
| F.S. Moy and M. Thien, Hospital Likas, Kota Kinabalu, Malaysia. |
| G. Jourdain, PHPT, Chiangmai, Chiang Mai, Thailand. |
| J. Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia. |
| K. Chokephaibulkit and W. Prasitsuebsai, Siriraj Hospital, Mahidol University, Bangkok, Thailand. |
| A.H. Sohn, J. Smith, and J. Pang, The Foundation for AIDS Research, Bangkok, Thailand.a |
| K. Razali and N.F. Abdul Rahman, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia. |
| L.K. Do and L.N. Oanh, Worldwide Orphans Foundation (WWO), Ho Chi Minh City, Vietnam. |
| N. Kumarasamy and S. Saghayam, YRG Centre for AIDS Research and Education, Chennai, India. |
| N. Kurniati and H.I. Satari, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. |
| N.K. Nik Yusoff and L.C. Hai, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia. |
| P. Lumbiganon and P. Tharnprisan, Khon Kaen University, Khon Kaen, Thailand. |
| R. Hansudewechakul and A. Khongponoi, Chiang Rai Regional Hospital, Chiang Rai, Thailand. |
| R. Nallusamy and K.C. Chan, Penang Hospital, Penang, Malaysia. |
| S. Seitaboth and K. Nearyroth, Angkor Hospital for Children, Siem Reap Cambodia. |
| T. Puthanakit and T. Suwanlerk, HIV/NAT, Bangkok, Thailand. |
| T.H. Khanh and L.P. Kim Thoa, Children Hospital no.1, Ho Chi Minh City, Vietnam. |
| U. Vibol and S. Sophan, National Pediatric Hospital, Phnom Penh, Cambodia. |
| V. Saphonn and S. Saramony, National Centre for HIV/AIDS, Dermatology and STI (NCHADS), Social Health Clinic, Phnom Penh, Cambodia. |
| V. Sirisanthana and L. Aurpibul, Chiang Mai University, Pediatric Department, Chiang Mai, Thailand. |
Not participating in the survey.
Acknowledgments
The TREAT Asia Pediatric Program is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with major support provided by the Austrian AIDS Life Association (AALA), and with additional support from the following institutes and offices of the U.S. National Institutes of Health (NIH): National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), and the Office of the Director (OD), as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; grant no. U01AI069907). The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
The authors thank PharmAccess for their support in conducting the Web-based survey, and for freely providing their platform. We acknowledge all pediatric sites of The TREAT Asia Pediatric HIV Program. We also thank Dr. Kulkanya Chokephaibulkit for her detailed review of the survey questions and this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS. Report on the global HIV/AIDS epidemic 2008. 2008. UNAIDS.
- 2.Children and AIDS: Third Stocktaking Report, 2008. Geneva: UNICEF; 2009. [Google Scholar]
- 3.Zhou J. Kumarasamy N. Ditangco R, et al. The TREAT Asia HIV Observational Database: Baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38:174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohn AH. Ananworanich J. How can we simplify antiretroviral therapy in children? Curr Opin HIV AIDS. 2007;2:426–430. doi: 10.1097/COH.0b013e3282ced12c. [DOI] [PubMed] [Google Scholar]
- 5.Arrive E. Kyabayinze DJ. Marquis B, et al. Cohort profile: The paediatric antiretroviral treatment programmes in lower-income countries (KIDS-ART-LINC) collaboration. Int J Epidemiol. 2008;37:474–480. doi: 10.1093/ije/dym216. [DOI] [PubMed] [Google Scholar]
- 6.Kagaayi J. Gray RH. Brahmbhatt H, et al. Survival of infants born to HIV-positive mothers, by feeding modality, in Rakai, Uganda. PLoS One. 2008;3:e3877. doi: 10.1371/journal.pone.0003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rollins NC. Becquet R. Bland RM, et al. Infant feeding, HIV transmission and mortality at 18 months: The need for appropriate choices by mothers and prioritization within programmes. AIDS. 2008;22:2349–2357. doi: 10.1097/QAD.0b013e328312c740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thior I. Lockman S. Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: The Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 9.Jourdain G. Mary JY. Coeur SL, et al. Risk factors for in utero or intrapartum mother-to-child transmission of human immunodeficiency virus type 1 in Thailand. J Infect Dis. 2007;196:1629–1636. doi: 10.1086/522009. [DOI] [PubMed] [Google Scholar]
- 10.Sohn AH. Thanh TC. Thinh le Q, et al. Failure of human immunodeficiency virus enzyme immunoassay to rule out infection among polymerase chain reaction-negative Vietnamese infants at 12 months of age. Pediatr Infect Dis J. 2009;28:273–276. doi: 10.1097/INF.0b013e31818e03b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teeraratkul A. Simonds RJ. Asavapiriyanont S, et al. Evaluating programs to prevent mother-to-child HIV transmission in two large Bangkok hospitals, 1999–2001. J Acquir Immune Defic Syndr. 2005;38:208–212. doi: 10.1097/00126334-200502010-00013. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Report of the WHO Technical Reference Group. Paediatric HIV/ART Care Guideline Group Meeting WHO Headquarters; Geneva, Switzerland: 2008. April 10–11. [Google Scholar]
- 13.Hazra R. Stoszek SK. Freimanis Hance L, et al. Cohort Profile: NICHD International Site Development Initiative (NISDI): A prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and Caribbean countries. Int J Epidemiol. 2009;38:1207–1214. doi: 10.1093/ije/dyn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Griensven J. De Naeyer L. Uwera J, et al. Success with antiretroviral treatment for children in Kigali, Rwanda: Experience with health center/nurse-based care. BMC Pediatr. 2008;8:39. doi: 10.1186/1471-2431-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaspan HB. Berrisford AE. Boulle AM. Two-year outcomes of children on non-nucleoside reverse transcriptase inhibitor and protease inhibitor regimens in a South African pediatric antiretroviral program. Pediatr Infect Dis J. 2008;27:993–998. doi: 10.1097/INF.0b013e31817acf7b. [DOI] [PubMed] [Google Scholar]
- 16.Chearskul P. Chokephaibulkit K. Chearskul S, et al. Effect of antiretroviral therapy in human immunodeficiency virus-infected children. J Med Assoc Thai. 2005;88(Suppl 8):S221–231. [PubMed] [Google Scholar]
- 17.Kumarasamy N. Venkatesh KK. Devaleenol B, et al. Safety, tolerability and effectiveness of generic HAART in HIV-infected children in South India. J Trop Pediatr. 2009;55:155–159. doi: 10.1093/tropej/fmn080. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F. Haberer JE. Zhao Y, et al. Chinese pediatric highly active antiretroviral therapy observational cohort: A 1-year analysis of clinical, immunologic, and virologic outcomes. J Acquir Immune Defic Syndr. 2007;46:594–598. doi: 10.1097/QAI.0b013e318158c08e. [DOI] [PubMed] [Google Scholar]
- 19.Sungkanuparph S. Apiwattanakul N. Thitithanyanont A. Chantratita W. Sirinavin S. HIV-1 drug resistance mutations in children who failed non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Southeast Asian J Trop Med Public Health. 2009;40:83–88. [PubMed] [Google Scholar]
- 20.Jittamala P. Puthanakit T. Chaiinseeard S. Sirisanthana V. Predictors of virologic failure and genotypic resistance mutation patterns in Thai children receiving non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J. 2009;28:826–830. doi: 10.1097/INF.0b013e3181a458f9. [DOI] [PubMed] [Google Scholar]
- 21.Cozzi-Lepri A. Phillips AN. Ruiz L, et al. Evolution of drug resistance in HIV-infected patients remaining on a virologically failing combination antiretroviral therapy regimen. AIDS. 2007;21:721–732. doi: 10.1097/QAD.0b013e3280141fdf. [DOI] [PubMed] [Google Scholar]