Abstract
Nerve growth factor-induced B (NGFI-B) genes are orphan nuclear receptors, and NGFI-Bα (Nur77, TR3) is overexpressed in bladder tumors and bladder cancer cells compared with nontumorous bladder tissue. 1,1-Bis(3′-indolyl)-1-(p-methoxyphenyl)-methane (DIM-C-pPhOCH3) and 1,1-bis(3′-indolyl)-1-(p-phenyl)methane have previously been identified as activators of Nur77, and both compounds inhibited growth and induced apoptosis of UC-5 and KU7 bladder cancer cells. The proapoptotic effects of methylene-substituted diindolylmethanes (C-DIMs) were unaffected by cotreatment with leptomycin B and were dependent on nuclear Nur77, and RNA interference with a small inhibitory RNA for Nur77 (iNur77) demonstrated that C-DIM-induced activation of apoptosis was Nur77-dependent. Microarray analysis of DIM-C-pPhOCH3-induced genes in UC-5 bladder cancer cells showed that this compound induced multiple Nur77-dependent proapoptotic or growth inhibitory genes including tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), cystathionase, p21, p8, and sestrin-2. DIM-C-pPhOCH3 (25 mg/kg/d) also induced apoptosis and inhibited tumor growth in athymic nude mice bearing KU7 cells as xenografts, demonstrating that Nur77-active C-DIMs exhibit potential for bladder cancer chemotherapy by targeting Nur77, which is overexpressed in this tumor type.
The nuclear receptor family of transcription factors includes the steroid and thyroid hormones, vitamin D, retinoid and ecdysone receptors, ligand-activated orphan receptors, and orphan receptors with no known ligands (Milbrandt, 1988; Mangelsdorf et al., 1995). Nuclear receptors influence diverse aspects of normal physiology in multiple tissues, and several receptors are drug targets for treating several diseases, including cancer. Nerve growth factor-induced B (NGFI-B) is part of a subfamily of orphan nuclear receptors; members of this subfamily include Nur77 (NGFI-Bα, TR3), Nurr1 (NGFI-Bβ), and Nor1 (NGFI-Bγ). Nur77 is expressed in multiple tissues; Nurr1 has been detected in thymus osteoblasts, liver, and pituitary gland; and Nor1 is highly expressed in the pituitary gland with low expression in other tissues (Milbrandt, 1988; Bandoh et al., 1997; Maruyama et al., 1997). The physiological roles for NGFI-B proteins are not fully understood; however, gene targeting knockout experiments demonstrate several important functions for these proteins. For example, Nurr1 knockout mice have severe impairments in midbrain neuronal development and dopamine expression, and these animals die soon after birth (Zetterstrom et al., 1997; Saucedo-Cardenas et al., 1998; DeYoung et al., 2003); Nor1 knockout animals die at gestational day 5 (DeYoung et al., 2003), whereas Nur77 knockout mice do not exhibit a specific phenotype (Lee et al., 1995), and this may be related to coexpression of both Nur77/Nor1, which exhibit some overlapping functions.
Several studies suggest that in cancer cells, Nur77 plays a role in cell death pathways activated by apoptosis-inducing agents (Li et al., 2000; Wu et al., 2002; Holmes et al., 2003a,b; Mu and Chang, 2003; Wilson et al., 2003a; Lin et al., 2004). Li et al. (2000) reported that treatment of LNCaP prostate cancer cells with agents such as retinoids, 12-O-tetradecanoylphorbol-13-acetate, and tumor necrosis factor α resulted in induction of Nur77 gene expression. Surprisingly, induction of apoptosis and cytochrome c release from the mitochondria was independent of the DNA binding domain of Nur77, and treatment with leptomycin B (a blocker of nuclear export) inhibited induction of Nur77-dependent apoptosis. Induction of apoptosis was accompanied by translocation of Nur77 from the nucleus to the mitochondria, and Nur77 specifically interacted with Bcl-2 and converted Bcl-2 into a proapoptotic factor in human embryonic kidney 293T and HCT116 cells (Lin et al., 2004). However, a study in colon cancer cells reported that butyrate-induced apoptosis was associated with nuclear-to-cytoplasmic translocation of Nur77, which was not accompanied by subsequent mitochondrial interactions (Wilson et al., 2003a).
Studies in this laboratory have characterized a series of methylene-substituted diindolylmethane (C-DIM) analogs as activators of orphan receptors (Chintharlapalli et al., 2004, 2005a; Qin et al., 2004; Kassouf et al., 2006; Cho et al., 2007; Inamoto et al., 2008). Two of these compounds, 1,1-bis(3′-indolyl)-1-(p-methoxyphenyl)methane (DIM-C-pPhOCH3) and 1,1-bis(3′-indolyl)-1-(p-phenyl)methane (DIM-C-pPh), activate Nur77 in colon and pancreatic cancer cells (Chintharlapalli et al., 2005a; Cho et al., 2007), and 1,1-bis(3′-indolyl)-1-(p-chlorophenyl)methane (DIM-C-pPhCl) activates NGFI-Bβ (Nurr1) orphan receptor in bladder cancer cells (Inamoto et al., 2008). Nur77 and Nurr1 are widely expressed in bladder cancer cells (Inamoto et al., 2008), and we now show overexpression of Nur77 in bladder tumors. Nur77-active C-DIMs, such as DIM-C-pPhOCH3, inhibit bladder cancer cell growth and induce apoptosis. DIM-C-pPhOCH3 activates nuclear Nur77, and results of RNA interference studies show that induction of apoptosis is due to induction of several proapoptotic genes and proteins, including tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). DIM-C-pPhOCH3 inhibited bladder tumor growth in a xenograft mouse model, suggesting that C-DIM-dependent activation of Nur77 is a promising new mechanism-based pathway for developing new agents for bladder cancer chemotherapy.
Materials and Methods
Cells, Biochemicals, and Antibodies.
Human bladder cancer cell lines KU7 and UC-5 were provided by author A.M.K. Cells were maintained in Dulbecco's modified Eagle's medium/Ham's F-12 (Sigma, St. Louis, MO) without phenol red supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 5% fetal bovine serum, and 10 ml/liter of 100× antibiotic/antimycotic solution (Sigma-Aldrich). Cells were maintained at 37°C in the presence of 5% CO2. Antibodies for cleaved PARP and cleaved caspase 8 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies for Nur77, TRAIL, Sp1, IgG, and β-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Nur77 antibodies from Imgenex (San Diego, CA) were also used for immunostaining and Western blots, and results were similar to those with Nur77 antibodies from Santa Cruz. Caspase inhibitor (Z-VAD-fmk) was obtained from Alexis Biochemicals (Lausen, Switzerland). Western Lightning chemiluminescence reagent was from PerkinElmer Life Sciences (Waltham, MA). For RNA interference assays, we used a nonspecific scrambled (iScr) oligonucleotide as described previously (Abdelrahim et al., 2002). The small inhibitory RNA for Nur77 (iNur77) was identical to the reported oligonucleotide (Lin et al., 2004), and these were purchased from Dharmacon RNA Technologies (Lafayette, CO). Leptomycin B was purchased from Sigma. The C-DIMs were synthesized in this laboratory as described previously (Qin et al., 2004).
Cell Proliferation Assay.
KU-7 and UC-5 cells were seeded in 12-well plates in Dulbecco's modified Eagle's medium/Ham's F-12 containing 2.5% charcoal-stripped fetal bovine serum for 24 and 48 h until plates reached 50 to 60% confluence, which was usually observed 24 h after seeding. Cells were then treated with different concentrations of the test compounds (in DMSO) or DMSO alone. KU7 and UC-5 cells were counted to evaluate the effect of C-DIMs and DMSO (solvent control) on viable cell number using a particle counter (Z1; Beckman Coulter, Fullerton, CA). Each experiment was carried out in triplicate, and results are expressed as means ± S.D. for each treatment group.
Western Blot Analysis.
Cells were treated with the C-DIM compounds, and a caspase inhibitor and leptomycin B were added simultaneously. For RNA interference studies, the oligonucleotides (iScr or iNur77) were transfected into bladder cancer cells; after 36 h, cells were treated with DMSO or C-DIM compounds as described previously (Cho et al., 2007). Cells were collected, and cell lysates were prepared using lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 1 mM sodium orthovanadate; 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, and 1 μg/ml aprotinin). After centrifugation of lysates at 15,000g for 20 min, the supernatants were recovered, and protein was quantified by the Bradford protein assay using reagent kit from Bio-Rad Laboratories (Hercules, CA). Protein samples (20 to 60 μg) were size-separated by electrophoresis on SDS-polyacrylamide gels under nonreducing conditions. Separated proteins were electroblotted onto nitrocellulose membranes. The blot was blocked by incubating in blocking buffer (5% skim milk, 10 mM Tris, pH 7.5, 10 mM NaCl, and 0.1% Tween 20) for 1 h at 20°C and was incubated with the primary antibody overnight at 4°C. Incubation with a horseradish peroxidase-conjugated anti-mouse or rabbit secondary antibody was then carried out at 37°C for 1 h. Antibody-bound proteins were detected by the enhanced chemiluminescence Western blotting analysis system.
Immunostaining.
Cells were fixed immediately in 4% paraformaldehyde, added with 0.3% Triton X-100 (Roche Molecular Biochemicals, Indianapolis, IN) for 10 min, and preincubated for 1 h with 10% normal goat serum (Vector Laboratories, Burlingame, CA). Cells were incubated with anti-Nur77 antibody (1:100) or anti-IgG (1:100) and were incubated with fluorescein isothiocyanate-conjugated secondary antibody (1:200; Vector Laboratories, Burlingame, CA). The four-well chambers were mounted with mounting medium (Vector Laboratories) and viewed on a fluorescence microscope (Olympus).
Reverse Transcriptase-Polymerase Chain Reaction.
Total RNA was extracted using RNeasy Mini Kit (Qiagen Inc., Valencia, CA), and 1 μg of RNA was used to synthesize cDNA using reverse transcription system (Promega, Madison, WI). The PCR conditions were as follows: initial denaturation at 94°C (2 min) followed by 28 cycles (NAG-1 and CSE), 30 cycles (p8 and Sestin-2), or 26 cycles (GAPDH) of denaturation for 1 min at 94°C, annealing for 1 min at 58°C (NAG-1) or 61°C (CSE, p8, Sestin-2, and GAPDH), extension at 72°C for 1 min, and a final extension step at 72°C for 5 min. The mRNA levels were normalized using GAPDH as an internal housekeeping gene. Primers obtained from Integrated DNA Technologies, Inc. (Coralville, IA) and used for amplification were as follows: NAG-1: sense, 5′-GTG CTC ATT CAA AAG ACC GAC ACC G-3′; antisense, 5′-ATA CAC AGT TCC ATC AGA CCA GCC CC-3′; p8: sense, 5′-ATG GCC ACC TTC CCA CCA GCA-3′; antisense, 5′-TCA GCG CCG TGC CCC TCG CT-3′; CSE: sense, 5′-GGC GAT CCA TGT GGG CCA GGA-3′; antisense, 5′-ATG TCT CCA TGC TTA TGG ACA AT-3′; sestrin-2: sense, 5′-GAC TCC GAG TGC CGC GCA GAG-3′; antisense, 5′-ATG GCG GGC GGC AGC CAT GAT-3′; and GAPDH: sense, 5′-ACG GAT TTG GTC GTA TTG GGC G-3′; antisense, 5′-CTC CTG GAA GAT GGT GAT GG-3′. PCR products were electrophoresed on 1% agarose gels containing ethidium bromide and visualized under UV transillumination.
Quantitative Real-Time PCR.
cDNA was prepared from the total RNA of cells using Reverse Transcription System (Promega). Each PCR was carried out in triplicate in a 20-μl volume using SYBR Green Mastermix (Applied Biosystems, Foster City, CA) for 15 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 30 s and 60°C for 1 min in the fast real-time PCR system (7900HT; Applied Biosystems). The ABI Dissociation Curves software was used after a brief thermal protocol (95°C for 15 s and 60°C for 15 s, followed by a slow ramp to 95°C) to control for multiple species in each PCR amplification. Values for each gene were normalized to expression levels of TATA-binding protein. The sequences of the primers used for real-time PCR were as follows: p21: sense 5′-GGC AGA CCA GCA TGA CAG ATT TC-3′; antisense, 5′-CGG ATT AGG GCT TCC TCT TGG-3′; p8: sense, 5′-CTA TAG CCT GGC CCA TTC CT-3′; antisense, 5′-TCT CTC TTG GTG CGA CCT TT-3′; sestrin 2: sense, 5′-CAA GCT CGG AAT TAA TGT GCC-3′; antisense, 5′-CTC ACA CCA TTA AGC ATG GAG-3′; and TATA-binding protein: sense, 5′-TGC ACA GGA GCC AAG ATG GAA-3′; antisense, 5′-CAC ATC ACA GCT CCC CAC CA-3′. The PCR primers for CSE, NAG1, and Nur77 were purchased from Qiagen.
Microarray Experiments.
Microarray studies focused on early-induced genes, and UC-5 cells were treated with DMSO or 15 μM DIM-C-pPhOCH3 for 2 and 6 h. RNA was isolated as described for the RT-PCR experiment and analyzed for gene expression using the Codelink Whole Genome Bioarrays (300026), and three replicates were determined for each time point and the DMSO control. The microarray data were analyzed using GeneSpring software version 7.2 (Agilent Technologies, Santa Clara, CA). The data were normalized in two steps. First, for each array, the expression value of each gene was divided by the median of all the values in that array. Second, for each gene, the expression value in each array was divided by the median value of that gene across all arrays. Genes with low-quality signals were excluded for statistical analysis. One-way ANOVA (assume equal variances) was carried out to identify differentially expressed genes. A gene was said to be differentially expressed if the Benjamini and Hochberg adjusted p values were less than 0.05.
Xenograft Studies in Athymic Mice.
Male athymic nude mice (Foxn1nu, aged 7–8 weeks) were purchased from Harlan (Indianapolis, IN). The mice were housed and maintained in laminar flow cabinets under specific pathogen-free conditions. A xenograft was established by subcutaneous injection of in vitro cultured KU-7 cells (107 cells/150 μl) into the flanks of individual mice. Tumors were allowed to grow for 7 days until tumors were palpable. Mice were then randomized into two groups of five mice per group and dosed by oral gavage with either corn oil or 25 mg/kg/day DIM-C-pPHOCH3 for 17 days. The mice were weighed, and tumor size was measured twice a week with calipers to permit calculation of tumor volumes, V = L × W2/2, where L and W were length and width. Final body, organ, and tumor weights were determined at the end of the dosing regimen, and both organ and tumor blocks were obtained for hematoxylin and eosin staining and histopathological analysis.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay.
For the TUNEL assay, tumor tissue was fixed in formalin and embedded in paraffin, TUNEL staining was carried out using DeadEnd Colorimetric TUNEL System (Promega). Paraffin-embedded sections (4–6 μm thick) were processed per manufacturer's protocol. In brief, sections were deparaffinized in xylene and then treated with a graded series of alcohol [100, 95, 85, 70, and 50% ethanol (v/v) in double-distilled water] and rehydrated in PBS, pH 7.5. Tissues were then treated with proteinase K solution for permeabilization and then refixed with 4% paraformaldehyde solution. Slides were then treated with recombinant terminal deoxynucleotidyl transferase reaction mix and incubated at 37°C for 1 h. Reaction was terminated by immersing the slides in 2× standard saline citrate solutions for 15 min at room temperature. After blocking the endogenous peroxidases activity (by 0.3% hydrogen peroxide), slides were washed with phosphate-buffered saline and then incubated with streptavidin horseradish peroxidase solution for 30 min at room temperature. After washing, slides were incubated with 3,3′-diaminobenzidine (substrate) solution until a light brown background appeared (10 min) and then rinsed several times in deionized water. After mounting, slides were observed by light microscope.
Statistical Analysis.
Statistical significance was assessed using Student's t test. A value of P < 0.05 compared with solvent control was considered statistically significant.
Results
The potential application of Nur77-active C-DIMs for bladder cancer chemotherapy is dependent on expression of this orphan receptor in bladder tumor tissue. Figure 1 summarizes immunostaining of Nur77 in bladder tumors from patients. Nur77 staining was observed in low-grade noninvasive tumors (Fig. 1A), in high-grade stage T2 bladder cancer (Fig. 1B), and in high-grade stage T3 bladder cancer (Fig. 1C) growing in adipose tissue outside the muscle of the bladder. This pattern of Nur77 staining was observed in multiple bladder tumor samples, whereas minimal to nondetectable staining of Nur77 was detected in nontumorous tissue. In addition, expression of Nur77 was also investigated in low-grade RT4 and high-grade invasive UC-14 tumors established in nude mice (Fig. 1D). Sections from the low-grade well differentiated RT4 tumors growing in the wall of the mouse bladder expressed Nur77 throughout the tumor, whereas staining for this protein in nontumorous tissue was not detected. Nur77 staining was also detected in the UC-14 cells, which are shown invading the wall of the mouse bladder, whereas Nur77 was not detected in nontumor tissue. Previous studies show that Western blot analysis of whole-cell lysates from several bladder cancer cell lines, including KU7 and UC-5 cells, indicates that all of these cell lines express Nur77 protein (Inamoto et al., 2008).
Fig. 1.
Nur77 expression in human bladder tumors and bladder cancer cell lines. Expression of Nur77 in low-grade (A), high-grade stage 2 (B), and high-grade stage 3 (C) bladder tumors and in RT4 and UC-14 (D) bladder cancer cell lines were determined by immunostaining with Nur77 antibodies as outlined under Materials and Methods.
Figure 2, A and B, illustrate the concentration-dependent effects of DIM-C-pPhOCH3 and DIM-C-pPh (5, 10, and 15 μM) on proliferation of UC-5 and KU7 bladder cancer cell lines, respectively. Both compounds inhibited growth of UC-5 and KU7 cells within 24 h after treatment, and the magnitude of growth inhibition was higher after 48 h. IC50 (48 h) values for DIM-C-pPhOCH3 in UC-5 and KU7 cells were 12.3 and 9.3 μM, and the corresponding values for DIM-C-pPh were 11.8 and 11.2 μM, respectively.
Fig. 2.
Nur77-active C-DIMs decrease cell proliferation in UC-5 and KU7 cells. UC-5 cells (A) and KU7 cells (B) were treated with DMSO or different concentrations of C-DIMs (5, 10 and 15 μM) for 24 and 48 h, and the percentages of cells in the treatment groups compared with the solvent control (DMSO; set at 100%) were determined. Columns, mean of three replicate experiments for each treatment group; error bars, S.D. ∗, p < 0.05 compared with DMSO treatment group.
We further investigated the subcellular location of this receptor in the absence or presence of DIM-C-pPhOCH3. Figure 3A illustrates the expression of Nur77, PARP, and Sp1 proteins in cytosolic (C) or nuclear (N) fractions in untreated cells and cells treated with 15 μM DIM-C-pPhOCH3, 0.05 ng/ml leptomycin B, or their combination for 24 h. In all treatment groups, Nur77, PARP (full-length or cleaved), and Sp1 proteins were observed only in the nuclear fraction, and this is consistent with the subcellular nuclear location of these proteins. Moreover, treatment with DIM-C-pPhOCH3 did not change the subcellular location of these proteins. A nonspecific band was observed in both cytosolic and nuclear fractions. DIM-C-pPhOCH3 also induced caspase-dependent PARP cleavage, and this was observed in the presence or absence of leptomycin B, which inhibits nuclear export, suggesting that induction of caspase-dependent PARP cleavage by DIM-C-pPhOCH3 was associated with nuclear Nur77 and did not require nuclear export of the receptor. The nuclear location of Nur77 in UC-5 cells treated with DMSO or 15 μM DIM-C-pPhOCH3 was also confirmed by immunostaining for Nur77, which was located in the nucleus of these cells (Fig. 3B). Both DIM-C-pPhOCH3 and DIM-C-pPh induced PARP and caspase-8 cleavage in UC-5 and KU7 cells after treatment for 24 h, and the decreased apoptosis using 15 μM DIM-C-Ph in KU7 cells was due to cytotoxicity in this cell line (Fig. 3C). In addition, DIM-C-pPhOCH3- and DIM-C-pPh-induced PARP cleavage in UC-5 cells was inhibited after cotreatment with the pancaspase inhibitor Z-VAD-fmk (Fig. 3D).
Fig. 3.
Nur77-active C-DIMs induce apoptosis in UC-5 and KU7 cells and also act through nuclear Nur77. A, effects of leptomycin B on PARP cleavage. UC-5 cells were treated for 24 h with DIM-C-pPhOCH3 alone or in the presence of leptomycin B (0.05 ng/ml), and Western blot analysis of nuclear and cytosolic cell lysates was used to detect Nur77 and PARP cleavage. Sp1, loading control for nuclear fraction; N.S., nonspecific band for loading control. B, immunostaining for Nur77. UC-5 cells were treated with DMSO or 15 μM DIM-C-pPhOCH3 for 24 h and immunostained with IgG or Nur77 antibodies. C, dose-dependent induction of apoptosis by C-DIMs. UC-5 and KU7 cells were treated with DMSO or different concentrations of DIM-C-pPhOCH3 or DIM-C-Ph for 48 h. D, inhibition of Nur77-active C-DIMs-induced apoptosis by Z-VAD-fmk. UC-5 cells were treated with Nur77-active C-DIMs alone or in combination with 10 μM Z-VAD-fmk, and whole-cell lysates were analyzed by Western blot analysis.
Previous studies in pancreatic and colon cancer cells showed that DIM-C-pPhOCH3 induced TRAIL and the extrinsic pathway of apoptosis (Chintharlapalli et al., 2005a; Cho et al., 2007). Because Nur77-active C-DIMs activated caspase-8, we also investigated activation of TRAIL in UC-5 cells, and treatment with 15 μM DIM-C-pPhOCH3 or DIM-C-pPh for 48 h induced TRAIL protein (Fig. 4A). Similar results were observed for TRAIL mRNA levels (data not shown). The role of Nur77 in mediating the induction of TRAIL and apoptosis in UC-5 cells by DIM-C-pPhOCH3 was investigated by transient transfection of a small inhibitory RNA for Nur77 (iNur77) as described previously (Cho et al., 2007). Cells were transfected with a nonspecific oligonucleotide (iScr) or iNur77 and treated with DMSO or 15 μM DIM-C-pPhOCH3, and whole-cell lysates were analyzed by Western blot analysis. RNA interference decreased Nur77 expression in the DMSO and DIM-C-pPhOCH3 treatment groups by approximately 60% in whole-cell lysates (Fig. 4B), indicating that both transfection efficiencies and knockdown of Nur77 by iNur77 were high. In a parallel experiment, RNA interference in UC-5 cells transfected with iNur77 and treated with DMSO or 15 μM DIM-C-pPhOCH3 also decreased TRAIL expression and PARP cleavage by 45 to 50% (Fig. 4C), confirming that these responses were also Nur77-dependent.
Fig. 4.
Nur77-dependent induction of TRAIL and apoptosis in UC-5 cells. A, induction of TRAIL by DIM-C-pPhOCH3 and DIM-C-Ph. Cells were treated with Nur77-active C-DIMs (15 μM) for 24 h, and whole-cell lysates were analyzed for TRAIL or β-tubulin. B and C, RNA interference with iNur77. UC-5 cells were transfected with iScr (nonspecific) or iNur77 and treated with DMSO or 15 μM DIM-C-pPhOCH3, and whole-cell lysates were analyzed by Western blot analysis for Nur77, PARP cleavage, TRAIL, and β-tubulin proteins. Columns, mean of three replicate experiments for each treatment group; error bar, S.D. Protein levels were normalized to β-tubulin. B, ∗, P < 0.05, significant inhibition of Nur77 expression. C, ∗, significant induction of TRAIL and PARP cleavage; ∗∗, decreased expression of these proteins by iNur77.
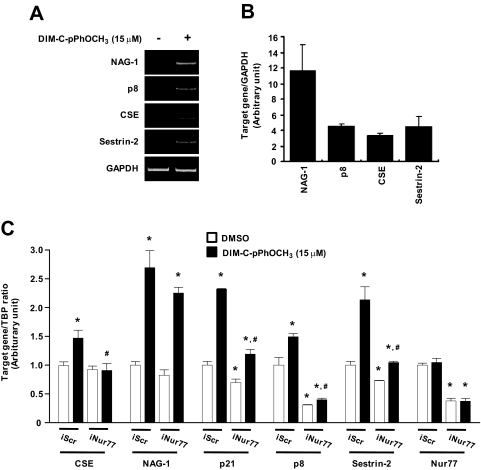
Although DIM-C-pPhOCH3 induced apoptosis through Nur77-dependent activation of TRAIL in UC-5 cells, this compound activates Nur77-dependent and -independent growth inhibition and proapoptotic genes in other cancer cell lines (Lei et al., 2006; Chintharlapalli et al., 2007; Cho et al., 2008). Therefore, we treated UC-5 bladder cancer cells for 2 or 6 h with DMSO or 12.5 μM DIM-C-pPhOCH3 and performed microarray analysis (three arrays for each treatment group) by using the CodeLink Human Whole Genome Bioarrays. Results for DIM-C-pPhOCH3 were compared with a DMSO control at each time point, and the microarray data were analyzed using GeneSpring software version 7.2 (Agilent Technologies). Several growth inhibitory and proapoptotic genes were induced by DIM-C-pPhOCH3 after treatment for 2 or 6 h, and these include the following genes (induction after 2 or 6 h indicated in brackets): nonsteroidal anti-inflammatory drug activated gene (NAG-1) (5.58- and 22.70-fold); p8 (1.50- and 5.88-fold); cystathionase (0.97- and 5.02-fold); sestrin-2 (1.65- and 4.59-fold); and p21 (2.01- and 2.28-fold) (Supplemental Table 1). In addition, we also observed activation of genes (by ≥2.5-fold) associated with metabolism and homeostasis, signal transduction, transcription, protein folding and modification, and genes with several other functions (Supplemental Table 1). Results in Fig. 5A illustrate that DIM-C-pPhOCH3 significantly induced NAG-1, p8, CSE, and sestrin-2 mRNA levels in UC-5 cells as determined by semiquantitative RT-PCR, and replicates of these experiments confirm the significance of these induction responses (Fig. 5B). DIM-C-pPhOCH3 and other C-DIMs induce receptor-independent responses in cancer cells (Lei et al., 2006; Chintharlapalli et al., 2007; Cho et al., 2008); therefore, we investigated the Nur77-dependent effects of DIM-C-pPhOCH3 on induction of several genes in UC-5 cells transfected with iScr or iNur77. DIM-C-pPhOCH3 significantly induced CSE, NAG-1, p21, p8, and sestrin-2; in the presence of iNur77, there was a significant decrease in CSE, p21, p8, and sestrin-2 mRNA levels, whereas NAG-1 mRNA levels were not affected by Nur77 knockdown. Levels of Nur77 mRNA were not affected by DIM-C-pPhOCH3; however, in UC-5 cells transfected with iNur77, there was decreased expression of Nur77 mRNA in the control and DIM-C-pPhOCH3-treated groups. This study identifies CSE, p21, p8, and sestrin-2 as Nur77-regulated genes in bladder cancer cells.
Fig. 5.
Induction of gene expression by DIM-C-pPhOCH3. A and B, induction of NAG-1, p8, CSE, and sestrin-2 in UC-5 cells. Cells were treated with DMSO or 15 μM DIM-C-pPhOCH3 for 6 h, and NAG-1, p8, CSE, sestrin-2, and GAPDH (reference mRNA) were determined by RT-PCR. Induction responses were determined in three separate experiments. ∗, P < 0.05, significantly induced mRNA expression. C, effects of iNur77 on induced gene expression. UC-5 cells were transfected with iScr or iNur77, treated with DMSO or 15 μM DIM-C-pPhOCH3 for 6 h, and analyzed by real-time PCR. Columns, mean of three replicate determinations for each treatment group; error bars, S.D. ∗, P < 0.05, significant induction by DIM-C-pPhOCH3; #, significant decrease after transfection with iNur77.
The in vivo antitumorigenic activity of DIM-C-pPhOCH3 was determined in athymic nude mice bearing KU7 cells as xenografts. At doses of 25 mg/kg, DIM-C-pPhOCH3 significantly inhibited tumor volumes and weights compared with the corn oil-treated solvent controls (Fig. 6, A and B). Histopathological examination of the treated animals revealed no significant pathological conditions, and changes in body or organ weights were not observed (data not shown). In addition, the TUNEL assay for apoptosis showed enhanced staining (Fig. 6C), and extraction of proteins from tumor tissue obtained from corn oil and DIM-C-pPhOCH3-treated mice showed that in the latter treatment group, there was a significant increase in PARP cleavage (Fig. 6D). These results demonstrate that Nur77-active C-DIMs inhibit bladder cancer cell and tumor growth and induce apoptosis in both in vitro and in vivo models.
Fig. 6.
Inhibition of tumor growth by DIM-C-pPhOCH3. Male athymic nude mice bearing KU7 cell xenografts were treated with DIM-C-pPhOCH3, and tumor volumes (A) and weight (B) were determined. The compound was given daily (25 mg/kg/day) in corn oil by oral gavage, and corn oil served as a solvent control. C, immunostaining for apoptosis. Tumor tissue from animals treated with solvent control or DIM-C-pPhOCH3 were stained, and a TUNEL assay was used to detect apoptosis. D, whole-cell lysates from tumor tissues was analyzed by Western blot analysis using PARP antibody to detect apoptosis. Columns, mean of five replicate determinations for each group; error bars, S.E. ∗, P < 0.05, significant induction by DIM-C-pPhOCH3.
Discussion
Nur77 is an orphan nuclear receptor that plays a unique role in mediating the proapoptotic activity of a wide variety of agents (Li et al., 2000; Wu et al., 2002; Holmes et al., 2003a,b; Mu and Chang, 2003; Wilson et al., 2003a; Lin et al., 2004; Chintharlapalli et al., 2005a; Cho et al., 2007). There are differences in the proposed mechanisms of action of various apoptosis inducers in mediating induction of Nur77-dependent cell death pathways that may involve interactions of this receptor with mitochondrial or cytosolic proteins. For example, in different cell lines treated with retinoids or phorbol ester, there is evidence that Nur77 translocates from the nucleus to the mitochondria and forms a proapoptitic complex with bcl-2 (Li et al., 2000; Lin et al., 2004). In all cases in which Nur77-dependent apoptosis requires extranuclear transport, the effects of the apoptosis-inducing agents are inhibited after cotreatment with leptomycin B, a nuclear export inhibitor. It has also been reported that phorbol ester induced expression of nuclear Nur77 in LNCaP prostate cancer cells and this resulted in enhanced expression of E2F1, which exhibits proapoptotic activity (Mu and Chang, 2003). In contrast, the Nur77-active C-DIMs used in this study did not induce E2F1 in bladder cancer cells (data not shown) or in pancreatic or colon cancer cells (Chintharlapalli et al., 2005a; Cho et al., 2007). Both DIM-C-pPhOCH3 and DIM-C-pPh represent a novel class of Nur77-active proapoptotic compounds. The Nur77-active C-DIMs decrease bladder cancer cell proliferation (Fig. 2A) and induce apoptosis in these cells through nuclear Nur77-dependent pathways that are not inhibited by leptomycin B (Fig. 3A).
Previous studies on Nur77-active C-DIMs in pancreatic and colon cancer cells show that DIM-C-pPhOCH3 induces both receptor-dependent and -independent apoptosis (Chintharlapalli et al., 2005a; Cho et al., 2007, 2008), and receptor independent responses include induction of other proapoptotic genes such as NAG-1. DIM-C-pPhOCH3 and other C-DIMs also induce endoplasmic reticulum stress, which results in activation of death receptor 5, and this response is also receptor-independent (Chintharlapalli et al., 2005a; Abdelrahim et al., 2006; Lei et al., 2008a,b). Results illustrated in Fig. 4 demonstrate that DIM-C-pPhOCH3 induces the death receptor ligand TRAIL (Fig. 4A) as previously reported in colon and pancreatic cancer cells (Chintharlapalli et al., 2005a; Cho et al., 2007). Nur77 knockdown in UC-5 cells was determined by transient transfection of iNur77, and the results obtained using whole-cell lysates suggest that transfection efficiencies were high and resulted in significant knockdown of Nur77 protein. Based on the comparison of the relative effects of iNur77 on Nur77, PARP cleavage, and decreased TRAIL protein expression (Fig. 4, B and C), the results suggest that induction of apoptosis by DIM-C-pPhOCH3 in UC-5 cells was primarily Nur77-dependent. Previous studies in pancreatic and colon cancer cell lines suggest that the contribution of Nur77-activated apoptosis by C-DIMs is variable and cell context dependent (Chintharlapalli et al., 2005a; Cho et al., 2007), and current studies are investigating this variability in other bladder cancer cell lines.
We also have used microarrays to investigate induction of gene expression by DIM-C-pPhOCH3 in bladder cancer cells, and we observed induction of several growth inhibitory and proapoptotic genes. A comparison of the effects of DIM-C-pPhOCH3 on patterns of gene expression in bladder, colon, pancreatic, and prostate cancer cells showed that among the several hundred genes induced in these cell lines, only 19 were genes induced in all 4 cell lines (data not shown). Two of these genes, NAG-1 and sestrin-2, are growth inhibitory/proapoptotic and are among the more highly induced in UC-5 cells (Fig. 5 and Supplemental Table 1). Among the five genes that we have examined by real-time PCR (Fig. 5C), results of RNA interference studies demonstrate that induction of NAG-1 by DIM-C-pPhOCH3 was Nur77-independent, and these results are consistent with previous studies showing that NAG-1 is induced by structurally diverse compounds, including some nuclear receptor agonists, and induction of this gene is receptor-independent (Baek et al., 2001, 2002, 2004a,b; Wilson et al., 2003b; Chintharlapalli et al., 2005b; Lee et al., 2005). However, we also observed that genes involved in cell cycle regulation and arrest (sestrin-2 and p21), stress and apoptosis (p8), and growth inhibition via hydrogen sulfide release (cystathionase) (Gartel and Tyner, 1999; Yang et al., 2004; Carracedo et al., 2006; Budanov and Karin, 2008) are induced by DIM-C-pPhOCH3 in a Nur77-dependent manner (Fig. 5C) and thereby represent novel genes induced by nuclear Nur77. In pancreatic cancer cells, we have shown that Nur77-dependent induction of p21 involves interactions with specificity proteins bound to proximal GC-rich regions of the p21 promoter (Lee et al., 2009). The molecular mechanisms of transactivation and interactions of Nur77 with cis elements in gene promoters are currently being investigated.
It is clear from results of in vivo studies that DIM-C-pPhOCH3 also inhibits bladder tumor growth in a mouse xenograft model with KU7 cells (Fig. 6). Analysis of the tumor tissue demonstrates that DIM-C-pPhOCH3 induces apoptosis in tumors and this complements in vivo studies in cell culture (Fig. 3). These data, coupled with expression of Nur77 in bladder cancer cells (Inamoto et al., 2008) and human tumors (Fig. 1) suggests that this receptor is a potential target for bladder cancer chemotherapy. Ongoing research is focused on development of additional C-DIM analogs that activate Nur77 and other NGFI-B receptors and their application for treatment of different stages of bladder cancer.
Supplementary Material
Acknowledgments
We gratefully acknowledge the Texas A&M AgriLife.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This research was supported by the National Institutes of Health National Cancer Institute [01R01-CA124998], the Korea Research Foundation Grant, MOEHRD Basic Research Promotion Fund [Grant KRF-2008-331-E00260].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061143
- NGFI-B
- nerve growth factor-induced B
- C-DIM
- methylene-substituted diindolylmethane
- DIM-C-pPhOCH3
- 1,1-bis(3′-indolyl)-1-(p-methoxyphenyl)methane
- DIM-C-pPhC
- 1,1-bis(3′-indolyl)-1-(p-chlorophenyl)methane
- TRAIL
- tumor necrosis factor-related apoptosis-inducing ligand
- IgG
- immunoglobulin G
- iNur77
- small inhibitory RNA for Nur77
- iScr
- nonspecific scrambled oligonucleotide
- NAG-1
- nonsteroidal anti-inflammatory drug activated gene
- DMSO
- dimethyl sulfoxide
- RT-PCR
- reverse transcriptase-polymerase chain reaction
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- TUNEL
- terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- Z-VAD-fmk
- N-benzyloxycarbonyl-Val-Ala-Asp fluoromethyl ketone
- PARP
- poly(ADP-ribose) polymerase.
References
- Abdelrahim et al., 2006.Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. (2006) 3,3′-Diindolylmethane (DIM) and derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis 27:717–728 [DOI] [PubMed] [Google Scholar]
- Abdelrahim et al., 2002.Abdelrahim M, Samudio I, Smith R, 3rd, Burghardt R, Safe S. (2002) Small inhibitory RNA duplexes for Sp1 mRNA block basal and estrogen-induced gene expression and cell cycle progression in MCF-7 breast cancer cells. J Biol Chem 277:28815–28822 [DOI] [PubMed] [Google Scholar]
- Baek et al., 2004a.Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. (2004a) Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis 25:2425–2432 [DOI] [PubMed] [Google Scholar]
- Baek et al., 2004b.Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE. (2004b) Expression of NAG-1, a transforming growth factor-β superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem 279:6883–6892 [DOI] [PubMed] [Google Scholar]
- Baek et al., 2001.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. (2001) Cyclooxygenase inhibitors regulate the expression of a TGF-β superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol 59:901–908 [PubMed] [Google Scholar]
- Baek et al., 2002.Baek SJ, Wilson LC, Eling TE. (2002) Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis 23:425–434 [DOI] [PubMed] [Google Scholar]
- Bandoh et al., 1997.Bandoh S, Tsukada T, Maruyama K, Ohkura N, Yamaguchi K. (1997) Differential expression of NGFI-B and RNR-1 genes in various tissues and developing brain of the rat: comparative study by quantitative reverse transcription-polymerase chain reaction. J Neuroendocrinol 9:3–8 [DOI] [PubMed] [Google Scholar]
- Budanov and Karin, 2008.Budanov AV, Karin M. (2008) p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo et al., 2006.Carracedo A, Gironella M, Lorente M, Garcia S, Guzmán M, Velasco G, Iovanna JL. (2006) Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res 66:6748–6755 [DOI] [PubMed] [Google Scholar]
- Chintharlapalli et al., 2005a.Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. (2005a) Activation of Nur77 by selected 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J Biol Chem 280:24903–24914 [DOI] [PubMed] [Google Scholar]
- Chintharlapalli et al., 2005b.Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. (2005b) 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and NAG-1. Mol Pharmacol 68:1782–1792 [DOI] [PubMed] [Google Scholar]
- Chintharlapalli et al., 2007.Chintharlapalli S, Papineni S, Safe S. (2007) 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes inhibit growth, induce apoptosis, and decrease the androgen receptor in LNCaP prostate cancer cells through PPARγ-independent pathways. Mol Pharmacol 71:558–569 [DOI] [PubMed] [Google Scholar]
- Chintharlapalli et al., 2004.Chintharlapalli S, Smith R, 3rd, Samudio I, Zhang W, Safe S. (2004) 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes induce peroxisome proliferator-activated receptor γ-mediated growth inhibition, transactivation and differentiation markers in colon cancer cells. Cancer Res 64:5994–6001 [DOI] [PubMed] [Google Scholar]
- Cho et al., 2008.Cho SD, Lei P, Abdelrahim M, Yoon K, Liu S, Guo J, Papineni S, Chintharlapalli S, Safe S. (2008) 1,1-Bis(3′-indolyl)-1-(p-methoxyphenyl)methane activates Nur77-independent proapoptotic responses in colon cancer cells. Mol Carcinog 47:252–263 [DOI] [PubMed] [Google Scholar]
- Cho et al., 2007.Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S, Khan S, Ramaiah SK, Safe S. (2007) Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res 67:674–683 [DOI] [PubMed] [Google Scholar]
- DeYoung et al., 2003.DeYoung RA, Baker JC, Cado D, Winoto A. (2003) The orphan steroid receptor Nur77 family member Nor-1 is essential for early mouse embryogenesis. J Biol Chem 278:47104–47109 [DOI] [PubMed] [Google Scholar]
- Gartel and Tyner, 1999.Gartel AL, Tyner AL. (1999) Transcriptional regulation of the p21 WAF1/CIP1 gene. Exp Cell Res 246:280–289 [DOI] [PubMed] [Google Scholar]
- Holmes et al., 2003a.Holmes WF, Soprano DR, Soprano KJ. (2003a) Comparison of the mechanism of induction of apoptosis in ovarian carcinoma cells by the conformationally restricted synthetic retinoids CD437 and 4-HPR. J Cell Biochem 89:262–278 [DOI] [PubMed] [Google Scholar]
- Holmes et al., 2003b.Holmes WF, Soprano DR, Soprano KJ. (2003b) Early events in the induction of apoptosis in ovarian carcinoma cells by CD437: activation of the p38 MAP kinase signal pathway. Oncogene 22:6377–6386 [DOI] [PubMed] [Google Scholar]
- Inamoto et al., 2008.Inamoto T, Papineni S, Chintharlapalli S, Cho SD, Safe S, Kamat AM. (2008) 1,1-Bis(3′-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder carcinogenesis. Mol Cancer Ther 7:3825–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassouf et al., 2006.Kassouf W, Chintharlapalli S, Abdelrahim M, Nelkin G, Safe S, Kamat AM. (2006) Inhibition of bladder tumor growth by 1,1-bis(3′-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor γ agonists. Cancer Res 66:412–418 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2005.Lee SH, Kim JS, Yamaguchi K, Eling TE, Baek SJ. (2005) Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun 328:63–69 [DOI] [PubMed] [Google Scholar]
- Lee et al., 1995.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. (1995) Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science 269:532–535 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2009.Lee SO, Chintharlapalli S, Liu S, Papineni S, Cho SD, Yoon K, Safe S. (2009) p21 Expression is induced by activation of nuclear nerve growth factor-induced Bα (NGFI-Bα, Nur77) in pancreatic cancer cells. Mol Cancer Res 7:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei et al., 2008a.Lei P, Abdelrahim M, Cho SD, Liu S, Chintharlapalli S, Safe S. (2008a) 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-Jun N-terminal kinase. Carcinogenesis 29:1139–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei et al., 2008b.Lei P, Abdelrahim M, Cho SD, Liu X, Liu X, Safe S. (2008b) Structure-dependent activation of endoplasmic reticulum stress-mediated apoptosis in pancreatic cancer by 1,1-bis(3′-indoly)-1-(p-substituted phenyl)methanes. Mol Cancer Ther 7:3363–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei et al., 2006.Lei P, Abdelrahim M, Safe S. (2006) 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl) methanes inhibit ovarian cancer cell growth through peroxisome proliferator-activated receptor-dependent and independent pathways. Mol Cancer Ther 5:2324–2336 [DOI] [PubMed] [Google Scholar]
- Li et al., 2000.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X. (2000) Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 289:1159–1164 [DOI] [PubMed] [Google Scholar]
- Lin et al., 2004.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. (2004) Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116:527–540 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf et al., 1995.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama et al., 1997.Maruyama K, Tsukada T, Bandoh S, Sasaki K, Ohkura N, Yamaguchi K. (1997) Expression of the putative transcription factor NOR-1 in the nervous, the endocrine and the immune systems and the developing brain of the rat. Neuroendocrinology 65:2–8 [DOI] [PubMed] [Google Scholar]
- Milbrandt, 1988.Milbrandt J. (1988) Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron 1:183–188 [DOI] [PubMed] [Google Scholar]
- Mu and Chang, 2003.Mu X, Chang C. (2003) TR3 orphan nuclear receptor mediates apoptosis through up-regulating E2F1 in human prostate cancer LNCaP cells. J Biol Chem 278:42840–42845 [DOI] [PubMed] [Google Scholar]
- Qin et al., 2004.Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R, 3rd, Phillips T, Abdelrahim M, Samudio I, Safe S. (2004) A new class of peroxisome proliferator-activated receptor γ (PPARγ) agonists that inhibit growth of breast cancer cells: 1,1-bis(3′-indolyl)-1-(p-substitutedphenyl)methanes. Mol Cancer Ther 3:247–259 [PubMed] [Google Scholar]
- Saucedo-Cardenas et al., 1998.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, Conneely OM. (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A 95:4013–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al., 2003a.Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH. (2003a) TR3/Nur77 in colon cancer cell apoptosis. Cancer Res 63:5401–5407 [PubMed] [Google Scholar]
- Wilson et al., 2003b.Wilson LC, Baek SJ, Call A, Eling TE. (2003b) Nonsteroidal anti-inflammatory drug-activated gene (NAG-1) is induced by genistein through the expression of p53 in colorectal cancer cells. Int J Cancer 105:747–753 [DOI] [PubMed] [Google Scholar]
- Wu et al., 2002.Wu Q, Liu S, Ye XF, Huang ZW, Su WJ. (2002) Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis 23:1583–1592 [DOI] [PubMed] [Google Scholar]
- Yang et al., 2004.Yang G, Cao K, Wu L, Wang R. (2004) Cystathionine gamma-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. J Biol Chem 279:49199–49205 [DOI] [PubMed] [Google Scholar]
- Zetterström et al., 1997.Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. (1997) Dopamine neuron agenesis in Nurr1-deficient mice. Science 276:248–250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.