Abstract
Tumor hypoxia or a reduction of the tissue oxygen tension is a key microenvironmental factor for tumor progression and treatment resistance in solid tumors. Because hypoxic tumor cells have been demonstrated to be more resistant to ionizing radiation, hypoxia has been a focus of laboratory and clinical research in radiation therapy for many decades. It is believed that proper detection of hypoxic regions would guide treatment options and ultimately improve tumor response. To date, most clinical efforts in targeting tumor hypoxia have yielded equivocal results due to the lack of appropriate patient selection. However, with improved understanding of the molecular pathways regulated by hypoxia and the discovery of novel hypoxia markers, the prospect of targeting hypoxia has become more tangible. This chapter will focus on the development of clinical biomarkers for hypoxia targeting.
Keywords: hypoxia, biomarkers, polarographic electrode, imaging, 2-Nitroimidazole compounds, endogenous markers, HIF, CA IX, Glut-1, Osteopontin, VEGF
Introduction
Hypoxia is a common phenomenon in solid neoplasms. It arises when tissue oxygen demands exceed the oxygen supply from the vasculature. Hypoxic regions develop within solid tumors due to aberrant blood vessel formation, fluctuations in blood flow and increasing oxygen demands from rapid tumor expansion.1 That hypoxia exists in human tumors was first demonstrated by Thomlinson and Gray in 1955.2 It was subsequently noted that hypoxia limits tumor cell response to radiation and chemotherapy and predisposes them to metastasis; these findings resulted in substantial laboratory and clinical efforts to overcomethis microenvironmental effect.1, 3–5 Unfortunately, most clinical trials targeting hypoxia have yielded inconclusive results to date.6–10 The lack of improved outcomes from hypoxia targeting could be partially attributed to poor patient selection for hypoxia targeted therapies.11 Therefore, considerable efforts have been devoted to identify clinical markers for tumor hypoxia. These hypoxic markers could be used to identify patients most likely to benefit from a hypoxia-sensitizing treatment regimens. Finally it has been proposed that measurement of hypoxia may also be a method to monitor treatment efficacy.
At the present time, there exist several clinical approaches for detecting tumor hypoxia. However, none of these approaches represents a clear “gold standard” as agreed by the experts in a recent hypoxia workshop that was convened by the National Cancer Institute.12 A reason for the lack of an ideal biomarker is that there exist extreme spatial and temporal heterogeneities in tissue oxygen levels due to the complex nature of blood supplies and cellular oxygen consumption, and none of the current methods can completely capture this heterogeneity. Existing methods for assessing hypoxia differ from one another in several aspects, including sampled tissue volumes (macroscopic versus microscopic), time intervals (seconds to hours), compartment (intracellular versus interstitial) and type of hypoxia (chronic versus acute). Despite their differences, these approaches or biomarkers can be categorized into 2 groups: direct and indirect. Their advantages and disadvantages are detailed below and summarized in Table 1.
Table 1.
Advantages and disadvantages for different approaches in assessing tumor hypoxia
| Method | Examples | Measure | Spatial resolution | Advantages | Disadvantages |
|---|---|---|---|---|---|
| PO2 Histography | Eppendorf electrode OxyLite fiber optic probe | pO2 | 0.5 mm (thousands of cells) |
|
|
| Direct imaging |
19F-MRI BOLD-MRI EPRI |
pO2 or deoxy- Hb | 0.2–1mm |
|
|
| Exogenous Markers | EF5 Pimonidazole |
Chronic hypoxia | 1.0 um (single cell) |
|
|
| Endogenous hypoxia marker | HIF-1 CA IX Glut-1 |
Biologic hypoxia | 1.0 um (single cell) |
|
|
| Secreted markers | OPN VEGF |
Biologic hypoxia | N/A |
|
|
| PET-based hypoxia imaging |
18F-MISO 18F-FAZA 18F-EF5 18FETNIM 60CuATSM 24I-IAZGP |
Chronic hypoxia | 2–10 mm |
|
|
Hb: Hemoglobin
Direct oxygen measurements in tissues
Needle Electrode
Direct approaches can be applied to tissue (needle electrodes, fiberoptic probes) or blood (measurements or imaging of oxyhemoglobin saturation and oxygen diffusion). Polarographic needle electrodes (pO2 histograph, Eppendorf, Hamburg, Germany) provided the first convincing evidence that hypoxia existed in human solid tumors.13, 14 The sensing electrode, mounted on the tip of a needle, is advanced via a step motor through the tissue, taking rapid measurements (1.4 s) to avoid spurious readings from pressure artifacts caused by the needle. A histogram of oxygen tensions (pO2) can then be obtained from multiple sampling points along different tracks. Normal tissues typically show a Gaussian pO2 distribution with the median value between 40–60 mm Hg; whereas tumors invariably show lower pO2 measurements (Figure 1). Clinical investigations with the microelectrodes have illustrated that regions of hypoxia can be found in a wide range of human tumors, including cancers of the brain, head and neck (HN), lung, breast, rectum, pancreas, cervix and prostate.15 Several studies have showed that low tumor pO2, defined by either the median value or the hypoxic fraction (% readings below 2.5 or 5 mm Hg), correlated with poor treatment outcomes in HN, cervical, prostate and lung cancers.16–23 One study also found that tumor pO2 predicted for pathologically persistent neck nodes in patients undergoing a neck dissection for clinical N2–3 necks after chemoradiation treatment.24 Pooled data from several institutions in 397 head and neck cancer (HNC) patients provided strong evidence that tumor pO2 is an independent predictor for survival.25 In cervical cancers and sarcomas, lower pO2 has been associated with increased risk of nodal and distant metastasis, respectively.21, 26, 27
Figure 1.
An example of the pO2 distribution as measured by the polarographic electrode in (a) Control normal subcutaneous tissue, (b) Involved neck node and (3) Primary head and neck cancer in the same patient with a head and neck squamous cell carcinoma.
Although the microelectrode technique can directly measure tumor pO2, it does suffer from several drawbacks that make it difficult for general use. These include high machine cost, invasiveness, tumor inaccessibility, pressure dependence, inter-observer variability, failure to distinguish necrosis from hypoxia, and the lack of spatial information on hypoxia. In addition, it primarily measures extracellular oxygen level at a low resolution of 500 cells or greater. Therefore, although the microelectrode has been the most studied approach for assessing hypoxia to date, it is unlikely that this technique can be used routinely to select for patients with hypoxic tumor in large phase III clinical trials.28
Direct imaging
Different techniques can be used to directly image oxygen levels in tissues or blood based on known properties of paramagnetic agents. One such technique is 19F MRI, which employs injectable perfluorocarbons (PFC), whose 19F nuclear magnetic resonance spin lattice is highly sensitive to oxygen, hence allowing measurement of vascular and tissue oxygenation.12 This approach, however, is limited by the requirement of local injection of PFC compounds directly into the tumor for imaging.
Another approach is the blood oxygen level-dependent magnetic resonance imaging (BOLD MRI), whose image contrast rests on the balance between paramagnetic deoxyhemoglobin and diamagnetic oxygehemoglobin and the effect of the latter on MR signals.12 Although BOLD MRI does not require injection of an exogenous contrast agent, its signal can be influence by factors other than hypoxia including blood flow, CO2 tension, hematocrit, pH and biphosphoglycerate. 29 In a recent study of 24 patients with prostate cancer undergoing radical prostatectomy, BOLD MRI was performed preoperatively and correlated with pimonidazole staining, an indirect marker for hypoxia (see below), using a co-registered histologic and imaging grid map of whole mount sections. R2 (MR relaxivity parameter) maps from BOLD MRI yielded high sensitivity but low specificity for defining hypoxic tumor regions stained with pimonidazole.30
EPR or electron paramagnetic resonance imaging detects species with unpaired electrons.12 Molecular oxygen, which has 2 unpaired electrons, can be imaged when a biologically compatible, inert free radical is introduced directly into the tumor. This approach is currently still in preclinical models and is limited by the requirement for direct tumoral injection or implantation of particulate paramagnetic materials.12 It does however, have a theoretical advantage of allowing repeated measurement over a long period of time. To date, clinical experience with these direct imaging approaches, specifically with regards to predicting prognosis is limited and their utility as biomarkers for tumor hypoxia needs to be validated in large clinical trials.
Indirect approach – Injectable markers
Immunohistochemical staining of 2- nitroimidazole compounds on tissue sections
Indirect approaches use injectable molecular reporters of oxygen as endpoints. These reporters include 2-nitroimidazole compounds such as misonidazole (1-(alpha-methoxymethyl ethanol)-2-nitroimidazole)31, pimonidazole (1-(2-nitro-1-imidazolyl)-3-N-piperidino-2-propanolol)32, and EF5 (nitroimidazole[2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentaflouropropyl) acetamide).33 These compounds form stable adducts with intracellular macromolecules and this binding is prohibited at higher oxygen levels.34 Detection of these adducts with antibodies can then provide quantitative information on the relative oxygenation at cellular resolution.35, 36 In general, 2-nitroimidazole markers stain for areas of chronic hypoxia, at pO2 levels below 10 mm Hg and are more sensitive at severe hypoxic conditions than the microelectrode.36, 37 Although these compounds share a common ring structure and the nitro group, which confer oxygen dependency, their behaviors are not the same due to the different side chains, which significantly affect the pharmacokinetics, accumulation rates and tissue distributions of these compounds. For examples, EF5 is significantly more lipophilic than other 2-nitroimidazole markers, resulting in more even biodistribution in the body with slower whole body drug elimination.38 Pimonidazole accumulation rate is more dependent on pH than other compounds, resulting in more pimonidazole binding on the vessel side in transient hypoxia.35 The differences in the pharmacokinetics and tissue distribution of these agents have been exploited to analyze the hypoxic fluctuation in individual tumors in experimental animal models. Double hypoxia marker assays, in which consecutive injections of two different 2-nitroimidazole compounds were administered before and after treatment, have been used to visualize spatial and temporal changes from carbogen breathing and hydralazine infusion.39, 40 For an excellent review of injectable markers, please see Ljungkvist et al.35
Though widely used in animal studies, there is minimal clinical data regarding the prognostic significance of this approach in cancer patients. In a small study evaluating pimonidazole, microvessel density count and carbonic anhydrase IX (CA IX) binding in 42 HNC, pimonidazole staining was more pronounced at distance > 100 μm from blood vessels than CA IX, suggesting that it is more specific for chronic hypoxia.41 High pimonidazole staining correlated with a higher risk of locoregional relapse in patients treated with radiotherapy (RT) alone but not in those treated with RT plus carbogen and nicotinamide, which were used to modulate tumor hypoxia. In a small study of 16 sarcoma patients, severe hypoxia, defined as ≥ 20% of EF5 binding in the primary tumors, correlated with increased risk of distant metastasis.42 In 18 patients with supratentorial gliomas, increasing EF5 binding was associated with higher tumor grade and shorter time to recurrence.43
This immunohistochemical (IHC) approach, though informative and elegant, is limited by the requirement for exogenous drug administration, additional tumor biopsies for staining and expertise in staining quantification. Because of these limitations, it has not been widely used clinically and needs to be validated in larger studies. An on-going phase III trial is evaluating the use of pimonidazole as a predictive marker. Patients with larynx cancer are randomized to receive accelerated radiation therapy alone or the same treatment with carbogen and nicotinamide.35 The result of this study will help to elucidate the role of pimonidazole as a clinical hypoxia biomarker.
Imaging studies using 2- nitroimidazole compounds
These 2-nitroimidazole compounds can also be labeled with 18F and employed as special tracers for hypoxia imaging using PET or SPECT imaging approaches. The most extensively investigated 2-nitroimidazole tracer is 18Fmisonidazole (F-MISO). Typically, a tumor to blood ratio of ≥ 1.2 has been used as a reasonable cut point between normoxia and hypoxia for F-MISO. Tumor to muscle ratio of F-MISO has been shown to significantly correlate with tumor hypoxic fraction as measured by the polarographic needle electrode in 16 HNC patients.44 Preliminary clinical data suggested that hypoxia imaging with F-MISO could be used to predict treatment outcome and assess treatment response to RT and chemotherapy in solid cancers.45–48 The largest series was in 73 HNC patients where pretreatment F-MISO imaging was an independent prognostic factor for survival when fluorodeoxyglucose standard uptake value, a measure of glucose metabolism, was removed from the model.45 More importantly, in 45 patients with pretreatment F-MISO PET imaging, hypoxic tumors were less likely to fail when treated with a combined regimen of chemoradiation and the hypoxic cytotoxic agent Tirapazamine (TPZ) when compared to a non-TPZ regimen.49 Serial F-MISO imaging at 3–4 weeks into the radiation course for HNC has also been performed in 2 studies, which showed either eliminated or decreased F-MISO uptake in most if not all patients.50, 51 However, the prognostic implication of decreased F-MISO uptake during treatment is not clear. Although all patients with persistent or increased F-MISO uptake failed, a substantial portion of those with improved uptake also recurred, making it hard to interpret the results of these studies.
Other PET tracers that have been investigated in patients are 18F-EF5, 18F-FAZA and 64Cu-ATSM. Clinical data on the 18F-EF5 and 18F-FAZA are minimal with small series reporting the feasibility of imaging patients with solid tumor.12, 52 Cu-ATSM (Copper (II) diacetyl-bis(N4)-methylthiosemicarbazone) is activated under hypoxia by a different mechanism than the 2-nitroimidazole compounds and mechanistic studies suggested the involvement of the mitochondria.53 It has been shown to correlate strongly with oxygen electrode measurements in animal tumors and to be feasible to use for imaging tumor hypoxia in cancer patients.54 It also enjoys the advantage of having a short half-life (23 minutes), which makes it possible to perform serial imaging studies on the same patients.12 In two small series of less than 20 patients each, Cu-ATSM has been shown to accumulate in tumors in cervical and non-small cell lung cancers (NSCLC) and its uptake pattern was predictive for treatment response in NSCLC and survival in cervical cancers.55, 56
From the radiation-targeting standpoint, PET imaging with hypoxic tracers can theoretically be combined with intensity-modulated radiotherapy (IMRT) for dose escalation to improve local control. The ability of IMRT for dose painting provides a tantalizing possibility of delivering higher doses to hypoxic regions visualized by PET tracers in the tumor without increasing normal tissue toxicity. The premise of such dose escalation requires that hypoxic regions in the tumor remain relatively stable before and during the course of IMRT treatment over several weeks. Serial F-MISO imaging at 3–4 weeks into the radiation course for HNC suggested that the regions of persistent hypoxia, if present, were located in the pre-treatment hypoxic volumes.50, 51 In addition, feasibility studies suggested that dose painting can be applied to target hypoxic regions in the tumor using F-MISO or 18F-FAZA PET/CT-guided IMRT.51, 57, 58 The main question is whether this is clinically achievable in patients, and clinical trials for dose escalation using hypoxia PET imaging is warranted. However, since PET-based hypoxia imaging requires expensive dedicated equipments for both imaging and tracer generation, it is only available at selective academic institutions, which may limit its general use.
Indirect approach – Tissue Endogenous markers
Endogenous molecular markers for tumor hypoxia represent proteins and genes whose expressions are induced by hypoxic exposure. One of the most studied oxygen response pathways is that mediated by the hypoxia inducible factor-1 (HIF-1), which regulates genes that are involved in cell metabolism, angiogenesis, invasion, metastasis and apoptosis. HIF-1 and several of its downstream targets such as Glut-1 (glucose transporter-1), CA IX and vascular endothelial growth factor (VEGF) have been widely investigated as prognostic markers in HNC with mixed results. Table 2 summarized representative large clinical series (>40 patients) that focused on the prognostic significance of HIF-1, CA IX and Glut-1 for certain solid tumors (HN, cervix, breast and lung cancers). It is by no mean an exhaustive list but it does show that in general, elevated expression of these markers portends poorer outcomes in patients treated with non-surgical, and for certain sites, surgical therapies.
Table 2.
Significance of HIF-1, CA IX & Glut-1 endogenous markers for selective solid cancers, including head and neck, cervical, breast & non-small cell lung cancers
| HIF Markers | Head & Neck Cancer | |||
|---|---|---|---|---|
| Author | Hypoxia Marker | # Pts | Treatment | Survival |
| Aebersold89 | HIF-1α | 98 | RT or RT+C | LRC, DFS, OS (Multivariate) |
| Koukourakis90 | HIF-1α, HIF-2α | 75 | RT+C | LRC, OS for HIF-2α only (Multivariate) |
| Beasley91 | HIF-1α | 69 | S | Improved DFS, OS (Multivariate) |
| Hui92 | HIF-1α, HIF-2α, CA IX, VEGF | 90 (NPC) | RT or RT+C | PFS for HIF-1α + CA IX & HIF-1α + VEGF but not individual marker (Multivariate) |
| Kyzas93 | HIF-1α, VEGF | 81 | S | OS for VEGF, not for HIF-1α (Univariate) |
| Winter94 | HIF-2α, CA IX | 140 | S | CSS & DFS for HIF-1α (multivariate); Improved with addition of HIF-2α; No significance for CAIX |
| Koukourakis95 | HIF-2α, CA IX | 198 | RT (CHART vs conventional) | LRC, OS for both (Multivariate) |
| HIF Markers | Cervical Cancers | |||
| Bachtiary96 | HIF-1α | 67 | RT | PFS, CSS (Multivariate) |
| Birner97 | HIF-1α | 91 | S ± RT | DFS, OS (Multivariate) |
| Burri98 | HIF-1α | 78 | RT ± C | LPFS (Univariate), OS (Multivariate) |
| Hutchison99 | HIF-1α | 99 | RT | No (DFS, MFS, LRFS as endpoints) |
| Haugland78 | HIF-1α | 42 | RT | No (DFS as endpoint) |
| HIF Markers | Breast Cancers | |||
| Dales100 | HIF-1α | 745 | S | DMFS, OS (Multivariate) |
| Kronblad101 | HIF-1α, VEGF | 377, Stage II, premenopause | S + RT+ C/Tam | RFS for low grade tumors (Multivariate) |
| Schindl102 | HIF-1α | 206 (LN +) | S ± C | DFS, OS (Multivariate) |
| Vleugel103 | HIF-1α, CA IX, Glut 1 | 166 | S + C | DFS (Only HIF-1α evaluated & Univariate) |
| Trastour104 | HIF-1α, CA IX | 132 | S ± R ± C/Tam | DFS, MFS for HIF-1α mainly (Multivariate) |
| HIF Markers | Non-Small Cell Lung Cancers | |||
| Giatromanolaki 105 | HIF-1α, HIF-2α,VEGF | 98 | S | OS for HIF-2α only (Multivariate) |
| Swinson106 | HIF-1α | 172 | S ± RT ± C | OS for CA IX only (Multivariate) |
| Kim107 | HIF-1α, CA IX | 74, Stage I-II | S | DFS for CA IX Only (Multivariate) |
| CA IX only | Head & Neck Cancer | |||
| Beasley108 | CA IX | 79 | Surgery | Not assessed |
| Koukourakis109 | CA IX | 75 | CRT | LRCS, OS (Univariate only) |
| Jonathan110 | CAIX, Glut-1, Glut-3 | 58 | RT + ARCON | Better LRC & MFS with stronger CA IX & Glut-3 (Univariate) |
| De Schutter111 | CA IX, Glut-1 | 67 | RT ± C | LRC, DFS only for CA IX + Glut-1 (multivariate) |
| CA IX only | Cervix | |||
| Hedley112 | CA IX | 102 | RT ± C | No prognostic significance |
| Loncaster113 | CA IX | 130 | RT | MFS, OS (Multivariate) |
| CA IX only | Breast Cancer | |||
| Span114 | CA IX (mRNA) | 253 | S ± RT ± C/Tam | More resistance to adjuvant systemic treatment (Multivariate) |
| Brennan115 | CA IX | 400, Stage II, premenopause | S + R ±Tam | CSS (Multivariate) |
| Hussain116 | CA IX | 144 | S | OS (Multivariate) |
| Chia | CA IX | ‘103 | S + RT ± C/Tam | OS (Multivariate) |
| CA IX only | Non Small Cell Lung Cancer | |||
| Giatromanolaki117 | CAIX, HIF-1α, HIF-2α | 107 | S | OS (Multivariate) |
| Kon-No118 | CA IX | 134 | S | OS, DFS (Univariate only, not multivariate) |
| Swinson119 | CA IX | 175 | S | OS for perinuclear staining pattern (Multivariate) |
| Glut-1 only | Head & Neck Cancer | |||
| Oliver120 | Glut-1 | 54, OC only | S | LRC, FFR, CSS (Univariate) |
| Kunkle121 | Glut-1 | 118, OC only | S ± RT | OS (multivariate) |
| Mineta122 | Glut-1 | 99, HP only | CRT | PFS (Multivariate) |
| Glut-1 only | Cervical Cancer | |||
| Airley123 | Glut-1 | 121 | RT | MFS (Multivariate) |
| Mayer76 | Glut-1 | 47 | S ± C or RT ± C | OS, PFS (Univariate only) |
| Glut-1 only | Breast Cancer | |||
| Stackhouse124 | Glut-1 | 141 | S ± RT ± C/Tam | No significance for recurrence |
| Glut-1 only | Non-Small Cell Lung Cancer | |||
| Minami125 | Glut-1 | 47 | S | OS (Multivariate) |
| Nguyen126 | Glut-1 | 53 | S ± R ± C | No significance for DFS |
Pt: patients; S: Surgery; RT: radiotherapy; C: chemotherapy; Tam: Tamoxifen; ARCON: Carbogen and nicotidamide; CHART: Continuous hyperfractionated accelerated radiotherapy
OC: Oral cavity cancer; NPC: Nasopharyngeal carcinoma; HP: Hypopharyngeal carcinoma; LN+: Lymph node positive; LRC: locoregional control; DFS: disease-free survival; PFS: Progression-free survival: OS: Overall survival; CSS: Cancer specific suvival; LPFS: Local progression-free survival; MFS: Metastasis-free survival; FFR: Freedom from relapse
Other endogenous tissue makers that have been studied in relation to hypoxia include VEGF, BNIP3 (Bcl-2/adenovirus E1B 19 kDA-interacting enzyme), Lysyl oxidase (LOX), Lactate Dehydrogenase isoenzyme-5 (LDH-5), Plasminogen activator inhibitor-1 (PAI-1) and Galectin-1.59–67 At the present time, the clinical relevance of these markers is unclear since results are either conflicting such as those for VEGF 67–69 or intriguing as for LOX and LDH-5 but would need further validation from larger, more uniform series.
The advantage of endogenous markers is that levels of these proteins can be assessed on archival materials, thereby allowing rapid correlation to treatment outcomes. In addition it requires neither the injection of foreign material nor any additional invasive procedure beyond that of a biopsy at diagnosis. A significant drawback to these approaches is that these proteins can be regulated by factors other than hypoxia. For example, HIF-1α expression can be influenced by several non-hypoxic stimuli including nitric oxide, cytokines (interleukin-β and tumor necrosis factor-α), trophic stimuli (serum, insulin, insulin-like-growth factors) and oncogenes (p53, Vsrc, PTEN, etc).70–73 Comparison of the staining patterns between endogenous and injectable markers showed that the former, in general, stained more diffusely and closer to the blood vessels than the latter, suggesting other modes of induction and activation at a wider range of oxygen concentration.41, 74 In addition, there is minimal correlation between intensity of endogeneous marker staining and tumor pO2. In the most vigorous studies where tumor biopsies were performed along the paths of the polarographic electrode and stained for HIF-1α, CA IX and Glut-1, there was no observed correlation between any staining parameter and measured pO2.75–77
Other major drawbacks of endogenous tissue makers include the lack of standardization for the IHC protocol and result interpretation for individual markers across different laboratories. For example, different studies used different HIF-1 antibodies with different sensitivities and binding affinities for the protein. The duration of tissue fixation is highly variable and extended fixation time has been shown to reduce HIF-1α expression.78 Comparisons of results are further hampered by diverse image evaluation techniques and different interpretations of positive staining. While some studies employed quantitative image analysis, others used visual estimation of positive stained cell number and intensity. Moreover, while most authors assessed only nuclear staining for transcriptional factors such as HIF-1α, others also included cytoplasmic expression. Finally, since only a very small portion of tumor is assessed with endogenous markers, this approach is prone to sampling bias and does not reflect hypoxia heterogeneity in the entire tumor. These drawbacks make it less desirable to use individual endogenous markers alone to select for patients with hypoxic tumors.
To circumvent this dilemma, suggestions have been made to combine several endogenous markers together to improve hypoxia specificity. For example, gene expression analysis has been used to generate a hypoxia gene signature or a hypoxia metagene to predict treatment outcomes in several solid tumors, including HNC.79, 80 Chi et al described a gene array signature for hypoxia in breast cancer patients that strongly predicted for poor outcomes.79 Using gene expression profiling of 59 HNC, Winter et al generated a hypoxia metagene by identifying genes whose expression clustered with 10 known hypoxia regulated genes.80 They found that this metagene was able to predict recurrence-free survival in an independent HNC data set as well as overall survival in another breast cancer series. We have also used a combination of gene expression and proteomic analyses to identify novel hypoxia induced proteins. After confirming their hypoxic inducibility in cell lines and animal models, we investigated their utility in combination with CA IX to predict outcomes by staining a HNC tissue array with known tumor pO2. These studies resulted in a panel of 4 hypoxia markers (CA IX, Lysyl oxidase (LOX), Galectin-1 and Ephrin A1) that can be used to predict treatment outcomes in terms of cancer-specific survival.60 (Figure 2) These endogenous hypoxia signatures, though promising, need to be validated in larger independent datasets before they can be used in the clinical settings.
Figure 2.
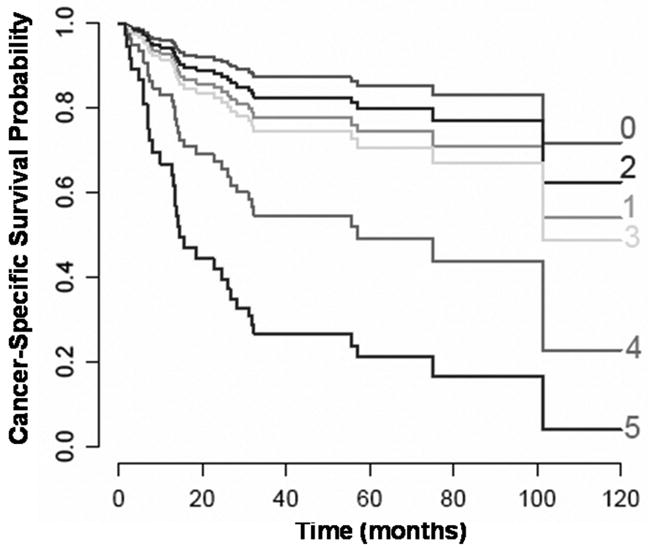
Cancer-specific survival by hypoxia marker score comprised of Galectin-1, Ephrin A1, Lysyl Oxidase, CA IX cytoplasmic and CA IX membrane staining, where a score of 1 was assigned to strong staining for each marker and a score of 0 to negative and week staining. This has been adjusted for age and hemoglobin levels, 2 other significant factors on univariate analysis.
Indirect approach - Secreted hypoxia markers
Our laboratory has focused on identifying secreted markers of hypoxia that can be rapidly and inexpensively measured in the blood. Two markers that have been tested clinically with mixed results are VEGF and osteopontin (OPN). Although circulating VEGF levels were elevated in cancer patients81, 82 and in those with acute hypoxia such as obstructive apnea83, the relationship between tumor hypoxia and systemic VEGF levels is unclear. Dunst et al found that serum VEGF levels independently correlated with hypoxic tumor subvolume in 56 HNC patients.84 However, it also correlated with total tumor volume, hemoglobin level and platelet counts. They did not report on the clinical significance of serum VEGF levels in terms of treatment outcomes. In contrast, we did not find a direct relationship between plasma VEGF and tumor pO2 in 48 HNC patients in our study (unpublished observations). We did however found a small but significant relationship between OPN level and tumor pO2 in our patient cohort.85 This was confirmed by Nordsmark et al.86 In addition, plasma OPN was an independent and significant predictor for treatment outcomes in these patients and another independent group of HNC patients.87 These results were confirmed by the DAHANCA group in a larger cohort of HNC patients treated with radiation therapy +/− nimorazole, a hypoxic cell radiosensititizer.88 Intriguingly, only patients with high pretreatment circulating OPN levels benefited from nimorazole whereas those with low-intermediate levels did not, suggesting that OPN may be use to select patients for hypoxia targeting. Further validation of this marker is ongoing in another set of HNC patients treated with or without Tirapazamine (TPZ), a hypoxic cell cytotoxin. Advantages of secreted markers for hypoxia is that they are non-invasive, easy to measure, inexpensive and allow for serial measurements through the course of therapies. However, they do suffer the same drawbacks faced by endogenous tissue markers including the lack of method standardization and regulation by factors other than hypoxia. In addition, spatial information is lost and contributions from non-cancerous tissues and other pathological processes such as inflammation cannot be ruled out. Therefore, combining one or multiple of these markers with another approach of assessing hypoxia such as imaging is more desirable than using them alone.
Conclusion
In summary there exist presently several ways or biomarkers for assessing hypoxia. However, none of these approaches, by itself, can capture all the intricacies of tumor hypoxia and its heterogeneity. Therefore, none is currently considered the “gold standard” biomarker for hypoxia. In theory, a combination of biomarkers is more robust than a single marker; yet, there is no current clinical data to support such a hypothesis. Further work is needed to validate the utility of incorporating multiple biomarkers such as imaging plus tissue or blood markers to identify patients with hypoxic tumors for future targeting.
Acknowledgments
This work was support by the National Institute of Health, 1 R01 CA118582-01 (QTL) and under Ruth L. Kirschstein National Research Service Award 5T32 CA09302 (DC). Its contents are solely the responsibility of the authors and do no necessarily represent the official views of the NIH.
Bibliography
- 1.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 2.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy KA, Teicher BA, Rockwell S, et al. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem Pharmacol. 1980;29:1–8. doi: 10.1016/0006-2952(80)90235-x. [DOI] [PubMed] [Google Scholar]
- 4.Graeber TG, Peterson JF, Tsai M, et al. Hypoxia induces the accumulation of p53 protein, but the activation of a G1-phase checkpoint by low oxygen conditions is independent of p53 status. Mol Cell Biol. 1994;14:6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 6.Fazekas JT, Pajak TF, Wasserman T, et al. Failure of misonidazole-sensitized radiotherapy to impact upon outcome among stage III–IV squamous cancers of the head and neck. Int J Radiat Oncol Biol Phys. 1987;13:1155–1160. doi: 10.1016/0360-3016(87)90188-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee DJ, Moini M, Giuliano J, et al. Hypoxic sensitizer and cytotoxin for head and neck cancer. Ann Acad Med Singapore. 1996;25:397–404. [PubMed] [Google Scholar]
- 8.Overgaard J, Hansen HS, Anderson AP, et al. Misonidazole combined with split course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA study. Int J Radiat Oncol Biol Phys. 1989;16:1065–1068. doi: 10.1016/0360-3016(89)90917-6. [DOI] [PubMed] [Google Scholar]
- 9.Van den Bogaert W, van der Schueren E, Horiot JC, et al. The EORTC randomized trial on three fractions per day and misonidazole (trial no. 22811) in advanced head and neck cancer: long-term results and side effects. Radiother Oncol. 1995;35:91–99. doi: 10.1016/0167-8140(95)01538-r. [DOI] [PubMed] [Google Scholar]
- 10.Lee DJ, Pajak TF, Stetz J, et al. A phase I/II study of the hypoxic cell sensitizer misonidazole as an adjunct to high fractional dose radiotherapy in patients with unresectable squamous cell carcinoma of the head and neck: a RTOG randomized study (#79-04) Int J Radiat Oncol Biol Phys. 1989;16:465–470. doi: 10.1016/0360-3016(89)90343-x. [DOI] [PubMed] [Google Scholar]
- 11.Hill RP. Targeted treatment: insights from studies of osteopontin and hypoxia. Lancet Oncol. 2005;6:733–734. doi: 10.1016/S1470-2045(05)70363-6. [DOI] [PubMed] [Google Scholar]
- 12.Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 13.Gatenby RA, Kessler HB, Rosenblum JS, et al. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988;14:831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- 14.Wendling P, Manz R, Thews G, et al. Heterogeneous oxygenation of rectal carcinomas in humans: a critical parameter for preoperative irradiation? Adv Exp Med Biol. 1984;180:293–300. doi: 10.1007/978-1-4684-4895-5_28. [DOI] [PubMed] [Google Scholar]
- 15.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Brizel DM, Dodge RK, Clough RW, et al. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 17.Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57:39–43. doi: 10.1016/s0167-8140(00)00223-1. [DOI] [PubMed] [Google Scholar]
- 18.Rudat V, Stadler P, Becker A, et al. Predictive value of the tumor oxygenation by means of pO2 histography in patients with advanced head and neck cancer. Strahlenther Onkol. 2001;177:462–468. doi: 10.1007/pl00002427. [DOI] [PubMed] [Google Scholar]
- 19.Le QT, Chen E, Salim A, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–1514. doi: 10.1158/1078-0432.CCR-05-2049. [DOI] [PubMed] [Google Scholar]
- 20.Hockel M, Schlenger K, Aral B, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 21.Hockel M, Schlenger K, Hockel S, et al. Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res. 1999;59:4525–4528. [PubMed] [Google Scholar]
- 22.Fyles A, Milosevic M, Hedley D, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol. 2002;20:680–687. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- 23.Movsas B, Chapman JD, Hanlon AL, et al. Hypoxic prostate/muscle pO2 ratio predicts for biochemical failure in patients with prostate cancer: preliminary findings. Urology. 2002;60:634–639. doi: 10.1016/s0090-4295(02)01858-7. [DOI] [PubMed] [Google Scholar]
- 24.Brizel DM, Prosnitz RG, Hunter S, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58:1418–1423. doi: 10.1016/j.ijrobp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 27.Nordsmark M, Alsner J, Keller J, et al. Hypoxia in human soft tissue sarcomas: adverse impact on survival and no association with p53 mutations. Br J Cancer. 2001;84:1070–1075. doi: 10.1054/bjoc.2001.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone HB, Brown JM, Phillips TL, et al. Oxygen in human tumors: correlations between methods of measurement and response to therapy. Radiat Res. 1993;136:422–434. [PubMed] [Google Scholar]
- 29.Howe FA, Robinson SP, McIntyre DJ, et al. Issues in flow and oxygenation dependent contrast (FLOOD) imaging of tumours. NMR Biomed. 2001;14:497–506. doi: 10.1002/nbm.716. [DOI] [PubMed] [Google Scholar]
- 30.Hoskin PJ, Carnell DM, Taylor NJ, et al. Hypoxia in prostate cancer: correlation of BOLD-MRI with pimonidazole immunohistochemistry-initial observations. Int J Radiat Oncol Biol Phys. 2007;68:1065–1071. doi: 10.1016/j.ijrobp.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Chapman JD. Hypoxic sensitizers--implications for radiation therapy. N Engl J Med. 1979;301:1429–1432. doi: 10.1056/NEJM197912273012606. [DOI] [PubMed] [Google Scholar]
- 32.Raleigh JA, Calkins-Adams DP, Rinker LH, et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998;58:3765–3768. [PubMed] [Google Scholar]
- 33.Evans SM, Hahn S, Pook DR, et al. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 2000;60:2018–2024. [PubMed] [Google Scholar]
- 34.Varghese AJ, Gulyas S, Mohindra JK. Hypoxia-dependent reduction of 1-(2-nitro-1-imidazolyl)-3-methoxy-2-propanol by Chinese hamster ovary cells and KHT tumor cells in vitro and in vivo. Cancer Res. 1976;36:3761–3765. [PubMed] [Google Scholar]
- 35.Ljungkvist AS, Bussink J, Kaanders JH, et al. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007;167:127–145. doi: 10.1667/rr0719.1. [DOI] [PubMed] [Google Scholar]
- 36.Evans SM, Koch CJ. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 2003;195:1–16. doi: 10.1016/s0304-3835(03)00012-0. [DOI] [PubMed] [Google Scholar]
- 37.Raleigh JA, Chou SC, Arteel GE, et al. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumors. Radiat Res. 1999;151:580–589. [PubMed] [Google Scholar]
- 38.Koch CJ, Hahn SM, Rockwell K, Jr, et al. Pharmacokinetics of EF5 [2-(2-nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide] in human patients: implications for hypoxia measurements in vivo by 2-nitroimidazoles. Cancer Chemother Pharmacol. 2001;48:177–187. doi: 10.1007/s002800100324. [DOI] [PubMed] [Google Scholar]
- 39.Ljungkvist AS, Bussink J, Rijken PF, et al. Changes in tumor hypoxia measured with a double hypoxic marker technique. Int J Radiat Oncol Biol Phys. 2000;48:1529–1538. doi: 10.1016/s0360-3016(00)00787-2. [DOI] [PubMed] [Google Scholar]
- 40.van Laarhoven HW, Klomp DW, Kamm YJ, et al. In vivo monitoring of capecitabine metabolism in human liver by 19fluorine magnetic resonance spectroscopy at 1.5 and 3 Tesla field strength. Cancer Res. 2003;63:7609–7612. [PubMed] [Google Scholar]
- 41.Kaanders JH, Wijffels KI, Marres HA, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–7074. [PubMed] [Google Scholar]
- 42.Evans SM, Fraker D, Hahn SM, et al. EF5 binding and clinical outcome in human soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2006;64:922–927. doi: 10.1016/j.ijrobp.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 43.Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10:8177–8184. doi: 10.1158/1078-0432.CCR-04-1081. [DOI] [PubMed] [Google Scholar]
- 44.Gagel B, Reinartz P, Dimartino E, et al. pO(2) Polarography versus positron emission tomography ([(18)F] fluoromisonidazole, [(18)F]-2-fluoro-2′-deoxyglucose). An appraisal of radiotherapeutically relevant hypoxia. Strahlenther Onkol. 2004;180:616–622. doi: 10.1007/s00066-004-1229-y. [DOI] [PubMed] [Google Scholar]
- 45.Rajendran JG, Schwartz DL, O’Sullivan J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehtio K, Eskola O, Viljanen T, et al. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59:971–982. doi: 10.1016/j.ijrobp.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Thorwarth D, Eschmann SM, Holzner F, et al. Combined uptake of [18F]FDG and [18F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother Oncol. 2006;80:151–156. doi: 10.1016/j.radonc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Eschmann SM, Paulsen F, Reimold M, et al. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med. 2005;46:253–260. [PubMed] [Google Scholar]
- 49.Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 50.Hicks RJ, Rischin D, Fisher R, et al. Utility of FMISO PET in advanced head and neck cancer treated with chemoradiation incorporating a hypoxia-targeting chemotherapy agent. Eur J Nucl Med Mol Imaging. 2005;32:1384–1391. doi: 10.1007/s00259-005-1880-2. [DOI] [PubMed] [Google Scholar]
- 51.Thorwarth D, Eschmann SM, Paulsen F, et al. Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol Biol Phys. 2007;68:291–300. doi: 10.1016/j.ijrobp.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 52.Souvatzoglou M, Grosu AL, Roper B, et al. Tumour hypoxia imaging with [(18)F]FAZA PET in head and neck cancer patients: a pilot study. Eur J Nucl Med Mol Imaging. 2007 doi: 10.1007/s00259-007-0424-3. [DOI] [PubMed] [Google Scholar]
- 53.Fujibayashi Y, Taniuchi H, Yonekura Y, et al. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38:1155–1160. [PubMed] [Google Scholar]
- 54.Chao KS, Bosch WR, Mutic S, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1171–1182. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 55.Grigsby PW, Malyapa RS, Higashikubo R, et al. Comparison of Molecular Markers of Hypoxia and Imaging with (60)Cu-ATSM in Cancer of the Uterine Cervix. Mol Imaging Biol. 2007;9:278–283. doi: 10.1007/s11307-007-0095-2. [DOI] [PubMed] [Google Scholar]
- 56.Dehdashti F, Mintun MA, Lewis JS, et al. In vivo assessment of tumor hypoxia in lung cancer with (60)Cu-ATSM. Eur J Nucl Med Mol Imaging. 2003 doi: 10.1007/s00259-003-1130-4. [DOI] [PubMed] [Google Scholar]
- 57.Lee NY, Mechalakos JG, Nehmeh S, et al. Fluorine-18-Labeled Fluoromisonidazole Positron Emission and Computed Tomography-Guided Intensity-Modulated Radiotherapy for Head and Neck Cancer: A Feasibility Study. Int J Radiat Oncol Biol Phys. 2007 doi: 10.1016/j.ijrobp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grosu AL, Souvatzoglou M, Roper B, et al. Hypoxia Imaging With FAZA-PET and Theoretical Considerations With Regard to Dose Painting for Individualization of Radiotherapy in Patients With Head and Neck Cancer. Int J Radiat Oncol Biol Phys. 2007;69:541–551. doi: 10.1016/j.ijrobp.2007.05.079. [DOI] [PubMed] [Google Scholar]
- 59.Le QT, Shi G, Cao H, et al. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le QT, Kong C, Lavori PW, et al. Expression and Prognostic Significance of a Panel of Tissue Hypoxia Markers in Head-and-Neck Squamous Cell Carcinomas. Int J Radiat Oncol Biol Phys. 2007;69:167–175. doi: 10.1016/j.ijrobp.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 61.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 62.Giatromanolaki A, Koukourakis MI, Gatter KC, et al. BNIP3 expression in endometrial cancer relates to active hypoxia inducible factor 1a pathway and prognosis. J Clin Pathol. 2007 doi: 10.1136/jcp.2007.046680. [DOI] [PubMed] [Google Scholar]
- 63.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway--a report of the Tumour Angiogenesis Research Group. J Clin Oncol. 2006;24:4301–4308. doi: 10.1200/JCO.2006.05.9501. [DOI] [PubMed] [Google Scholar]
- 64.Giatromanolaki A, Sivridis E, Gatter KC, et al. Lactate dehydrogenase 5 (LDH-5) expression in endometrial cancer relates to the activated VEGF/VEGFR2(KDR) pathway and prognosis. Gynecol Oncol. 2006;103:912–918. doi: 10.1016/j.ygyno.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 65.Giatromanolaki A, Koukourakis MI, Sowter HM, et al. BNIP3 expression is linked with hypoxia-regulated protein expression and with poor prognosis in non-small cell lung cancer. Clin Cancer Res. 2004;10:5566–5571. doi: 10.1158/1078-0432.CCR-04-0076. [DOI] [PubMed] [Google Scholar]
- 66.de Witte JH, Sweep CG, Klijn JG, et al. Prognostic impact of urokinase-type plasminogen activator (uPA) and its inhibitor (PAI-1) in cytosols and pellet extracts derived from 892 breast cancer patients. Br J Cancer. 1999;79:1190–1198. doi: 10.1038/sj.bjc.6690191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linderholm BK, Lindh B, Beckman L, et al. Prognostic correlation of basic fibroblast growth factor and vascular endothelial growth factor in 1307 primary breast cancers. Clin Breast Cancer. 2003;4:340–347. doi: 10.3816/cbc.2003.n.039. [DOI] [PubMed] [Google Scholar]
- 68.De Paola F, Granato AM, Scarpi E, et al. Vascular endothelial growth factor and prognosis in patients with node-negative breast cancer. Int J Cancer. 2002;98:228–233. doi: 10.1002/ijc.10118. [DOI] [PubMed] [Google Scholar]
- 69.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11:1434–1440. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 70.Stroka DM, Burkhardt T, Desbaillets I, et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. Faseb J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 71.Zundel W, Schindler C, Haas-Kogan D, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- 72.Zelzer E, Levy Y, Kahana C, et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. Embo J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 74.Janssen HL, Haustermans KM, Sprong D, et al. HIF-1A, pimonidazole, and iododeoxyuridine to estimate hypoxia and perfusion in human head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2002;54:1537–1549. doi: 10.1016/s0360-3016(02)03935-4. [DOI] [PubMed] [Google Scholar]
- 75.Mayer A, Hockel M, Vaupel P. Carbonic anhydrase IX expression and tumor oxygenation status do not correlate at the microregional level in locally advanced cancers of the uterine cervix. Clin Cancer Res. 2005;11:7220–7225. doi: 10.1158/1078-0432.CCR-05-0869. [DOI] [PubMed] [Google Scholar]
- 76.Mayer A, Hockel M, Wree A, et al. Microregional expression of glucose transporter-1 and oxygenation status: lack of correlation in locally advanced cervical cancers. Clin Cancer Res. 2005;11:2768–2773. doi: 10.1158/1078-0432.CCR-04-2344. [DOI] [PubMed] [Google Scholar]
- 77.Mayer A, Wree A, Hockel M, et al. Lack of correlation between expression of HIF-1alpha protein and oxygenation status in identical tissue areas of squamous cell carcinomas of the uterine cervix. Cancer Res. 2004;64:5876–5881. doi: 10.1158/0008-5472.CAN-03-3566. [DOI] [PubMed] [Google Scholar]
- 78.Haugland H, Vukovic V, Pintilie M, et al. Expression of hypoxia-inducible factor-1alpha in cervical carcinomas: correlation with tumor oxygenation. Int J Radiat Oncol Biol Phys. 2002;53:854–861. doi: 10.1016/s0360-3016(02)02815-8. [DOI] [PubMed] [Google Scholar]
- 79.Chi JT, Wang Z, Nuyten DS, et al. Gene Expression Programs in Response to Hypoxia: Cell Type Specificity and Prognostic Significance in Human Cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winter SC, Buffa FM, Silva P, et al. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 2007;67:3441–3449. doi: 10.1158/0008-5472.CAN-06-3322. [DOI] [PubMed] [Google Scholar]
- 81.Riedel F, Gotte K, Schwalb J, et al. Serum levels of vascular endothelial growth factor in patients with head and neck cancer. Eur Arch Otorhinolaryngol. 2000;257:332–336. doi: 10.1007/s004059900208. [DOI] [PubMed] [Google Scholar]
- 82.Salven P, Manpaa H, Orpana A, et al. Serum vascular endothelial growth factor is often elevated in disseminated cancer. Clin Cancer Res. 1997;3:647–651. [PubMed] [Google Scholar]
- 83.Imagawa S, Yamaguchi Y, Higuchi M, et al. Levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea--hypopnea syndrome. Blood. 2001;98:1255–1257. doi: 10.1182/blood.v98.4.1255. [DOI] [PubMed] [Google Scholar]
- 84.Dunst J, Stadler P, Becker A, et al. Tumor hypoxia and systemic levels of vascular endothelial growth factor (VEGF) in head and neck cancers. Strahlenther Onkol. 2001;177:469–473. doi: 10.1007/pl00002428. [DOI] [PubMed] [Google Scholar]
- 85.Le QT, Sutphin PD, Raychaudhuri S, et al. Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res. 2003;9:59–67. [PMC free article] [PubMed] [Google Scholar]
- 86.Nordsmark M, Eriksen JG, Gebski V, et al. Differential risk assessments from five hypoxia specific assays: The basis for biologically adapted individualized radiotherapy in advanced head and neck cancer patients. Radiother Oncol. 2007 doi: 10.1016/j.radonc.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Petrik D, Lavori PW, Cao H, et al. Plasma osteopontin is an independent prognostic marker for head and neck cancers. J Clin Oncol. 2006;24:5291–5297. doi: 10.1200/JCO.2006.06.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Overgaard J, Eriksen JG, Nordsmark M, et al. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 2005;6:757–764. doi: 10.1016/S1470-2045(05)70292-8. [DOI] [PubMed] [Google Scholar]
- 89.Aebersold DM, Burri P, Beer KT, et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. [PubMed] [Google Scholar]
- 90.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:1192–1202. doi: 10.1016/s0360-3016(02)02848-1. [DOI] [PubMed] [Google Scholar]
- 91.Beasley NJ, Leek R, Alam M, et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62:2493–2497. [PubMed] [Google Scholar]
- 92.Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 93.Kyzas PA, Stefanou D, Batistatou A, et al. Hypoxia-induced tumor angiogenic pathway in head and neck cancer: an in vivo study. Cancer Lett. 2005;225:297–304. doi: 10.1016/j.canlet.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 94.Winter SC, Shah KA, Han C, et al. The relation between hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression with anemia and outcome in surgically treated head and neck cancer. Cancer. 2006;107:757–766. doi: 10.1002/cncr.21983. [DOI] [PubMed] [Google Scholar]
- 95.Koukourakis MI, Bentzen SM, Giatromanolaki A, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006;24:727–735. doi: 10.1200/JCO.2005.02.7474. [DOI] [PubMed] [Google Scholar]
- 96.Bachtiary B, Schindl M, Potter R, et al. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9:2234–2240. [PubMed] [Google Scholar]
- 97.Birner P, Schindl M, Obermair A, et al. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–4696. [PubMed] [Google Scholar]
- 98.Burri P, Djonov V, Aebersold DM, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:494–501. doi: 10.1016/s0360-3016(02)04579-0. [DOI] [PubMed] [Google Scholar]
- 99.Hutchison GJ, Valentine HR, Loncaster JA, et al. Hypoxia-inducible factor 1alpha expression as an intrinsic marker of hypoxia: correlation with tumor oxygen, pimonidazole measurements, and outcome in locally advanced carcinoma of the cervix. Clin Cancer Res. 2004;10:8405–8412. doi: 10.1158/1078-0432.CCR-03-0135. [DOI] [PubMed] [Google Scholar]
- 100.Dales JP, Garcia S, Meunier-Carpentier S, et al. Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int J Cancer. 2005;116:734–739. doi: 10.1002/ijc.20984. [DOI] [PubMed] [Google Scholar]
- 101.Kronblad A, Jirstrom K, Ryden L, et al. Hypoxia inducible factor-1alpha is a prognostic marker in premenopausal patients with intermediate to highly differentiated breast cancer but not a predictive marker for tamoxifen response. Int J Cancer. 2006;118:2609–2616. doi: 10.1002/ijc.21676. [DOI] [PubMed] [Google Scholar]
- 102.Schindl M, Schoppmann SF, Samonigg H, et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8:1831–1837. [PubMed] [Google Scholar]
- 103.Vleugel MM, Greijer AE, Shvarts A, et al. Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. J Clin Pathol. 2005;58:172–177. doi: 10.1136/jcp.2004.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trastour C, Benizri E, Ettore F, et al. HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer. 2007;120:1451–1458. doi: 10.1002/ijc.22436. [DOI] [PubMed] [Google Scholar]
- 105.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Swinson DE, Jones JL, Cox G, et al. Hypoxia-inducible factor-1 alpha in non small cell lung cancer: relation to growth factor, protease and apoptosis pathways. Int J Cancer. 2004;111:43–50. doi: 10.1002/ijc.20052. [DOI] [PubMed] [Google Scholar]
- 107.Kim SJ, Rabbani ZN, Dewhirst MW, et al. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer. 2005 doi: 10.1016/j.lungcan.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 108.Beasley NJ, Wykoff CC, Watson PH, et al. Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res. 2001;61:5262–5267. [PubMed] [Google Scholar]
- 109.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Hypoxia-regulated carbonic anhydrase-9 (CA9) relates to poor vascularization and resistance of squamous cell head and neck cancer to chemoradiotherapy. Clin Cancer Res. 2001;7:3399–3403. [PubMed] [Google Scholar]
- 110.Jonathan RA, Wijffels KI, Peeters W, et al. The prognostic value of endogenous hypoxia-related markers for head and neck squamous cell carcinomas treated with ARCON. Radiother Oncol. 2006;79:288–297. doi: 10.1016/j.radonc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 111.De Schutter H, Landuyt W, Verbeken E, et al. The prognostic value of the hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head and neck squamous cell carcinoma treated by radiotherapy +/− chemotherapy. BMC Cancer. 2005;5:42. doi: 10.1186/1471-2407-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hedley D, Pintilie M, Woo J, et al. Carbonic anhydrase IX expression, hypoxia, and prognosis in patients with uterine cervical carcinomas. Clin Cancer Res. 2003;9:5666–5674. [PubMed] [Google Scholar]
- 113.Loncaster JA, Harris AL, Davidson SE, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- 114.Span PN, Bussink J, Manders P, et al. Carbonic anhydrase-9 expression levels and prognosis in human breast cancer: association with treatment outcome. Br J Cancer. 2003;89:271–276. doi: 10.1038/sj.bjc.6601122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brennan DJ, Jirstrom K, Kronblad A, et al. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res. 2006;12:6421–6431. doi: 10.1158/1078-0432.CCR-06-0480. [DOI] [PubMed] [Google Scholar]
- 116.Hussain SA, Ganesan R, Reynolds G, et al. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer. 2007;96:104–109. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–7998. [PubMed] [Google Scholar]
- 118.Kon-no H, Ishii G, Nagai K, et al. Carbonic anhydrase IX expression is associated with tumor progression and a poor prognosis of lung adenocarcinoma. Lung Cancer. 2006;54:409–418. doi: 10.1016/j.lungcan.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 119.Swinson DE, Jones JL, Richardson D, et al. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol. 2003;21:473–482. doi: 10.1200/JCO.2003.11.132. [DOI] [PubMed] [Google Scholar]
- 120.Oliver RJ, Woodwards RT, Sloan P, et al. Prognostic value of facilitative glucose transporter Glut-1 in oral squamous cell carcinomas treated by surgical resection; results of EORTC Translational Research Fund studies. Eur J Cancer. 2004;40:503–507. doi: 10.1016/j.ejca.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 121.Kunkel M, Reichert TE, Benz P, et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–1024. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 122.Mineta H, Miura K, Takebayashi S, et al. Prognostic value of glucose transporter 1 expression in patients with hypopharyngeal carcinoma. Anticancer Res. 2002;22:3489–3494. [PubMed] [Google Scholar]
- 123.Airley R, Loncaster J, Davidson S, et al. Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res. 2001;7:928–934. [PubMed] [Google Scholar]
- 124.Stackhouse BL, Williams H, Berry P, et al. Measurement of glut-1 expression using tissue microarrays to determine a race specific prognostic marker for breast cancer. Breast Cancer Res Treat. 2005;93:247–253. doi: 10.1007/s10549-005-5158-y. [DOI] [PubMed] [Google Scholar]
- 125.Minami K, Saito Y, Imamura H, et al. Prognostic significance of p53, Ki-67, VEGF and Glut-1 in resected stage I adenocarcinoma of the lung. Lung Cancer. 2002;38:51–57. doi: 10.1016/s0169-5002(02)00108-3. [DOI] [PubMed] [Google Scholar]
- 126.Nguyen XC, Lee WW, Chung JH, et al. FDG uptake, glucose transporter type 1, and Ki-67 expressions in non-small-cell lung cancer: correlations and prognostic values. Eur J Radiol. 2007;62:214–219. doi: 10.1016/j.ejrad.2006.12.008. [DOI] [PubMed] [Google Scholar]



