Abstract
Glomerular dysfunction and proteinuria characterize focal segmental glomerulosclerosis (FSGS) associated with chronic kidney disease. FSGS is resistant to treatment and a circulating permeability factor (FSPF) frequently causes post-renal transplantation recurrence. In order to explore the role of 5,6-, 8,9-, 11,12- and 14,15-epoxyeicosatrienoic acids (EETs), we determined their effect on FSPF-induced increase in glomerular albumin permeability (Palb) using an in vitro assay. Exogenous 8,9-EET (1–1000nM) dose-dependently prevented the FSPF–induced increase in Palb. The other three EET regioisomers, 8,9-EET metabolite 8,9-Dihydroxyeicosatrienoic acid and unrelated 11,14-eicosadienoic acid (100 nM each) were not effective suggesting specificity of the observed glomerular protection by 8,9-EET. Synthetic analogs of 8,9-EET containing one double bond antagonized the effect of 8,9-EET on the FSPF-induced increase in Palb. Analogs containing two double bonds did not antagonize the effect of 8,9-EET and significantly blocked the FSPF-induced increase in Palb. These novel findings suggest a unique protective role for 8,9-EET in the glomerulus. Stable analogs of 8,9-EET may be valuable in developing effective management/treatment of glomerular dysfunction.
Keywords: Epoxyeicosatrienoic acids; 8,9-EET; Glomerular filtration barrier; FSGS; Albumin permeability; Chronic kidney disease
1. Introduction
The kidney glomerulus is responsible for plasma filtration and formation of primary urine. The glomerular filtration barrier restricts albumin and larger proteins to plasma space and minimizes their passage with urine. Increased glomerular permeability to plasma proteins is an early event in the development of albuminuria/proteinuria associated with chronic renal disease leading to end-stage renal disease. Glomerular dysfunction is characterized by sclerosis, loss of capillary lumen, effacement of podocyte foot processes and increased passage of albumin into the urinary space.
Focal and segmental glomerulosclerosis (FSGS) is associated with idiopathic renal disease and systemic diseases that lead to chronic kidney disease. FSGS occurs in adults and children of both sexes and its incidence has increased during the past two decades. Putative risk factors include morbid obesity, racial background and family history. Viral infections or toxins have been implicated in severe and rapidly progressing forms such as the collapsing variant of FSGS or HIV nephropathy. FSGS is characterized by nephrotic syndrome that includes heavy proteinuria, hypoalbuminemia, hyperlipidemia and hypertension [1, 2].
The precise etiology of idiopathic FSGS remains unclear, however, the post-transplant recurrence of proteinuria in 30–40% patients with idiopathic FSGS appears to be caused by a circulating factor termed the FSGS permeability factor (FSPF). The presence and potency of this factor can be estimated in vitro by measurement of glomerular albumin permeability (Palb) [3]. Increased Palb is one of the earliest detectable markers for subsequent proteinuria and renal injury in vivo. The plasma fraction containing FSPF increases Palb in vitro [4] and induces proteinuria in rats in vivo [5]. Extracorporeal treatments such as plasma exchange (plasmapheresis) or immunoadsorption result in transient attenuation of proteinuria in patients with primary FSGS and decrease the ability of their sera to raise Palb in vitro. Current treatment regimens to treat FSGS are empirical and largely ineffective necessitating continued search for new therapeutic molecules [6].
Epoxyeicosatrienoic acid (EET, EpETrE) regioisomers, namely, 5,6-, 8,9-, 11,12- and 14,15-EET, are epoxides of arachidonic acid biosynthesized by cytochrome P-450 (CYP450) epoxygenases and are readily metabolized to respective vicinal diols dihydroxyepoxyeicosatrienoic acids (DiHETrEs, DiHETs) by soluble epoxide hydrolase activity. EETs function as autocrine and paracrine modulators of several cellular events including molecular signaling, anti-inflammation, ion channel opening, mitogenesis, angiogenesis and protection against ischemia-reperfusion injury [7]. The ability of the kidney to synthesize EETs was demonstrated in 1981 but the role of these metabolites in the glomerular barrier, the site of plasma filtration and primary urine formation, is not known. We have recently shown that MS-PPOH, an inhibitor of the CYP450 epoxygenase activity, decreases the levels of EETs and increases Palb [8].
In this report, we describe for the first time a unique protective effect of 8,9-EET on the glomerular filtration barrier by demonstrating that it prevents the increase in Palb induced by FSPF in the plasma from patients with FSGS. To elucidate the structure-activity-relationships (SARs) of 8,9-EET and to expedite the development of more stable analogs, we synthesized structural variants of 8,9-EET and studied their protective effects on Palb. These novel findings suggest that EETs play a role in glomerular protein permeability barrier function and, that 8,9-EET or its analogs may be useful in further studies to develop new molecules for preserving the glomerular protein permeability barrier.
2. Materials and Methods
2.1. FSGS and Normal Plasma
Discarded plasma specimens obtained following plasmapheresis of a male patient with recurrent FSGS and allograft loss in 1985, since treated with thrice-weekly hemodialysis, were the source of the FSPF used in these studies. This patient’s serum, plasma, and plasmapheresis fluid have consistently increased Palb in vitro. This patient was not on any immunosuppressive medications, and was not nephrotic at the time of plasmapheresis. Plasmapheresis was performed using citrate anticoagulation, and plasma removed was replaced with 0.9% sodium chloride and 5% human albumin solution. We have used discarded plasma from this patient in our studies as a reference for consistency of our assays and purification protocols approved by the Institutional Review Board at the Medical College of Wisconsin, Milwaukee. Details of the criteria used for the diagnosis of FSGS have been described earlier [3, 9]. Discarded normal plasma specimens from the Blood Center of Southeastern Wisconsin (Milwaukee, WI) were obtained as controls. Each sample was stored at −20°C until use. Plasma from 35 normal individuals was pooled and designated normal pooled plasma (NPP).
2.2. Experimental Animals and Preparation of Glomeruli
Animals were cared for according to the NIH guidelines. All protocols were reviewed and approved by the Institutional Committee for Care and Use of Animals at the Medical College of Wisconsin. Rats were maintained at the Biomolecular Resource Center with free access to diet and drinking water.
Glomeruli were isolated from kidneys of normal male Sprague-Dawley rats (200–220 g). Rats were anesthetized with halothane vapor (Halocarbon Laboratories, River Edge, NJ), the kidneys removed, and the animals immediately euthanized. Renal medulla was removed and small pieces of the cortical slices were passed through stainless steel sieves of decreasing pore sizes. Glomeruli were collected from atop the 200-mesh sieve (75 micron pore size). Glomeruli were suspended in the isolation medium containing 5% bovine serum albumin (BSA). Glomeruli isolated from 2 kidneys were resuspended in 1.5 ml of buffer (~10,000 glomeruli/ml). Further details of the reagents, techniques and conditions have been described [9, 10].
2.3. Synthesis of 8,9-EET Analogs
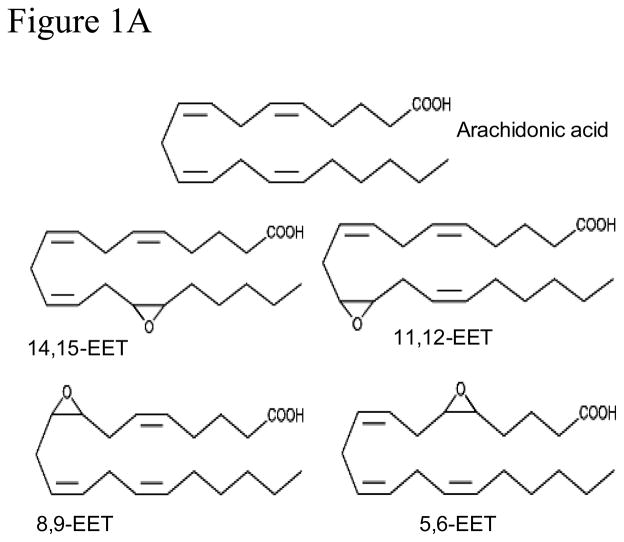
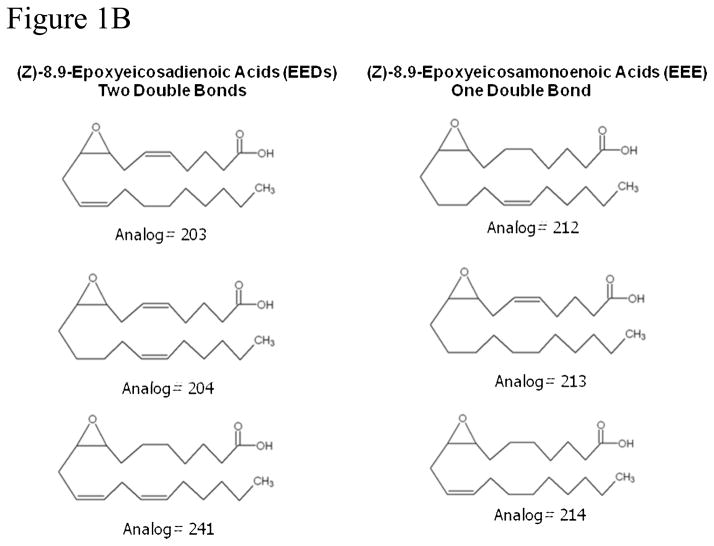
EETs are generated by the CYP450 epoxygenase catalyzed metabolism of arachidonic acid. Chemical structures of the four EET regioisomers are shown in Figure 1A. The presence of three double bonds renders these molecules prone to auto-oxidation and/or secondary metabolism which account in large part for their short half-life. Reduction in the number of double bonds generally improves the stability of polyenoic compounds, albeit with partial loss of biological activity. Analogs that provide a suitable compromise between metabolic/chemical stability and residual activity are highly desirable substitutes for the parent metabolite. We synthesized several analogs of 8,9-EET containing one or two double bonds instead of three to study their effects on Palb. For convenience and to develop consistent terminology, we have provided the full chemical name and a suggested abbreviation for each numerically identified analog (Table 1 and Figure 1B). General methods for the synthesis of analogs have been described [11].
Figure 1.
Figure 1A and 1B: Structures of arachidonic acid, 8,9-EET and synthetic analogs of 8,9-EET. Analogs of 8,9-EET contain a similar carbon chain with differing numbers and positions of double bonds between carbons 5 and 15.
Table 1.
Names and abbreviations for 8,9-EET analogs.
| Analog | Chemical Name | Abbreviated name |
|---|---|---|
| # | Analogs containing 2 double bonds | Epoxyeicosadienoic acid (EED) |
| 203 | (Z)-8,9-Epoxyeicosa-5(Z),11(Z)-dienoic acid | 8,9-EE-5,11-DA |
| 204 | (Z)-8,9-Epoxyeicosa-5(Z),14(Z)-dienoic acid | 8,9-EE-5,14-DA |
| 241 | (Z)-8,9-Epoxyeicosa-11,(Z),14(Z)-dienoic acid | 8,9-EE-11,14-DA |
| Analogs containing 1 double bond | Epoxyeicosaenoic acid (EEE) | |
| 212 | (Z)-8,9-Epoxyeicosa-14(Z)-enoic acid | 8,9-EE-14-EA |
| 213 | (Z)-8,9-Epoxyeicosa-5(Z)-enoic acid | 8,9-EE-5-EA |
| 214 | (Z)-8,9-Epoxyeicosa-11(Z)-enoic acid | 8,9-EE-11-EA |
2.4. Experimental treatment of glomeruli and incubation conditions
2.4a. Epoxyeicosatrienoic acid (EET) regioisomers 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET
To compare the effect of the four EET regioisomers, aliquots from stock solutions of 5,6-, 8,9-, 11,12- or 14,15-EET in ethanol were diluted to final concentrations by adding glomerular isolation medium containing 5% BSA (890 μL). Based on preliminary experiments, we used each EET regioisomer at 100 nM final concentration to compare their protective effect against the FSPF-induced increase in Palb. FSGS plasma (20 μL) alone or with EETs (100 nM) was incubated for 15 minutes at 37°C. NPP (20 μL) was used as plasma control. An equal volume of ethanol (10 μL) was present in all incubation mixtures.
2.4b. Dose-dependent effect of 8,9-EET
In separate experiments we used 8,9-EET at 1 to 1000 nM concentration to determine whether it protected the glomerular barrier function in a dose-dependent manner. FSGS plasma (20 μL) was incubated alone or with 8,9-EET (1, 10, 100 or 1000 nM) for 15 minutes at 37°C in a final volume of 1 mL. NPP (20 μL) was used as control. An equal volume of ethanol (10 μL) vehicle was included in all groups.
2.4c. Protective effect of structurally unrelated fatty acid all-cis-11,14-eicosadienoic acid (11,14-EDA)
We confirmed the specificity of the observed effect of 8,9-EET using a structurally unrelated fatty acid namely, all-cis-11,14-eicosadienoic acid. In a series of experiments we compared the effect of 8,9-EET (100 nM) and all-cis-11,14-eicosadienoic acid (100 nM) on FSPF-induced increase in Palb. FSGS plasma (20 μL) was incubated alone or with one of the fatty acids for 15 minutes at 37°C. NPP (20 μL) was used as the control group. An equal volume of ethanol (10 μL) was used in all groups.
2.4d. 8,9-EET and its vicinal diol 8,9-Dihydroxyeicosatrienoic acid (8,9-DiHETrE, 8,9-DiHET)
EETs are efficiently metabolized to their respective diols by soluble epoxide hydrolase (sEH) and the observed effect of 8,9-EET may be through its metabolite 8,9-DiHETrE. We compared the effect of 8,9-EET (100 nM) with its vicinal diol 8,9-DiHETrE (100 nM) on FSPF-induced increase in Palb. FSGS plasma (20 μL) was incubated with or without these agents for 15 minutes at 37°C. NPP (20 μL) was used as the control group. An equal volume of ethanol (10 μL) was used in all groups.
2.4e. FSGS plasma and normal pooled plasma
Glomerular suspension (100 μL) in isolation medium was added to the samples and gently mixed to facilitate dispersion (final volume 1 mL). The mixture was incubated for 15 minutes at 37°C with or without FSGS-plasma (20 μL). NPP (20 μL) was used as the negative control as described. Plasma aliquots were added within 1 minute after EETs or 8,9-EET analogs to glomerular suspension. Vehicle (10 μL ethanol) was included with FSGS plasma or NPP. Preliminary results indicated that 10 μL did not significantly alter the effect of NPP (Palb NPP alone 0.01±0.059 vs. NPP + ethanol 0.01±0.065, NS) or FSGS plasma (FSGS alone 0.83±0.08 vs. FSGS + ethanol 0.77±0.069, NS) on glomerular filtration barrier.
2.4f. Structural analogs of 8,9-EET
Structure-activity relationship was further explored using analogs of 8,9-EET. We used six structural analogs of 8,9-EET containing one or two double bonds to determine the structural requirements for the observed biological effect of 8,9-EET on glomerular filtration barrier. Three EEEs, analogs containing 1 double bond, designated as #212, 213 and 214, and 3 EEDs, analogs containing two double bonds, designated as #203, 204 and 241, were compared for their efficacy in attenuating the effect of FSPF on Palb (Table 1).
Studies on 8,9-EET analogs were carried out in three stages. First, we compared the effect of 8,9-EET (100 nM) and its analogs (300 nM) on Palb by incubating each compound with isolated glomeruli for 15 minutes at 37°C. Analogs were used at higher concentration than 8,9-EET to identify and exclude the analogs that increased Palb. 8,9-EET at 100 nM or 1000 nM concentrations does not increase Palb (Section 2.4b). Second, we determined whether any of the analogs (300 nM) attenuated the protective effect of 8,9-EET (100 nM) on the FSPF-induced increase in Palb. Finally, based on results of these series, we selected an analog containing two double bonds (#241) to determine its effect on the FSPS-induced increase in Palb at 100 and 300 nM concentrations.
2.5. In vitro assay of Palb
Following incubation, glomeruli were held and initial images of at least 5 glomeruli from one rat were recorded by video-microscopy. The BSA concentration of the medium was changed from 5% to 1% to generate an oncotic gradient across the glomerular capillary wall causing movement of fluid into the capillaries resulting in an increase in glomerular size. The final image of each glomerulus was recorded after 60 seconds. Each video image was measured along four diameters 45 degrees apart and the average diameter was used to calculate initial and final volumes (Vinitial and Vfinal ). The change in volume (ΔV) of each glomerulus due to the oncotic gradient was calculated as: ΔV = [Vfinal−Vinitial/Vinitial] × 100.
The effective force produced by an albumin oncotic gradient across the capillary wall (about 16 mm Hg) is measured as the change in glomerular volume. The reflection coefficient (σ) of a membrane measured in relation to a given solute is defined by the ratio of the oncotic force exerted by that solute to its theoretical oncotic force. It is a function both of the membrane and the physical characteristics of the solute including molecular radius, configuration and charge. Albumin reflection coefficient (σalb) was calculated as: σalb= ΔVexperimental/ΔVcontrol. Convectional permeability (Palb) was calculated as: Palb=1−σalb. When alb is zero, albumin moves across the membrane at the same rate as water and Palb equals 1.0. When σalb is one, albumin cannot cross the membrane with water and Palb equals zero. Details of the method for determination of Palb have been described previously [9, 10].
2.6. Statistical Analysis
Fifteen glomeruli from three rats (5 glomeruli per rat) were observed in each group. Values of Palb were expressed as mean ± SEM. Results were compared using unpaired t-test analysis and P values described significance of difference between groups.
3. Results
3.1. 8,9-EET but no other regioisomers protects the glomerular filtration barrier
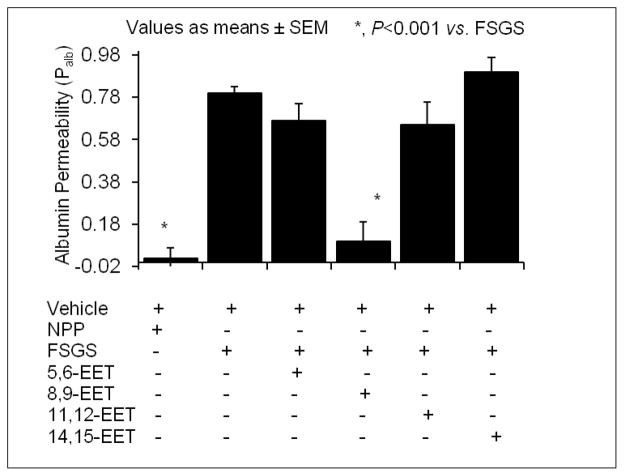
Figure 2 shows the effect of individual EET regioisomers on the FSPF-induced increase in Palb. FSGS plasma alone increased Palb compared to the normal pooled plasma (0.8±0.03 and 0.02±0.05, P<0.001). Results show that identical concentrations (100 nM) of 5,6-, 11,12- and 14,15-EET did not attenuate the effect of FSPS on the glomerular filtration barrier (Palb 5,6-EET 0.67±0.08, NS vs. FSGS; 11,12-EET 0.65±0.11, NS vs. FSGS; 14,15-EET-0.9±0.07, NS vs. FSGS). Only 8,9-EET blocked the effect of FSPF (Palb 0.1±0.09, P<0.001 vs. FSGS plasma). Thus, 8,9-EET appears to have a unique protective effect on the glomerular filtration barrier.
Figure 2. 8,9-EET protects against FSGS plasma-induced increase in glomerular albumin permeability in vitro.
Freshly isolated rat glomeruli were incubated with FSGS plasma or normal pooled plasma (NPP, 20 μL) for 15 minutes at 37°C. Parallel groups of glomeruli were incubated with FSGS plasma (20 μL) and 100 nM of each EET regioisomers, 5,6-, 8,9-, 11,12- or 14,15-EET. FSGS plasma caused increased albumin permeability compared to the vehicle control (P<0.001). NPP did not induce change in Palb. 8,9-EET (100 nM) blocked the increase in permeability induced by the FSGS-plasma (P<0.001 vs. FSGS). None of the other regioisomers block the FSGS plasma-induced in Palb.
3.2. 8,9-EET protects the glomerular filtration barrier in a dose-dependent manner
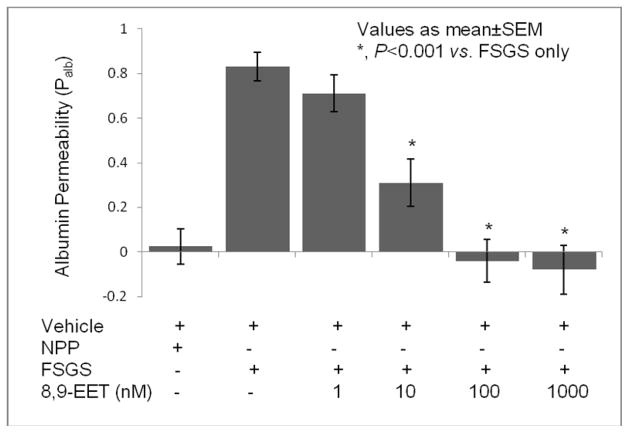
Figure 3 shows the effect of increasing concentrations of 8,9-EET (1–100 nM) on the FSPF-induced increase in Palb. FSGS plasma caused a marked increase in Palb compared to NPP (FSGS 0.81±0.06 vs. NPP −0.025±0.08, P< 0.001). Increasing concentrations of 8,9-EET gradually attenuated the FSPF-induced increase in Palb. Thus, FSPF-induced increased Palb at 8,9-EET concentrations 1, 10, 100 and 1000 nM was 0.71±0.08, NS vs. FSGS; 0.31±0.1, P<0.001 vs. FSGS plasma; −0.04±0.09, P < 0.001 vs. FSGS plasma; and −0.1±0.06, P < 0.001 vs. FSGS plasma, respectively. Increasing concentration of 8,9-EET did not affect Palb by itself (Palb 100 nM 8,9-EET −0.08±0.11 and 1 μM 8,9-EET −0.145±0.11). Thus, 8,9-EET blocked the effect of FSGS plasma in a dose-dependent manner and it did not increase Palb at 100 nM or 1000 nM.
Figure 3. The protective effect of 8,9-EET on the glomerular filtration barrier is dose-dependent.
Freshly isolated rat glomeruli were incubated with FSGS plasma (20 μL) alone or with different concentrations of 8,9-EET (1–100 nM) for 15 minutes at 37°C. FSPF in the FSGS plasma caused increased albumin permeability compared to the vehicle control (P<0.001). Significant protection against FSGS plasma-induced increase in Palb observed at 10 nM 8,9-EET and complete protection at 100 nM (P<0.001 vs. FSGS).
3.3. All-cis-11,14-eicosadienoic acid (11,14-EDA) does not block the FSPF-induced increase in Palb
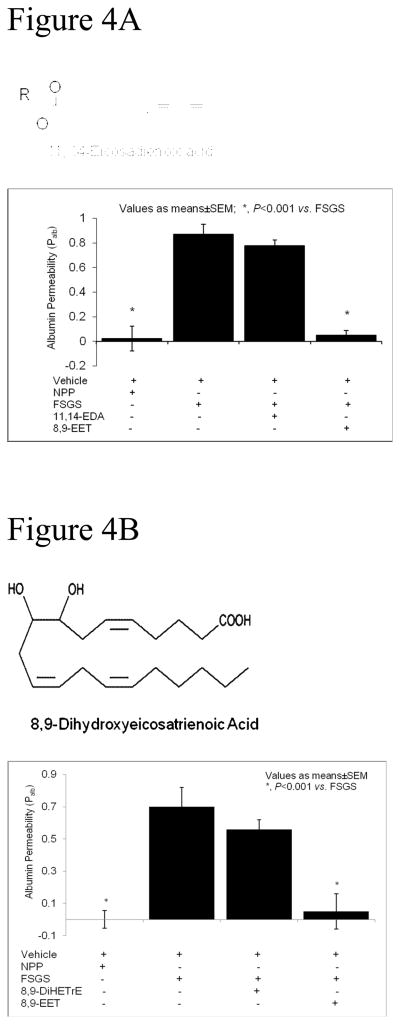
11,14-EDA is a twenty carbon-long fatty acid, same as 8,9-EET, but lacks the epoxide of the latter. Figure 4A shows that 11,14-EDA did not alter the FSPF-induced increase in Palb (0.78±0.045, NS vs. FSGS plasma) while 8,9-EET completely blocked the FSPF-induced increase in Palb (0.05±0.04, P<0.001 vs. FSGS plasma). Thus, identical number of carbon atoms in the fatty acid chain length does not appear sufficient to block the effect of FSGS plasma on glomerular filtration barrier. These data suggest a structure-specific protective effect of 8,9-EET.
Figure 4.
Figure 4A: The protective effect of 8,9-EET on the glomerular filtration barrier is specific-Structurally unrelated fatty acid is not protective. Freshly isolated rat glomeruli were incubated with FSGS plasma alone (20 μL), FSGS plasma + 8,9-EET (100 nM) or FSGS plasma + 11,14-eicosadienoic acid (100 nM) for 15 minutes at 37°C. 11,14-eicosadienoic acid is a 20 carbon fatty acid containing 2 double bonds that is not normally found in mammalian tissues. FSGS plasma-induced increase in Palb was blocked by 8,9-EET (P<0.001 vs. FSGS) but not by 11,14-eicosadienoic acid.
Figure 4B: The protective effect of 8,9-EET on the glomerular filtration barrier is specific-8,9-EET vic diol 8,9-DiHETrE is not protective. Freshly isolated rat glomeruli were incubated with FSGS plasma alone (20 μL), FSGS plasma + 8,9-EET (100 nM) or FSGS plasma + 8,9-diHETrE (100 nM) for 15 minutes at 37°C. 8,9-DiHETrE is generated from 8,9-EET in a reaction catalyzed by soluble epoxide hydrolase. 8,9-diHETrE contains two hydroxyl groups in place of the epoxide group. FSGS plasma-induced increase in Palb was blocked by 8,9-EET (P<0.001 vs. FSGS) but not by 8,9-DiHETrE.
3.4. 8,9-DiHETrE (8,9-DiHET), vicinal diol of 8,9-EET, does not block the FSPF-induced increase in Palb
EETs are readily metabolized to their respective diols by soluble epoxide hydrolase activity. Therefore, the observed protective effect of 8,9-EET may be due to its vicinal diol. Figure 4B shows the results of incubating glomeruli with FSGS plasma with 100 nM 8,9-DiHETrE or 8,9-EET. While 8,9-EET completely blocked the effect of FSPF (Palb 0.05±0.11 vs. FSGS plasma alone 0.70±0.12, P<0.001) its diol 8,9-DiHETrE did not have a significant blocking effect (Palb 0.56±0.06, NS vs. FSGS plasma). These results suggest that the epoxide group is required for the protective effect of 8,9-EET.
3.5. 8,9-EET analogs containing one double bond (8,9-EEEs) increase Palb
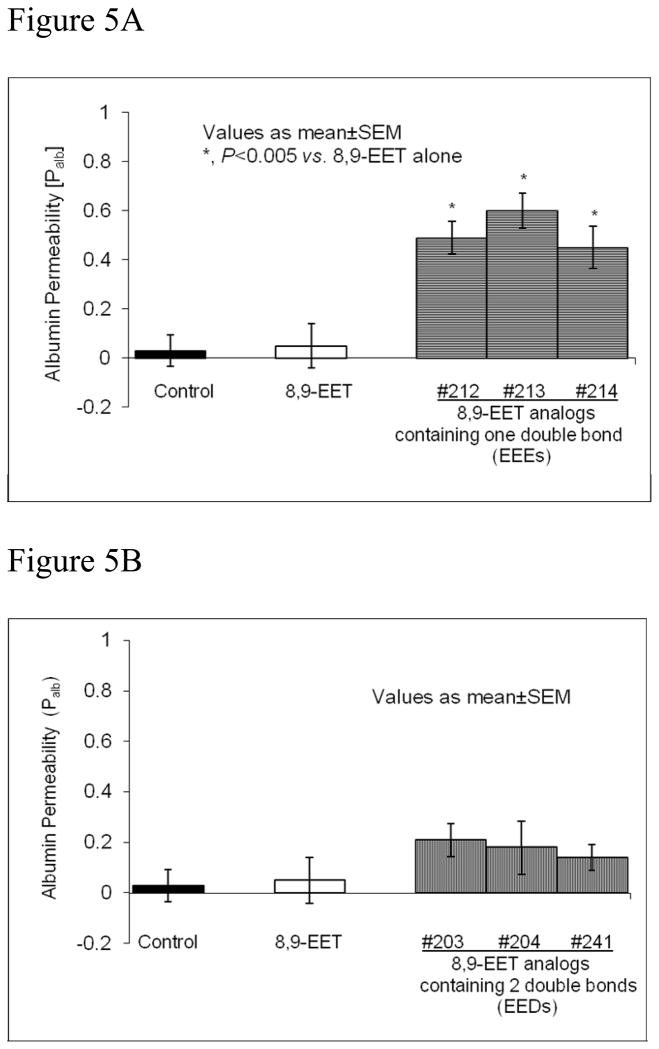
Figure 5A shows results to compare the effect of three 8,9-EEEs (300 nM) with that of 8,9-EET (100 nM) on Palb. Each 8,9-EEE (#212, 213 and 214, Table 1) or 8,9-EET was incubated separately with glomeruli for 15 minutes. Each 8,9-EEE namely, #212, 213 and 214 (Table 1), induced a significant increase in Palb (0.49±0.066, 0.6±072 and 0.45±0.087, respectively; P<0.005 vs. 8,9-EET alone). Thus, at three times higher concentration than 8,9-EET (100 nM), the 1-double bond containing analogs caused a significant increase in Palb.
Figure 5.
Figure 5A: Analogs of 8,9-EET containing one double bond increase Palb. Freshly isolated rat glomeruli were incubated with vehicle, 8,9-EET (100nM) or its analogs containing one double bond (EEE) # 212, 213 or 214 (300nM) for 15 minutes at 37°C. Each of the three EEEs (#212, 213 or 214) caused significant increase in glomerular albumin permeability (P<0.005 vs. 8,9-EET).
Figure 5B: Analogs of 8,9-EET containing two double bonds (EEDs) do not alter Palb. Freshly isolated rat glomeruli were incubated with vehicle, 8,9-EET (100nM) or its analogs containing two double bonds (8,9-EED) # 203, 204 or 241 (300nM) for 15 minutes at 37°C. Neither 8,9-EET nor EEDs caused significant change in glomerular albumin permeability.
3.6. 8,9-EET analogs containing two double bonds (8,9-EEDs) do not change Palb
Figure 5B shows the results comparing the effects of three 8,9-EEDs with that of 8,9-EET on Palb. Each 8,9-EED (#203, 204 or 241, see Table 1) or 8,9-EET was incubated separately with glomeruli for 15 minutes. Results show that 8,9-EET (100 nM) did not alter Palb (0.05±0.09 vs. vehicle control 0.03±0.064, NS). Higher concentrations of 8,9-EET up to 1 μM also did not increase Palb (Section 3.2). Two double bond containing 8,9-EEDs, analog #203, 204 and 241 (300 nM each), resulted in Palb 0.21±0.065, 0.18±0.105 and 0.1± 0.05, respectively. Thus, at three times higher concentration than 8,9-EET (100 nM), the 2-double bond containing analogs did not cause a significant increase in Palb.
3.7. Analogs containing one double bond (8,9-EEE) antagonize the protective effect of 8,9-EET on Palb
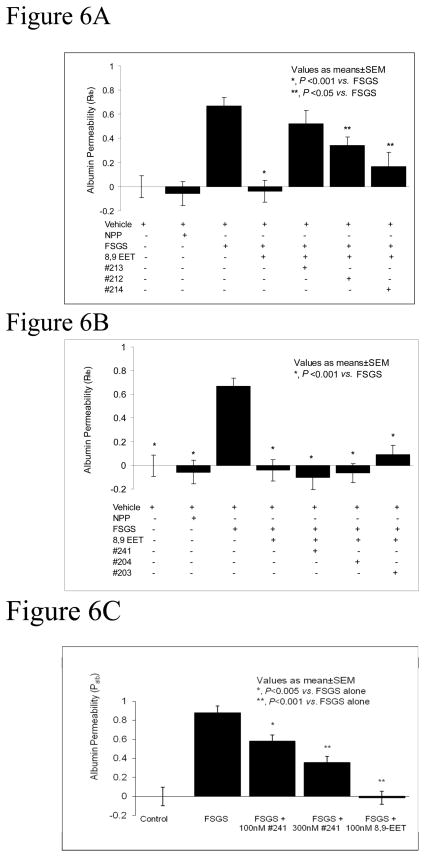
Figure 6A shows that one double bond containing analogs (8,9-EEEs) #213, 212 and 214 (Table 1) attenuated the protective effect of 8,9-EET on FSPF-induced increase in Palb. FSPF-induced increase in Palb (0.67±0.07) was blocked by 8,9-EET (100 nM) alone (Palb −0.04±0.09, P<0.001 vs. FSGS plasma alone). Addition of one double bond containing 8,9-EEE, analog #213, 212 and 214 (300 nM each) with FSGS plasma and 8,9-EET, resulted Palb 0.52±0.11, 0.34±0.07 and 0.167±0.12, respectively. These Palb values were higher than that obtained with FSGS plasma + 8,9-EET (−0.04±0.09) and suggest an antagonistic effect of EEEs against 8,9-EET. The extent of antagonistic effect appeared to vary (Palb for #213 > 212 > 214) suggesting its association with the position of the double bond.
Figure 6.
Figure 6A: Analogs containing one double bond (8,9-EEE) antagonize the protective effect of 8,9-EET on Palb. Freshly isolated rat glomeruli were incubated with vehicle, NPP, FSGS plasma, FSGS + 8,9-EET (100nM), FSGS + 8,9-EET + analogs containing one double bond (EEE) # 212, 213 or 214 (300nM) for 15 minutes at 37°C. FSPF-induced increase in Palb blocked by 8,9-EET (P<0.001 vs. FSGS alone). 8,9-EEEs appeared to antagonize the effect of 8,9 EET to varying (213 > 212 > 214) degree (P<0.001, 0.05 vs. 8,9-EET).
Figure 6B: Analogs containing two double bonds (8,9-EED) do not antagonize the protective effect of 8,9-EET on Palb. Freshly isolated rat glomeruli were incubated with vehicle, NPP, FSGS plasma, FSGS plasma + 8,9-EET (100nM), FSGS plasma +8,9-EET + analogs containing two double bonds (8,9-EED) # 241, 204 or 203 (300nM) for 15 minutes at 37°C. FSPF-induced increase in Palb was blocked by 8,9-EET alone (P<0.001 vs. FSGS alone). Inclusion of 8,9-EED #241, 204 or 203 did not significantly change the protective effect of 8,9-EET. Analog #241 appeared to be the most effective representative of this class of molecules.
Figure 6C: An 8,9-EED (Analog# 241) blocks the effect of FSPF on the glomerular filtration barrier. Freshly isolated rat glomeruli were incubated with vehicle, FSGS plasma, FSGS plasma + 8,9-EED Analog# 241 (100nM or 300 nM), FSGS plasma + 8,9-EET (100 nM) for 15 minutes at 37°C. Analog #241 attenuated the effect of FSGS plasma on Palb in a dose-dependent manner (100 nM P<0.005 vs. FSGS alone; 300 nM P<0.001 vs. FSGS alone). 8,9-EET blocked the FSPF-induced increase in Palb (P<0.001 vs. FSGS alone).
3.8. Analogs containing two double bonds (8,9-EED) do not antagonize the protective effect of 8,9-EET on Palb
Figure 6B shows that two double bond containing analogs of 8,9-EET (8,9-EEDs) i.e., analog # 241, 204 or 203 (Table 1), did not attenuate the protective effect of 8,9-EET. The FSPF-induced increase in Palb (0.67±0.07) was blocked by 8,9-EET (100 nM) alone (Palb −0.04±0.09, P<0.001 vs. FSGS plasma alone). Inclusion of 8,9-EED #241, 204 or 203 with 8,9-EET and FSGS plasma resulted in Palb −0.104±0.1, −0.065±0.08; and 0.09±0.08, respectively. These results were not significantly different from 8,9-EET + FSGS plasma (Palb −0.04±0.09). An apparent structure-related trend in efficacy (#241 > 204 > 203) suggested analog #241 as the most effective representative of this class for the next series of experiments.
3.9. 8,9-EED (Analog# 241) blocks the effect of FSPF on the glomerular filtration barrier
Figure 6C shows the effect of analog #241 (100 nM and 300 nM) or 8,9-EET (100 nM) on the FSPF-induced increase in Palb. FSGS plasma increased Palb (0.88±0.07) was blocked by 8,9-EET (Palb −0.15±0.07). Analog #241 also attenuated the effect of FSGS plasma on Palb in a dose-dependent manner 100 nM (Palb 0.58±0.064, P<0.005 vs. FSGS plasma alone) and 300 nM (0.36±0.065, P<0.001 vs. FSGS plasma alone). Thus, the analogs containing two double bonds may be useful candidates for further studies.
4. Discussion
FSGS is one of the most challenging problems in modern medicine. Increasing incidence, post-transplantation recurrence in about 30% of renal allograft recipients and poorly understood etiology add to the complexity of FSGS. Current therapeutic regimens are empirical and generally ineffective necessitating an ongoing search for potential therapeutic agents. We have focused on the role of arachidonic acid metabolites in the glomerular barrier function. Results of the present studies indicate that 8,9-EET attenuates the FSGS plasma-induced increase in Palb. This protective effect appears to be unique to the 8,9-regioisomer since 5,6-, 11,12- or 14,15-EET did not attenuate the increase in Palb. Analogs of 8,9-EET containing two double bonds also significantly attenuated the increase in Palb and did not antagonize the protective effect of 8,9-EET. On the other hand, analogs containing only one double bond showed varying degrees of antagonism to the protective effect of 8,9-EET. Thus, 8,9-EET may have a unique and specific protective role in the glomerular filtration barrier with a strong structure-activity relationship.
The filtration barrier is constituted by glomerular capillary endothelial cells, basement membrane and visceral epithelial cells (podocytes). Podocyte foot processes cover the basement membrane in an interdigitating fashion and form slit pore junctions. The resulting 3-layer structure resists the outward capillary pressure and restricts the passage of plasma macromolecules. Mutations in several podocyte proteins including nephrin and podocin that are associated with slit-pore junction result in hereditary FSGS. For example, mutation in nephrin gene NPHS1 has been shown to cause the Finnish-type congenital FSGS. Defective interaction of podocyte molecules with effector molecules and changes in signaling may also cause foot process effacement, increased protein permeability and result in FSGS [2, 12, 13, 14].
Etiology of FSGS is not clear but a circulating permeability factor (FSPF) is strongly associated with post-transplantation recurrence. FSPF in the serum, plasma or plasma fractions increases glomerular Palb in vitro and induces transient proteinuria in rats [4]. FSPF appears to be a low molecular weight anionic, hydrophobic and glycosylated protein(s) that alters glomerular protein phosphorylation and down regulates expression of nephrin [5]. FSPF has strong affinity for galactose and intravenously or orally administered galactose blocks the effect of FSPF on Palb [15]. We have developed and extensively used an in vitro functional assay for our studies on purification and characterization of the FSPF.
The in vitro permeability assay is a sensitive and reliable technique to study changes in the glomerular filtration barrier without the influence of hemodynamic and neuro-humoral factors. Increased glomerular Palb is a subtle indicator of glomerular injury and precedes proteinuria in several animal models of chronic disease including salt-induced hypertension [16], PAN nephrosis [17] and FSGS [4, 5]. We have applied the in vitro assay to isolate and identify FSPF and to ascertain the presence of FSPF in sera from FSGS patients during pre-transplantation evaluation and post-transplantation follow-up. We have also used this assay to study the effect of a wide range of molecules on the glomerular filtration barrier including cytokines (TNFα and TGF-β1) and free radicals superoxide and nitric oxide [18, 19, 20]. We have reported that indomethacin protects against the glomerular injury caused by FSPF and cyclooxygenase products PGE2, PGF2α or thromboxane A2 mimetic [21]. We have also reported that 20-hydroxyeicosatetraenoic acid (20-HETE), a product of the CYP450 hydroxylase activity, protects the glomerular barrier against puromycin aminonucleoside (PAN). PAN-induced injury develops into PAN-nephrosis, a well-known model of minimal change disease [17]. Following our initial work on PGE2, PGF2α, thromboxane A2 mimetic and 20-HETE we started studies on the role of EETs in the glomerulus and found that inhibition of CYP450 epoxygenase activity increases Palb that is prevented by 8,9-EET [8].
Liquid chromatography mass spectrometry confirmed that EETS are synthesized in glomeruli [8]. Four EET regioisomers, 5,6-, 8,9-, 11,12- and 14,15-EET are generated by epoxidation of arachidonic acid catalyzed by NAD(P)H-dependent CYP450 monooxygenases (epoxygenases). The mammalian epoxygenase subfamilies include CYP1A, CYP2B, CYP2C, CYP2D, CYP2G, CYP2J, CYP2N, and CYP4A. CYP2C isoforms are predominant in human and rat renal tissues [22]. CYP450 2C23 is an abundant epoxygenase isoform in the kidney [23]. Our preliminary results using LC-MS/MS also indicate the presence of CYP450 2C23 (EC 1.14.14.1) in rat glomerular proteins (unpublished data). Regioisomer selectivity, stereoisomer selectivity and efficiency of epoxygenation vary with the enzyme. The tissue, circulating and urinary levels of EETs depend upon the genetically determined cell-specific expression of epoxygenases and nutritional factors such as salt and cholesterol [22].
Cellular EETs occur in free as well as phospholipid-bound forms and are principally metabolized by soluble epoxide hydrolase (sEH) to corresponding vicinal diols, DiHETrE (DiHETs). Widely recognized as the endothelium derived hyperpolarizing factor (EDHF), EETs modulate an increasing number of biological events in an autocrine or paracrine manner [7, 24, 25]. EETs are anti-inflammatory and promote recovery after myocardial infarction and stroke [26, 27, 28]. EETs also have pro-angiogenic [29, 30, 31] and anti-apoptotic effects [32, 33]. EETs promote proliferation and activate Na+/H+ exchange in mesangial cells [34, 35]. Selective effects of EETs include pre-glomerular endothelium-dependent vasoconstriction (5,6-EET) or participate in adenosine-induced vasodilation (11,12-EET) through smooth muscle cells [36, 37] or vasoconstriction (8,9-EET) [38]. Our preliminary observations that led to these studies showed that 8,9-EET is glomeruloprotective [39] and that inhibition of the CYP450 epoxygenase activity results in increased glomerular albumin permeability [8]. These diverse tissue protective effects have made EETs an attractive target for developing new therapeutic molecules.
8,9-EET prevented the effect of FSPF in a dose-dependent manner (Figures 2, 3). A significant effect was observed at concentrations as low as 10 nM but at 100 nM 8,9-EET completely attenuated the FSPF-induced increase in Palb (Figure 3). We compared the effect of 8,9-EET with that of 5,6-EET, 11,12-EET and 14,15-EET. None of the other regioisomers attenuated the effect of the FSPF (Figure 2). These experiments showed that positions of the double bonds provide the molecular conformation required for the observed glomerular effect of 8,9-EET.
We confirmed the specificity of this unique protective effect using an unrelated fatty acid (all-cis 11, 14-eicosadienoic acid) and a metabolite of 8,9-EET (8,9-DiHETrE). 11,14-eicosadienoic acid (11, 14-EDA), a 20 carbon long fatty acid containing two double bonds, is not commonly present as free fatty acid or bound with tissue lipids. EDA at 100 nM concentration did not attenuate the effect of FSPF (Figure 4A). These results showed that identical number of carbons in the molecule were not sufficient to block the FSPF-induced increase in Palb. Structural specificity required for glomerular protection was further demonstrated in experiments using 8,9-DiHETrE, the vicinal diol of 8,9-EET generated in sEH catalyzed reaction. Vicinal diols of EETs are not incorporated into membrane lipids but may show biological activity (40, 41). Results showed that replacing the epoxy group by two hydroxyls at carbons 8 and 9 results in a loss of activity (Figure 4B). Thus, the presence of an epoxy group and the positions of double bonds are structural determinant of the biological activity of 8,9-EET.
Exogenously applied 8,9-EET may exert its protective effects via one or more mechanisms, inter alia, binding with a membrane receptor, calcium mobilization, calcium activated potassium channels, protein phosphorylation/dephosphorylation, peroxisome proliferator-activated receptors and gene activation [7, 28]. Diverse autocrine and paracrine effects suggest that EETs also perform second messenger functions in addition to modulation of calcium-activated potassium channels. EETs have been shown to interact with a number of molecules in intracellular signaling pathways and transcription factors. EETs activate many signaling molecules including protein kinase A [42], tyrosine kinases and phosphatases [43], p38 MAP kinase [43], ERK1/2, MAP kinase phosphatatses. EETs also inhibit cJun N-terminal kinases (JNK) [44]. EETs also modulate intracellular signaling through binding with transcription factors such as the cyclic AMP-response element-binding protein (CREB) [45], nuclear factor-κB (NF-κB) [46], peroxisome proliferator-activated receptor α(PPAR-α[47] or forkhead box O3a (FOXO3a) [48]. One or more of these signaling mechanisms may be invoked by 8,9- and other EETs in the glomerular filtration barrier. Availability of suitable analogs and antagonists of 8,9-EET will be essential to explore the signaling events in vitro or in vivo.
We speculate that up regulation of the glomerular levels of 8,9-EET will be useful in preserving the filtration barrier function under a variety of pathological conditions including FSGS. However, the therapeutic usefulness of native 8,9-EET would be limited due to several factors including high reactivity, rapid conversion into DiHETrE by the epoxide hydrolase activity, efficient esterification with membrane glycerol at the sn-2 position [48] and inactivated by auto-oxidation. Further, it is difficult to test the beneficial effect of 8,9-EET in biological systems in vivo without determining the conditions that would promote its stability. Nevertheless, inhibition of metabolism, development of structural analogs or up-regulation of in vivo synthesis may lead to success in increasing the levels of 8,9-EET.
Up regulation of EETs levels through inhibition of the sEH activity or administration of stable analogs may result in increased tissue and circulating levels of 8,9-EET or its active analogs. Highly effective sEH inhibitors have been developed and their potential therapeutic application is being tested [49, 50]. Alternatively, compounds with analogous biological effects may serve as effective substitutes. Structural analogs are commonly used to study the role of transient metabolites and for developing new drugs. Since the presence of three double bonds renders 8,9-EET highly reactive and therefore unstable, we have initiated the use of synthetic analogs (see Methods) with a goal to identify mimetic(s) of 8,9-EET that are stable in biological environment and effective at the molecular level (Figure 1b, Table 1).
We synthesized and used three analogs containing 2 double bonds each, i.e., 8,9-EEDs (# 203, 204 and 241), and three analogs with 1 double bond each, i.e., 8,9-EEEs (# 212, 213 and 214). Using the Palb assay we demonstrated that analogs with two double bonds did not increase Palb of untreated glomeruli, significantly blocked the effect of the FSPF and did not antagonize the effect of 8,9-EET on Palb. Thus presence of two double bonds may provide a degree of stability to molecular molecule and retain the biological effect of the parent molecule.
Analogs containing only one double bond (EEEs, Table 1) increased Palb of untreated glomeruli in the absence of FSPS and antagonized the protective effect of 8,9-EET to varying degrees (Figures 5A, 6A). The number and position of double bonds are key determinants of fatty acid chemistry and biology. The biological differences among fatty acids and metabolites derived from linolenic acid (ω-3), linoleic Acid (ω-6) and oleic acid (ω-9) are fundamental to our understanding of physiology and several pathological conditions. The incidence of several cardiovascular diseases is believed to be due to differences in dietary intakes of linoleic and linolenic acids resulting in altered ratios of fatty acids derived from these parent molecules (51). Several classical studies successfully used structural analogs based on co-enzyme A to characterize steps in the biosynthesis and regulation of fatty acids [52]. We have also developed structural analogs of EETs by introducing sub-molecular changes. For example 14,15-Epoxyeicosa-5(Z)-enoic acid is a specific antagonist of 14,15-EET [53]. Therefore, the observed loss of protective effect in one double bond containing analogs (8,8-EEEs) also suggests a potential gain of another function e.g., feed-back modulation of 8,9-EET. As suggested by one of the reviewers of this manuscript, such reversal of function may exist as mechanism to regulate the biological effect(s) of 8,9-EET.
In conclusion, we have demonstrated for the first time that 8,9-EET, a naturally occurring epoxide of arachidonic acid, protects the glomerular protein permeability barrier from injury caused by FSPF. None of the other regioisomers attenuated the FSPF-induced glomerular injury. At least two double bonds appear to be required for the glomerulo-protective function of 8,9-EET. We have recently shown that 20-HETE also protects the glomerular filtration barrier [17]. This raises the possibility that such metabolites are required to maintain normal glomerular barrier function and that metabolic dysregulation of these molecules plays an important role in the development of proteinuric states. Angiotensin II converting enzyme inhibitors, corticosteroids, cyclosporine and tacrolimus are clinically used to manage/treat chronic renal disease. However, FSGS is highly resistant to regimens based on these and other currently used therapeutic agents. Besides, most therapies also cause side effects including nephrotoxicity and immunosuppression that may complicate the disease and add to poor outcomes. Thus, there is a need for new and effective therapeutic agents. Development of more stable analogs of certain P450 metabolites may provide a new class of specific therapeutic tools to treat patients with glomerular dysfunction associated with chronic kidney disease.
Acknowledgments
We wish to thank Jaya Bhojak, M.S. and Dr. R.S. Reddy, D.V.M., Ph.D. for excellent technical support. We thank Dr. Elizabeth Jacobs, Chief, Pulmonary and Critical Care Medicine, Department of Medicine, for her generous support of these studies. We thank Dr. Richard Roman, Director, Kidney Disease Center, Medical College of Wisconsin, Milwaukee, for a critical review of the results presented at the 41st Annual Meeting of the American Society of Nephrology and Renal Week, Philadelphia, November 4th–9th, 2008.
Financial support for these studies was provided in part by NIH/NIDDK 061588 (ETM), NIH/NIDDK 064969 (ETM), NIH HL 69996 (MM), GM31278 (JRF), the Robert A. Welch Foundation (JRF) and the Department of Medicine, Medical College of Wisconsin, Milwaukee (MS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hogg R, Middleton J, Vehaskari VM. Focal segmental glomerulosclerosis - epidemiology aspects in children and adults. Pediatr Nephrol. 2007;22:183–186. doi: 10.1007/s00467-006-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Agati VD. The spectrum of focal segmental glomerulosclerosis: new insights. Curr Opin Nephrol Hypertens. 2008;17:271–281. doi: 10.1097/MNH.0b013e3282f94a96. [DOI] [PubMed] [Google Scholar]
- 3.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 4.Sharma M, Sharma R, Reddy SR, McCarthy ET, Savin VJ. Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation. 2002;73:366–372. doi: 10.1097/00007890-200202150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Sharma M, Sharma R, McCarthy ET, Savin VJ. The focal segmental glomerulosclerosis permeability factor: biochemical characteristics and biological effects. Exp Biol Med (Maywood) 2004;229:85–98. doi: 10.1177/153537020422900111. [DOI] [PubMed] [Google Scholar]
- 6.Savin VJ, McCarthy ET, Sharma M. Permeability factor in FSGS. Semin Nephrol. 2003;23:147–160. doi: 10.1053/snep.2003.50024. [DOI] [PubMed] [Google Scholar]
- 7.Spector A. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2008 doi: 10.1194/jlr.R800038-JLR200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams JM, Sharma M, Anjaiah S, Falck J, Roman RJ. Role of Endogenous CYP450 Metabolites of AA in Maintaining the Glomerular Protein Permeability Barrier. Am J Physiol. 2007;293:F501–505. doi: 10.1152/ajprenal.00131.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma M, Sharma R, McCarthy ET, Savin VJ. ‘‘The FSGS factor’’: Enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol. 1999;10:552–561. doi: 10.1681/ASN.V103552. [DOI] [PubMed] [Google Scholar]
- 10.Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient in isolated rat glomeruli. J Am Soc Nephrol. 1992;3:1260–1269. doi: 10.1681/ASN.V361260. [DOI] [PubMed] [Google Scholar]
- 11.Corey EJ, Niwa H, Falck JR. Selective epoxidation of eicosa-cis-5,8,11,14-tetraenoic (arachidonic) acid and eicosa-cis-8,11,14-trienoic acid. J Am Chem Soc. 1979;101:1586–1587. [Google Scholar]
- 12.Ballermann BJ, Stan RV. Resolved: capillary endothelium is a major contributor to the glomerular filtration barrier. J Am Soc Nephrol. 2007;18:2432–2438. doi: 10.1681/ASN.2007060687. [DOI] [PubMed] [Google Scholar]
- 13.Garg P, Verma R, Holzman LB. Slit diaphragm junctional complex and regulation of the cytoskeleton. Nephron Exp Nephrol. 2007;106:e67–72. doi: 10.1159/000101795. [DOI] [PubMed] [Google Scholar]
- 14.Pollak MR. Inherited podocytopathies: FSGS and nephrotic syndrome from a genetic viewpoint. J Am Soc Nephrol. 2002;13:3016–3023. doi: 10.1097/01.asn.0000039569.34360.5e. [DOI] [PubMed] [Google Scholar]
- 15.Savin VJ, McCarthy ET, Sharma R, Sharma M. Galactose binds to focal segmental glomerulosclerosis permeability factor and inhibits its activity. Translational Research. 2008;151:288–292. doi: 10.1016/j.trsl.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming Growth Factor-β, 20-HETE Interaction, and Glomerular Injury in Dahl Salt-Sensitive Rats. Hypetension. 2005;45:1–6. doi: 10.1161/01.HYP.0000153791.89776.43. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy ET, Sharma R, Sharma M. Protective effect of 20-hydroxy- eicosatetraenoic acid (20-HETE) on glomerular protein permeability barrier. Kidney Int. 2005;67:152–156. doi: 10.1111/j.1523-1755.2005.00065.x. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy ET, Sharma R, Sharma M, Savin VJ. TNF-α increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol. 1998;9:433–438. doi: 10.1681/ASN.V93433. [DOI] [PubMed] [Google Scholar]
- 19.Sharma R, Sharma M, Khanna AK, Savin VJ. Transforming Growth factor increases glomerular albumin permeability via hydroxyl radicals. Kidney Int. 2000;58:131–136. doi: 10.1046/j.1523-1755.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharma M, McCarthy ET, Savin VJ, Lianos EA. Nitric oxide preserves the glomerular filtration barrier by antagonizing superoxide. Kidney Int. 2005;68:2735–2744. doi: 10.1111/j.1523-1755.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy ET, Sharma M. Indomethacin protects permeability barrier from focal segmental glomerulosclerosis serum. Kidney Int. 2002;61:534–541. doi: 10.1046/j.1523-1755.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- 22.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 23.Holla VR, Makita K, Zaphiropoulos PG, Capdevila JH. The kidney cytochrome P-450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest. 1999;104:751–760. doi: 10.1172/JCI7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 2007;72(6):683–689. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- 25.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 26.Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, Hurn PD. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke. 2002;33:1677–1684. doi: 10.1161/01.str.0000016332.37292.59. [DOI] [PubMed] [Google Scholar]
- 27.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wray J, Bishop-Bailey D. Epoxygenases and peroxisome proliferator-activated receptors in mammalian vascular biology. Exp Physiol. 2008;93:148–154. doi: 10.1113/expphysiol.2007.038612. [DOI] [PubMed] [Google Scholar]
- 29.Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, Harder DR. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H215–224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- 30.Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- 31.Fleming I. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat. 2007;82:60–67. doi: 10.1016/j.prostaglandins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Chen JK, Capdevila J, Harris RC. Cytochrome P450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Mol Cell Biol. 2001;21:6322–6331. doi: 10.1128/MCB.21.18.6322-6331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhanasekaran A, Al Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora MM. Protective effect of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol. 2006;291:H517–H531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 34.Harris RC, Homma T, Jacobson HR, Capdevila J. Epoxyeicosatrienoic acids activate Na+/H+ exchange and are mitogenic in cultured rat glomerular mesangial cells. J Cell Physiol. 1990;144:429–437. doi: 10.1002/jcp.1041440310. [DOI] [PubMed] [Google Scholar]
- 35.Homma T, Zhang JY, Shimizu T, Prakash C, Blair IA, Harris RC. Cyclooxygenase-derived metabolites of 8,9-epoxyeicosatrienoic acid are potent mitogens for cultured rat glomerular mesangial cells. Biochem Biophys Res Commun. 1993;191:282–288. doi: 10.1006/bbrc.1993.1214. [DOI] [PubMed] [Google Scholar]
- 36.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;11:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 37.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141:441–448. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katoh T, Takahashi K, Capdevila J, Karara A, Falck JR, Jacobson HR, Badr KF. Glomerular stereospecific synthesis and hemodynamic actions of 8,9-epoxyeicosatrienoic acid in rat kidney. Am J Physiol. 1991;261:F578–86. doi: 10.1152/ajprenal.1991.261.4.F578. [DOI] [PubMed] [Google Scholar]
- 39.Sharma M, Reddy DS, Falck JR, Harland DR, McCarthy ET, Savin VJ, Jacobs ER, Medhora MM. 8,9-EET Protects Against FSGS Factor-Induced Glomerular Injury. 8th Annual Winter Eicosanoid Conference; 2006, March 12–15. [Google Scholar]
- 40.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Cir Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 41.Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- 42.Imig JD, Inscho EW, Deichamnn PC, Reddy KM, Falck JR. Afferent arteriolar dilation to the sulfonamide analog of 11,12-epoxyeicosatrienoic acid involves protein kinase A. Hypertension. 1999;33:408–413. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- 43.Fleming I, Fissthaler B, Michaelis UR, Kiss l, Popp R, Busse R. The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signaling pathways and proliferation in vascular cells. Pflugers Arch. 2001;442:511–518. doi: 10.1007/s004240100565. [DOI] [PubMed] [Google Scholar]
- 44.Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-I, inhibition of the c-Jun N-terminal kinase, and upregulation of cyclin D1. J Biol chem. 2002;277:15671–15676. doi: 10.1074/jbc.M110806200. [DOI] [PubMed] [Google Scholar]
- 45.Michaelis UR, Falck JR, Schmidt R, Busse R, Fleming I. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce the expression of cyclooxygenase-1 in endothelial cells. Arterioscler Thromb vasc Biol. 2005;25:321–326. doi: 10.1161/01.ATV.0000151648.58516.eb. [DOI] [PubMed] [Google Scholar]
- 46.Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelail proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–29625. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- 47.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of Cytochrome P450 epoxygenas-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capdevila JH, Kishore V, Dishman E, Blair IA, Falck JR. A novel pool of rat liver inositol and ethanolamine phospholipids contains epoxyeicosatrienoic acids (EETs) Biochem Biophys Res Commun. 1987;146:638–644. doi: 10.1016/0006-291x(87)90576-6. [DOI] [PubMed] [Google Scholar]
- 49.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 50.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 51.Weber PC, Fischer S, schacky CV, Lorenz r, Strasser T. The conversion of dietary eicosapentaenoic acid to prostanoids and leukotrienes in man. Prog Lipid Res. 1986;25:273–276. doi: 10.1016/0163-7827(86)90056-1. [DOI] [PubMed] [Google Scholar]
- 52.Robinson JD, Brady RO, Bradley RM. Biosynthesis of fatty acids: IV. Studies with inhibitors. J Lipid Res. 1963;4:144–150. [PubMed] [Google Scholar]
- 53.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]