Abstract
Ovarian tumors are formed either in the absence of Bam (bag-of-marbles) in germ-line cells or the overexpression of Dpp (decapentaplegic) in ovarian somatic cells. These tumor cells contain spectrosomes characteristic of ovarian germ-line stem cells and the immediate descendents called cystoblasts. We show that pole cells can successfully populate the gonad after transplantation to the dorsal mesoderm of host embryos following germ-band extension. By using this approach, we demonstrate that bam– cells can populate the gonad and become established as germ-line stem cells. Tumor cells containing the wild-type bam gene under heat shock transcriptional control are able to produce functional oocytes. Thus, stem cells/cystoblasts of the adult ovary are capable of forming stem cells in the embryonic ovary and recapitulating the development of the female germ line.
Recent studies have established the Drosophila ovary as an outstanding subject for the analysis of factors required to establish and maintain stem cells (1, 2). Each ovary consists of ≈15 ovarioles or egg tubes. At the anterior tip of each ovariole in the region called the germarium, a small number of somatic cells form a “niche” in which two to three stem cells are located (3). When a stem cell divides, it produces another stem cell and a daughter cystoblast. The cystoblast undergoes four synchronous nuclear divisions with incomplete cytokinesis, leading to the formation of a 16-cell cyst, with the cells interconnected by 15 intercellular bridges or ring canals. Fifteen of the cyst cells differentiate as nurse cells and one becomes the oocyte. The subsequent stages of differentiation of the oocyte are described by Spradling (4).
Stem cells can be identified by the characteristic spherical spectrosome, a cytoplasmic organelle composed of membrane cytoskeletal proteins such as the spectrins, adducin-like protein (Hts), actin, and the critical bag-of-marbles (Bam) protein (5). Ovarian stem cells are first seen at the end of larval growth, adjacent to the newly forming terminal filaments and cap cells that form the niche (6). Each stem cell is attached to the somatic cells of the niche by adherens junctions (7) and gap junctions (8), both of which are essential for maintaining the stem cell population. In the absence of either type of junction stem cells disappear. In addition, maintenance of stem cells requires several other germ-line functions, such as nos and pumilio (9), piwi (10), although piwi plays a more important role in the soma, and a somatic niche, characterized by the expression of fs (1)Yb (11) and decapentaplegic (12). In each instance, mutations in these genes lead to a loss of ovarian stem cells.
Several genes are known to produce an ovarian phenotype commonly called an “ovarian tumor” (13). In the absence of Sxl function in the germ line (14), each ovariole becomes filled with diploid cells that resemble testicular cells. A similar phenotype is seen when several genes required for Sxl function are mutated (reviewed in ref. 15). Because many male germ-line functions are activated in these ovarian tumors (16), the activation of Sxl is apparently required to establish or maintain female sexual identity in the germ line.
A second class of ovarian tumors forms in ovaries mutant for bam (bag-of-marbles) and bgcn (benign gonial cell neoplasm). In a wild-type ovary, after a stem cell division, bam becomes activated in the daughter cell and functions with bgcn to complete the four synchronous divisions leading to the 16-cell cyst and subsequent stages of oogenesis (17, 18). In the absence of either gene function, the products of the stem cell division appear arrested in a stem cell-like state with spectrosomes (5). The germarium of ovaries mutant for either of these genes fills up with hundreds of diploid cells, each containing a stem-cell-like spectrosome.
The proper level of expression of dpp is also required for normal stem cell function in the ovary (12). Too little Dpp produced by the somatic cells of the niche leads to loss of stem cells, whereas too much Dpp leads to an overproliferation of spectrosome-containing cells. Overexpression of Dpp leads to even larger “tumorous” ovaries than found in ovaries lacking either bam or bgcn function.
The critical role of bam in the progression of germ-line cells from the stem cell to a differentiated 16-cell cyst suggests that the cells accumulating in bam– ovarioles are arrested in a state resembling a stem cell or its immediate descendant. Activation of bam with an hsp70 promoter is sufficient to lead to normal differentiation of ovarian follicles and subsequent fertility (19). We have been pursuing experiments to determine whether these bam– stem-like cells might be cultured as a model system for studying stem cell biology. As part of these studies, we have also explored whether bam– cells are committed to a poststem cell fate, or whether they are capable of reverting to a primordial germ-cell state, repopulating the embryonic gonad, reestablishing a stem cell population, and ultimately differentiating into functional gametes (after wild-type bam+ activity has been restored).
Here we report our experiments demonstrating that bam– ovarian tumor cells can repopulate the ovary, where they apparently reestablish stem cells and, after activation of bam+ by heat shock, produce functional gametes.
Materials and Methods
Fly Stocks and Genetic Crosses. All fly stocks used in this study were maintained under standard culture conditions. Oregon-RP was used as the wild-type strain. bamΔ86 ry e, Sb e/TM3, Sb e, and w1118;P[w+ hsp-70 bam+]11–d bamΔ86 ry e/TM3, Sb e were kindly supplied by D. McKearin (University of Texas Southwest, Dallas). bamΔ86 ovo-lacZ/TM3 strain was constructed by crossing bamΔ86 ry e/TM3, Sb e with ovo-lacZ (obtained from B. Oliver, National Institutes of Health, Bethesda). As recipient embryos for transplantation experiments, embryos were derived from the crossing of oskar301and oskarCE4 (provided by R. Lehmann, New York University, New York) or from P–M dysgenic crossing of Oregon R females and males of a strong P strain, IG280. About 95% of the progeny from this P–M dysgenic mating are sterile at 25°C. The IG280 strain was provided by E. Matsuura (Ochanomizu University, Tokyo). As a control for the P–M crosses, we transplanted pole cells into Oregon R embryos. We found no difference between the P–M and Oregon R embryonic hosts.
Microscopy and Histochemistry. 5-Bromo-4-chloro-3-indolyl β-d-galactoside staining of ovaries was performed as described by Margolis and Spradling (20).
Heat-Shock Protocol. Flies were heat-shocked at 37°C for 1 h according to the method of Ohlstein and McKearin (19).
Transplantation Experiments. Transplantation of pole cells was carried out as described (21). For transplanting bam– ovarian tumor cells, ovaries of homozygous bam– females at ages of 20–40 days after emergence were used. To facilitate the isolation of individual ovarioles before drawing ovarian tumor cells into a glass needle, the ovaries were initially dissected in divalent cation-free medium (22) and then transferred to Chan and Gehring buffered medium (23), where the ovarioles were fragmented with tungsten needles. The bam– ovarian tumor cells were drawn into a glass needle with an inner diameter of ≈12 μm. Two to 10 pole cells or bam– ovarian tumor cells were transplanted either to the posterior pole of blastoderm-stage embryos or into the presumptive embryonic gonadal region on the dorsal side of the recipient embryo.
Adult flies from transplantation experiments using the ovolacZ transgene were aged appropriately, and their ovaries were stained for β-galactosidase activity. Some females were mated to verify that the host embryos were sterile, unless they had received transplanted pole cells.
Results
In Drosophila, germ cells originate from pole cells, which form at the posterior tip of the preblastoderm embryo as the first population of cells formed (Fig. 1). These cells are transported by the posterior midgut invagination to the interior of the embryo, where they migrate through the midgut epithelium into the dorsal mesodermal region. Subsequently, they aggregate to form the embryonic gonad (details in Fig. 1; reviewed in ref. 25). Pole cells can be transplanted successfully to the posterior tip of a host blastoderm-staged embryo, and the transplanted cells will subsequently populate the embryonic gonad (26). Other blastoderm cells do not have the capacity to differentiate as germ cells. To increase the possibility that the transplanted cells will populate the embryonic gonad, several means can be used to reduce or eliminate host pole cells, such as oskar mutations (27), UV irradiation (28), or hybrid dysgenesis (21). In preliminary experiments, bam– tumor cells were transplanted to the posterior tip of blastoderm-staged embryos, but we were unsuccessful in recovering adult flies with bam– cells in the ovary. This outcome led us to explore an alternative approach to obtain integration of tumor cells into the gonad.
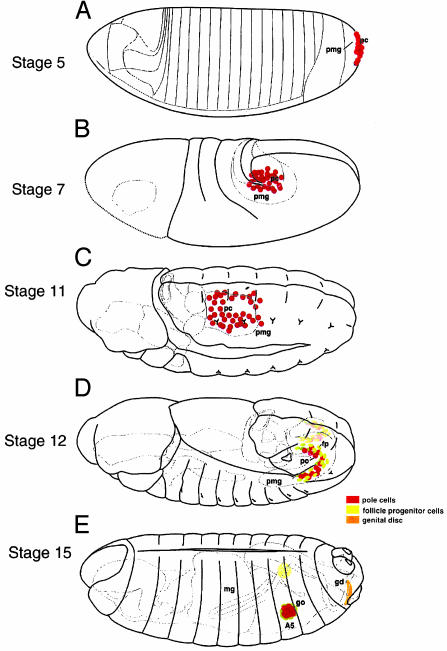
Fig. 1.
Diagrams of early gastrulation and postgastrulation stages of Drosophila development to illustrate the formation and migration of pole cells into the embryonic gonad. At stage 5, the pole cells form at the posterior tip of the blastoderm embryo. The blastoderm cells below the pole cells form the precursor of the posterior midgut invagination. At stage 7 the germ band has extended around the posterior tip of the embryo, pushing the posterior midgut invagination, within which are located the pole cells, anteriorly along the dorsal side of the embryo. At stage 11 the pole cells migrate out of the midgut lumen into the dorsal mesoderm. When tumor cells are transplanted at this stage, both gonads might become populated by tumor cells, indicating that a clear left–right boundary does not exist at this time. At stage 12 the germ band is undergoing contraction, resulting in the posterior movement of the abdominal segments. During this time the gonadal mesodermal cells are aggregating to form the embryonic gonad. At stage 15 germ-band contraction is complete and the embryonic gonad has formed. [Reproduced with permission from Rongo et al. (24) (Copyright 1997, Cold Spring Harbor Laboratories).]
Earlier studies had shown that pole cells, transplanted into the midventral furrow at early gastrulation, could reach the embryonic gonad with efficiency similar to pole cells transplanted to the pole-cell region of the blastoderm (29). Assuming that ovarian tumor cells might bypass the requirement for many critical stems in the migration of pole cells to the embryonic gonad if they were transplanted directly into the appropriate mesoderm site for gonad formation, we transplanted wild-type pole cells directly into the dorsal mesoderm of oskar embryos (Table 1). With high frequency, we found that cells transplanted into the dorsal mesoderm were able to successfully establish the germ-line lineage and produce functional gametes. Because some of the donor embryos were probably male, and male pole cells in a female gonad produce tumors (14), the appearance of flies with tumorous ovaries was expected. These transplantations confirmed the earlier result in which pole cells were transplanted into the ventral furrow and indicated that transit through the posterior midgut can be bypassed.
Table 1. Transplantation of wild-type pole cells (expressing lacZ) to the dorsal mesoderm of oskar embryos.
| Age of recipient embryos | Injected embryos, n | Hatched embryos, n (%) | Emerged adults, n (%) | Fertile adults, n (% of emerged adults) | Tumorous ovaries,*n (% of ovaries with tumors) |
|---|---|---|---|---|---|
| Stage 11 | 228 | 157 (68.9) | 42 (18.4) | 23/37 (62.2) | 9/13 (69.2) |
| Stages 11-12 | 96 | 72 (75) | 18 (18.8) | 6/11 (54.5) | 2/4 (50) |
| Stages 12-13 | 141 | 111 (78.7) | 33 (23.4) | 16/29 (55.2) | 6/10 (60) |
The tumorous ovaries occur when male pole cells are transplanted into female embryos (14).
We then transplanted bam– ovarian tumor cells into the posterior dorsal mesoderm of stage 11 to stage 15 embryos (Table 2). Although the frequency of successful incorporation into the gonad was substantially less than that seen with pole cells, in a few instances, ovarian stem cells/cystoblasts were able to populate the embryonic gonad and establish ovarian tumors (Fig. 2). Because several ovarioles became filled with tumor cells, we assume that the transplanted cells were able to populate the stem cell niche and produce a large number of progeny cells. The failure of cells to incorporate into the gonad of stage 15 embryos is probably due to the appearance of the basal lamina around the newly formed embryonic gonad, thus ending the time when cells can incorporate into the gonad.
Table 2. Transplantation of homozygous bamΔ86 ovo-lacZ tumor cells into oskar embryos.
| Ages of recipient | Injected embryos, n | Hatched embryos, n (%) | Adults emerged, n (%) | Adults with germ cells, n (%) |
|---|---|---|---|---|
| Stage 11 | 136 | 101 (74.3) | 37 (27.2) | 1/36 (2.8) |
| Stages 11-12 | 78 | 59 (75.6) | 17 (21.8) | 3/14 (21.4) |
| Stages 14-15 | 233 | 174 (74.7) | 50 (21.5) | 0/50 (0.0) |
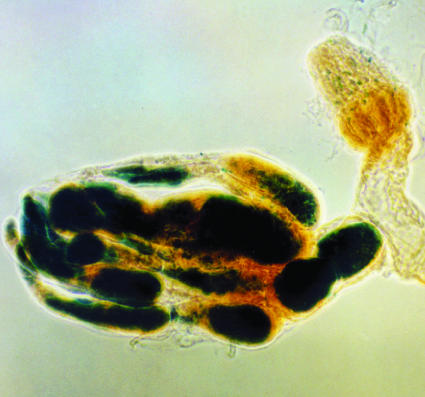
Fig. 2.
A pair of ovaries derived from oskar embryos that had received ovarian tumor cells from bam–;ovo–lacZ+ flies. One ovary has no germ cells (the residual β-galactosidase activity is due to native Drosophila activity), whereas the other has many ovarioles filled with LacZ+ tumor cells, indicating that the transplanted cells had populated the embryonic gonad and formed stem cells in several ovarioles.
Although these results demonstrated that ovarian tumor cells can populate the embryonic gonad and proliferate, we do not know whether these cells can recapitulate oogenesis, because they lacked the critical Bam protein. Ohlstein and McKearin (19) have shown that forced expression of bam+ (by the hsp70 promoter) was sufficient to induce differentiation of tumor cells into functional oocytes. We have repeated these experiments and found that we could reproducibly obtain fertile adults after induction of the hs bam+ transgene in bam– flies (Fig. 3). Because these oocytes were produced even though the ovarioles were filled with tumor cells before the induction of the transgene by temperature, it is clear that the appearance of Bam protein is sufficient to initiate normal oogenesis in these bam– flies (19).
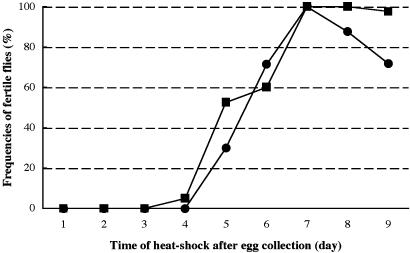
Fig. 3.
Graph representing the frequency of fertile flies after heat shock treatment of flies homozygous for p[hsp-bam+] bamΔ86 (▪) and carrying p[hsp-bam+] bamΔ86/bamΔ86 ovo-lacZ (•), respectively. Fertility is equivalently restored in flies with either one or two copies of the hsp-bam+ transgene.
To determine whether bam– cells that had repopulated the ovary might function normally, we transplanted bam–hsp70-bam+/bam–ovo-lacZ tumor cells into the dorsal mesoderm of embryos lacking host pole cells (Table 3). Although the frequency with which cystocytes successfully populate the ovary is low, the bam– and bam–;hsp70-bam+ cells are equally effective in populating the embryonic ovary. In Table 4 we show that some of these flies are capable of producing fertile oocytes after activation of bam+ by the heat shock promoter. Thus, cells from a tumorous bam– ovary clearly are capable of repopulating the embryonic gonad, establishing a stem cell population, and functioning normally to produce offspring.
Table 3. Transplantation of bam- P[hsp-bam+] tumor cells into either oskar or hybrid dysgenic recipient embryos (at stage 11).
| Genotype of donor cells | Genotype of recipient | Eggs injected, n | Larvae hatched, n (%) | Adults emerged, n (%) | Females emerged, n (%) | Females with germ cells, n (%) |
|---|---|---|---|---|---|---|
| bam- ovo-lacZ* | Oregon R × IG280 | 1,074 | 440 (41) | 231 (21.5) | 108 (10.1) | 7/108 (6.5) |
| bam- P[hsp-bam+]/bam- ovo-lacZ† | Oskar301/oskarC84 | 971 | 755 (77.8) | 550 (56.6) | 278 (28.6) | 15/278 (5.4) |
| bam- P[hsp-bam+]/bam- ovo-lacZ† | Oregon R × IG280 | 657 | 295 (44.9) | 190 (28.9) | 100 (15.2) | 6/100 (6) |
The genotypes of donor tumors cells are homozygous for bam ovo-lacZ.
The genotypes of donor tumor cells are homozygous for w1118;P[w+ hsp-70 bam+]11-d bamΔ86/bamΔ86 ovo-lacZ.
Table 4. Induction of fertility of P[w+ hsp-70 bam]11-d;bam by heat shock.
| Females with germ cells, n | Females with tumorous cells, n (%) | Females with immature oocytes, n (%) | Females with mature oocytes, n (%) | Females with functional oocytes, n (%) |
|---|---|---|---|---|
| 15 | 2 (13.3) | 8 (53.3) | 3 (20.0) | 2 (13.3) |
| 6 | 1 (16.7) | 2 (33.3) | 0 | 3 (50) |
Discussion
These experiments demonstrate that bam– ovarian tumor cells can be introduced into embryos, populate the embryonic gonad, produce stem cells resulting in ovarian tumors, and, if wild-type bam activity is provided, differentiate into functional ovaries. The developmental status of the “tumor” cells in bam– ovaries is unclear. Because stem cells apparently require attachment to the somatic niche by both adherens (7) and gap (8) junctions, and the bam– tumor cells have lost these attachments, it is usually assumed that these cells are cystoblasts, committed to differentiate although arrested because of the lack of the critical Bam protein (17, 19). Cystoblasts, however, are programmed for a set number of cell divisions and, after each nuclear division, cytokinesis is incomplete, resulting in the formation of intercellular bridges or ring canals between daughter cells (13). Stem cells, on the other hand, are not limited in the number of mitoses they will complete, and, after nuclear division, cytokinesis is complete. Both of these latter properties fit more closely with the phenotype of bam– ovarian tumors. The failure to maintain contact with the niche, which is thought to be an essential feature of stem cells, suggests that tumor cells are not the exact equivalent of the stem cells. The simplest interpretation is that without Bam (and Bgcn; see ref. 18) proteins, the daughters of stem cells are not able to proceed to the next stage of differentiation, namely the cystocyte. Consequently, they retain some properties of stem cells, namely the capacity to continue to divide.
The ability to populate the embryonic gonad may require several critical steps (reviewed in ref. 25). At the time pole cells migrate out of the posterior midgut lumen, pole cells show pseudopodial extensions, suggesting that they actively migrate (30). The neighboring mesodermal cells also express attractants that are required to draw pole cells into the dorsal mesoderm (31, 32). The absence of a typical membrane skeleton composed of spectrins and associated proteins (33) may affect the mobility of ovarian tumor cells to leave the midgut.
The gonadal mesoderm forms from clusters of cells in parasegments 10–12. These cells contact the pole cells after they have migrated into dorsal mesoderm, and the clusters of cells coalesce in segment 10 to form the embryonic gonad (34). Both the formation and aggregation of these mesodermal gonadal precursors occurs in the absence of pole cells (35, 36), indicating that the pole cells do not play an active role in the formation of the gonad. Both cell-adhesion cadherins and the fear of intimacy protein are required for the formation of the gonad (36). Ovarian tumor cells must have distinctive surface properties that enable the aggregating gonadal mesodermal cells to incorporate them into the embryonic gonad. This property must be distinctive for germ-line cells, because no other embryonic cell can replace either pole cells or these ovarian tumor cells in populating the germ line of the embryo.
Extensive genetic screens for mutations affecting pole-cell migration (37, 24) have not yet identified any genes required in the pole cells to effect this migration. We have transplanted wild-type pole cells into the dorsal mesoderm shortly before the final aggregation of the embryonic mesoderm, and these cells have become incorporated into the gonad. Because pole cells are not active in transcription until after their migration into the posterior midgut invagination (38), this result suggests that new pole-cell transcription is not required for the incorporation of pole cells into the gonad. Thus, some surface property of pole cells (or ovarian tumor cells) is sufficient to identify these cells as “germ line” and lead to their incorporation into the gonad.
The discovery that bam– tumor cells are capable of repopulating the germ line suggests that it might be possible to use these cells in gene-replacement experiments, similar to those used in mammals by means of embryo stem cells (39), or in the replacement of testis stem cells (40). To attempt this type of experiment, we have explored a variety of culture conditions for growing ovarian tumor cells as a preliminary condition for obtaining gene replacements. These studies will be reported separately.
Acknowledgments
We acknowledge the help of John Perrino, Olga Issaenko, and Takafumi Yamaguchi. We thank Allan Spradling and Dennis McKearin for helpful comments on earlier versions of this manuscript, and Dennis McKearin for providing the bam– and hs-bam; bam– flies. We thank the National Institutes of Health for support (A.P.M.) and the Japanese government for support (Y.N.).
References
- 1.Lin, H. (2002) Nat. Rev. Genet. 3, 931–940. [DOI] [PubMed] [Google Scholar]
- 2.Xie, T. & Spradling, A. (2001) in Stem Cell Biology, eds. Marshak, D. R., Gardner, R. L. & Gottlieb, D. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 129–148.
- 3.Xie, T. & Spradling, A. C. (2000) Science 290, 328–330. [DOI] [PubMed] [Google Scholar]
- 4.Spradling, A. C. (1993) in The Development of Drosophila, eds. Bate, M. & Maartinez-Arias, A. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 1–70.
- 5.Lin, H., Yue, L. & Spradling, A. C. (1994) Development (Cambridge, U.K.) 120, 947–956. [DOI] [PubMed] [Google Scholar]
- 6.Zhu, C.-H. & Xie, T. (2003) Development (Cambridge, U.K.) 130, 2579–2588. [DOI] [PubMed] [Google Scholar]
- 7.Song, X., Zhu, C.-H., Doan, C. & Xie, T. (2002) Science 296, 1855–1857. [DOI] [PubMed] [Google Scholar]
- 8.Tazuke, S. I., Schulz, C., Gilboa, L., Fogarty, M., Mahowald, A. P., Guichet, A., Ephrussi, A., Wood, C. G., Lehmann, R. & Fuller, M. T. (2002) Development (Cambridge, U.K.) 129, 2529–2539. [DOI] [PubMed] [Google Scholar]
- 9.Forbes, A. & Lehmann, R. (1998) Development (Cambridge, U.K.) 125, 679–690. [DOI] [PubMed] [Google Scholar]
- 10.Cox, D. N., Chao, A. & Lin, H. (2000) Development (Cambridge, U.K.) 127, 503–514. [DOI] [PubMed] [Google Scholar]
- 11.King, F. J. & Lin, H. (1999) Development (Cambridge, U.K.) 126, 1833–1844. [DOI] [PubMed] [Google Scholar]
- 12.Xie, T. & Spradling, A. C. (1998) Cell 94, 251–260. [DOI] [PubMed] [Google Scholar]
- 13.King, R. C. (1970) Ovarian Development in Drosophila melanogaster (Academic, New York).
- 14.Schüpbach, T. (1985) Genetics 109, 529–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver, B. (2002) in International Review of Cytology, ed. Jeon, K. W. (Academic, San Diego), pp. 1–60.
- 16.Wei, G., Oliver, B., Pauli, D. & Mahowald, A. P. (1994) Dev. Biol. 161, 318–320. [DOI] [PubMed] [Google Scholar]
- 17.McKearin, D. & Ohlstein, B. (1995) Development (Cambridge, U.K.) 121, 2937–2947. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie, C., Ohlstein, B. & McKearin, D. M. (1999) Dev. Biol. 212, 405–413. [DOI] [PubMed] [Google Scholar]
- 19.Ohlstein, F. & McKearin, D. (1997) Development (Cambridge, U.K.) 124, 3651–3662. [DOI] [PubMed] [Google Scholar]
- 20.Margolis, J. & Spradling, A. C. (1995) Development (Cambridge, U.K.) 121, 3797–3807. [DOI] [PubMed] [Google Scholar]
- 21.Niki, Y. (1986) Dev. Biol. 113, 255–258. [Google Scholar]
- 22.Wu, C.-F., Suzuki, N. & Poo, M.-H. (1983) J. Neurosci. 3, 1888–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan, L.-N. & Gehring, W. (1971) Proc. Natl. Acad. Sci. USA 68, 2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rongo, C., Broihier, H. T., Moore, L., Van Doren, M., Forbes, A. & Lehmann, R. (1997) Cold Spring Harb. Symp. Quant. Biol. 62, 1–11. [PubMed] [Google Scholar]
- 25.Starz-Gaiano, M. & Lehmann, R. (2001) Mech. Dev. 105, 5–18. [DOI] [PubMed] [Google Scholar]
- 26.Illmensee, K. (1973) Roux' Arch. Dev. Biol. 171, 331–343. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann, R. & Nüsslein-Volhard, C. (1986) Cell 47, 141–152. [DOI] [PubMed] [Google Scholar]
- 28.Illmensee, K., Mahowald, A. P. & Loomis, M. R. (1976) Dev. Biol. 49, 40–65. [DOI] [PubMed] [Google Scholar]
- 29.Illmensee, K. & Mahowald, A. P. (1976) Exp. Cell Res. 97, 127–140. [DOI] [PubMed] [Google Scholar]
- 30.Jaglarz, M. K. & Howard, K. R. (1995) Development (Cambridge, U.K.) 121, 3495–3503. [DOI] [PubMed] [Google Scholar]
- 31.Starz-Gaiano, M., Cho, N. K., Forbes, A. & Lehmann, R. (2001) Development (Cambridge, U.K.) 128, 983–991. [DOI] [PubMed] [Google Scholar]
- 32.Van Doren, M., Broihier, H. T., Moore, L. A. & Lehmann, R. (1998) Nature 396, 466–469. [DOI] [PubMed] [Google Scholar]
- 33.Lin, H. & Spradling, A. C. (1997) Development (Cambridge, U.K.) 124, 2463–2476. [DOI] [PubMed] [Google Scholar]
- 34.Boyle, M. & DiNardo, S. (1995) Development (Cambridge, U.K.) 121, 1815–1825. [DOI] [PubMed] [Google Scholar]
- 35.Geigy, R. (1931) Rev. Suisse Zool. 38, 187–288. [Google Scholar]
- 36.Van Doren, M., Mathews, W. R., Samuels, M., Moore, L. A., Broihier, H. T. & Lehmann, R. (2003) Development (Cambridge, U.K.) 130, 2355–2364. [DOI] [PubMed] [Google Scholar]
- 37.Moore, L. A., Broihier, H. T., Van Doren, M., Lunsford, L. B. & Lehmann, R. (1998) Development (Cambridge, U.K.) 125, 667–678. [DOI] [PubMed] [Google Scholar]
- 38.Seydoux, G. & Dunn, M. A. (1997) Development (Cambridge, U.K.) 124, 2191–2201. [DOI] [PubMed] [Google Scholar]
- 39.Schwartzberg, P. L., Robertson, E. J. & Goff, S. P. (1989) Science 246, 799–803. [DOI] [PubMed] [Google Scholar]
- 40.Brinster, R. L. (2002) Science 296, 2174–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]