Abstract
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, is a cytokine of central importance for the angiogenesis associated with cancers and other pathologies. Because angiogenesis often involves endothelial cell (EC) migration and proliferation within a collagen-rich extracellular matrix, we investigated the possibility that VEGF promotes neovascularization through regulation of collagen receptor expression. VEGF induced a 5- to 7-fold increase in dermal microvascular EC surface protein expression of two collagen receptors—the α1β1 and α2β1 integrins—through induction of mRNAs encoding the α1 and α2 subunits. In contrast, VEGF did not induce increased expression of the α3β1 integrin, which also has been implicated in collagen binding. Integrin α1-blocking and α2-blocking antibodies (Ab) each partially inhibited attachment of microvascular EC to collagen I, and α1-blocking Ab also inhibited attachment to collagen IV and laminin-1. Induction of α1β1 and α2β1 expression by VEGF promoted cell spreading on collagen I gels which was abolished by a combination of α1-blocking and α2-blocking Abs. In vivo, a combination of α1-blocking and α2-blocking Abs markedly inhibited VEGF-driven angiogenesis; average cross-sectional area of individual new blood vessels was reduced 90% and average total new vascular area was reduced 82% without detectable effects on the pre-existing vasculature. These data indicate that induction of α1β1 and α2β1 expression by EC is an important mechanism by which VEGF promotes angiogenesis and that α1β1 and α2β1 antagonists may prove effective in inhibiting VEGF-driven angiogenesis in cancers and other important pathologies.
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, is a potent angiogenic cytokine that stimulates endothelial cells (EC) through two receptor tyrosine kinases, Flt-1 and KDR/Flk-1 (reviewed in ref. 1). Although there are potentially numerous angiogenesis factors (reviewed in ref. 2), considerable evidence has accumulated indicating that VEGF is particularly important. VEGF induces angiogenesis in a variety of experimental models (3–5); and conversely, antagonism of VEGF function or VEGF expression inhibits angiogenesis (6–9). Also, targeted inactivation of a single VEGF allele disrupted normal blood vessel development resulting in embryonic death in utero (10). Finally, elevated expression of VEGF and its receptors has been shown to correlate with the neovascularization associated with embryogenesis (11, 12), wound healing (13), cancer (reviewed in ref. 1), rheumatoid arthritis (14), psoriasis (15), delayed hypersensitivity reactions (16), and proliferative retinopathy (17).
Angiogenesis is a complex process that involves extracellular matrix remodeling, EC migration and proliferation, and the functional maturation of new EC into mature blood vessels (reviewed in ref. 18). Cell surface integrins, which are the major receptors for extracellular matrix, have been implicated in all of these processes (reviewed in ref. 19). Consistent with the importance of integrin function during angiogenesis, targeted deletion of α5 and αv integrin subunits in mice resulted in embryonic vascular defects (20), and an antibody (Ab) that broadly inhibits members of the β1 integrin family inhibited development of the embryonic vasculature (21). Furthermore, an αvβ3 integrin-blocking Ab inhibited angiogenesis in several experimental models (22–24). We reported previously (25) that VEGF induces expression of the αvβ3 integrin in dermal microvascular EC; αvβ3 is a receptor for several ligands including vitronectin, fibronectin, fibrin, and osteopontin (19) that are present in the provisional extracellular matrix during VEGF-driven angiogenesis (26). However, angiogenesis often proceeds in a microenvironment consisting predominantly of interstitial collagens. For example, collagens account for ≈75% of the dry weight of the skin and most of this collagen in the adult is type I (27). Although denatured collagen is recognized by αvβ3 (28), native collagen is not bound significantly by this integrin. Therefore, we investigated whether VEGF also induces expression of the α1β1, α2β1, and α3β1 integrins that are receptors for native collagens (19). Moreover, we investigated the importance of collagen receptors for VEGF-driven angiogenesis in vivo with specific integrin-blocking Abs.
MATERIALS AND METHODS
Cells, Cell Culture, and VEGF Stimulation.
Human dermal microvascular EC were isolated from neonatal foreskins (29, 30) and cultured as described (25). For experiments involving Northern blot analysis, cells were shifted to EC basal medium (Clonetics, San Diego, CA) supplemented with 2% fetal calf serum and antibiotics 24 h prior to stimulation with VEGF. For experiments involving stimulation with VEGF for 72 h or longer, cells were shifted to this medium when VEGF was added. Recombinant human VEGF165, which is the principal VEGF isoform, was purchased from R & D Systems and added to cultures as indicated in the figure legends. All experiments were performed at least twice with similar results.
RNA Isolation and Northern Blot Analyses.
Total cellular RNA was isolated and Northern blot analyses performed as previously described (25). 32P-labeled cDNA probes were prepared as described (25) with purified cDNA inserts isolated from the following: human α2 integrin plasmid (clone 2.72F) and human α3 integrin plasmid (clone 3.10) from the American Type Culture Collection, human α1 integrin plasmid (clone 3RA) (31), generously provided by Eugene Marcantonio (Columbia University, New York), and a plasmid containing a 2.5-kb human β1 cDNA insert, generously provided by Larry Fitzgerald (University of Utah, Salt Lake City). A purified 2.0-kb human β-actin cDNA was purchased from CLONTECH.
Cell Surface Biotinylation and Immunoprecipitation Analyses.
Surface labeling with biotin was performed essentially as described (32) except that cells were suspended at a final concentration of 2 × 106 cells/ml and NHS-LC-biotin (Pierce) was dissolved in PBS and added to cells at a final concentration of 1 mM. The labeling reaction was allowed to proceed for 30 min at room temperature with gentle agitation to maintain cells in suspension. After washing twice in PBS with 50 mM ammonium chloride to eliminate and quench the biotinylating reagent, cells were lysed in detergent-containing immunoprecipitation buffer as described previously (25). After extraction for 30 min at 4°C, 1.0 ml lysates were centrifuged (29,000 × g) at 4°C for 30 min. To control for differences in cell recovery and/or biotinylation efficiency, equal volumes of lysates were subjected to polyacrylamide gel electrophoresis and transferred to poly(vinylidene difluoride) membrane (Millipore) and total biotinylated protein was visualized with chemiluminescence (32). Images were captured on x-ray film and quantitated with a Gel Doc 1000 Imaging Densitometer (Bio-Rad). Differences, if any, were minor, and lysate volumes were normalized accordingly for immunoprecipitation.
Immunoprecipitation was performed as described previously (25). Specific rabbit polyclonal Abs to α1 integrin, α2 integrin, and α3 integrin subunits were purchased from Chemicon. Rabbit polyclonal Ab to the β1 subunit (33) was generously provided by Richard Hynes (Massachusetts Institutes of Technology, Cambridge). All of these Abs were raised to synthetic peptides representing C-terminal sequences of the respective integrin subunits. Immunoprecipitates were subjected to electrophoresis, transferred to polyvinylidene difluoride membrane, visualized with chemiluminescence, and protein bands were quantitated as above. Biotinylated protein standards purchased from Bio-Rad included myosin (Mr 200,000), β-galactosidase (Mr 116,000), and phosphorylase B (Mr 97,400).
Cell Attachment and Spreading Assays.
For cell attachment assays, 96-well plates (catalog no. 3603, Corning Costar) were coated with matrix proteins at a concentration of 10 μg/ml for 1 h followed by 100 mg/ml BSA (catalog no. A9306, Sigma) for 2 h to block remaining protein binding sites. Matrix proteins included human placental collagen I and mouse EHS laminin-1 (Life Technologies, Grand Island, NY) and human placental collagen IV (Collaborative Biomedical Products, Bedford, MA). Cells were prelabeled with fluorescent Cell Tracker Dye (Molecular Probes) at a concentration of 3 μM for 30 min and then incubated with fresh medium for 60 min to remove unincorporated dye. Labeled cells were gently trypsinized and suspended in serum-free medium at 1.5 × 105 cells/ml, mixed with Ab (see below) as indicated for 15 min, and 100 μl of cell suspension was added to each well. After 45 min, unattached cells were removed by washing, and attached cells were quantitated with a fluorescence plate reader. Attachment of cells to wells coated with BSA alone was negligible. Control mouse IgG and mouse monoclonal blocking Ab to the human β1 integrin subunit (clone P4C10) were purified from control serum and P4C10 ascites (Life Technologies), respectively, with the MAPS II Ab purification kit (Bio-Rad). Purified mouse monoclonal blocking Abs to the human α1 integrin subunit (clone 5E8D9) and α2 integrin subunit (clone A2-IIE10) were purchased from Upstate Biotechnology (Lake Placid, NY).
To assess cell spreading on collagen I gels, Vitrogen (bovine dermal collagen I, Collagen Corp.) was neutralized according to the manufacturer’s instructions, diluted to a final concentration of 500 μg/ml with serum-free medium, and added to 24 well plates (500 μl per well). After the diluted Vitrogen had polymerized at 37°C, 1.2 × 105 cells were added to each well with Abs (see above).
Mouse Angiogenesis Assays and Analyses of Angiogenesis Inhibition by Integrin Abs.
The assay used was essentially as described (34) with the following modifications. Athymic NCr nude mice (7–8 week old, females) were injected subcutaneously midway on the right and left back sides with 0.25 ml Matrigel (Collaborative Biomedical Products) at a final concentration of 10 mg/ml together with 2.5 × 106 VEGF-transfected SK-MEL-2 cells (5). Soon after injection, the Matrigel implant solidified and persisted without apparent deterioration throughout the 6-day assay interval. Animals were treated (see Results) with the following purified, low endotoxin (≤0.01 ng/μg protein) hamster mAbs (PharMingen): α1-blocking Ab (clone Ha31/8) and α2-blocking Ab (clone Hmα2), or control isotype standard anti-TNP Ab (clone G235–2356). After six days, the animals were euthanized and dissected, and the implants were photographed.
Implants together with associated skin were fixed for 60 min in 10% formalin and embedded in paraffin. Sections were cut, deparaffinized, and treated with 0.1% trypsin for 30 min at 37°C to enhance antigen availability to CD31 rat mAb (clone MEC13.3, PharMingen). Bound rabbit anti-rat secondary Ab, coupled to horseradish peroxidase (Vector Laboratories), was visualized with True Blue peroxidase substrate (Kirkegaard & Perry Laboratories). Sections were counterstained with Eosin Y (Richard-Allan Scientific, Kalamazoo, MI). Cross-sectional diameters of individual new blood vessels at the implant/host interface were measured from representative photographs (obtained from three specimens of each group) and data expressed as average diameter ± standard deviation (n = 60 for each group). Also, total new blood vessel cross-sectional area was measured from digitized representative photographic images obtained from four specimens of each group with the N.I.H. Image Program 1.61 (n = 26 for each group). To determine statistical significance, data were subjected to the unpaired t test.
RESULTS
VEGF Induction of α1β1 and α2β1 Expression by Human Dermal Microvascular EC.
EC were stimulated with VEGF165 (20 ng/ml) for up to 24 h, and mRNAs encoding α1, α2, α3, and β1 integrin subunits were quantitated by Northern blot analysis. VEGF stimulation resulted in a >6-fold induction of α1 and α2 mRNAs as compared with unstimulated EC; however, no induction of α3 mRNA or β1 mRNA was detected (Fig. 1A and B). As reported by us previously (25), α5 mRNA was not induced by VEGF stimulation (data not shown).
Figure 1.
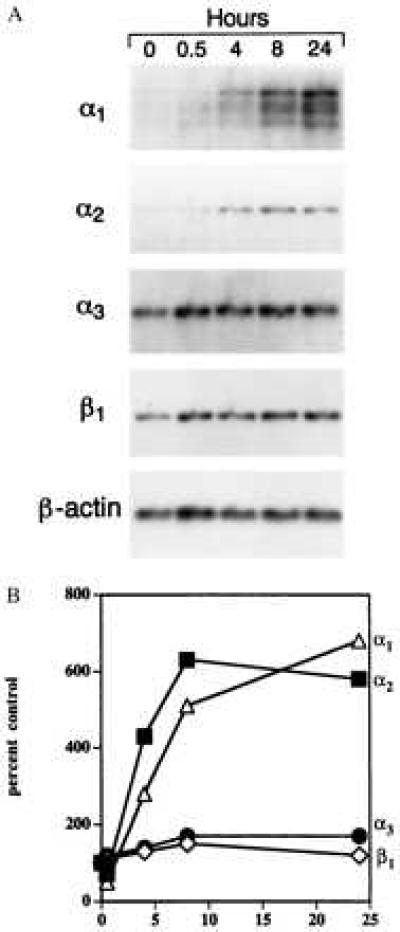
(A) Northern blot analyses of integrin subunit mRNAs in human dermal microvascular EC stimulated with VEGF (20 ng/ml) for up to 24 h. Ten micrograms of total cellular RNA was loaded in each well. (B) Densitometric quantitation of Northern blot analyses. The signal associated with each integrin mRNA was normalized to the internal β-actin mRNA standard to adjust for minor differences in RNA loading.
To determine whether induction of α1 and α2 mRNAs by VEGF translated to increased expression of α1β1 and α2β1 heterodimers at the EC surface, we stimulated cells with VEGF for 72 h or 96 h, labeled cell surface proteins, and subjected equal numbers of cells to surface biotinylation. Minor differences in cell recovery and biotinylation were controlled for by quantitating incorporated biotin (see Materials and Methods). As shown in Fig. 2, stimulation of EC with VEGF resulted in markedly increased expression of α1β1 and α2β1 at the cell surface. The induction of α1β1 and α2β1 was confirmed in multiple experiments (>5), and densitometric quantitation indicated 5- to 7-fold induction for both integrins. In contrast, expression of the α3β1 integrin was not induced by VEGF stimulation (Fig. 2).
Figure 2.
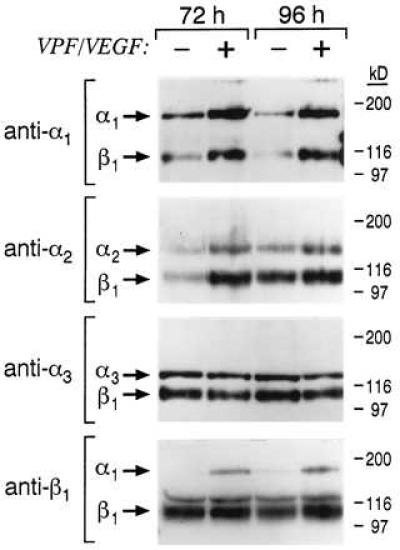
Integrin expression at the surface of dermal microvascular EC following stimulation with VEGF (20 ng/ml) for 72 h and 96 h. Lysates from biotinylated cells were subjected to immunoprecipitation, and immunoprecipitates were subjected to electrophoresis in 7.5% polyacrylamide gels under nonreducing conditions. Control cells were cultured and biotinylated in parallel. As determined by densitometry, α1β1 and α2β1 typically were induced 5- to 7-fold by VEGF treatment.
EC Attachment Mediated by α1β1 and α2β1 Integrins.
The α1β1 and α2β1 integrins bind collagens and laminin-1 (35, 36), and α2β1 also has been reported to bind tenascin (37). However, the ligand binding specificities of these integrins are not absolute and differ among cell types (35, 38). Therefore, we tested attachment of 72 h VEGF-stimulated microvascular EC to collagens I and IV and laminin-1 in the presence of α1-blocking Ab and/or α2-blocking Ab in comparison with β1-blocking Ab and control IgG. As shown in Fig. 3, the α1 Ab and α2 Ab each partially blocked cell attachment to collagen I, and the two Abs in combination inhibited attachment >90%.
Figure 3.
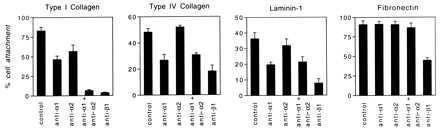
Attachment assays performed with dermal microvascular EC and integrin-blocking Abs. Cells were stimulated with VEGF (20 ng/ml, 72 h) before assay for maximal induction of α1β1 and α2β1. Control IgG and Abs were used at a concentration of 10 μg/ml.
The β1 Ab similarly inhibited attachment >95%. Although α1 Ab and β1 Ab inhibited attachment of VEGF-stimulated cells to collagen IV and laminin-1, attachment to these ligands was not inhibited significantly by α2 Ab. As expected, we observed no inhibition of cell attachment to fibronectin with α1 Ab or α2 Ab. Thus, these experiments indicated that both the α1β1 and α2β1 integrins present on the surface of VEGF-stimulated microvascular EC were important for mediating cell attachment to collagen I and that the α1β1 integrin also mediated attachment to collagen IV and laminin-1.
VEGF-Induced Expression of α1β1 and α2β1: Consequences for EC Interactions with Three-Dimensional Collagen Gels in Vitro.
Interactions between microvascular EC and three-dimensional collagen gels (i.e., polymeric collagen) presumably are more relevant to angiogenesis than interactions between cells and collagen-coated plastic (i.e., planar collagen) (39). Therefore, we investigated the consequences of increased α1β1 and α2β1 expression for interactions between microvascular EC and polymeric collagen. Unstimulated control and 72 h VEGF prestimulated EC were plated on type I collagen gels in the presence of control or integrin-blocking Abs. As shown in Fig. 4, 72 h VEGF prestimulation promoted EC spreading on polymeric collagen as compared with unstimulated EC that attached but did not spread significantly. We obtained similar results with EC embedded in type I collagen (data not shown). Addition of α1-blocking Ab in combination with α2-blocking Ab completely inhibited spreading of the VEGF-stimulated cells (Fig. 4). Addition of α1 Ab and α2 Ab separately resulted in intermediate inhibition of cell spreading indicating that both α1β1 and α2β1 participate in interactions between microvascular EC and polymeric collagen I (not shown). Thus, basal expression of α1β1 and α2β1 by microvascular EC was not sufficient to promote cell spreading on collagen I gels, and VEGF induction of α1β1 and α2β1 expression correlated with EC spreading on collagen I gels that was abolished by a combination of α1-blocking and α2-blocking Abs.
Figure 4.
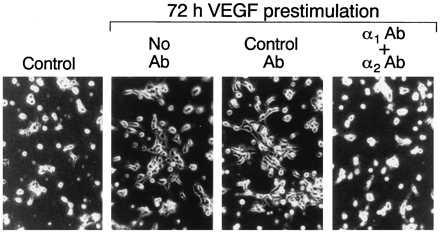
Spreading of dermal microvascular EC on type I collagen gels after 4 h. Control = unstimulated cells cultured in parallel with cells prestimulated with VEGF (20 ng/ml) for 72 h. A combination of α1-blocking Ab and α2-blocking Ab (10 μg/ml of each) abolished cell spreading of the VEGF prestimulated cells; control IgG (20 μg/ml) was without effect.
Inhibition of VEGF-Driven Angiogenesis in Vivo by Abs that Block α1 and α2 Integrins.
To test directly the importance of α1β1 and α2β1 integrins for VEGF-driven angiogenesis in vivo, we used a modified version of a mouse angiogenesis model described previously (34), involving subcutaneous injection of athymic nude mice with Matrigel containing human SK-MEL-2 tumor cells stably transfected for expression of murine VEGF164 (5). Untransfected SK-MEL-2 tumor cells do not provoke an angiogenic response (5), and therefore angiogenesis induced by the VEGF transfectants was entirely or predominantly attributable to VEGF. Each animal received implants by subcutaneous injection, midway on the right and left back sides on day zero. Isotype-matched control hamster mAb (300 μg) or a combination of hamster monoclonal α1 Ab and α2 Ab (150 μg each) were administered to five animals per group by i.p. injection on days 1, 3, and 5. These Abs do not recognize the respective human integrin subunits and, therefore, did not interact with the transfected SKMEL-2 cells. On day 6 animals were sacrificed and implants were photographed and processed for immunohistochemical analyses. Thus, a total of 10 implants per group were analyzed. Findings were highly consistent within each of the two groups, and typical examples are shown in Fig. 5. In the α1 Ab + α2 Ab treatment group, the overlying skin adjacent to the implants contained substantially reduced numbers of grossly visible small tortuous blood vessels in comparison with controls (Fig. 5, Upper). We observed no effects of Abs on the larger pre-existing blood vessels. Consistent with these gross observations, immunohistochemical staining for the EC marker CD31 (40) demonstrated that the average cross-sectional diameter of individual new blood vessels adjacent to the angiogenic stimulus was significantly (P < .001) reduced to 8.4 ± 1.5 μm in the α1 Ab + α2 Ab treatment group, in comparison with 26.2 ± 6.6 μm in the control Ab group (Fig. 5, Lower). This reduction in average new blood vessel diameter translated into a 90% reduction in average cross-sectional area. Similarly, average total new blood vessel cross-sectional area was reduced by 82% in the α1 Ab + α2 Ab treatment group (P < .0001). Thus, the combination of α1-blocking and α2-blocking Abs potently inhibited VEGF-driven angiogenesis in vivo without detectable adverse effects on the pre-existing vasculature.
Figure 5.
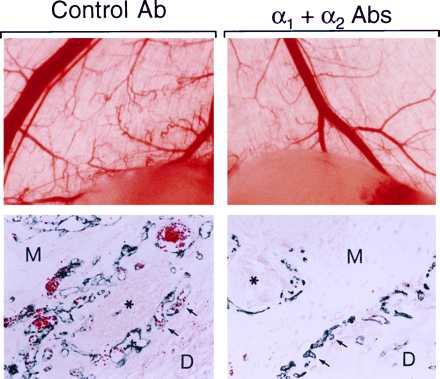
Inhibition of VEGF-driven angiogenesis in adult skin by a combination of α1-blocking Ab and α2-blocking Ab. (Upper) Gross observation after dissection: Matrigel implants, visible at bottom of photographs, together with overlying skin. Small tortuous blood vessels, typical of neovascularization, were visible in the skin adjacent to the implant in animals treated with control Ab (left). These vessels were absent or substantially less visible in animals treated with α1 Ab + α2 Ab (right). In contrast, the larger pre-existing blood vessels appear unaffected by α1 Ab + α2 Ab. (Lower) Light microscopy of paraffin sections: Immunohistochemical staining for CD31 (blue color) illustrates that new blood vessels at the interface between the Matrigel implant (M) and host dermis (D), and in association with large nerves (∗), were reduced in cross-sectional area 90% in the α1 Ab + α2 Ab treated animals (Right), in comparison with controls (Left). Similarly, total new vascular area was reduced 82% (see text). In each panel, two representative vessels are marked with arrows.
DISCUSSION
In vitro, the α1β1 and α2β1 integrins have been shown to function in cell migration (38, 41–43) and in reorganization and contraction of collagen (41, 44). Also, the α2β1 integrin has been implicated in capillary lumen and tube formation by EC (45), EC proliferation in collagen (46), and cell survival (47). Thus, the previously established functions of these integrins raised the possibility of important roles for these integrins in angiogenesis in vivo.
Findings reported here indicate that the angiogenesis factor VEGF potently induces microvascular EC expression of both the α1β1 and α2β1 integrins and that each serves significantly as a receptor for collagen I on this cell type. Accordingly, VEGF induction of α1β1 and α2β1 substantially promoted EC spreading on collagen I gels in vitro. Furthermore, we found that together α1- and α2-blocking mAbs markedly inhibited VEGF-driven angiogenesis in vivo, directly implicating the α1β1 and α2β1 integrins functionally in VEGF-driven angiogenesis.
Previously, deletion of integrin α1 by homologous recombination was shown to be permissive for normal murine development, indicating that α1β1 expression is not essential for development of the vasculature (38). However, that observation is not inconsistent with those reported here because our experiments involved angiogenesis in adult animals rather than embryos and because the α2β1 integrin may compensate for α1β1 during angiogenesis. This latter possibility is supported by our findings that a combination of α1 Ab + α2 Ab was required to abolish both EC attachment to collagen I (Fig. 3) and spreading of VEGF-stimulated EC on collagen I gels (Fig. 4), whereas each Ab alone was only partially inhibitory. Also, because integrins can exhibit trans-dominant effects over other integrins (48), the consequences of blocking an integrin with a specific Ab need not correlate with the phenotype of mice that lack expression of that integrin. Thus, integrin antagonists such as blocking Abs may produce effects that are more severe than those predicted by the phenotype of corresponding null mice.
It has been reported previously that an αvβ3-blocking Ab inhibited tumor angiogenesis (22, 23) and development of the normal vasculature (24) indicating that αvβ3 is important for neovascularization. In addition to α1β1 and α2β1 as reported here, VEGF also induces expression of αvβ3 (3- to 4-fold) (25), suggesting an additional mechanism by which VEGF regulates angiogenesis. In multiple experiments, we found that VEGF-induction of α1β1 and α2β1 was nearly 2-fold greater than VEGF-induction of αvβ3. Future investigations are required to establish the relative contribution of α1β1/α2β1 vs. αvβ3 in angiogenesis assays and in the pathological angiogenesis that occurs in human neoplastic and inflammatory diseases.
Because the normal vasculature is generally quiescent and because angiogenesis is required for neoplastic tumor growth, there is much interest in developing cancer therapies designed to inhibit angiogenesis (49), and our findings suggest that the α1β1 and α2β1 integrins are attractive targets for therapeutic intervention. It could be argued that because α1β1 and α2β1 are expressed normally by microvascular EC (50) and a variety of other cell types (38, 51), considerable toxicity might be associated with systemic administration of α1β1 and α2β1 antagonists. However, integrin function can require activation through signaling pathways inside the cell, and therefore expression of a particular integrin need not correlate with functional activity (32, 52). Furthermore, and consistent with findings reported here, α1β1 was found to be overexpressed by tumor blood vessels (53) and increased expression of α2β1 was demonstrated at the sprouting tips of neonatal blood vessels (54). Thus, it is an intriguing possibility that α1β1 and α2β1 integrins are expressed by quiescent microvascular endothelium in low abundance relative to stimulated endothelium and also that they are less active and therefore less influenced by α1β1 and α2β1 antagonists. Furthermore, because α1β1 and α2β1 integrins promote cell migration, proliferation, and matrix reorganization—none of which are relevant to quiescent cells—it is reasonable to expect that antagonists of α1β1 and α2β1 would most strongly influence dynamic situations such as angiogenesis, where cell proliferation, cell migration, and matrix reorganization are critical. Thus, our findings that Abs to α1β1 and α2β1 selectively inhibited VEGF-driven angiogenesis in vivo without any detectable adverse consequences for the pre-existing vasculature are entirely consistent with these possibilities and suggest that α1β1 and α2β1 antagonists may prove effective in inhibiting the VEGF-driven angiogenesis associated with cancers and other pathologies.
Acknowledgments
We thank Eugene Marcantonio for α1 cDNA, Larry Fitzgerald for β1 cDNA, Richard Hynes for β1 Ab, and Carol Foss for help in preparing the manuscript. This work was supported by a grant from Biochem Therapeutic, National Institutes of Health Grants HL54465, CA69184, and CA64436, and the V. Kann Rasmussen Foundation.
ABBREVIATIONS
- VEGF
vascular endothelial growth factor
- EC
endothelial cell
- Ab
antibody
References
- 1.Dvorak H F, Brown L F, Detmar M, Dvorak A M. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J, Shing Y. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 3.Wilting J, Christ B, Weich H A. Anat Embryol. 1992;186:251–257. doi: 10.1007/BF00174147. [DOI] [PubMed] [Google Scholar]
- 4.Takeshita S, Zheng L P, Brogi E, Kearney M, Pu L-Q, Bunting S, Ferrara N, Symes J F, Isner J M. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claffey K P, Brown L F, del Aguila L F, Tognazzi K, Yeo K-T, Manseau E J, Dvorak H F. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 6.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 7.Millauer B, Longhi M P, Plate K H, Shawver L K, Risau W, Ullrich A, Strawn L M. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- 8.Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E H. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson G S, Pierce E A, Rook S L, Foley E, Webb R, Smith L E H. Proc Natl Acad Sci USA. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Nature (London) 1996;380:435–442. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 11.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller N P H, Risau W, Ullrich A. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 12.Peters K G, De Vries C, Williams L T. Proc Natl Acad Sci USA. 1993;90:8915–8919. doi: 10.1073/pnas.90.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown L F, Yeo K-T, Berse B, Yeo T-K, Senger D R, Dvorak H F, Van De Water L. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fava R A, Olsen N J, Spencer-Green G, Yeo K-T, Yeo T-K, Berse B, Jackman R W, Senger D R, Dvorak H F, Brown L F. J Exp Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detmar M, Brown L F, Claffey K P, Yeo K-T, Kocher O, Jackman R W, Berse B, Dvorak H F. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown L F, Olbricht S M, Berse B, Jackman R W, Matsueda G, Tognazzi K A, Manseau E J, Dvorak H F, Van De Water L. J Immunol. 1995;154:2801–2807. [PubMed] [Google Scholar]
- 17.Pierce E A, Avery R L, Foley E D, Aiello L P, Smith L E H. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breier G, Risau W. Trends Cell Biol. 1996;6:454–456. doi: 10.1016/0962-8924(96)84935-x. [DOI] [PubMed] [Google Scholar]
- 19.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 20.Hynes R O. Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- 21.Drake C J, Davis L A, Little C D. Dev Dyn. 1992;193:83–91. doi: 10.1002/aja.1001930111. [DOI] [PubMed] [Google Scholar]
- 22.Brooks P C, Montgomery A M P, Rosenfeld M, Reisfeld R A, Hu T, Klier G, Cheresh D A. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 23.Brooks P C, Stromblad S, Klemke R, Visscher D, Sarkar F H, Cheresh D A. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake C J, Cheresh D A, Little C D. J Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- 25.Senger D R, Ledbetter S R, Claffey K P, Papadopoulos-Sergiou A, Perruzzi C A, Detmar M. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 26.Senger D R. Am J Pathol. 1996;149:1–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzpatrick T B, Eisen A Z, Wolff K, Freedberg I M, Austen K F. In: The Structure and Development of Skin. Jeffers J D, Scott E, White J, editors. New York: McGraw-Hill; 1987. [Google Scholar]
- 28.Davis G E. Biochem Biophys Res Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 29.Detmar M, Imcke E, Ruszczak Z, Orfanos C E. J Invest Dermatol. 1990;39:219S–222S. doi: 10.1111/1523-1747.ep12875807. [DOI] [PubMed] [Google Scholar]
- 30.Richard L, Velasco P, Detmar M. In: Methods in Tissue Engineering. Morgan J R, Yarmuch M, editors. Totowa, NJ: Humana; 1997. , in press. [Google Scholar]
- 31.Briesewitz R, Epstein M R, Marcantonio E E. J Biol Chem. 1993;268:2989–2996. [PubMed] [Google Scholar]
- 32.Shaw L M, Lotz M M, Mercurio A M. J Biol Chem. 1993;268:11401–11408. [PubMed] [Google Scholar]
- 33.Marcantonio E E, Hynes R O. J Cell Biol. 1988;106:1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passaniti A, Taylor R M, Pili R, Guo Y, Long P V, Haney J A, Pauly R R, Grant D S, Martin G R. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 35.Elices M J, Hemler M E. Proc Natl Acad Sci USA. 1989;86:9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ignatius M J, Large T H, Houde M, Tawil J W, Barton A, Esch F, Carbonetto S, Reichardt L F. J Cell Biol. 1990;111:709–720. doi: 10.1083/jcb.111.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriramarao P, Mendler M, Bourdon M A. J Cell Sci. 1993;105:1001–1012. doi: 10.1242/jcs.105.4.1001. [DOI] [PubMed] [Google Scholar]
- 38.Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 39.Vernon R B, Sage E H. Am J Pathol. 1995;147:873–883. [PMC free article] [PubMed] [Google Scholar]
- 40.Berger R, Albelda S M, Berd D, Ioffreda M, Whitaker D, Murphy G F. J Cutaneous Pathol. 1993;20:399–406. doi: 10.1111/j.1600-0560.1993.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 41.Gotwals P J, Chi-Rosso G, Lindner V, Yang J, Ling L, Fawell S E, Koteliansky V E. J Clin Invest. 1996;11:2469–2477. doi: 10.1172/JCI118693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skinner M P, Raines E W, Ross R. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- 43.Keely P J, Fong A M, Zutter M M, Santoro S A. J Cell Sci. 1995;108:595–607. doi: 10.1242/jcs.108.2.595. [DOI] [PubMed] [Google Scholar]
- 44.Schiro J A, Chan B M C, Roswit W T, Kassner P D, Pentland A P, Hemler M E, Eisen A Z, Kupper T S. Cell. 1991;67:403–410. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- 45.Davis G E, Camarillo C W. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 46.Gamble J R, Matthias L J, Meyer G, Kaur P, Russ G, Faull R, Berndt M C, Vadas M A. J Cell Biol. 1993;121:931–943. doi: 10.1083/jcb.121.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saelman E U M, Kelly P J, Santoro S A. J Cell Sci. 1995;108:3531–3540. doi: 10.1242/jcs.108.11.3531. [DOI] [PubMed] [Google Scholar]
- 48.Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg M H. Mol Biol Cell. 1996;7:1939–1951. doi: 10.1091/mbc.7.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folkman J. New Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 50.Defilippi P, van Hinsbergh V, Bertolotto A, Rossino P, Silengo L, Tarone G. J Cell Biol. 1991;114:855–863. doi: 10.1083/jcb.114.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zutter M M, Santoro S A. Am J Pathol. 1990;137:113–120. [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 53.Fabbri M, Castellani P, Gotwals P J, Kotelianski V, Zardi L, Zocchi M R. Tissue Antigens. 1996;48:47–51. doi: 10.1111/j.1399-0039.1996.tb02604.x. [DOI] [PubMed] [Google Scholar]
- 54.Enenstein J, Kramer R H. J Invest Dermatol. 1994;103:381–386. doi: 10.1111/1523-1747.ep12395390. [DOI] [PubMed] [Google Scholar]


