Abstract
Objective
Centralized adiposity, insulin resistance, excess iron, and elevated oxidative stress place postmenopausal women at risk for atherosclerotic cardiovascular disease (CVD). The objective of this study was to determine the relationship among excess iron, oxidative stress, and centralized fat mass in healthy postmenopausal women.
Methods
The parent project recruited healthy women for a randomized, double-blind, clinical trial designed to examine the effect of soy isoflavones on bone. At baseline (n = 122), we measured three antioxidant enzymes, iron status indices (serum ferritin among others), oxidative stress indices (oxidized low-density lipoprotein [oxLDL], urinary isoprostanes [PGF2α], protein carbonyls, DNA damage), and waist, hip, and thigh fat mass using dual-energy x-ray absorptiometry (DXA). We calculated insulin resistance using the homeostasis model assessment (HOMA). Multiple regression analysis was used to determine the CVD risk factors that contributed to oxidative stress and centralized fat mass (waist + hip/thigh = AndGynFM ratio).
Results
Almost 14% (p < 0.0005) of the variability in oxLDL was accounted for by AndGynFM ratio (6.1%, p < 0.0005), age (4.0%, p = 0.012), and serum iron (2.8%, p = 0.053). Similarly, 16% (p < 0.0001) of the variability in PGF2α was accounted for by the AndGynFM ratio (4.8%, p = 0.011), HOMA (3.9%, p = 0.021), and serum iron (2.7%, p = 0.054). We accounted for 33% (p ≤ 0.0001) of the variability in AndGynFM ratio by high-density lipoprotein cholesterol (HDL-C) (4.3%, p = 0.008), ferritin (4.9%, p = 0.005), HOMA (4.5%, p = 0.006), oxLDL (2.6%, p = 0.04), and PGF2α (3.0%, p = 0.025).
Conclusions
Our study suggests that reducing centralized fat mass and maintaining a favorable lipid profile, antioxidant status, and iron status all may be important in protecting postmenopausal women from atherosclerotic CVD.
Introduction
Oxidative stress, body iron stores, blood lipids, and body fat typically increase with age, especially after menopause, because of the loss of endogenous estrogen production. Oxidative stress or compromised antioxidant status is thought to be involved in the etiology of diseases, including cancer, atherosclerotic cardiovascular disease (CVD), and cataracts, as well as the aging process.1 Favorable oxidative status is defined as a balance between reactive oxygen species (ROS) and the antioxidant defense system. Iron through free radicals causes oxidation of lipids and proteins, contributing to atherosclerosis or vasoconstriction by promoting oxidized low-density lipoprotein (oxLDL) and by promoting isoprostanes.2 The cessation of menses contributes to a general increase in body iron, translating into elevated serum ferritin,3 as evidenced by 3-fold greater ferritin in postmenopausal compared with premenopausal women.4 Although controversial,5–7 some epidemiological studies show a positive relationship between body iron stores and atherosclerotic CVD risk.8,9 Elevated ferritin concentration in postmenopausal women was associated with an increased risk of ischemic stroke,9,10 possibly due to the interaction of other CVD risk factors, particularly adiposity, that accelerate atherogenesis by stimulating oxidative stress.
Some studies do not support the role of iron in oxidative stress. Iron supplementation did not increase oxLDL,11,12 and body iron stores were not related to coronary heart disease (CHD) or oxLDL.13 However, women with high iron stores and elevated total cholesterol (TC) were shown to be at greater risk for carotid atherosclerosis than those with only elevated iron stores.14 Thus, in a healthy population, iron excess may not be a major concern; however, in persons with high oxidative stress, hyperlipidemia, and centralized adiposity, iron excess may place them at greater CVD risk.15,16 The hypothesis that elevated iron stores are causally linked to CVD17 suggests that oxidative imbalance is the central biological mechanism for the greater incidence of heart disease in postmenopausal compared with premenopausal women.
Research is limited on the relationship among excess iron, oxidative stress, and body fat distribution, despite the independent association of each factor with CVD risk. A positive association between serum ferritin and indices of central adiposity,16,18 insulin resistance,19,20 and metabolic syndrome21 suggests a role of excess iron in obesity and CVD. The objective of our study was to determine the relationship among atherosclerotic CVD risk factors, particularly oxidative stress indices, centralized fat mass, and body iron status in healthy, nonobese postmenopausal women.
Subjects and Methods
Study design
Postmenopausal women were recruited for the Soy Isoflavones for Reducing Bone Loss (SIRBL) clinical trial, a randomized, double-blind, 3-year intervention that was designed to examine the effects of two doses of soy isoflavones on bone loss in postmenopausal women. The aim of this ancillary study was to determine the relationship among specific atherosclerotic CVD risk factors at baseline, including body iron stores, oxidative stress, and centralized fat mass. The study protocol, consent form, and subject-related materials were approved by the Institutional Review Board (IRB) at ISU. Approval for the dual-energy x-ray absorptiometry (DXA) procedure was obtained from our institution's IRB and appropriate safety board. We obtained informed consent from all women at the start of prebaseline screening.
Subject selection
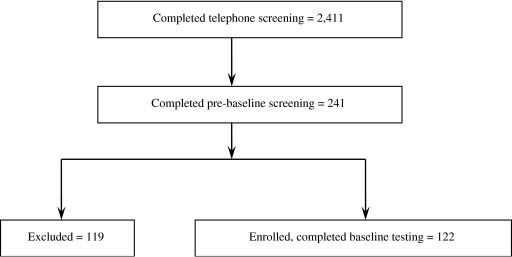
We recruited subjects throughout the state of Iowa primarily through direct mailing, stories in local newspapers, regional radio advertisements, and other recruiting avenues (Fig. 1). Telephone interviews (n = 2411) were conducted to ensure that women met inclusion/exclusion criteria: postmenopausal women ≤65 years of age, cessation of menses (≥1 but ≤8 years), body mass index (BMI) (kg/m2) ranging from18.5 through 29.9. We excluded vegans, smokers, and high alcohol consumers (>7 servings/week). Based on a medical history questionnaire and blood chemistry profile, we also excluded women with bone disease or any other chronic disease and those women who had a first-degree relative with breast cancer. We also excluded women who chronically used medications, such as cholesterol-lowering or antihypertensive medications, oral or topical hormone or estrogen therapy, selective estrogen receptor modulators, calcitonin, frequent use of antibiotics, or any previous use of bisphosphonates.
FIG. 1.
Subject enrollment flow chart.
Data collection
At baseline, we obtained from each subject a health and medical history and a nutrition history using interviewer-administered questionnaires. We assessed usual dietary intake using a food frequency questionnaire (FFQ) from Block Dietary Data Systems (Berkeley, CA). Fasted blood samples were drawn between 7 and 8 am and processed as serum, plasma, and erythrocyte samples. We separated serum and plasma from whole blood by centrifuging for 15 minutes at 4°C at 1000g and stored aliquots at −80°C until analyses. Each subject collected a 24-hour urine sample; we measured total volume and froze aliquots at −20°C until analysis. A certified clinical reference laboratory (Laboratory Corporation of America, Kansas City, MO) analyzed each subject's serum for the blood lipid profile (TC, LDL cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], triacylglycerol [TG]) and other blood chemistry markers. We measured oxidative stress indices (protein carbonyls, oxLDL, urinary isoprostanes, specifically 15-isoprostane F2α [PGF2α], 8-hydroxy-2′-deoxyguanosine [8-OHdG]), antioxidant enzymes (catalase, glutathione peroxidase [GPX], superoxide dismutase [SOD]), insulin, and serum ferritin in the laboratory at Iowa State University. We calculated the percentage intraassay and interassay coefficient of variation (CV) for each method using a pooled sample as a quality control. The intraassay CV (%) was 2.5, 4.8, 3.2, and 2.5, and the interassay CV (%) was 3.4, 5.2, 17, and 3.2, for protein carbonyls, oxLDL, PGF2α, and 8-OHdG, respectively. The intraassay CV (%) was 5.3, 4.0, 8.7, and 4.9, and the interassay CV (%) was 8.4, 7.5, 10.9, and 5.6, for catalase, GPX, SOD, and ferritin, respectively.
We used erythrocyte lysate to measure antioxidant enzyme (catalase, GPX, SOD) activity and plasma to determine protein carbonyls (per mg creatinine) (Cayman Chemical Company, Ann Arbor, MI) and oxLDL (ALPCO Diagnostics, Windham, NH). We determined urinary PGF2α (Oxford Biomedical Research, Oxford, MI) using enzyme-linked immunoassay (ELISA) kits. Urine is better than blood to quantify PGF2α because some factors, such as aspirin, may suppress the circulating concentration,22 and urine is more stable than blood. We measured urinary 8-OHdG to assess DNA damage using an ELISA kit (Stressgen Bioreagents, Victoria, BC, Canada). We measured serum ferritin to determine iron stores using a radioimmunoassay (RIA) kit (Ramco Diagnostics, Stafford, TX). Fasting insulin was measured using an RIA kit (LINCO Research, St. Charles, MO), and the homeostatic model assessment (HOMA) of insulin resistance for each subject was calculated using the following formula.23
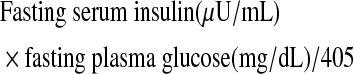 |
A trained anthropometrist measured standing height and weight; waist circumference and sagittal diameter (Holtain-Kahn abdominal caliper, Crosswell, Crymych Dyfed, U.K.) and these were used as anthropometric indices of centralized adiposity. Sagittal diameter was measured at the narrowest section between the small of the back and the navel, with subjects relaxed in the supine position. Centralized adiposity was also assessed using DXA (Delphi W Hologic Inc, Waltham, MA) by a certified DXA operator who conducted a whole body scan on each subject. One evaluator sectioned each whole body DXA scan into waist, hip, and thigh regions based on bone landmarks,24,25 and these subregions were analyzed using special software (Discovery Version 12.3:7). The androidal/gynoidal fat mass (AndGynFM) ratio was calculated for each subject:
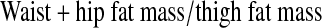 |
Statistical analysis
Statistical analyses were performed with SAS (version 9.0, Cary, NC), with p < 0.05 as the level of significance for all analyses. Descriptive statistics included medians and range for these data, as most were not normally distributed. Pearson correlation analysis was used initially to examine the correlations (data not shown) among the atherosclerotic CVD risk factors (oxidative stress, antioxidant status, iron status, and centralized adiposity). Classes of variables in modeling the outcomes of interest (oxidative stress indicators and centralized adiposity) included all covariates that were biologically plausible or significantly related based on the Pearson correlation analysis. Multiple regression analysis with backward selection was used to determine the CVD risk factors that contributed to oxidative stress and centralized fat mass in these healthy postmenopausal women.
Results
Subject characteristics
The subjects' descriptive characteristics and daily nutrient intakes at baseline are shown in Table 1. The median age of subjects was 55 years, and time since menopause was 2.7 years. The BMI of women ranged from normal to overweight, and 47% had a BMI >25.0 kg/m2. Median dietary intake of macronutrients and selected antioxidant nutrients (vitamins A, C, E, and selenium) for these women was within their dietary recommended intakes (DRIs). Great variability was noted in dietary intake, as reflected by extreme minimum and maximum values, a common limitation of memory-dependent methods of assessment. Descriptive data for adiposity indices, oxidative stress indices, antioxidant enzymes, iron status indices, blood lipid profile, glucose and insulin concentrations, and insulin resistance (HOMA) are presented in Table 2.
Table 1.
Subject Characteristics (n = 122)
| Measurement | Mediana | Range (minimum–maximum) |
|---|---|---|
| Age (years) | 55.2 | (47.3–61.8) |
| Time since menopause (years) | 2.7 | (1.0–7.9) |
| Weight (kg) | 68.1 | (47.8–89.2) |
| Height (cm) | 165.6 | (150.6–178.4) |
| BMI (kg/m2) | 24.9 | (18.9–29.8) |
| Dietary intake/dayb | ||
| Energy (KJ) | 6649 | (1771–19109) |
| Protein (g) | 64 | (20–168) |
| Carbohydrate (g) | 195 | (33–476) |
| Total fat (g) | 67 | (17–247) |
| Saturated fat (g) | 20 | (5–65) |
| Polyunsaturated fatty acids (g) | 16.1 | (2.5–70.4) |
| Iron (mg) | 11.4 | (3.1–45.6) |
| Vitamin A (RE) | 1286 | (360–5163) |
| Vitamin C (mg) | 100 | (19–325) |
| Vitamin E (mg) | 9 | (2–38) |
| Selenium (μg) | 75 | (23–227) |
Median values are reported for subject characteristics and dietary intake, as most were not normally distributed.
Dietary intake was assessed using a food frequency questionnaire (Block Dietary Data Systems).
Table 2.
Baseline Descriptive Measurements (n = 122)
| Measurement | Mediana | Range (minimum-maximum) |
|---|---|---|
| Iron indices | ||
| Ferritin (ng/mL) | 53.1 | (4.8–175.1) |
| Hemoglobin (g/dL) | 13.7 | (11.4–16.0) |
| Hematocrit (%) | 40.8 | (33.5–47.6) |
| Serum iron (μg/dL) | 80.0 | (31.0–177.0) |
| Transferrin saturation (%) | 24.0 | (7.0–56.0) |
| Oxidative stress indices | ||
| Urinary isoprostanes (ng/mL) | 2.44 | (1.26–5.82) |
| Oxidized LDL (U/L) | 73.7 | (31.8–130.6) |
| 8-Hydroxy-2′-deoxyguanosine (ng/mL) | 43.7 | (14.5–194.8) |
| Protein carbonyls (nmol/mg protein) | 6.0 | (0.06–67.7) |
| Antioxidant enzymesb | ||
| Catalase (nmol/min/mL) | 41.3 | (5.9–112.5) |
| Superoxide dismutase (U/mL) | 755.6 | (27.3–1874.2) |
| Glutathione peroxidase (nmol/min/mL) | 188.5 | (91.7–430.4) |
| Central adiposity indices | ||
| Waist circumference (cm)c | 76.7 | (62.8–98.6) |
| Sagittal diameter (cm)c | 18.6 | (14.4–24.8) |
| Waist + hip fat mass (kg)d | 5.37 | (1.09–10.1) |
| Thigh fat mass(kg)d | 3.26 | (2.44–8.97) |
| AndGynFM ratiod,e | 1.00 | (0.45–2.20) |
| Blood lipids/lipoproteins | ||
| Total cholesterol (TC) (mg/dL) | 205 | (142–285) |
| Total triacylglycerides (mg/dL) | 81 | (31–290) |
| LDL-C (mg/dL) | 122 | (62–173) |
| HDL-C (mg/dL) | 69 | (42–103) |
| TC/HDL-C ratio | 3.0 | (1.9–4.9) |
| Other | ||
| Fasting insulin (mU/mL) | 12.8 | (4.5–47.2) |
| Fasting glucose (mg/dL) | 87.0 | (66.0–108.0) |
| HOMA6 | 2.72 | (0.83–10.8) |
Median values are reported for baseline descriptive data, as most were not normally distributed.
Data for 5 subjects are missing (n = 117) because of processing errors of erythrocytes at the time of collection.
Assessed by anthropometric measurements.
Assessed by DXA.
AndGynFM ratio, andriodal (waist + hip)/gynoidal (thigh) fat mass ratio.
Homeostasis model assessment of insulin resistance (HOMA) calculated as follows: Fasting insulin (mU/mL) × fasting glucose (mg/dL)/405.23
Oxidative status
The median values for the oxidative stress and antioxidant status indices were within the normal range, based on the manufacturer's assay guidelines. Because the normal range is not firmly established, we cannot determine the proportion of women with abnormal values. There was a wide range (4- to 7-fold) of antioxidant enzyme activity (catalase, SOD, GPX) among the subjects, particularly for catalase and SOD. Similarly, the oxidative stress indices (oxLDL, PGF2α, 8-OHdG, protein carbonyls) varied widely (4- to 100-fold) among subjects, being particularly high for protein carbonyls.
Iron indices
Median values for ferritin, hemoglobin (Hb), serum iron, and transferrin saturation were within the normal range, suggesting that these women did not have iron excess. However, a small percentage (3%) of women had ferritin values >140 ng/mL, and 9% had transferrin saturation <15%. Only 1 woman had anemia, as indicated by Hb = 11.4 g/dL.
Adiposity and insulin resistance
Only 7.4% of women had waist circumference values above the cutoff (88 cm); thus, the majority of these women were not considered at high risk of chronic diseases.26 Although the median fasting insulin was within normal limits, it varied almost 10-fold, whereas the glucose concentration did not vary widely. Four of 122 women had an insulin concentration >20 mU/mL, and 8 had a glucose concentration >100 mg/dL, but these values were not in the diabetic range. The calculated HOMA values ranged from 0.83 to 10.8.
Contributors to oxidative stress and centralized fat mass
Multiple regression analysis was performed to examine the potential contributors to lipid peroxidation and centralized fat mass (Tables 3 and 4). The following variables were included in both the oxLDL and PGF2α models: age, ferritin, serum iron, transferrin saturation, HOMA, intracellular antioxidants (catalase, SOD, GPX), and dietary intake (vitamin E, vitamin C, iron, energy). The variables included in the AndGynFM ratio model were age, ferritin, serum iron, transferrin saturation, HOMA, intracellular antioxidants (catalase, SOD, GPX, oxLDL), oxidative stress indicators (protein carbonyls, oxLDL, 8-OHdG), blood lipids (HDL-C and LDL-C), C-reactive protein (CRP), and energy intake. Almost 14% (p < 0.0005) of the overall variability in oxLDL was accounted for by the AndGynFM ratio (6.1%, p < 0.0005), age (4.0%, p = 0.021), and serum iron (2.8%, p = 0.053). Similarly, 16% (p < 0.0001) of the overall variability in PGF2α was explained by the AndGynFM ratio (4.8%, p = 0.011), HOMA (3.9%, p = 0.021), and serum iron (2.7%, p = 0.054). To determine the contributing factors to centralized fat mass, we chose the ratio of AndGynFM as the dependent variable because this ratio emerged as a significant contributor in the oxidative stress models and also provided a better model than the AndFM. We accounted for 33% (p ≤ 0.0001) of the overall variability in the AndGynFM ratio by HDL-C (4.3%, p = 0.008), ferritin (4.9%, p = 0.005), HOMA (4.5%, p = 0.006), oxLDL (2.6%, p = 0.04), and PGF2α (3.0%, p = 0.025).
Table 3.
Factors Related to Oxidative Stress (n = 122)a
|
oxLDLb: Overall model R2 = 14.0% (Adj R2 = 11.81%), F = 6.40 (p = 0.0005)c | ||||
|---|---|---|---|---|
| Independent variable | Parameter estimate | Percentage varianced | p value | Variance inflation |
| Intercept | −11.40 | 0.674 | ||
| AndGynFM ratio | 13.62 | 6.16 | <0.0044 | 1.03 |
| Age | 1.135 | 4.00 | 0.021 | 1.03 |
| Serum iron | 0.103 | 2.79 | 0.053 | 1.00 |
|
PGF2α: Overall model R2 = 15.87% (Adj R2 = 13.73%), F = 7.42 (p = 0.0001)e | ||||
|---|---|---|---|---|
| Independent variable | Parameter estimate | Percentage varianced | p value | Variance inflation |
| Intercept | 1.142 | 0.0012 | ||
| AndGynFM ratio | 0.617 | 4.81 | 0.011 | 1.19 |
| HOMA | 0.158 | 3.86 | 0.021 | 1.21 |
| Serum iron | 0.005 | 2.71 | 0.054 | 1.02 |
Multiple regression analysis with stepwise selection.
oxLDL, oxidized low-density lipoproteins; PGF2α, urinary isoprostanes; AndGynFM ratio, androidal (waist + hip)/gynoidal (thigh) fat mass ratio; HOMA, homeostasis model assessment of insulin resistance.
Variables eliminated (p > 0.10): age, serum ferritin, transferrin saturation, HOMA, intracellular antioxidants (catalase, SOD, GPX), dietary antioxidants (vitamins E and C), iron, energy intake.
Squared semipartial type II correlation coefficient, which accounts for shared variance among variables.
Variables eliminated (p > 0.10): age, serum ferritin, total cholesterol, intracellular antioxidants (catalase, SOD, GPX), dietary antioxidants (vitamins E and C), iron, and energy intake.
Table 4.
Factors Related to Central Adiposity (n = 122)a
|
AndGynFM ratiob: Overall model R2 = 32.67% (Adj R2 = 29.76%), F = 11.25 (p ≤ 0.0001)c | ||||
|---|---|---|---|---|
| Independent variable | Parameter estimate | Percentage varianced | p value | Variance inflation |
| Intercept | 0.697 | 0.0005 | ||
| Serum ferritin | 0.002 | 4.90 | 0.004 | 1.02 |
| HOMA | 0.066 | 4.52 | 0.006 | 1.21 |
| HDL-C | −0.005 | 4.27 | 0.008 | 1.21 |
| PGF2α | 0.072 | 3.02 | 0.024 | 1.14 |
| OxLDL | 0.003 | 2.62 | 0.035 | 1.06 |
Multiple regression analysis with backward selection.
AndGynFM ratio, androidal (waist + hip)/gynoidal (thigh) fat mass ratio; HOMA, homeostasis model assessment of insulin resistance; PGF2α, urinary isoprostanes; oxLDL, oxidized LDL.
Variables eliminated (p > 0.10): age, serum iron, intracellular antioxidants (catalase, SOD, GPX), oxidative stress indicators (protein carbonyls, oxLDL, 8-OHdG), CRP, energy intake.
Squared semipartial type II correlation coefficient, which accounts for shared variance among variables.
Discussion
Postmenopausal women are at increased risk of atherosclerotic CVD, which may be attributed to dyslipidemia, oxidative stress, elevated iron stores, centralized adiposity, insulin resistance, and other factors. We examined the relationship of oxidative stress with circulating blood lipids, iron, and obesity by including various markers of oxidative damage related to lipid, protein, and DNA. However, the oxidative damage to lipids as indicated by oxLDL and PGF2α were the only markers that emerged as significant contributors. This supports the idea that lipid damage is involved in obesity and the pathogenesis of CVD, whereas oxidatively modified DNA and proteins may be more involved in carcinogenesis27 and cataract formation,28 respectively. Although we measured three indicators of antioxidant enzymes (catalase, SOD, GPX), none of them accounted for the variability in oxidative stress indicators. Perhaps this was because of the tremendous variability in the antioxidant enzyme values in these women.
Adipose tissue is highly metabolic, and abdominal fat induces oxidative stress, which in turn has been shown to cause insulin resistance in men.17 The previous study indicated that circulating PGF2α was related to adiposity and insulin resistance in men, with obese men having a higher concentration than their nonobese counterparts. In both men and women, a high waist circumference was associated with elevated oxLDL, independent of BMI,29 illustrating that abdominal fat mass may induce a greater degree of oxidative stress than overall fat mass. The DXA-derived centralized fat mass (AndGynFM) is advantageous because it is specific for fat and encompasses both internal and external fat, whereas waist circumference reflects both fat and lean mass. Our results support the idea that centralized adiposity was one of the key factors related to both elevated oxLDL and PGF2α, with age being important for oxLDL and HOMA for PGF2α. Our study results support the observed relationship18 between HOMA and lipid-related oxidative stress.
Interestingly, serum iron was positively related (albeit not significantly) to both oxidative stress indices, suggesting that high circulating iron may induce oxidative stress based on the role of iron in generating free radicals. Although the relationship is only suggestive, it does not support the previous study showing an association between iron status and LDL oxidative susceptibility in healthy men and women.11 Perhaps the association between serum iron and oxLDL was not striking in our study because the women were not markedly hypercholesterolemic (LDL-C ≤160 mg/dL), nor did they have elevated iron stores (only 3 women had serum ferritin >140 ng/mL); thus, they were not very susceptible to oxidative damage.
A recent cross-sectional study, showing an association between abdominal adiposity (waist girth and waist/hip ratio) in young adults with early atherosclerosis as evidenced by coronary artery calcification,30 suggests the importance of centralized fat in predicting CHD. Hence, it is important to determine the factors that contribute to centralized adiposity. As expected, our study showed that ferritin, HOMA, HDL-C, PGF2α, and oxLDL contributed to centralized fat mass (Table 4) (AndGynFM ratio). As centralized adiposity is a well-known risk factor for atherosclerotic CVD, the association of ferritin with centralized fat mass suggested a role for iron in androidal fat accumulation. Perhaps excess iron exacerbates centralized adiposity by aggravating insulin resistance,16,31 but it is also possible that centralized adiposity may contribute to elevated ferritin. As an acute-phase protein, ferritin may be elevated in inflammatory conditions, other than excess body iron. Overweight and obese individuals are characterized by low-grade inflammation.32 Thus, increased ferritin may reflect obesity-induced inflammation, a relatively new factor in the etiology of atherosclerotic CVD, with elevations placing postmenopausal women at increased risk of ischemic stroke.9 Our results are contrary to the NHANES III findings that the prevalence of iron deficiency is higher in obese children.33 Obesity has been shown to be negatively associated with circulating iron in younger groups due to the inflammatory response34; however, we did not find this association between adiposity and indicators of circulating iron in our postmenopausal women. As CRP and serum iron were not significant contributors to central adiposity, our results suggest that the relationship between adiposity and ferritin may not be a result of inflammation in these postmenopausal women. Differences in findings may be attributed to the criterion that subjects in our study were not obese and were also not at risk for developing iron deficiency. Hence, obesity-induced iron deficiency and iron excess may be of concern, and consequences may be different in younger vs. older individuals.
Our results are supported by data indicating a direct relation between elevated ferritin and visceral and subcutaneous fat, using computed tomography (CT).20 Although a meta-analysis showed no significant association between excess iron stores and CHD,7 other studies have reported such an association.8,9 The Bruneck study14 reported ferritin as one of the strongest indicators of carotid artery disease. The role of iron in CVD also may be mediated through induced insulin resistance,19 thus increasing centralized adiposity. The median ferritin concentration was 2-fold higher (86 vs. 42 ng/mL) in metabolic syndrome patients with insulin resistance (defined by HOMA) compared with patients without insulin resistance,31 and waist circumference was significantly higher in women with elevated ferritin (>75 ng/mL). Although we did not find a significant relationship between HOMA and ferritin (data not shown), there is evidence that excess iron may lead to insulin resistance.35,36
HOMA is a useful tool to assess insulin resistance because it takes both circulating glucose and insulin into account. Although the subjects in our study were deemed healthy, we found that centralized fat mass was positively associated with HOMA and oxidative stress and negatively associated with HDL-C. Central adiposity has been linked not only to insulin resistance and oxidative stress but also to dyslipidemia, specifically low HDL-C and high LDL-C. In a cross-sectional study with healthy men and women (n = 20,021),37 researchers reported that the waist/hip ratio, independent of BMI, was positively associated with LDL-C and negatively associated with HDL-C. Similar results were published from another cross-sectional study,38 although the association between LDL-C and waist circumference, once adjusted for age and BMI, was significant only in men.
Oxidative stress has been shown to be responsible for endothelial dysfunction, an early event in atherosclerosis.39 The PGF2α concentration was reported to be significantly higher in obese compared with nonobese men.18 Circulating lipids in humans were susceptible to oxidative damage with generalized obesity,40 and body weight and waist circumference were significantly related to oxLDL after adjusting for lifestyle and CVD risk factors.29 Similar to previous studies,29,40 our results showed that lipid oxidation indicators, but not antioxidant status or protein (protein carbonyls) and DNA (8-OHdG) damage indicators, were positively associated with centralized adiposity in these women.
Conclusions
We demonstrated an association among oxidative stress, iron status, lipids, insulin resistance, and centralized adiposity. Iron status was positively associated with centralized adiposity, possibly mediating insulin resistance or oxidative stress in these postmenopausal women. Although this is an observational study, reducing centralized fat mass and maintaining a favorable lipid profile, antioxidant status, and iron status all may be important in protecting postmenopausal women from atherosclerotic CVD. Further studies should elucidate the purported mechanism by which iron status might influence centralized adiposity and insulin resistance.
Acknowledgments
The project described was supported by a grant from the American Heart Association (AHA-Heartland Affiliate, 0350550Z), by a grant (RO1 AR046922 A2) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), by a grant (P01 ES012020) from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), and by a grant (95P50AT004155) from the National Center of Complementary and Alternative Medicine (NCCAM) and ODS, the National Institutes of Health. There are no conflicts of interest.
Disclosure Statement
No competing financial interests exist.
References
- 1.Institute of Medicine. National Academy of Sciences, Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds 2000: Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 2.Cracowski JL. Devillier P. Durand T. Stanke-Labesque F. Bessard G. Vascular biology of the isoprostanes. J Vasc Res. 2001;38:93–103. doi: 10.1159/000051036. [DOI] [PubMed] [Google Scholar]
- 3.Nordbo L. Bonaa KH. Nordoy A. Serum ferritin, sex hormones, and cardiovascular risk factors in healthy women. Arterioscler Thromb. 1994;14:857–861. doi: 10.1161/01.atv.14.6.857. [DOI] [PubMed] [Google Scholar]
- 4.Masse PG. Dosy J. Cole DEC. Evroski J. Allard J. D'Astous M. Is serum ferritin an additional cardiovascular risk factor for all postmenopausal women? Ann Nutr Metab. 2004;48:381–389. doi: 10.1159/000082366. [DOI] [PubMed] [Google Scholar]
- 5.Corti MC. Gaziano M. Hennekens CH. Iron status and risk of cardiovascular disease. Ann Epidemiol. 1997;7:62–68. doi: 10.1016/s1047-2797(96)00112-3. [DOI] [PubMed] [Google Scholar]
- 6.Eichner JE. Qi H. Moore WE. Schechter E. Iron measures in coronary angiography patients. Atherosclerosis. 1998;136:241–245. doi: 10.1016/s0021-9150(97)00215-3. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J. Appleby P. Coronary heart disease and iron status: Meta-analyses of prospective studies. Circulation. 1999;99:852–854. doi: 10.1161/01.cir.99.7.852. [DOI] [PubMed] [Google Scholar]
- 8.Salonen JT. Kyyssonen K. Korpela H. Tuomilehto J. Seppanen R. Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86:803–811. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- 9.Klipstein-Grobusch K. Koster JF. Grovvee DE. Lindemans J. Boeing H. Hofman A. Serum ferritin and risk of myocardial infarction in the elderly: The Rotterdam Study. Am J Clin Nutr. 1999;69:1231–1236. doi: 10.1093/ajcn/69.6.1231. [DOI] [PubMed] [Google Scholar]
- 10.van der DL. Grobbee DE. Roest M. Marx JJ. Voorbij HA. van der Schouw YT. Serum ferritin is a risk factor for stroke in postmenopausal women. Stroke. 2005;36:1637–1641. doi: 10.1161/01.STR.0000173172.82880.72. [DOI] [PubMed] [Google Scholar]
- 11.Derstine JL. Murray-Kolb LE. Yu-Poth S. Hargrove RL. Kris-Etherton PM. Beard JL. Iron status in association with cardiovascular disease risk in 3 controlled feeding studies. Am J Clin Nutr. 2003;77:56–62. doi: 10.1093/ajcn/77.1.56. [DOI] [PubMed] [Google Scholar]
- 12.Binkoski AE. Kris-Etherton PM. Beard JL. Iron supplementation does not affect the susceptibility of LDL to oxidative modification in women with low iron status. J Nutr. 2004;134:99–103. doi: 10.1093/jn/134.1.99. [DOI] [PubMed] [Google Scholar]
- 13.Sempos CT. Looker AC. Iron status and the risk of coronary heart disease: An example of the use of nutritional epidemiology in chronic disease research. J Nutr Biochem. 2001;12:170–182. doi: 10.1016/s0955-2863(00)00153-4. [DOI] [PubMed] [Google Scholar]
- 14.Kiechl S. Aichner F. Gerstenbrand F, et al. Body iron stores and presence of carotid athersclerosis: Results from the Bruneck study. Arterioscler Thromb. 1994;14:1625–1630. doi: 10.1161/01.atv.14.10.1625. [DOI] [PubMed] [Google Scholar]
- 15.Reddy MB. Clark L. Iron, oxidative stress, and disease risk. Nutr Rev. 2004;62:120–124. doi: 10.1111/j.1753-4887.2004.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 16.Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—The Third National Health and Nutrition Examination Survey. Int J Obes. 2001;25:639–645. doi: 10.1038/sj.ijo.0801561. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 18.Urakawa H. Katsuki A. Sumida Y, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4376. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 19.Sheu WH. Chen Y. Lee W. Wang C. Lin L. A relationship between serum ferritin and the insulin resistance syndrome is present in non-diabetic women but not in non-diabetic men. Clin Endocrinol. 2003;58:380–385. doi: 10.1046/j.1365-2265.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki T. Nakajima A. Yoneda M, et al. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care. 2005;28:2486–2491. doi: 10.2337/diacare.28.10.2486. [DOI] [PubMed] [Google Scholar]
- 21.Jehn M. Clark JM. Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27:2422–2428. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- 22.Reilly M. Delanty N. Lawson JA. FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in men. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Moeller LE. Peterson CT. Hanson KB, et al. Isoflavone-rich soy protein prevents loss of hip lean mass, but does not prevent the shift in regional fat distribution in menopausal women. Menopause. 2003;10:322–331. doi: 10.1097/01.GME.0000054763.94658.FD. [DOI] [PubMed] [Google Scholar]
- 25.Glickman SG. Marn CS. Supiano MA. Dengel DR. Validity and reliability of dual-energy x-ray absorptiometry for the assessment of abdominal adiposity. J Appl Physiol. 2004;97:509–514. doi: 10.1152/japplphysiol.01234.2003. [DOI] [PubMed] [Google Scholar]
- 26.National Cholesterol Education Program. Third report of the expert panel: Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Bethesda, MD: National Heart Lung and Blood Institute; 2002. NIH Pub. No. 02-5215. [Google Scholar]
- 27.Poulsen HE. Prieme H. Loft S. Role of oxidative DNA damage in cancer initiation and promotion. Eur J Cancer Prev. 1998;7:9–16. [PubMed] [Google Scholar]
- 28.Taylor A. Cataract: Relationship between nutrition and oxidation. J Am Coll Nutr. 1993;12:138–146. doi: 10.1080/07315724.1993.10718294. [DOI] [PubMed] [Google Scholar]
- 29.Weinbrenner T. Schroder H. Escurriol V, et al. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr. 2006;83:30–35. doi: 10.1093/ajcn/83.1.30. [DOI] [PubMed] [Google Scholar]
- 30.Lee C-D. Jacobs DR. Schreiner PJ. Iribarren C. Hankinson A. Abdominal obesity and coronary artery calcification in young adults: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86:48–54. doi: 10.1093/ajcn/86.1.48. [DOI] [PubMed] [Google Scholar]
- 31.González AS. Guerrero DB. Soto MB. Díaz SP. Martinez-Olmos M. Vidal O. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur J Clin Nutr. 2006;60:802–809. doi: 10.1038/sj.ejcn.1602384. [DOI] [PubMed] [Google Scholar]
- 32.Vega GL. Obesity and the metabolic syndrome. Min Endocrinol. 2004;29:47–54. [PubMed] [Google Scholar]
- 33.Nead KG. Halterman JS. Kaczorowski JM. Auinger P. Weitzman M. Overweight children and adolesecents: A risk group for iron deficiency. Pediatrics. 2004;114:104–108. doi: 10.1542/peds.114.1.104. [DOI] [PubMed] [Google Scholar]
- 34.Yanoff LB. Menzie CM. Denkinger B, et al. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes. 2007;31:1412–1419. doi: 10.1038/sj.ijo.0803625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Real JM. Ricart-Engel W. Arroyo E, et al. Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care. 1998;21:62–68. doi: 10.2337/diacare.21.1.62. [DOI] [PubMed] [Google Scholar]
- 36.Niederau C. Berger M. Stremmel W, et al. Hyperinsulinaemia in non-cirrhotic hemochromatosis: Impaired hepatic insulin degradation? Diabetologia. 1984;26:441–444. doi: 10.1007/BF00262217. [DOI] [PubMed] [Google Scholar]
- 37.Canoy D. Wareham N. Luben R, et al. Serum lipid concentration in relation to anthropometric indices of central and peripheral fat distribution in 20,021 British men and women: Results from the EPIC-Norfolk population-based cohort study. Atherosclerosis. 2006;189:420–427. doi: 10.1016/j.atherosclerosis.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 38.Seidell JC. Pérusse L. Després JP. Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: The Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 39.Perticone F. Ceravolo R. Candigliota M, et al. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: Protective effect of vitamin C. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 40.Davi G. Guagnano MT. Ciabattoni G, et al. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]