Abstract
In the cochlea, spiral ganglion neurons play a critical role in hearing as they form the relay between mechanosensory hair cells in the inner ear and cochlear nuclei in the brainstem. The proneural basic helix-loop-helix transcription factors Neurogenin1 (Neurog1) and NeuroD1 have been shown to be essential for the development of otocyst-derived inner ear sensory neurons. Here, we show neural competence of nonsensory epithelial cells in the cochlea, as ectopic expression of either Neurog1 or NeuroD1 results in the formation of neuronal cells. Since the high-mobility-group type transcription factor Sox2, which is also known to play a role in neurogenesis, is expressed in otocyst-derived neural precursor cells and later in the spiral ganglion neurons along with Neurog1 and NeuroD1, we used both gain- and loss-of-function experiments to examine the role of Sox2 in spiral ganglion neuron formation. We demonstrate that overexpression of Sox2 results in the production of neurons, suggesting that Sox2 is sufficient for the induction of neuronal fate in nonsensory epithelial cells. Furthermore, spiral ganglion neurons are absent in cochleae from Sox2Lcc/Lcc mice, indicating that Sox2 is also required for neuronal formation in the cochlea. Our results indicate that Sox2, along with Neurog1 and NeuroD1, are sufficient to induce a neuronal fate in nonsensory regions of the cochlea. Finally, we demonstrate that nonsensory cells within the cochlea retain neural competence through at least the early postnatal period.
Introduction
During inner ear development, the first cellular lineage to differentiate from the otocyst is the neuroblast lineage that gives rise to the ganglia of the VIII cranial nerve. After closure of the otic cup, which occurs at embryonic day 9 (E9) in the mouse, neuroblast precursors begin to delaminate from the ventral region of the otocyst and migrate a short distance away where they coalesce to form the primary neurons of the auditory and vestibular divisions of the VIII nerve (Rubel and Fritzsch, 2002). In the auditory system, neuronal precursors become distributed in a spiral pattern (referred to as the spiral ganglion) that mirrors the distribution of their peripheral targets, the mechanosensory hair cells located along the cochlea. Spiral ganglion neurons extend dendritic processes that innervate both inner and outer hair cells with 95% of all afferent synapses occurring on inner hair cells. Loss of spiral ganglion neurons is believed to contribute to decreased hearing acuity and the presence of intact spiral ganglion neurons is required for cochlear implant function (Linthicum et al., 1991; Khan et al., 2005). Despite their crucial role in auditory function, the molecular pathways that mediate spiral ganglion neuron formation are not fully understood.
Members of the basic helix-loop-helix (bHLH) family of transcription factors are known to play critical roles in cellular commitment and differentiation in many neuronal systems including the spiral ganglion (for review, see Lefebvre et al., 2007). In particular, the bHLH transcription factors, Neurogenin1 (Neurog1) and NeuroD1 are required for the formation of vestibulocochlear neuroblasts (Ma et al., 1998, 2000; Kim et al., 2001). Both factors are expressed in neuroblasts before delamination from the otocyst and deletion of either gene results in a complete (Neurog1) or nearly complete (NeuroD1) loss of vestibulocochlear neurons. Based on these findings it has been suggested that Neurog1 plays a role in specifying proneural fate while NeuroD1 regulates neuronal differentiation (Liu et al., 2000; Kim et al., 2001). However, in most systems the transition of a neuroblast from active proliferation to a progenitor cell state primed for commitment and differentiation through the expression of bHLH factors requires additional signaling molecules. In particular, members of the SoxB1 group (Sox1, Sox2, Sox3) of high-mobility-group type (HMG) box domain transcription factors have been shown to play a role in this transition in other systems (Collignon et al., 1996; Pevny et al., 1998; Wood and Episkopou, 1999). Specifically, mice carrying a null mutation of either Sox1 or Sox3 or a hypomorphic allele for Sox2 exhibit various neuronal defects (Malas et al., 2003; Ferri et al., 2004; Rizzoti et al., 2004; Ekonomou et al., 2005), suggesting significant roles for all three genes in neurogenesis. In addition to the role in neurogenesis, Sox2 has been shown to be involved in the maintenance of neural stem cells as well as neurons (Episkopou, 2005).
Here, we examined the molecular factors that are instructive for neuronal formation within the inner ear. We show that nonsensory epithelial cells within the cochlear duct are competent to develop as neurons since ectopic expression of Neurog1 or NeuroD1 within these cells is sufficient to induce a neuronal phenotype. Furthermore, we demonstrate that Sox2 is expressed in spiral ganglion neurons and that mutations in the otocyst-specific promoter for Sox2 (Sox2Lcc/Lcc; light coat circling) (Kiernan et al., 2005) in mice leads to the loss of spiral ganglion neurons. Finally, ectopic expression of Sox2 in nonsensory regions of the cochlea is sufficient to induce neurons, demonstrating an essential role for Sox2 in neurogenesis in the inner ear.
Materials and Methods
Generation of Sox1LacZ/LacZ, Sox2Lcc/Lcc, and Sox3−/− mice.
Sox1LacZ/LacZ (Kan et al., 2007), Sox2Lcc/Lcc (Kiernan et al., 2005), and Sox3−/− mice (Rizzoti et al., 2004) were generated by crossing Sox1LacZ/+, Sox2Lcc/+, and Sox3+/− mice, respectively. Newborn pups and embryos from pregnant ICR/CD1 (outbred albino strain) mice were killed in accordance with NIH guidelines.
DNA constructs.
A pCLIG-NeuroD1 expression vector was kindly provided by R. Kageyama (Kyoto University, Kyoto, Japan) (Inoue et al., 2002). The pCLIG vector uses a CMV-IE enhancer/chicken β-actin promoter and an Internal Ribosomal Entry Site (IRES) to drive expression of NeuroD1 and EGFP as independent transcripts. A Neurog1 expression vector was generated by cloning the open reading frame for Neurog1 into the multiple cloning site of the pIRES2-EGFP vector (Clontech). Expression vectors for Sox1 and Sox2 were kindly provided by L. Kan (Northwestern University, Evanston, IL) (Kan et al., 2007) and L. Pevny (University of North Carolina, Chapel Hill, NC) (Taranova et al., 2006), respectively. In both cases, the expression constructs also expressed EGFP as a separate transcript.
Electroporation of cochlear explant cultures.
Individual cells in mouse cochlear explants were transfected using square-wave electroporation as described previously (Jones et al., 2006). In brief, cochlear explants were dissected between E13 and postnatal day 3 (P3), electroporated, and maintained for at least 6 days in vitro (DIV) and processed by immunohistochemistry. For immunolabeling with Glial Fibrillary Acidic Protein (GFAP), cochlear explant cultures were transfected at E13 and maintained for 10 DIV. For drug treatment, electroporated explant cultures were treated with 5 μm neurodazine (Calbiochem) after 1 DIV and maintained for 8 DIV.
Immunohistochemistry.
Cochleae from embryos and newborn pups were removed, isolated, and processed as either whole mounts or sectioned in a cryostat at a thickness of 12 μm. Immunocytochemistry was performed on cochleae and cochlear explant cultures as described previously (Jones et al., 2006). Cells were labeled with primary antibodies against TuJ1 (Sigma, 1:200), Sox2 (Millipore Bioscience Research Reagents, 1:1000; Santa Cruz Biotechnology, 1:250), Neurofilament 200 (Sigma, 1:200), Neurog1 (Affinity BioReagents, 1:100), NeuroD1 (Abcam, 1:100), Sox10 (Santa Cruz Biotechnology, 1:250), β-gal (Promega, 1:250), Map2 (Sigma, 1:300), Myosin 6 and 7a (Proteus Biosciences, 1:1000), and Phalloidin (Invitrogen, 1:100).
Electrophysiology.
For electrophysiological recordings, transfected cochlear explants after 1 DIV were transferred to a recording chamber perfused at 2 ml/min with an artificial CSF consisting of (in mm): 150 NaCl, 1.25 NaH2PO4, 2.5 KCl, 5 HEPES, 10 glucose, 1 MgCl2 and 0.2 CaCl2, pH 7.3 with NaOH. Cells were visualized with differential interference contrast optics and epifluorescence was used to distinguish control and transfected cells. Patch-clamp recordings were made with borosilicate glass electrodes (4–6 MΩ) containing an intracellular solution consisting of (in mm): 114 potassium methanesulfonate, 10 HEPES, 1 KCl, 4 Mg-ATP, 0.4 Na-GTP, 14 phosphocreatine, pH 7.3 with KOH. Recordings were made in both voltage-clamp and current-clamp configurations (Multiclamp 700B, Molecular Devices). Data were filtered at 3 kHz, sampled at 10 kHz and acquired using custom software written in Matlab (MathWorks).
Results
Neurogenin1 and NeuroD1 induce cochlear nonsensory epithelial cells to develop as ectopic neurons
Previous studies have shown that Neurog1 and NeuroD1 are expressed in the spiral ganglion (Fig. 1A–D) and are required for formation of all (Neurog1) or most (NeuroD1) neurons within the vestibulocochlear ganglion (Ma et al., 1998, 2000). This finding led us to investigate whether the expression of NeuroD1 or Neurog1 is sufficient to induce a neuronal identity in nonsensory inner ear epithelial cells that would not normally develop as neurons. To test this hypothesis, electroporation-mediated DNA transfection was used to induce ectopic expression of Neurog1 or NeuroD1 in nonsensory epithelial cells located within Kolliker's organ (KO) or the lesser epithelial ridge (LER) of cochlear explant cultures. Cochleae were dissected from E13 embryos and electroporated. After 6 DIV, development of neuronal phenotypes was assayed based on morphology and expression of the neuronal markers TuJ1 (β-tubulin III) (Lee et al., 1990; Hallworth and Luduena, 2000) and microtubule-associated-protein 2 (Map2) (Hafidi et al., 1992), both of which are expressed in spiral ganglion neurons but not in glial cells marked by Sox10 immunolabeling (Fig. 1A,B,E,F). As previously reported, electroporation resulted in multiple transfected cells in both KO and the LER. In addition, a subset of epithelial cells transfected with either Neurog1.EGFP or NeuroD1.EGFP was positive for ΤuJ1 (Fig. 2A and data not shown). Moreover, most of the ΤuJ1-positive cells, regardless of whether they expressed Neurog1.EGFP or NeuroD1.EGFP, extended long processes (Fig. 2B–E), many of which ended in growth cones (Fig. 2D), a morphology that is consistent with developing neurites. Transfected cells with neuronal morphologies were also positive for Map2 (Fig. 2F,G). To determine how rapidly expression of either NeuroD1 or Neurog1 induced a neuronal fate, explants were analyzed after only 1 DIV. A comparable number of TuJ1-positive transfected cells were detected in these explants (data not shown), suggesting a rapid induction of neuronal fate. In contrast, cells transfected with a Control.EGFP vector were consistently negative for expression of either TuJ1 or Map2 (Fig. 2H and data not shown). Moreover, the morphology of these cells, which included a centrally located nucleus with basal and lumenal projections, was consistent with that of epithelial cells (Fig. 2I,J).
Figure 1.
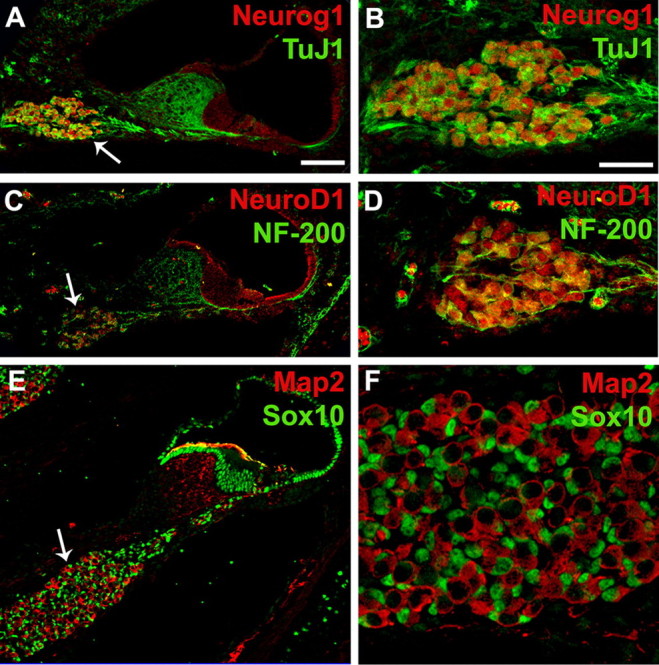
Neurog1 and NeuroD1 are expressed in spiral ganglion neurons. A, Cross-section through the cochlea at P0 illustrating expression of Neurog1 (red) and ΤuJ1 (green) in the spiral ganglion (arrow). B, High-magnification view of the spiral ganglion labeled as in A. Note that TuJ1 and Neurog1 are coexpressed in neuronal cells. C, Cross-section as in A illustrating expression of NeuroD1 (red) and NF-200 (green) in the spiral ganglion (arrow). D, High-magnification view of the spiral ganglion labeled as in C. NeuroD1 and NF-200 are colocalized in spiral ganglion neurons. E, Cross-section as in A, illustrating expression of Map2 (red) and Sox10 (green). Map2 is expressed in neurons while Sox10 is expressed in nuclei of spiral ganglion glia (arrow) and nonsensory cells in the cochlear duct. F, High-magnification view of the spiral ganglion labeled as in E. Large Map2-positive neurons are surrounded by smaller Sox10-positive glial cells. Scale bars: A (for A, C, E) 50 μm; B (for B, D, F) 20 μm.
Figure 2.

NeuroD1 and Neurog1 induce neuronal phenotypes in nonsensory cells within the cochlea. A, Low-magnification image of a cochlear explant transfected with NeuroD1.EGFP (green) and labeled with anti-TuJ1 (red). The sensory epithelium (SE, dashed line) and two nonsensory regions, KO and the LER, are indicated. Arrows indicate multiple transfected cells that are positive for TuJ1. B, High-magnification view of three cells located in KO that have been transfected with Neurog1.EGFP (green). Each cell has extended a neurite (arrowheads). C, The same image as in B, except with TuJ1 labeling in red. Note that each cell body and neurite is positive for TuJ1. D, E, Example of a cluster of NeuroD1.EGFP-transfected cells (green) that are TuJ1 positive (red) and have developed neuronal phenotypes including extension of neurites (D, arrowheads) and the formation of a growth cone (arrow and inset in D). F, G, Cells transfected with either Neurog1.EGFP (green in F) or NeuroD1.EGFP (green in G; NeuroD1.EGFP abbreviated as NeuroD1) were also positive for Map2 (red). H, In contrast, cells transfected with Control.EGFP (green) alone were not positive for TuJ1 (red). I, J, In addition, control transfected cells had morphologies that were consistent with epithelial cells including lumenal and basal extensions. K, NeuroD1.EGFP transfected cells (green) were negative for the hair cell marker Myo6 (red) demonstrating that NeuroD1 does not induce a hair cell fate. L, M, In contrast, cells transfected with Atoh1.EGFP (green) were consistently positive for hair cell markers such as Myo7a (red in L) but were negative for TuJ1 (red in M). Scale bars: A, 20 μm; B (for B–G), 10 μm; H, 20 μm; I (for E, J, K–M) 10 μm.
To determine the efficiency of induction of a neuronal fate, the percentage of transfected cells that also expressed TuJ1 was determined for cells transfected with Control.EGFP, Neurog1.EGFP or NeuroD1.EGFP. As shown in Table 1, 73% of cells (n = 156) transfected with NeuroD1.EGFP and 26% of cells transfected with Neurog1.EGFP (n = 111) were positive for ΤuJ1. Since a relatively large percentage of transfected cells, in particular cells transfected with Neurog1.EGFP, failed to develop as neurons, we wanted to determine whether these cells might have been induced to develop with an alternative cell fate. Previous results have demonstrated that nonsensory cells within KO or the LER are competent to develop into hair cells or supporting cells (Zheng and Gao, 2000; Woods et al., 2004). Therefore, expression of the hair cell markers Myosin6 (Myo6) and Myosin7a (Myo7a) and the support cell marker p27Kip1 were determined in cells transfected with Neurog1.EGFP or NeuroD1.EGFP. No induction of either hair cell or support cell markers was observed in any of these cells (Fig. 2K; supplemental Fig. S1A,B, available at www.jneurosci.org as supplemental material; and data not shown). Finally, to determine whether the ability to induce nonsensory cells to develop with a neuronal fate is specific to Neurog1 or NeuroD1, nonsensory cells were transfected with another bHLH transcription factor, Atoh1, and then assayed for expression of neuronal markers. Consistent with previous results (Zheng and Gao, 2000; Woods et al., 2004; Jones et al., 2006), 98% of Atoh1.EGFP transfected cells were positive for Myo7a (Fig. 2L). In contrast, none of the transfected cells were positive for TuJ1 (Fig. 2M).
Table 1.
Efficiency of neuronal induction by NeuroD1, Neurog1, and Sox2 at different ages
| cDNA | Total number of explants (T); totalnumber of transfected cells (N) | TuJ1-positive cells(% of total) |
|---|---|---|
| Control.EGFP (E13.5) | T = 4; N = 166 | 1 (0.6%) |
| NeuroD1.EGFP (E13.5) | T = 5; N = 156 | 114 (73%) |
| NeuroD1.EGFP (E16.5) | T = 4; N = 160 | 67 (41%) |
| NeuroD1.EGFP (P1) | T = 3; N = 130 | 33 (25%) |
| Neurog1.EGFP (E13.5) | T = 4; N = 111 | 29 (26%) |
| Neurog1.EGFP (E16.5) | T = 5; N = 253 | 40 (15%) |
| Neurog1.EGFP (P1) | T = 7; N = 143 | 11 (7%) |
| Sox2.nucEGFP (E13.5) | T = 6; N = 505 | 199 (39%) |
| Sox2.nucEGFP (E16.5) | T = 4; N = 526 | 82 (16%) |
| Sox2.nucEGFP (P1) | T = 5; N = 338 | 11 (3%) |
Nonsensory cells located in either Kolliker's organ or the lesser epithelial ridge that were transfected with the indicated constructs were identified based on expression of GFP. Neuronal identity was established based on expression of TuJ1.
To further characterize the ectopic neurons induced by overexpression of either Neurog1.EGFP or NeuroD1.EGFP, we examined the expression of several markers of mature neurons. Cells transfected with Neurog1.EGFP or NeuroD1.EGFP failed to express markers for more mature neurons including islet1, neurofilament-200, neurofilament-L, 2H3, synaptophysin, and GAP-43. Furthermore, preliminary experiments in which Neurog1.EGFP transfected explants were treated with different factors known to enhance neuronal differentiation, including FGF2, retinoic acid, nerve growth factor, or neurodazine—an inducer of neurogenesis (Williams et al., 2007) failed to induce the expression of additional neuronal markers. However, treatment with neurodazine did lead to an increased percentage of induction of TuJ1-positive neurons from 26% (n = 111) to 53% (n = 116), suggesting that the environment within KO and the LER may not be conducive for neuronal formation.
To confirm that transfected that were positive for TuJ1 or Map2 were in fact neurons, whole-cell recordings were made from transfected cells within cochlear explant cultures. Transfected cells which are selected based on GFP expression, while non-transfected cells within the same explant were used as controls. Examples of Neurog1.EGFP-transfected cells showing neuronal characteristics are shown in Figure 3A,C. In the voltage-clamp recording shown in Figure 3A, positive voltage steps activated a transient inward current and a persistent outward current, consistent with expression of voltage-gated sodium and potassium channels, respectively. In a current-clamp recording from another cell shown in Figure 3C, depolarizing current injections evoked action potentials, as indicated by their abrupt activation threshold, inflection in their rising phase, and peak membrane potential that exceeded 0 mV. Responses exhibiting evidence of expression of voltage-gated sodium and potassium channels were observed in 3 of 9 Neurog1.EGFP transfected cells from which recordings were made, demonstrating in vitro acquisition of electrophysiological properties characteristic of neurons. In the remaining 6 of 9 cells, a purely passive membrane response was observed in both voltage and current-clamp recordings (Fig. 3B,D). Similar passive responses were observed in all non-transfected cells (n = 3).
Figure 3.
Ectopic neurons exhibit electrophysiological characteristics consistent with neurons. Whole-cell recordings from cochlear explant cultures. A, B, Voltage-clamp recording from a Neurog1.EGFP-transfected cell (A) and a non-transfected cell (B). Voltage steps of −70 mV to +10 mV were delivered for duration of 100 or 20 ms (right panel in A). C, D, Current clamp recording from a different Neurog1.EGFP-transfected cell (C) and a non-transfected cell (D) demonstrate the presence of both inward currents and an outward current. Current injections of 50–250 pA were delivered for a duration of 20 ms.
It has been suggested that NeuroD1 is a direct target of Neurog1 signaling since targeted deletion of Neurog1 results in a disruption of NeuroD1 expression in the developing inner ear (Ma et al., 1998). To test this hypothesis directly, we examined the expression of NeuroD1 in Neurog1-transfected cells. No induction of NeuroD1 was observed in any Neurog1-transfected cell (data not shown). These results suggest that Neurog1 and NeuroD1 could function in different signaling cascades or that Neurog1 requires specific cofactors to induce NeuroD1 expression.
Sox2 is expressed in the developing spiral ganglion neurons
As discussed, members of the SoxB1 family play a significant role in neural development in the spinal cord and elsewhere. To investigate the potential role of SoxB1 genes in the development of spiral ganglion neurons, the expression of Sox1, Sox2 and Sox3 was compared with Neurog1 and NeuroD1 in the otocyst and early postnatal cochlea. Expression of Sox2 was observed along with Neurog1 and NeuroD1 in otocyst-derived neuroblasts as early as E10.5 (Fig. 4A–C). To confirm that Sox2 is expressed in neurons within the spiral ganglion, localization was confirmed by double labeling with TuJ1 in P0 cochleae (Fig. 4D,E).
Figure 4.
SoxB1 gene expression correlates with spiral ganglion development. A–C, Cross-sections through the otocyst from E10.5 mouse embryos demonstrating the expression of Neurog1 (green in A), NeuroD1 (green in B), and Sox2 (green in C) in delaminating neuroblasts of the developing spiral ganglion (arrow in each). Filamentous actin is labeled with Phalloidin (red) in each panel. Note that Neurog1 and Sox2 are also expressed in cells within the otocyst while expression of NeuroD1 is restricted to delaminating neuroblasts. Orientation for sections A–C is indicated to the right of panel C. D–G, Low- and high-magnification cross-sections of the cochlear duct (D, F) or spiral ganglion (E, G). Coimmunolabeling of Sox2 (green in D, E) or Sox1 (green in F, G) with the neuronal marker TuJ1 (red) demonstrates that Sox2 and Sox1 are expressed in spiral ganglion neurons (arrows in D, F) at P0. H, I, Cross-sections as in D,E demonstrating the specificity of TuJ1 as a neuronal marker. There is no overlap between cells labeled with TuJ1 (green) and the glial marker Sox10 (red) in the ganglion. Scale bars: A (for A–C), 50 μm; D (for D, F, H), 50 μm; E (for E, G, I) 20 μm.
Expression of Sox1 was also detected in spiral ganglion neurons at P0 based on coexpression of β-galactosidase and TuJ1 in a Sox1LacZ/+ reporter mouse (Fig. 4F,G). Finally, restriction of TuJ1 to neurons within the spiral ganglion was confirmed by doubling labeling with glial marker, Sox10, at P0. The nuclei of Sox10-positive glial cells were noticeably smaller than those of TuJ1-positive neuronal cells and the two populations of cells were non-overlapping (Fig. 4H,I). Since both Sox1 and Sox2 colocalize with TuJ1, these results strongly suggest that Sox1 and Sox2 are only expressed in neurons within the spiral ganglion. In contrast, Sox3 was not observed in spiral ganglion neurons at P0, and instead was restricted to a subset of cells within the epithelial cell layer of Reissner's membrane (data not shown).
Sox2 promotes neurogenesis
The expression of Sox1 and Sox2 in the spiral ganglion neurons suggested a role for one or both in neuronal development and/or maintenance. To ascertain whether Sox1 plays a role in any of these events, expression of ΤuJ1 was examined in Sox1LacZ/LacZ mutant cochlea at P0. As shown in Figure 5, A and B, ΤuJ1 expression and ganglion cell morphology are unaffected in the absence of Sox1 suggesting that Sox1 is not required for ganglion cell formation or maintenance. Moreover, labeling of spiral ganglion neurites with anti-NF-200 in cochlear whole-mounts from Sox1+/LacZ and Sox1LacZ/LacZ mice indicated no effects on the pattern of innervation in the absence of Sox1 (Fig. 5A,B, inset). In addition, no glial abnormalities were apparent based on immunolabeling with Sox10. Consistent with these results, nonsensory cells transfected with Sox1. EGFP did not develop a neuronal phenotype (Fig. 5C), suggesting that overexpression of Sox1 alone is not sufficient to induce a neuronal fate in the cochlea.
Figure 5.
Sox1 is neither necessary nor sufficient for spiral ganglion neuron formation. A, B, Low-magnification cross-sections through the cochlea of Sox1+/lacZ (control) or Sox1lacZ/lacZ (mutant) mice at P0 double-immunolabeled for ΤuJ1 (green) and Sox10 (red). Spiral ganglion neurons, with processes extending to the sensory epithelium, are present and surrounded by glia in both control and mutant cochleae. Inset, Whole-mount immunolabeling of Sox1+/lacZ (control) (A) or Sox1lacZ/lacZ (mutant) (B) cochlea using anti-NF-200 demonstrates no defects in the pattern of innervation in the absence of Sox1. C, Transfection of nonsensory cells with Sox1.EGFP (green) is not sufficient to induce the expression of TuJ1 (red) or development of a neuronal phenotype. Scale bars: B (for A, B), 50 μm; C, 20 μm.
In contrast with Sox1LacZ/LacZ mutant cochleae, expression of ΤuJ1 and Sox10 were absent in the spiral ganglion region of Sox2Lcc/Lcc mutant cochleae at E15.5 (Fig. 6A–D). In fact, labeling with DAPI demonstrated a complete absence of cell nuclei in the spiral ganglion region (Fig. 6A,B), suggesting a defect in neurogenesis. To confirm that the absence of cells within the spiral ganglion in these mutant cochleae is not due to delay in development, expression of TuJ1 and Sox10 were also analyzed at P0 (Fig. 6E,F). As was the case at E15.5, Tuj1 and Sox10 labeling were completely absent in cochleae from Sox2Lcc/Lcc mutants. These results demonstrate that Sox2 is necessary for spiral ganglion formation.
Figure 6.
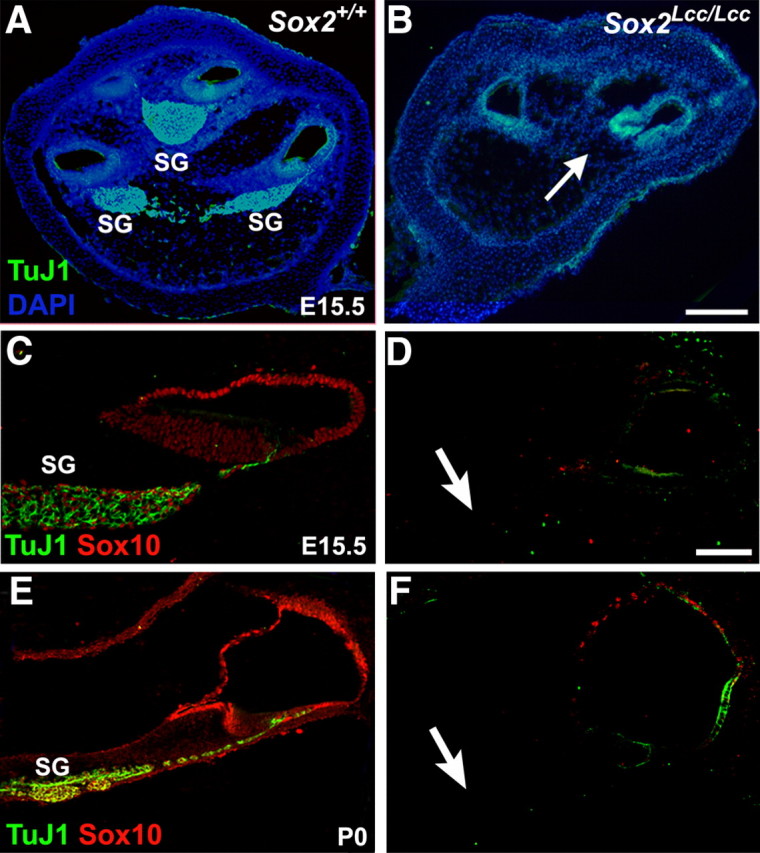
Sox2 is required for formation of the spiral ganglion. A, B, Low-magnification cross-sections through the cochleae of Sox2+/+ (control) and Sox2Lcc/Lcc (mutant) mice at E15.5. Spiral ganglion neurons (SG) are labeled with ΤuJ1 (green) and all cell nuclei are labeled with DAPI (blue). Note the complete absence of spiral ganglion neurons, including cell nuclei (arrow) in the Sox2Lcc/Lcc cochlea. C–F, Double-immunolabeling for TuJ1 (green) and Sox10 (red) on cross-sections of Sox2+/+ (control) and Sox2Lcc/Lcc (mutant) cochlea at E15.5 (C, D) and P0 (E, F) demonstrates the absence of spiral ganglion neurons and glial cells in Sox2 mutants. Arrows in D and F indicate expected position of the spiral ganglion. Scale bars: B (for A, B), 50 μm; D (for C–F), 20 μm.
To determine whether Sox2 is also sufficient to induce neurogenesis, nonsensory cells in cochlear explants were transfected with Sox2.nucEGFP, and development of neuronal identity was analyzed as described for NeuroD1 and Neurog1. Approximately 39% of Sox2.nucEGFP-positive cells (n = 505) were positive for TuJ1 (Table 1, Fig. 7A,B). Moreover, as was observed in response to the overexpression of Neurog1 or NeuroD1, these cells also extended processes that appeared consistent with developing neurites (Fig. 7B). Sox2-transfected cells that were positive for Map2 were also observed (Fig. 7C). These ectopic neurons were able to survive for at least 15 d after transfection (data not shown). In addition, as was observed for Neurog1 and NeuroD1 transfections, expression of Sox2 did not induce hair cell, support cell or glial cell phenotypes (supplemental Fig. S1C,D, available at www.jneurosci.org as supplemental material; and data not shown).
Figure 7.

Sox2 induces neuronal phenotypes in cochlear nonsensory cells. A, B, Low- (A) and high-magnification (B) images of Sox2.nucEGFP transfected cells (green) located in KO of cochlear explants labeled with anti-TuJ1 (red in A, B) or anti-Map2 (red in C). A subset of transfected cells are labeled with anti-TuJ1 and have developed a neuronal phenotype. Asterisk in A indicates a Sox2.nucEGFP transfected cell that is TuJ1-positive and has extended a single neurite (enlarged in inset). In contrast with Neurog1.EGFP or NeuroD1.EGFP, the Sox2.nucEGFP construct carries a nuclear localization signal sequence and is therefore restricted to the nucleus. Scale bars: A, 20 μm; B (for B, C), 10 μm.
The observation that Sox2 failed to induce expression of a hair cell phenotype is consistent with recent results demonstrating that Sox2 acts to antagonize Atoh1 expression in the cochlea (Dabdoub et al., 2008). Furthermore, forced expression of Sox2 induces Prox1 expression in nonsensory epithelial cells in the cochlea (Dabdoub et al., 2008). While Prox1, a homeobox transcription factor, is expressed in the spiral ganglion neurons (Bermingham-McDonogh et al., 2006); forced expression of Prox1 in nonsensory cochlear cells did not induce a neuronal fate (data not shown). To determine whether the ability of Sox2, Neurog1, and NeuroD1 to induce a neuronal fate in cochlear nonsensory cells is dependent on the age of the cells at the time of transfection, nonsensory cells were transfected with each construct in cochleae isolated at E16 or P1. A progressive decrease in the percentage of cells that develop as neurons was observed (Table 1).
To determine whether Sox2 acts upstream, downstream, or in parallel with NeuroD1 and Neurog1, expression of all three molecules was assessed in cells transfected with each construct. Sox2.nucEGFP-transfected cells did not express either NeuroD1 or Neurog1 nor were Neurog1.EGFP transfected cells positive for Sox2 (data not shown). Similar results were obtained for cells transfected with NeuroD1.EGFP. These results suggest that despite overlapping expression within the otocyst and developing spiral ganglion, Neurog1 and Sox2 act through independent pathways within the developing inner ear.
Discussion
Neurog1 and NeuroD1 are sufficient to induce neuronal identity
All of the cells within the membranous labyrinth of the inner ear and the neurons located within the associated vestibulocochlear ganglion are derived from otocyst epithelial cells. As development of the inner ear progresses, otocyst cells become partitioned into three broadly defined categories, neuroblasts that will go on to develop as auditory and vestibular neurons, prosensory cells that will develop as hair cells, and supporting cells and nonsensory cells that will form all of the regions of the labyrinth that lack hair cells and supporting cells. Previous results have suggested that the Notch signaling pathway and Sox2 play key roles in specifying prosensory regions (Kiernan et al., 2005, 2006) while deletion of either Neurog1 or NeuroD1 leads to the absence of all or most vestibulocochlear neurons (Ma et al., 1998, 2000; Kim et al., 2001), suggesting that Neurog1 and NeuroD1 are necessary for neuronal development. Here, we demonstrate that ectopic expression of Neurog1 or NeuroD1 is sufficient to convert nonsensory epithelial cells within the cochlear duct into neurons, indicating that Neurog1 and NeuroD1 are also sufficient to induce neuronal identity within the inner ear. These results are consistent with previous gain-of-function studies which demonstrated that ectopic expression of Neurog1 or NeuroD1 is also sufficient to convert ectodermal cells into neurons (Lee et al., 1995; Ma et al., 1996).
Approximately 30% of cochlear nonsensory cells transfected with Neurog1.EGFP became positive for the neuronal markers TuJ1 and Map2 and developed morphological features that were consistent with neuronal phenotypes. Moreover, an approximately equivalent percentage of transfected cells acquired electrophysiological properties characteristic of neurons. Whole-cell voltage-clamp recordings demonstrated classical neuronal responses, with both inward and outward currents typical of neurons expressing voltage-gated sodium and potassium channels. In current clamp, these cells also fired action potentials in response to current injections. In contrast, non-transfected cells exhibited purely passive membrane responses in both voltage- and current-clamp recordings. However, these cells were never observed to express more mature neuronal markers, such as Neurofilament-200, Neurofilament-L, Synaptophysin, GAP-43, or Islet1. Nor did co-transfection of Neurog1 and Sox2 or NeuroD1 and Sox2 induce expression of these markers. The reasons for this are unclear; however, it is possible that the absence of required cofactors, such as E12 or E47 (Kageyama et al., 1997; Chu et al., 2001), or the presence of neuronal inhibitory signals within KO and the LER could act to prevent the further maturation of these cells.
Sox2 is required for neuronal formation in the inner ear
Sox2, and other members of the SoxB1 group, have been shown to play diverse roles during neuronal development (for review, see Episkopou, 2005). For example, overexpression of Sox1 in the developing mouse CNS is sufficient to induce neuronal lineage commitment and promote neuronal differentiation in vitro (Kan et al., 2004). In the inner ear, Sox2 was already known to be required for prosensory formation, but the results presented here demonstrate that Sox2 is also necessary and sufficient for neuronal formation. The initial onset of Sox2 expression is nearly simultaneous with Neurog1 (Fig. 4) (B. Fritzsch, personal communication). This, along with the ability of Sox2 to induce a neuronal fate in nonsensory regions, strongly suggests that Sox2 plays a role in the initial specification of a neuronal fate within the inner ear. Sox2 expression persists in spiral ganglion neurons through at least the early postnatal period, suggesting a possible role in maintenance of spiral ganglion neurons as well. A similar role has been suggested for Sox2; neurons in the thalamus, striatum, and septum that express Sox2 beyond the period of initial development undergo degeneration in Sox2 deficient mice by 3–6 months of age (Ferri et al., 2004). However, considering that all spiral ganglion neurons are absent in Sox2Lcc/Lcc mice by E15, presumably as a result of the requirement of Sox2 for neuronal specification, any examination of the potential role of Sox2 in neuronal maintenance within the inner ear will require a specific deletion of Sox2 after the period of neuronal specification.
Interactions between Neurog1 and Sox2
The expression of Neurog1 and Sox2 in delaminating neuroblasts contrasts with the sequential expression of Sox and bHLH factors in the developing CNS (Bylund et al., 2003) and in the developing sensory epithelia of the cochlea where Sox2 expression occurs before expression of the bHLH factor Atoh1 in the same cells (Kiernan et al., 2005). Moreover, functional studies in both developing spinal cord and inner ear sensory epithelia have demonstrated antagonistic interactions between Sox and bHLH molecules (Bylund et al., 2003; Dabdoub et al., 2008). However, a study by Kan et al. (2004) has shown that Sox1 upregulates the expression of Neurog1 in cultured neural progenitor cells suggesting that the interactions between Sox and bHLH factors are variable and context dependent. This seems to be particularly true for the otocyst where both cooperative and antagonistic interactions between Sox2 and bHLH factors apparently occur in neuroblast and prosensory cell populations that are adjacent or, possibly, intermingled. These results suggest that a complex network of context-dependent transcriptional regulators is responsible for specification of neuronal versus prosensory cell fates within the otocyst.
Plasticity of otocyst derived cells
The demonstration that nonsensory epithelial cells within KO or the LER of the cochlear duct can be induced to develop as neurons through forced expression of Sox2, Neurog1, or NeuroD1 expands the known potential fates of these cells, which already included hair cells and supporting cells (Zheng and Gao, 2000; Woods et al., 2004). Our results along with previous findings demonstrate that these cells retain the potential to develop as any of the three possible otocyst-derived cell fates, neuronal, sensory or nonsensory, at least through the early postnatal period (E13–P3). However, the ability of Sox2, Neurog1, or NeuroD1 to induce neuronal fates decreases markedly between E13 and P1. These results, along with previous work demonstrating that supporting cells within the organ of Corti retain the ability to develop as hair cells until ∼P10 (White et al., 2006), suggest that the commitment of otocyst-derived cells is an extended process that may not be completed until after birth in the mouse. Our results also argue against the presence of committed progenitors in different regions of the otocyst since cell fates can be changed even at relatively late time points in development.
In addition to the correlation between increasing age and a progressive decrease in the number of transfected cells that developed as neurons, variability was also observed in the efficiency of neuronal induction between NeuroD1 (73%), Neurog1 (26%), and Sox2 (39%) at the same developmental time point (E13.5). The basis for these differences is unclear but could be related to either methodological limitations such as differences in overall levels of gene expression or unknown post-translational modifications. Alternatively, as discussed above, the presence or absence of specific binding partners could modulate the overall efficiency of each factor.
Implications for human health
Studies of human temporal bones indicate that loss of spiral ganglion neurons is highly correlated with sensorineural hearing loss (Doyle et al., 1998). Similarly, in vivo animal studies have demonstrated that spiral ganglion cells are lost as a secondary effect of the loss of hair cells in response to treatment with ototoxic drugs, acoustic trauma, or aging (for review, see Miller, 2001). The most prevalent existing therapeutic strategies for the amelioration of hearing loss are based on either increasing hair cell stimulation (hearing aids) or introducing an electronic substitute for the hair cells (cochlear implants). In either case, the presence of functional spiral ganglion neurons is absolutely required for a successful outcome. Therefore, it seems likely that the development of methodologies that could be used to induce the formation of spiral ganglion neurons have the potential to significantly increase the likelihood of positive outcomes for hearing impaired individuals. Since as few as 10% of the normal number of spiral ganglion neurons is sufficient for the success of a cochlear implant (Linthicum et al., 1991; Khan et al., 2005), the ability to induce the formation of even a small number of these cells in a damaged ear has critical implications for further advances in cochlear implant technology and the treatment of hearing loss. Together, we believe that our results provide a necessary and important first step toward the induction of spiral ganglion regeneration in mature cochleae.
Footnotes
This work was supported by the intramural program at National Institute on Deafness and Other Communication Disorders. We thank Drs. S. Raft, A. Groves, B. Fritzsch, and A. Ryan for reading an earlier version of this manuscript and C. W. Kramer and Drs. W. Chang and B. Jacques for technical assistance. We are grateful to Drs. L. Kan for providing the Sox1 expression vector and Sox1LacZ/LacZ tissue, K. S. E. Cheah for the Sox2Lcc/Lcc tissue, R. Kageyama for the NeuroD1 expression vector, R. Lovell-Badge for Sox3−/− tissue, and L. H. Pevny for providing us with the Sox2 expression vector.
References
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Chu K, Nemoz-Gaillard E, Tsai MJ. BETA2 and pancreatic islet development. Recent Prog Horm Res. 2001;56:23–46. doi: 10.1210/rp.56.1.23. [DOI] [PubMed] [Google Scholar]
- Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development. 1996;122:509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KJ, Sininger Y, Starr A. Auditory neuropathy in childhood. Laryngoscope. 1998;108:1374–1377. doi: 10.1097/00005537-199809000-00022. [DOI] [PubMed] [Google Scholar]
- Ekonomou A, Kazanis I, Malas S, Wood H, Alifragis P, Denaxa M, Karagogeos D, Constanti A, Lovell-Badge R, Episkopou V. Neuronal migration and ventral subtype identity in the telencephalon depend on SOX1. PLoS Biol. 2005;3:e186. doi: 10.1371/journal.pbio.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episkopou V. Sox2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Hafidi A, Fellous A, Ferhat L, Romand MR, Romand R. Developmental differentiation of MAP2 expression in the central versus the peripheral and efferent projections of the inner ear. J Comp Neurol. 1992;323:423–431. doi: 10.1002/cne.903230309. [DOI] [PubMed] [Google Scholar]
- Hallworth R, McCoy M, Polan-Curtain J. Tubulin expression in the developing and adult gerbil organ of Corti. Hear Res. 2000;139:31–41. doi: 10.1016/s0378-5955(99)00165-3. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26:550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ishibashi M, Takebayashi K, Tomita K. bHLH transcription factors and mammalian neuronal differentiation. Int J Biochem Cell Biol. 1997;29:1389–1399. doi: 10.1016/s1357-2725(97)89968-2. [DOI] [PubMed] [Google Scholar]
- Kan L, Israsena N, Zhang Z, Hu M, Zhao LR, Jalali A, Sahni V, Kessler JA. Sox1 acts through multiple independent pathways to promote neurogenesis. Dev Biol. 2004;269:580–594. doi: 10.1016/j.ydbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Kan L, Jalali A, Zhao LR, Zhou X, McGuire T, Kazanis I, Episkopou V, Bassuk AG, Kessler JA. Dual function of Sox1 in telencephalic progenitor cells. Dev Biol. 2007;310:85–98. doi: 10.1016/j.ydbio.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB., Jr Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115:672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PloS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Lee MK, Rebhun LI, Frankfurter A. Posttranslational modification of class III beta-tubulin. Proc Natl Acad Sci U S A. 1990;87:7195–7199. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Dumitriu B, Penzo-Méndez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39:2195–2214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum FH, Jr, Fayad J, Otto SR, Galey FR, House WF. Cochlear implant histopathology. Am J Otol. 1991;12:245–311. [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. Erratum (2000) 1:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malas S, Postlethwaite M, Ekonomou A, Whalley B, Nishiguchi S, Wood H, Meldrum B, Constanti A, Episkopou V. Sox1-deficient mice suffer from epilepsy associated with abnormal ventral forebrain development and olfactory cortex hyperexcitability. Neuroscience. 2003;119:421–432. doi: 10.1016/s0306-4522(03)00158-1. [DOI] [PubMed] [Google Scholar]
- Miller AL. Effects of chronic stimulation on auditory nerve survival in ototoxically deafened animals. Hear Res. 2001;151:1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36:247–255. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Williams DR, Lee MR, Song YA, Ko SK, Kim GH, Shin I. Synthetic small molecules that induce neurogenesis in skeletal muscle. J Am Chem Soc. 2007;129:9258–9259. doi: 10.1021/ja072817z. [DOI] [PubMed] [Google Scholar]
- Wood HB, Episkopou V. Comparative expression of the mouse Sox1, Sox2, and Sox3 genes from pre-gastrulation to early somite stages. Mech Dev. 1999;86:197–201. doi: 10.1016/s0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]





