Abstract
The concept of functional selectivity has now thoroughly supplanted the previously entrenched notion of intrinsic efficacy by explaining how agonists and antagonists exhibit a range of efficacies for distinct receptor-mediated responses. It is noteworthy that functional selectivity accommodates significant changes in efficacy resulting from differential expression of G protein-coupled receptor modifying proteins (i.e., “conditional efficacy”)—a phenomenon with profound implications for drug discovery. We have uncovered a novel regulatory mechanism whereby p90 ribosomal S6 kinase 2 (RSK2) interacts with 5-hydroxytryptamine2A (5-HT2A) serotonin receptors and attenuates receptor signaling via direct receptor phosphorylation (Proc Natl Acad Sci U S A 103:4717–4722, 2006; J Biol Chem 284:5557–5573, 2009). This discovery, together with the mounting evidence for conditional efficacy, suggested to us that 5-HT2A agonist signaling might be disproportionately affected by alterations in RSK2 expression. To test this hypothesis, we evaluated a chemically diverse set of 5-HT2A agonists at three readouts of 5-HT2A receptor activation in both wild-type (WT) and RSK2 knock-out (KO) mouse embryonic fibroblasts (MEFs). Here we report that 5-HT2A receptor agonist efficacies were significantly and variably augmented in RSK2 KO MEFs compared with WT MEFs. As a result, relative agonist efficacies were significantly altered, and even reversed, between WT and RSK2 KO MEFs for a single effector readout. This study provides the first evidence that deletion of a single kinase can elicit profound changes in patterns of agonist functional selectivity.
Ligand “intrinsic efficacy” or “intrinsic activity” (i.e., relative to a reference agonist) defines the magnitude of response that a given ligand imparts to a biological system (Stephenson, 1956). The classic view of intrinsic efficacy assumes that a ligand's ability to impart (or reduce) a stimulus once bound to the receptor is an inherent property of the ligand-receptor complex and is system-independent (i.e., rank orders of efficacy are static across all receptor responses) (Kenakin, 2002). However, a plethora of recent studies at receptor tyrosine kinases and G protein-coupled receptors (GPCRs) have unequivocally demonstrated that ligands exhibit a wide range of efficacies for different receptor behaviors (i.e., rank orders of efficacy are dynamic across various receptor responses) (Roth and Chuang, 1987; Mailman, 2007; Urban et al., 2007; Wilson et al., 2009). To address these observations and to provide a unifying conceptual framework, the related concepts of “functional selectivity” (Ghosh et al., 1996), “agonist-directed trafficking of receptor stimulus” (Kenakin, 1995; Berg et al., 1998b), “biased agonism” (Kenakin, 2007), and “pluridimensionality of signaling” (Galandrin and Bouvier, 2006) (collectively referred to here as “functional selectivity”) have emerged.
The capacity for ligands to elicit a spectrum of receptor behaviors is well documented for the Gαq-coupled 5-HT2A and 5-HT2C serotonin receptors. 5-HT2A and 5-HT2C receptors are essential for mediating various functions of 5-HT in both central and peripheral tissues (e.g., modulation of mood and perception, regulation of appetite, and platelet aggregation) and are targeted by multiple drugs (Kroeze and Roth, 1998; Berger et al., 2009). In what are now considered classic studies, the lab of Clarke and Berg (Berg et al., 1998a; Moya et al., 2007) convincingly demonstrated that the relative rank orders of efficacy for chemically diverse agonists at 5-HT2A and 5-HT2C receptors were reversed between phospholipase C β-mediated inositol phosphate (IP) accumulation and phospholipase A2-mediated arachidonic acid (AA) release. Likewise, Kurrasch-Orbaugh et al. (2003) reported that rank orders of efficacy were reversed for several classes of 5-HT2A agonists comparing IP accumulation and AA release. Significantly, the pleiotropic nature of 5-HT2 ligands was highlighted in a recent study wherein the 5-HT2C selective “antagonist” SB242084 both antagonizes 5-HT2C-mediated AA release and promotes IP accumulation (De Deurwaerdère et al., 2004). In addition, in vitro and in vivo findings have demonstrated that 5-HT2A-selective ligands behave as “inverse agonists” at IP accumulation while simultaneously acting as agonists by promoting receptor internalization (Berry et al., 1996; Willins et al., 1999; Bhatnagar et al., 2001; Gray and Roth, 2001). Such pathway-specific reversals in relative efficacy are incompatible with classic notions of intrinsic efficacy and are considered benchmark examples of functional selectivity.
As seen for 5-HT2A and 5-HT2C receptors, bona fide receptor-based functional selectivity manifests as a reversal in relative efficacies at different pathways. This behavior is not predicted by the classic concept of intrinsic efficacy and can only be explained by agonists stabilizing/promoting different receptor active states (Kenakin, 2007). It follows that the functional selectivity concept, unlike the concept of ligand intrinsic efficacy, ascribes quality to efficacy. Thus, ligand-specific receptor conformations can elicit multiple effector responses, including G protein activation; phosphorylation, desensitization, and internalization; formation of receptor dimers and oligomers; and interaction with auxiliary membrane and cytosolic proteins (Kenakin, 2002; Urban et al., 2007). In fact, GPCR-interacting proteins such as protein-coupling factors and receptor activity-modifying proteins (Christopoulos et al., 2003) have been shown to have profound effects on ligand efficacy (Kenakin, 2002). In addition, recent in vitro and in vivo evidence suggests that β-arrestins, in addition to their classic roles as GPCR-interacting proteins and negative regulators of GPCR signaling, are required for the signaling and functionally selective responses of several ligands (Lefkowitz and Shenoy, 2005; Abbas and Roth, 2008; Schmid et al., 2008). Accordingly, ligand efficacy is clearly conditional upon the expression of auxiliary modifying proteins within the cellular milieu (a phenomenon referred to as “conditional efficacy”) (Kenakin, 2002). Therefore, it is conceivable that cell type-specific expression of additional GPCR-interacting proteins such as kinases could result in differential modulation of ligand efficacy (Allen et al., 2008), although unequivocal evidence for such a phenomenon is not yet available.
We have recently uncovered a novel regulatory mechanism whereby the downstream extracellular signal regulated kinase (ERK)/mitogen-activated protein kinase effector, RSK2, interacts with 5-HT2A serotonin receptors and attenuates receptor signaling via direct receptor phosphorylation (Sheffler et al., 2006; Strachan et al., 2009). Together with the mounting evidence for conditional efficacy and with reports of phosphorylation-mediated stabilization of individual receptor conformations, we hypothesized that 5-HT2A agonist signaling would thus be disproportionately affected by changes in RSK2 expression. More explicitly, we wanted to determine whether genetic deletion of RSK2 differentially affected ligand efficacy. To this end, we performed a focused screen evaluating the effect of RSK2 expression (i.e., in WT and RSK2 KO MEFs) on the signaling of a chemically diverse panel of 5-HT2A receptor agonists at several readouts of 5-HT2A signaling (i.e., IP accumulation, Ca2+ release, and ERK1/2 phosphorylation).
In this study, we provide evidence to both support and extend the emerging concepts of functional selectivity and conditional efficacy by showing that the relative efficacies of 5-HT2A agonists are reversed 1) between different pathways in WT MEFs and 2) between WT and RSK2 KO MEFs at a single pathway. It is noteworthy that genetic deletion of RSK2 results in significant and variable increases in the relative efficacy, but not potency, of a diverse panel of 5-HT2A agonists. These data demonstrate that the signaling of 5-HT2A receptor agonists is disproportionately regulated by RSK2. Significantly, this study provides the first evidence that deletion of a single kinase modulates patterns of agonist functional selectivity at a GPCR. Moreover, this finding has profound implications for drug discovery in particular and molecular pharmacology in general.
Materials and Methods
Materials.
Cell culture reagents were supplied by Invitrogen (Carlsbad, CA) and Cambrex (East Rutherford, NJ). 5-HT, (±)DOI, quipazine, 5-methoxy-DMT, m-CPP, SCH-23390, α-Me-5-HT, LiCl, probenecid, porcine gelatin, paraformaldehyde, Triton X-100, and all other standard reagents were supplied by Sigma-Aldrich (St. Louis, MO). MK212 was obtained from Tocris Bioscience (Ellisville, MO). MDL-100907 and lisuride [1,1-diethyl-3-((6aR,9S)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinolin-9-yl)urea] were acquired as detailed previously (Willins et al., 1999). [myo-3H]inositol (21.7 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Multiwell imaging plates (96- and 384-well) were supplied by Greiner Bio-One (Monroe, NC). Hoechst, concanavalin-A conjugated to Alexa Fluor 488 nm, and goat anti-rabbit secondary antibody conjugated to Alexa Fluor 594 nm were supplied by Invitrogen. Normal goat serum was obtained from Millipore (Billerica, MA).
Cell Culture.
This study used polyclonal populations of WT and RSK2 KO MEFs stably expressing similar amounts of recombinant 5-HT2A receptors (WT Bmax = 1058 ± 53 fmol/mg protein; RSK2 KO Bmax = 731 ± 57 fmol/mg protein) as determined via radioligand binding assays (Gray et al., 2001). These were generated previously by Sheffler et al. (2006) and were shown to express similar amounts of surface receptors. Although MEFs endogenously express low levels of 5-HT2A receptors, the overexpressing MEFs were chosen for this study given their robust performance in scintillation proximity assays. Fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 4 μg/ml puromycin. Cells were grown at 37°C in a humidified environment in the presence of 5% CO2.
Fluorometric Imaging Plate Reader Analysis of Intracellular Ca2+ Release.
Intracellular Ca2+ release was measured using a Fluorometric Imaging Plate Reader (FLIPRTetra) and Ca2+ assay kit (Molecular Devices, Sunnyvale, CA) as detailed previously (Strachan et al., 2009). In brief, 30,000 cells were plated into black-walled, clear-bottomed 96-well tissue culture plates in dialyzed culture medium (DMEM, 5% FBS dialyzed to <0.05 nM 5-HT, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin). The following day, cells were treated with Ca2+ assay buffer (20 mM HEPES, 1× Hanks' balanced salt solution, 2.5 mM probenecid, and Ca2+ assay reagent, pH 7.4) for 1 h at 37°C and equilibrated to room temperature before drug addition. The FLIPRTetra was programmed to add agonist approximately 10 s after establishing baseline relative fluorescence unit (RFU) values (excitation, 470–495; emission, 515–575 nm). RFU values were collected every second for 5 min, and average baseline values were subtracted from maximum RFU values for each well. Baseline-subtracted values were normalized to cell number, analyzed by nonlinear regression (GraphPad Software Inc., San Diego, CA), and expressed relative to maximal 5-HT signaling at WT MEFs (set to 100%). The F test was used to determine statistical significance of the fit parameters EC50 and Emax (defined as p < 0.05). Agonist specificity was confirmed in control experiments wherein the selective 5-HT2A receptor antagonist MDL-100907 (1 μM) completely blocked the Ca2+ response elicited by an EC80 of agonist (data not shown).
Analysis of Inositol Phosphates.
Inositol phosphate (IP) accumulation was measured using the scintillation proximity assay method exactly as detailed previously (Bourdon et al., 2006; Strachan et al., 2009). In brief, 30,000 cells were plated into 96-well tissue culture plates in dialyzed culture medium. The cells were inositol-starved for 1.5 h and incubated with 100 μl of labeling medium (inositol-free basal medium Eagle's, 5% dialyzed FBS, and 0.01 μCi/μl [myo-3H]inositol) for 18 h at 37°C. The labeling medium was removed, and agonists were diluted in assay buffer (1× Hanks' balanced salt solution, 24 mM NaHCO3, 11 mM glucose, and 35 mM LiCl, pH 7.4) and added to the cells for 1 h at 37°C. The assay was terminated by the addition of 50 mM formic acid, and the supernatant was incubated with 0.2 mg yttrium silicate beads (Amersham, Chalfont St. Giles, Buckinghamshire, UK). Radioactivity in the form of [3H]inositol phosphate was measured via scintillation counting (Wallac Microbeta TriLux; PerkinElmer Life and Analytical Sciences). Baseline-subtracted values were normalized to cell number, analyzed by nonlinear regression (GraphPad Software), and expressed relative to maximal 5-HT signaling at WT MEFs (set to 100%). The F test was used to determine statistical significance of the fit parameters EC50 and Emax (defined as p < 0.05). Agonist specificity was confirmed in control experiments, wherein the selective 5-HT2A receptor antagonist MDL-100907 (1 μM) completely blocked IP accumulation elicited by an EC80 of agonist (data not shown).
High-Content Immunofluorescence Microscopy.
Here, we developed a novel high-content microscopic/automated image analysis approach to generate concentration-response curves for ERK1/2 phosphorylation in WT and RSK2 KO MEFs. In particular, we developed an extremely versatile triple-fluorescence labeling method that uses information gained from nuclear (Hoechst, 320 nm) and plasma membrane (concanavalin-A, 488 nm) staining to generate cellular masks (i.e., segmentation during image processing), which are then used to quantify the fluorescence intensity of the third fluorophore representing the protein of interest (referred to here as the “signal channel,” 594 nm) in distinct cellular compartments. It is noteworthy that after the testing of several lectins, concanavalin-A produced the most reliable cellular masks. Thus, the use of concanavalin-A was crucial to the success and general applicability of this approach. In this study, we detected ERK1/2 phosphorylation using a primary antibody specific for ERK1/2 phosphorylation and an Alexa Fluor 594-conjugated secondary antibody. In brief, WT and RSK2 KO MEFs were plated onto 0.2% gelatin-coated black-walled, clear- and thin-bottomed 384-well tissue culture plates (Greiner Bio-One, Monroe, NC) in dialyzed culture medium at a density of 25,000 and 15,000 cells/well, respectively. Twenty-four hours later, the cells were washed with serum-free medium (DMEM, 100 U/ml penicillin, and 100 μg/ml streptomycin) and then serum-starved for 18 h. For the initial time course experiments (0–30 min), cells expressing 5-HT2A receptors were stimulated with 10 μM drug. For all subsequent experiments, we used the liquid handling capability of a FLIPRTetra to simultaneously dispense concentrated drug solutions (final concentration range of 10 μM to 10 pM, performed in duplicate) into 384-well plates. Cells were treated with agonist for 5 min at 37°C, which we determined from time course experiments to produce maximal ERK1/2 phosphorylation for all agonists in both cell lines. After stimulation, the cells were immediately placed on ice, rinsed with ice-cold phosphate-buffered saline (PBS) wash buffer (PBS + 0.5 mM CaCl2, pH 7.4), and incubated with fixative (4% paraformaldehyde, PBS + 0.5 mM CaCl2, pH 7.4) to terminate activation. After 30 min at 25°C, the cells were washed and then permeabilized with 0.3% Triton X-100 for 30 min on ice. The permeabilized cells were incubated with blocking buffer (5% normal goat serum and PBS + 0.5 mM CaCl2, pH 7.4) for 1 h at 25°C and subsequently incubated with blocking buffer containing a phospho-ERK1/2-specific antibody (Thr202/Tyr204, 1:1000; Cell Signaling Technology, Inc., Danvers, MA) for 18 h at 4°C. The following day, the cells were extensively washed and incubated for 1 h at 25°C with blocking buffer containing Hoechst (5 μg/ml), concanavalin-A conjugated to Alexa Fluor 488 (20 μg/ml), and a goat anti-rabbit secondary antibody conjugated to Alexa Fluor 594 (1:200). The cells were extensively washed and incubated with fixative for 20 min at 4°C. Plates were then stored at 4°C in wash buffer before imaging.
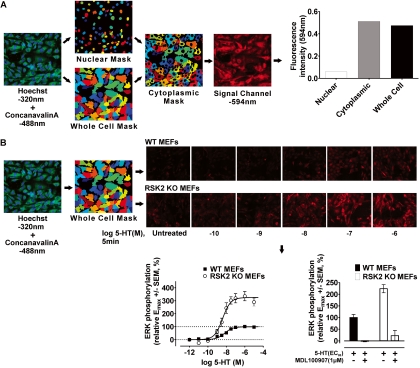
Imaging was performed on a BD Pathway 855 High Content Bioimager (BD Biosciences, San Jose, CA) using the Olympus UAPO40X/340 objective lens (Olympus, Tokyo, Japan). We developed a workflow that used infrared laser autofocusing, triple excitation/emission parameters (nuclear, 380/435 nm; plasma membrane, 488/515 nm; signal channel, 555/645 nm), and montaging of nine adjacent fields to produce superimposable nuclear, plasma membrane, and signal-channel images. The images were then exported to CellProfiler (Broad Institute Imaging Platform, Cambridge, MA) for image processing and analysis. In particular, we developed a macropipeline within CellProfiler that produced reliable cell segmentation wherein Hoechst and concanavalin-A intensities are used stepwise to generate nuclear, whole-cell, and cytoplasmic cell masks (Fig. 1A). Using this approach, we could measure the 594 nm intensity within defined cellular regions. Baseline-subtracted, whole-cell 594 nm mean intensity values corresponding to ERK1/2 phosphorylation were then analyzed via nonlinear regression (GraphPad Software) and expressed relative to the Emax for 5-HT in WT MEFs. Statistical significance of ERK1/2 time course data were determined by one-tailed paired t test (defined as p < 0.05). The F test was used to determine statistical significance of the fit parameters EC50 and Emax (defined as p < 0.05). Assay quality was assessed using the Z′ factor calculation of Zhang et al. (1999). Agonist specificity was confirmed in control experiments wherein the selective 5-HT2A receptor antagonist MDL-100907 (1 μM) completely blocked ERK1/2 phosphorylation elicited by an EC80 concentration of agonist (shown for 5-HT in Fig. 1B).
Fig. 1.
Development of a novel triple-fluorescence high-content microscopic assay in 384-well plates to rapidly measure ERK1/2 phosphorylation in WT and RSK2 KO MEFs. A, diagram showing stepwise generation of nuclear (Hoechst 320), whole-cell (concanavalin-A 488), and cytoplasmic cell masks subsequently used to measure fluorescence intensity of the signal channel (594 nm) in distinct cellular regions. This approach incorporates the nearly limitless application of state-specific antibodies (e.g., phospho-specific) and fluorescently labeled proteins to quantify receptor responses. Moreover, segmenting the cell into distinct regions allows us to extract a variety of signaling phenotypes with customizable CellProfiler image analysis software. B, the triple-fluorescence technique was used to generate full concentration-response curves for ERK1/2 phosphorylation in WT and RSK2 KO MEFs after 5-min agonist treatment. The workflow shown here describes the steps used to measure ERK1/2 phosphorylation (594 nm, see representative images) within whole-cell masks after 5-HT treatment. Concentration-response curves for 5-HT-induced ERK1/2 phosphorylation in WT (■) and RSK2 KO (○) MEFs highlight the robustness (Z′ factor = 0.54) and reproducibility of the triple-fluorescence technique. Values represent the mean ± S.E.M. of 18 independent experiments performed in duplicate. Also shown are representative results (bottom right, mean ± S.E.M.) in which the 5-HT2A-selective antagonist MDL-100907 (1 μM) blocked the response to 5-HT (EC80 concentration). Identical results were obtained for all agonists at both cell lines (data not shown).
Immunoblotting.
Western blot measurements of ERK1/2 phosphorylation were performed according to Sheffler et al. (2006). In brief, WT and RSK2 KO MEFs were plated onto six-well plates in dialyzed culture medium at a density of 150,000 cells/well and serum-starved for approximately 18 h before the experiment. During the experiment, the cells were treated with 10 μM 5-HT for various times (0–30 min) at 37°C and then immediately placed on ice, washed twice with cold PBS, and lysed (50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 1.0% CHAPS, EDTA-free protease inhibitor cocktail (Roche Applied Science, Branford, CT), 50 mM NaF, 50 mM β-glycerol phosphate, 0.1 mM Na3VO4, and 5 mM Na4P2O7, pH 7.5). The supernatants were collected after centrifugation (16,000g at 4°C for 20 min) and stored at −20°C until further use. To measure ERK1/2 phosphorylation, equal amounts of protein were separated on 10% SDS-polyacrylamide gel electrophoresis gels and immunoblotted using standard procedures (Gray et al., 2001). Nitrocellulose blots were incubated with blocking buffer (Tris-buffered saline, 0.1% Tween 20, and 5% nonfat dehydrated milk) and then probed with the phospho-ERK1/2-specific antibody (Thr202/Tyr204, 1:1000; Cell Signaling Technology, Inc.) diluted in phospho-blocking buffer (Tris-buffered saline, 0.1% Tween 20, and 5% bovine serum albumin) or the ERK1/2 antibody (p44/42, 1:1000; Cell Signaling Technology, Inc.) diluted in standard blocking buffer. The primary antibodies were detected using the goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:1000; Vector Laboratories, Burlingame, CA) and the SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific, Rockford, IL). Immunoreactive bands were quantified using Kodak imaging software (Eastman Kodak, New Haven, CT).
Statistical Analysis of Ligand Rank Order of Efficacy.
Relative agonist efficacies were analyzed using one-way ANOVA (significance set as p < 0.05) and significant differences between groups (set as p < 0.05) were subsequently identified via the Tukey-Kramer unplanned multiple comparisons test adjusted for unequal sample size (GraphPad Software). Mean relative agonist efficacies were then arranged in descending order and assigned to statistically homogeneous groups (labeled “a” through “f” in Table 4) such that significant differences in rank order were denoted by changes in group membership. Thus, agonists with nonoverlapping group assignments were considered to be significantly different.
TABLE 4.
Relative agonist rank order of efficacy for IP accumulation, Ca2+ release, and ERK1/2 phosphorylation in WT and RSK2 KO MEFs
Letters in parentheses indicate group assignment. Mean relative efficacies were analyzed via one-way ANOVA, and significant differences were determined via the Tukey-Kramer multiple comparison post-test (significance set at p < 0.05). Agonists were arranged in descending order (i.e. 1 through 9) and placed into statistically homogeneous groups (i.e., a through f) such that significant differences in rank order are denoted by changes in group membership. Agonists with nonoverlapping group assignments were considered to be significantly different.
| Rank Order | IP Accumulation |
Ca2+ Release |
ERK1/2 Phosphorylation |
|||
|---|---|---|---|---|---|---|
| WT MEFs | RSK2 KO MEFs | WT MEFs | RSK2 KO MEFs | WT MEFs | RSK2 KO MEFs | |
| 1 | 5-HT (a) | 5-Methoxy DMT (a) | a-Me5-HT (a) | a-Me5-HT (a) | Quipazine (a) | DOI (a) |
| 2 | a-Me5-HT (a,b) | Quipazine (a) | 5-HT (a,b) | 5-HT (a) | m-CPP (a) | 5-Methoxy DMT (a) |
| 3 | Quipazine (b,c) | DOI (a,b) | Quipazine (a,b,c) | MK212 (a) | 5-HT (a) | a-Me5-HT (a,b) |
| 4 | MK212 (c) | 5-HT (a) | DOI (a,b,c) | Quipazine (a,b) | DOI (a,b) | 5-HT (a) |
| 5 | DOI (c,d) | MK212 (b,c) | 5-Methoxy DMT (b,c,d) | 5-Methoxy DMT (a,b,c) | Lisuride (a,b) | Lisuride (a,b) |
| 6 | 5-Methoxy DMT (d) | a-Me5-HT (c) | MK212 (c,d) | DOI (a,b,c) | 5-Methoxy DMT (a,b) | Quipazine (a,b) |
| 7 | m-CPP (e) | m-CPP (c,d) | m-CPP (d) | m-CPP (a,b,c) | a-Me5-HT (a,b,c) | MK212 (a,b) |
| 8 | Lisuride (e,f) | Lisuride (d) | Lisuride (e) | Lisuride (b,c) | MK212 (b,c) | m-CPP (a,b) |
| 9 | SCH-23390 (f) | SCH-23390 (d) | SCH-23390 (e) | SCH-23390 (c) | SCH-23390 (c) | SCH-23390 (b) |
Results
Validation of a Novel High-Content Microscopic Approach for ERK1/2 Phosphorylation.
In this study, we developed a novel high content microscopic/automated image analysis approach to generate concentration-response curves for ERK1/2 phosphorylation in WT and RSK2 KO MEFs. We devised a versatile triple-fluorescence labeling method that uses information gained from nuclear (320 nm) and plasma membrane (488 nm) staining to reliably generate cellular masks, which were subsequently used to quantify the fluorescence intensity of the signal channel (594 nm) representing the protein of interest (i.e., phosphorylated ERK1/2) (Fig. 1A).
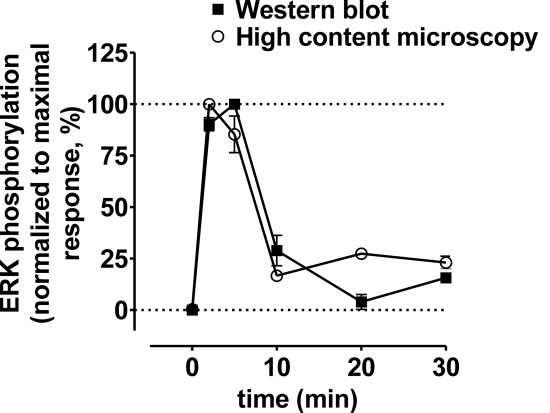
As shown in Fig. 1B, our approach using whole-cell masks yielded highly reproducible concentration-response curves (Z′ factor = 0.54). We also confirmed that the results obtained using the high-content approach were indistinguishable from those obtained using a standard Western blotting technique (Fig. 2), and the findings agree with our results published previously (Sheffler et al., 2006). As an additional control that was also repeated for measures of Ca2+ release and IP accumulation, we demonstrated that 5-HT2A-mediated ERK1/2 phosphorylation was blocked in both cell lines by the selective 5-HT2A antagonist MDL-100907 (shown for 5-HT in Fig. 1B).
Fig. 2.
Time course of 5-HT-induced ERK1/2 phosphorylation is similar between the techniques of Western blotting and high-content microscopy. As shown for both Western blotting (■) and triple-fluorescence high-content microscopy (○) in WT MEFs, maximal 5-HT-induced ERK1/2 phosphorylation occurred within 5 min. We also determined via high-content microscopy that ERK1/2 phosphorylation was maximal within 5 min in both WT and KO RSK2 MEFs for the agonists (10 μM) 5-HT, DOI, quipazine, 5-methoxy-DMT, lisuride, m-CPP, SCH-23390, α-Me-5-HT, and MK212 (data not shown).
5-HT2A Agonist Responses for Diverse Agonists Are Differentially Altered by Genetic Deletion of RSK2.
We next determined how genetic deletion of RSK2 might affect the signaling of a structurally diverse panel of 5-HT2A receptor agonists. In agreement with our recent studies (Strachan et al., 2009), genetic deletion of RSK2 significantly increased the relative efficacies of most 5-HT2A agonists at multiple effector pathways. As expected from our previous work (Sheffler et al., 2006; Strachan et al., 2009), the reference full agonist 5-HT, along with α-Me-5-HT and DOI, elicited significantly greater maximal increases in IP accumulation, Ca2+ release, and ERK1/2 phosphorylation in RSK2 KO MEFs relative to WT MEFs (Tables 1–3). In addition, the relative efficacies of quipazine, 5-methoxy-DMT, lisuride, and m-CPP were significantly increased at all three effector readouts in RSK2 KO MEFs (Tables 1–3). In contrast, the partial agonists SCH-23390 and MK212 were unique in that their signaling was significantly potentiated at measures of IP accumulation and ERK1/2 phosphorylation but not at Ca2+ release.
TABLE 1.
Potency and relative efficacy values for 5-HT2A-mediated IP accumulation
Agonist potencies (EC50) and relative efficacies (Emax) represent the average of four to five independent experiments. pEC50 values are represented as −log of EC50, given as molar values.
| Agonist | Agonist Potency EC50 (pEC50 ± S.E.M.) |
Relative Agonist Efficacy (Emax ± S.E.M.) |
EmaxRSK2KO/EmaxWT | ||||
|---|---|---|---|---|---|---|---|
| WT MEFs | RSK2 KO MEFs | F Test, p Value | WT MEFs | RSK2 KO MEFs | F Test, p Value | ||
| nM | % | ||||||
| 5-HT | 134 (6.87 ± 0.03) | 57.8 (7.24 ± 0.11) | 0.0309 | 99.1 ± 1.2 | 209 ± 8.5 | <0.0001 | 2.1 |
| DOI | 12.4 (7.91 ± 0.10) | 8.51 (8.07 ± 0.10) | 0.7145 | 71.7 ± 2.4 | 211 ± 13 | <0.0001 | 2.9 |
| Quipazine | 188 (6.73 ± 0.09) | 140 (6.85 ± 0.15) | 0.7010 | 82.7 ± 2.9 | 220 ± 14 | <0.0001 | 2.7 |
| 5-Methoxy-DMT | 590 (6.23 ± 0.13) | 386 (6.41 ± 0.15) | 0.6487 | 66.6 ± 4.0 | 224 ± 14 | <0.0001 | 3.4 |
| Lisuride | 3.52 (8.45 ± 0.57) | 5.99 (8.22 ± 0.20) | 0.7450 | 17.1 ± 2.6 | 63.1 ± 3.8 | <0.0001 | 3.7 |
| m-CPP | 167 (6.78 ± 0.19) | 231 (6.64 ± 0.09) | 0.5364 | 27.8 ± 2.4 | 90.0 ± 3.6 | <0.0001 | 3.2 |
| SCH-23390 | 16.3 (7.79 ± 0.30) | 24.6 (7.61 ± 0.08) | 0.5342 | 14.0 ± 1.5 | 33.9 ± 1.1 | <0.0001 | 2.4 |
| α-Me-5-HT | 178 (6.75 ± 0.04) | 68.9 (7.16 ± 0.05) | <0.0001 | 95.4 ± 1.9 | 134 ± 3.0 | <0.0001 | 1.4 |
| MK212 | 3390 (5.47 ± 0.09) | 2330 (5.63 ± 0.05) | 0.1148 | 80.4 ± 5.7 | 152 ± 5.0 | <0.0001 | 1.9 |
TABLE 2.
Potency and relative efficacy values for 5-HT2A-mediated intracellular Ca2+ release
Agonist potencies (EC50) and relative efficacies (Emax) represent the average of four to five independent experiments. pEC50 values are represented as −log of EC50, given as molar values.
| Agonist | Agonist Potency EC50 (pEC50 ± S.E.M.) |
Relative Agonist Efficacy Emax ± S.E.M. |
EmaxRSK2KO/EmaxWT | ||||
|---|---|---|---|---|---|---|---|
| WT MEFs | RSK2KO MEFs | F Test, p Value | WT MEFs | RSK2 KO MEFs | F Test, p Value | ||
| nM | % | ||||||
| 5-HT | 7.79 (8.11 ± 0.07) | 10.2 (7.99 ± 0.17) | 0.6969 | 97.0 ± 2.2 | 202 ± 12.5 | <0.0001 | 1.5 |
| DOI | 2.81 (8.55 ± 0.12) | 4.68 (8.33 ± 0.11) | 0.2309 | 87.3 ± 2.9 | 141 ± 4.6 | <0.0001 | 1.6 |
| Quipazine | 22.8 (7.64 ± 0.12) | 62.3 (7.21 ± 0.09) | 0.0114 | 88.1 ± 3.6 | 157 ± 5.7 | <0.0001 | 1.8 |
| 5-Methoxy-DMT | 60.0 (7.22 ± 0.08) | 58.2 (7.24 ± 0.14) | 0.9509 | 83.2 ± 2.6 | 145 ± 7.8 | <0.0001 | 1.7 |
| Lisuride | 985 (6.01 ± 0.17) | 1023 (5.99 ± 0.12) | 0.9405 | 29.1 ± 2.7 | 58.7 ± 3.8 | <0.0001 | 2.0 |
| m-CPP | 155 (6.81 ± 0.18) | 234 (6.63 ± 0.31) | 0.6959 | 60.5 ± 5.0 | 116 ± 17 | 0.0039 | 1.9 |
| SCH-23390 | 329 (6.48 ± 0.32) | 197 (6.71 ± 0.34) | 0.6864 | 25.7 ± 4.0 | 33.2 ± 5.2 | 0.2974 | 1.3 |
| α-Me-5-HT | 25.0 (7.60 ± 0.12) | 21.9 (7.66 ± 0.41) | 0.9401 | 106 ± 4.5 | 212 ± 32 | 0.0046 | 2.0 |
| MK212 | 817 (6.09 ± 0.18) | 846 (6.07 ± 0.33) | 0.9793 | 72.5 ± 7.8 | 168 ± 33 | 0.1035 | 2.3 |
TABLE 3.
Potency and relative efficacy values for 5-HT2A-mediated ERK1/2 phosphorylation
Agonist potencies (EC50) and relative efficacies (Emax) represent the average of four to five independent experiments. pEC50 values are represented as −log of EC50, given as molar values.
| Agonist | Agonist Potency EC50 (pEC50 ± S.E.M.) |
Relative Agonist Efficacy Emax ± S.E.M. |
EmaxRSK2KO/EmaxWT | ||||
|---|---|---|---|---|---|---|---|
| WT MEFs | RSK2 KO MEFs | F Test p Value | WT MEFs | RSK2 KO MEFs | F Test p Value | ||
| nM | % | ||||||
| 5-HT | 6.51 (8.19 ± 0.08) | 3.92 (8.41 ± 0.11) | 0.4729 | 99.1 ± 3.5 | 325 ± 15 | <0.0001 | 3.3 |
| DOI | 0.370 (9.43 ± 0.16) | 0.889 (9.05 ± 0.22) | 0.4866 | 99.0 ± 3.9 | 348 ± 24 | <0.0001 | 3.5 |
| Quipazine | 2.42 (8.62 ± 0.17) | 4.24 (8.37 ± 0.20) | 0.4796 | 108 ± 7 | 270 ± 22 | <0.0001 | 2.5 |
| 5-Methoxy-DMT | 8.23 (8.08 ± 0.19) | 7.45 (8.13 ± 0.19) | 0.9356 | 89.8 ± 7.7 | 343 ± 29 | <0.0001 | 3.8 |
| Lisuride | 7.39 (8.13 ± 0.21) | 2.27 (8.64 ± 0.32) | 0.5414 | 90.9 ± 8.3 | 278 ± 32 | 0.0010 | 3.1 |
| m-CPP | 21.6 (7.67 ± 0.18) | 21.6 (7.67 ± 0.19) | 0.9976 | 105 ± 9 | 245 ± 25 | <0.0001 | 2.3 |
| SCH-23390 | 21.2 (7.67 ± 0.29) | 3.65 (8.44 ± 0.23) | 0.3295 | 51.3 ± 7.4 | 202 ± 19 | 0.0100 | 3.9 |
| α-Me5-HT | 3.07 (8.51 ± 0.19) | 3.91 (8.41 ± 0.17) | 0.8488 | 83.4 ± 6.3 | 327 ± 23 | <0.0001 | 3.9 |
| MK212 | 133 (6.88 ± 0.13) | 42.5 (7.37 ± 0.14) | 0.2023 | 71.2 ± 4.6 | 261 ± 18 | 0.0005 | 3.7 |
Contrary to effects on maximal signaling, relative agonist potencies were not globally potentiated in RSK2 KO MEFs, with few exceptions. These included the full agonists 5-HT and α-methyl-5-HT, which were significantly more potent for IP accumulation in RSK2 KO MEFs, and the partial agonist quipazine, which was significantly more potent for Ca2+ release in WT MEFs (Tables 1–3). Taken together, these data showed that RSK2 modulates agonist efficacies, whereas agonist potencies remain largely unaffected.
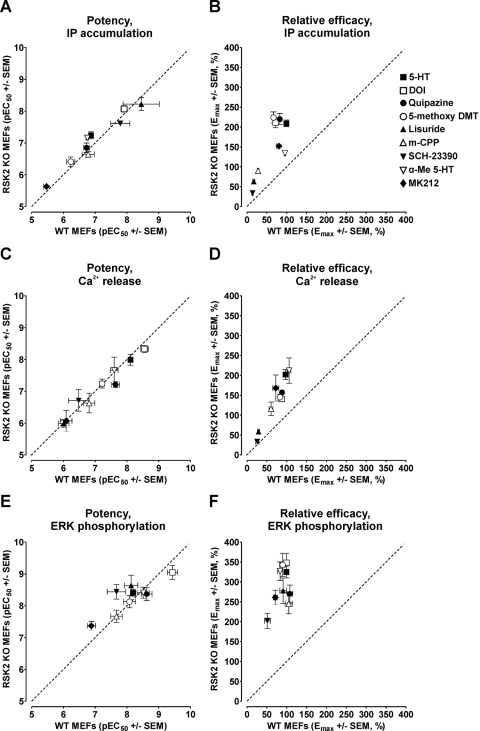
Indeed, compared with a line of identity, RSK2 deletion significantly potentiated agonist efficacies (Fig. 3, B, D, and F), with few changes in agonist potency (Fig. 3, A, C, and E). We also observed that genetic deletion of RSK2 resulted in a global potentiation of agonist-mediated ERK1/2 phosphorylation with little distinction between full and partial agonists (Fig. 3, E and F). This global potentiation of ERK1/2 phosphorylation in RSK2 KO MEFs is, in fact, consistent with removal of feedback inhibition on the ERK/mitogen-activated protein kinase pathway, because it is known that RSK2 phosphorylates Sos, thereby decreasing Ras activation (Frödin and Gammeltoft, 1999).
Fig. 3.
The relative efficacies of 5-HT2A receptor agonists are globally augmented by genetic deletion of RSK2. Agonist potencies (pEC50) and relative efficacies (Emax) for IP accumulation (A and B), Ca2+ release (C and D), and ERK phosphorylation (E and F) in WT and RSK2 KO MEFs were plotted as X–Y correlations. Relative to a line of identity (dashed line), Emax values were consistently higher in RSK2 KO MEFs, whereas pEC50 values were similar between cell lines. Emax and pEC50 values were calculated via nonlinear regression as reported in Tables 1 to 3. Values represent the mean ± S.E.M. of three to six independent experiments performed in duplicate. Agonists tested were 5-HT (■), DOI (□), quipazine (●), 5-methoxy-DMT (○), lisuride (▴), m-CPP (▵), SCH-23390 (▾), α-Me-5-HT (▿), and MK212 (♦).
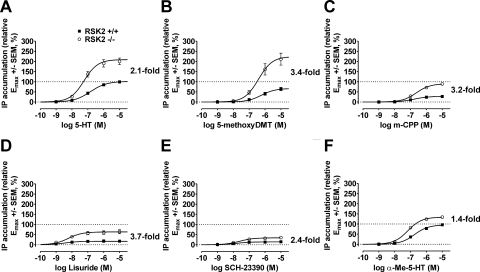
Because a major aim was to determine whether genetic deletion of RSK2 differentially modulates ligand efficacy, we generated relative efficacy ratios (i.e., EmaxRSK2KO/EmaxWT values) for each response to quantify an agonist's propensity to signal in the absence of RSK2. It is interesting that we found that EmaxRSK2KO/EmaxWT values differed considerably for each agonist and response (Tables 1–3), with the largest changes observed for IP accumulation and ERK1/2 phosphorylation. The partial agonists 5-methoxy-DMT (3.4-fold), m-CPP (3.2-fold), and lisuride (3.7-fold) differed substantially from the reference full agonist 5-HT (2.1-fold) with regard to their abilities to stimulate IP accumulation (Fig. 4, A–D). In fact, m-CPP and lisuride, whose relative efficacies were extremely low in WT MEFs, behaved as moderate to full agonists in RSK2 KO MEFs (i.e., compared with the reference agonist 5-HT in WT MEFs; Fig. 4).
Fig. 4.
5-HT2A receptor agonists are differentially responsive to RSK2 deletion. Concentration-response curves for IP accumulation in WT (■) and RSK2 KO (○) MEFs in response to 60-min treatment with 5-HT (A), 5-methoxy-DMT (B), m-CPP (C), lisuride (D), SCH-23390 (E), and α-Me-5-HT (F) show differential sensitivity to RSK2 expression. Relative efficacy values (Emax, 5-HT set to 100%) were determined via nonlinear regression and were significantly potentiated for all agonists in RSK2 KO MEFs, as shown in Table 1. Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate. EmaxRSK2KO/EmaxWT values were calculated for each agonist and are shown next to each plot as a measure of an agonist's propensity to signal in the absence of RSK2.
Considering that differences in stimulus-response coupling manifest as large increases in partial agonist efficacy and are often encountered comparing different cell lines or tissues, it was conceivable that partial agonists, as a class, signal more effectively in RSK2 KO MEFs. However, as shown in Fig. 4E, the relative efficacy of the weak partial agonist SCH-23390, which has an efficacy and potency comparable with lisuride in WT MEFs, increased only 2.4-fold in RSK2 KO MEFs. We also observed a small increase in the relative efficacy of the partial agonist MK212 in RSK2 KO MEFs (1.9-fold; data not shown). This suggested that increased receptor responsiveness was not exclusive to partial agonists, thus eliminating the influence of differences in stimulus-response coupling comparing WT with RSK2 KO MEFs.
Similar to our observations with partial agonists, highly efficacious ligands such as 5-HT (2.1-fold) and α-Me-5-HT (1.4-fold) were differentially sensitive to genetic deletion of RSK2. α-Me-5-HT behaved as a full agonist in WT MEFs, whereas it was substantially less responsive for IP accumulation in RSK2 KO MEFs (Fig. 4F). This behavior could not be explained by saturation of the response system because 5-HT was significantly more efficacious than α-Me-5-HT in RSK2 KO MEFs (relative Emax = 134 ± 3.0% versus 209 ± 8.5% for α-Me-5-HT and 5-HT, respectively; p < 0.05). Together with the discrepancies seen with partial agonists, these data suggest that 5-HT2A receptor agonists are differentially responsive to RSK2 deletion. Furthermore, in the case of IP accumulation, these discrepancies underlie reversals in relative rank order of efficacy between WT and RSK2 KO MEFs (see below).
5-HT2A Agonists Are Functionally Selective for ERK1/2 Phosphorylation in WT MEFs.
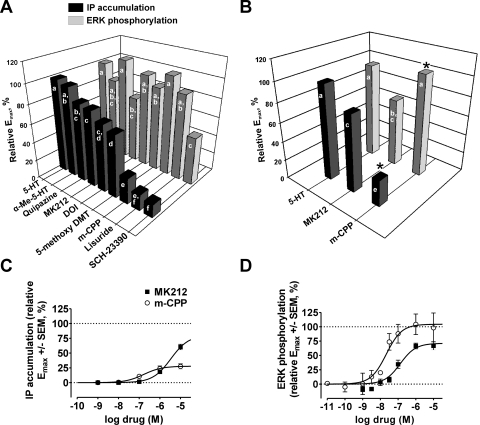
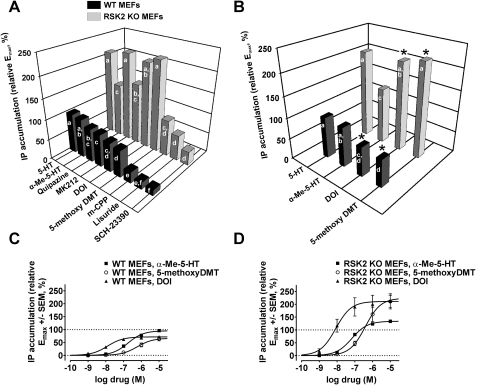
To identify novel instances of functional selectivity within our data set, we generated statistically rigorous rank orders of efficacy for each receptor response in WT and RSK2 KO MEFs. This approach enabled us to identify changes in rank orders of efficacy between receptor responses in WT MEFs and between WT and RSK2 KO MEFs at each receptor response. Fundamental to this approach was the assignment of agonists to statistically homogeneous groups such that significant differences in rank order (set as p < 0.05) were denoted by changes in group membership (Table 4 and Figs. 5 and 6). Therefore, agonists with nonoverlapping group assignments were considered to be significantly different.
Fig. 5.
Evidence of functional selectivity between measures of IP accumulation and ERK1/2 phosphorylation in WT MEFs. A, relative rank orders of efficacy (Emax, 5-HT set to 100%) were significantly altered between measures of IP accumulation (■) and ERK1/2 phosphorylation ( ) for 5-HT2A agonists in WT MEFs. Statistical ranking of relative Emax values was performed via one-way ANOVA and Tukey-Kramer multiple comparison post-tests in which agonists were assigned to statistically homogeneous groups (designated “a” through “f” in Table 4, labeled bars). Significant differences in rank order were denoted by changes in group membership, and agonists with nonoverlapping group assignments were considered to be significantly different. Values represent the mean of four to five independent experiments performed in duplicate. B, the relative efficacies of MK212 and m-CPP were reversed between measures of IP accumulation (■) and ERK1/2 phosphorylation (
) for 5-HT2A agonists in WT MEFs. Statistical ranking of relative Emax values was performed via one-way ANOVA and Tukey-Kramer multiple comparison post-tests in which agonists were assigned to statistically homogeneous groups (designated “a” through “f” in Table 4, labeled bars). Significant differences in rank order were denoted by changes in group membership, and agonists with nonoverlapping group assignments were considered to be significantly different. Values represent the mean of four to five independent experiments performed in duplicate. B, the relative efficacies of MK212 and m-CPP were reversed between measures of IP accumulation (■) and ERK1/2 phosphorylation ( ) in WT MEFs (∗, significantly different from MK212, p < 0.05). Labeled bars represent statistically homogeneous groups (designated “a” through “f” in Table 4). C, concentration-response curves showing the relative abilities of MK212 (■) and m-CPP (○) to stimulate IP accumulation via 5-HT2A receptors in WT MEFs. Relative Emax values were significantly different (p < 0.05). Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate. D, concentration-response curves showing the relative abilities for MK212 (■) and m-CPP (○) to stimulate ERK phosphorylation via 5-HT2A receptors in WT MEFs. Relative Emax values were significantly different (p < 0.05). Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate.
) in WT MEFs (∗, significantly different from MK212, p < 0.05). Labeled bars represent statistically homogeneous groups (designated “a” through “f” in Table 4). C, concentration-response curves showing the relative abilities of MK212 (■) and m-CPP (○) to stimulate IP accumulation via 5-HT2A receptors in WT MEFs. Relative Emax values were significantly different (p < 0.05). Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate. D, concentration-response curves showing the relative abilities for MK212 (■) and m-CPP (○) to stimulate ERK phosphorylation via 5-HT2A receptors in WT MEFs. Relative Emax values were significantly different (p < 0.05). Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate.
Fig. 6.
Genetic deletion of RSK2 significantly alters the relative rank order efficacy of 5-HT2A agonists: evidence for functional selectivity between WT and RSK2 KO MEFs. A, relative rank orders of efficacy (Emax, 5-HT set to 100%) were significantly altered between WT (■) and RSK2 KO MEFs ( ) for 5-HT2A-mediated IP accumulation. Statistical ranking of relative Emax values was performed via one-way ANOVA and Tukey-Kramer multiple comparison post-tests in which agonists were assigned to statistically homogeneous groups (designated “a” through “f” in Table 4, labeled bars). Significant differences in rank order were denoted by changes in group membership, and agonists with nonoverlapping group assignments were considered to be significantly different. Values represent the mean of four to five independent experiments performed in duplicate. B, the relative efficacies of α-Me-5-HT, 5-methoxy-DMT, and DOI were reversed between WT (■) and RSK2 KO MEFs (
) for 5-HT2A-mediated IP accumulation. Statistical ranking of relative Emax values was performed via one-way ANOVA and Tukey-Kramer multiple comparison post-tests in which agonists were assigned to statistically homogeneous groups (designated “a” through “f” in Table 4, labeled bars). Significant differences in rank order were denoted by changes in group membership, and agonists with nonoverlapping group assignments were considered to be significantly different. Values represent the mean of four to five independent experiments performed in duplicate. B, the relative efficacies of α-Me-5-HT, 5-methoxy-DMT, and DOI were reversed between WT (■) and RSK2 KO MEFs ( ) for 5-HT2A-mediated IP accumulation (∗, statistically different from α-Me-5-HT, p < 0.05). Labeled bars represent statistically homogeneous groups (designated “a” through “f” in Table 4). C, concentration-response curves showing the relative abilities of α-Me-5-HT (■), 5-methoxy-DMT (○), and DOI (▴) to stimulate IP accumulation via 5-HT2A receptors in WT MEFs. Relative Emax values were significantly different between α-Me-5-HT and both 5-methoxy-DMT and DOI (p < 0.05). Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate. D, concentration-response curves showing the relative abilities of α-Me-5-HT (■), 5-methoxy-DMT (○), and DOI (▴) to stimulate IP accumulation via 5-HT2A receptors in RSK2 KO MEFs. Relative Emax values were significantly different between α-Me-5-HT and both 5-methoxy-DMT and DOI (p < 0.05). Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate.
) for 5-HT2A-mediated IP accumulation (∗, statistically different from α-Me-5-HT, p < 0.05). Labeled bars represent statistically homogeneous groups (designated “a” through “f” in Table 4). C, concentration-response curves showing the relative abilities of α-Me-5-HT (■), 5-methoxy-DMT (○), and DOI (▴) to stimulate IP accumulation via 5-HT2A receptors in WT MEFs. Relative Emax values were significantly different between α-Me-5-HT and both 5-methoxy-DMT and DOI (p < 0.05). Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate. D, concentration-response curves showing the relative abilities of α-Me-5-HT (■), 5-methoxy-DMT (○), and DOI (▴) to stimulate IP accumulation via 5-HT2A receptors in RSK2 KO MEFs. Relative Emax values were significantly different between α-Me-5-HT and both 5-methoxy-DMT and DOI (p < 0.05). Values represent the mean ± S.E.M. of four to five independent experiments performed in duplicate.
A comparison of agonist responses in WT MEFs revealed that the relative efficacies did not differ substantially between the dependent measures of IP accumulation and Ca2+ release, in agreement with previous studies (Berg et al., 1998a) (Table 4). By contrast, relative rank orders of efficacy differed significantly comparing measures of IP accumulation and ERK1/2 phosphorylation (Fig. 5A, and Table 4). For example, lisuride and m-CPP, which were weak to moderate partial agonists for IP accumulation and Ca2+ release, maximally activated ERK1/2 in WT MEFs. In agreement with several other studies (Berg et al., 1998a; Kurrasch-Orbaugh et al., 2003), lisuride only weakly activated 5-HT2A-mediated IP accumulation (relative Emax = 17.1 ± 2.6%, n = 4) and Ca2+ release (relative Emax = 29.1 ± 2.7%, n = 4). However, despite its behavior as a weak partial agonist for IP accumulation and Ca2+ release, lisuride stimulated ERK phosphorylation similarly to the reference full agonist 5-HT (relative Emax = 90.9 ± 8.3 and 99.1 ± 3.5% for lisuride and 5-HT, respectively; p > 0.05). Similar observations were made for the partial agonist m-CPP, which was a partial agonist for IP accumulation (relative Emax = 27.8 ± 2.4%, n = 5) and Ca2+ release (relative Emax = 60.5 ± 5.0%). By contrast, m-CPP and 5-HT were equal in their abilities to induce ERK phosphorylation (relative Emax = 105 ± 9.0 and 99.1 ± 3.5% for m-CPP and 5-HT, respectively; p > 0.05).
Similar to the observations made comparing agonist signaling in WT and RSK2 KO MEFs, the relative efficacies of partial agonists were not uniformly increased for ERK1/2 phosphorylation in the WT MEFs. This suggested that the ERK1/2 pathway was not more efficiently coupled compared with IP accumulation. In support of this, the partial agonist MK212 signaled similarly between measures of IP accumulation and ERK phosphorylation (Fig. 5, A and B). In fact, the relative efficacies of MK212 and m-CPP were significantly reversed between measures of IP accumulation and ERK1/2 phosphorylation, which is consistent with classic examples of functional selectivity at 5-HT2 family receptors (Fig. 5, B–D).
Genetic Deletion of RSK2 Alters the Relative Rank Order of Efficacy of 5-HT2A Receptor Agonists.
We next determined whether genetic deletion of RSK2, a novel GPCR kinase that is known to modulate 5-HT2A agonist signaling, results in significant reversals in 5-HT2A agonist efficacies. To test this hypothesis, we compared relative rank orders of efficacy between WT and KO MEFs at each effector readout. As shown in Table 4, genetic deletion of RSK2 significantly affected the relative rank orders of efficacy at each effector readout. Further statistical analysis revealed that, in addition to a general reordering of rank orders of efficacy, genetic deletion of RSK2 resulted in significant reversals in relative agonist efficacies for IP accumulation (Table 4 and Fig. 6). We observed that α-Me-5-HT signaled as a full agonist in WT MEFs (relative Emax = 95.4 ± 1.9% versus 99.1 ± 1.2% for α-Me-5-HT and 5-HT, respectively; p > 0.05), whereas 5-methoxy-DMT and DOI signaled as partial agonists in WT MEFs (relative Emax = 66.6 ± 4.0 and 71.7 ± 2.4% for 5-methoxy-DMT and DOI, respectively; p < 0.05 for both ligands versus α-Me-5-HT) (Fig. 6, B and C). However, despite signaling as a full agonist in WT MEFs, α-Me-5-HT was significantly less efficacious than 5-methoxy-DMT and DOI in RSK2 KO MEFs (relative Emax = 134 ± 3.0 versus 224 ± 14% for α-Me-5-HT and 5-methoxy-DMT, respectively; relative Emax = 134 ± 3.0 versus 211 ± 13% for α-Me-5-HT and DOI, respectively; p < 0.05) (Fig. 6, B and D). Altogether, it is clear that the relative rank order of efficacy switched from α-Me-5-HT > DOI = 5-methoxy-DMT in WT MEFs to 5-methoxy-DMT = DOI > α-Me-5-HT in RSK2 KO MEFs. These results thus provide the first evidence indicating that a relatively minor change in the cellular kinome is sufficient to elicit profound alterations in relative agonist efficacy.
As reported above, striking variations in EmaxRSK2KO/EmaxWT values for IP accumulation suggested that the responses to some agonists were differentially sensitive to genetic deletion of RSK2. Furthermore, these discrepancies could not be explained by agonist class, because RSK2 deletion did not similarly potentiate all partial agonists or full agonists. For example, EmaxRSK2KO/EmaxWT values for the partial agonists 5-methoxy-DMT and DOI were highly responsive to RSK2 deletion, as exhibited by 3.4- and 2.9-fold increases in IP accumulation in RSK2 KO MEFs, respectively. In contrast, the full agonist α-Me-5-HT was the least responsive to RSK2 deletion, resulting in a meager 1.4-fold increase in IP accumulation in RSK2 KO MEFs. As a result, rank position significantly increased for 5-methoxy-DMT and DOI but not α-Me-5-HT in RSK2 KO MEFs. Taken together, the unique responsiveness of 5-methoxy-DMT and DOI in the absence of RSK2 most likely explains the conditional efficacy observed for IP accumulation in RSK2 KO MEFs.
Discussion
The major finding of this article is that patterns of 5-HT2A agonist functional selectivity are modulated by genetic deletion of a single kinase. Via high-throughput and high-content technologies, we identified global increases in agonist efficacies but not potencies for 5-HT2A-mediated IP accumulation, Ca2+ release, and ERK1/2 phosphorylation in the absence of RSK2. These findings imply that 5-HT2A receptors are more responsive in the absence of RSK2 (i.e., less desensitized) and confirm our previous reports showing that RSK2 attenuates 5-HT2A receptor signaling (Sheffler et al., 2006; Strachan et al., 2009). It is noteworthy that this study shows that patterns of functional selectivity vary depending upon the cellular milieu.
In agreement with many studies demonstrating that ligands elicit a spectrum of receptor behaviors (Urban et al., 2007; Mailman, 2007), including studies at 5-HT2A receptors (Berg et al., 1998a; Kurrasch-Orbaugh et al., 2003; Moya et al., 2007), we uncovered novel examples of functional selectivity between 5-HT2A-mediated IP accumulation and ERK1/2 phosphorylation in WT MEFs, and between WT and RSK2 KO MEFs at 5-HT2A-mediated IP accumulation.
First, we documented functional selectivity in WT MEFs. We identified significant reversals in relative agonist efficacies between effector readouts in WT MEFs. These changes in relative efficacy could be explained either by increased system responsiveness (i.e., cell-based functional selectivity) or by changes in the agonist-receptor complex (i.e., receptor-based functional selectivity). Kenakin (2007) has proposed that relative measures of efficacy are system-independent and are solely functions of agonist efficacy. It follows, then, that a reversal in the relative efficacies of two agonists, a hallmark of receptor-based functional selectivity, requires a change in the agonist-receptor complex (i.e., multiple receptor active states). Consistent with reports of receptor-based functional selectivity in the literature, we found that the relative efficacies of m-CPP and MK212 were significantly reversed between measures of IP accumulation and ERK1/2 phosphorylation.
It is apparent that the functional selectivity observed for m-CPP and MK212 in WT MEFs could be explained by a single activated receptor state and pathway-specific differences in stimulus-response coupling (e.g., receptor reserve for ERK1/2 phosphorylation). It follows, then, that if stimulus-response coupling was primarily enhanced for one pathway (e.g., ERK1/2) over another (e.g., IP accumulation), we would expect to observe increased efficacy for all partial agonists at the more efficiently coupled pathway. This assumption is central to the system-independence of the “intrinsic efficacy” concept, because the strength of signal imparted to the receptor between two agonists is reflected by the effector response. In functional terms, enhanced stimulus-response coupling manifests as increases in the efficacies of all agonists (i.e., until the response system is saturated), wherein the rank order of efficacy is retained, not reversed (Kenakin, 2009). As presented here, the relative efficacy of the partial agonist MK212 remained unchanged between measures of IP accumulation and ERK1/2 phosphorylation, whereas in the same cells, the relative efficacy of the partial agonist m-CPP was 4-fold higher for ERK1/2 phosphorylation than for IP accumulation. These data challenge the system-independent notion of intrinsic efficacy. Moreover, these data agree with previous reports of functional selectivity at 5-HT2A receptors, in which partial agonist efficacies (e.g., of quipazine and TFMPP) were not uniform comparing different pathways (Berg et al., 1998b; Kurrasch-Orbaugh et al., 2003).
The second and most intriguing example of functional selectivity at 5-HT2A receptors resulted from a comparison between effector readouts in WT and RSK2 KO MEFs. Our findings show that genetic deletion of RSK2 elicits a reversal in the relative rank order of efficacy for IP accumulation. We observed that the relative efficacies for IP accumulation were potentiated to different extents in RSK2 KO MEFs, as illustrated by different EmaxRSK2KO/EmaxWT values. This suggests that agonist responses are differentially regulated by RSK2. Modest differences between WT and RSK2 KO cell lines cannot account for reversals in relative efficacies because α-Me-5-HT, which is a full agonist in WT MEFs, exhibited weak partial agonist activity in RSK2 KO MEFs.
From a conceptual perspective, differences in EmaxRSK2KO/EmaxWT values and reversals in agonist relative efficacy between RSK2 KO and WT MEFs are not entirely surprising, because auxiliary GPCR-interacting proteins, of which there are many (Bockaert et al., 2004; Allen et al., 2008), have the potential to alter ligand activity at target receptors (Christopoulos et al., 2003). This new set of pharmacological behaviors is believed to arise from interactions between ligand-enriched GPCR conformations and auxiliary proteins and has been tentatively termed “conditional efficacy.” Indeed, we have shown previously that RSK2 interacts with the 5-HT2A receptor third intracellular loop and induces receptor phosphorylation, thereby attenuating receptor signaling (Sheffler et al., 2006; Strachan et al., 2009). Thus, it is conceivable that agonists are disproportionately affected by RSK2-mediated receptor phosphorylation. To support this concept, recent studies have shown evidence of 1) agonist-specific GPCR phosphorylation (Zhang et al., 1998; Roush et al., 1999; Li et al., 2003; Trester-Zedlitz et al., 2005), 2) agonist-specific third intracellular loop conformational changes (Swaminath et al., 2004, 2005), 3) phosphorylation-dependent functional responses (Tobin, 2008), and 4) phosphorylation-mediated stabilization of individual receptor conformations (Francesconi and Duvoisin, 2000; Thomas et al., 2000; Palanche et al., 2001). Although plausible, it remains to be determined how receptor phosphorylation differentially affects agonist signaling. Nevertheless, this is an intriguing hypothesis and warrants further study.
Zidar et al. (2009) demonstrated recently that endogenous CCR7 chemokine receptor ligands differentially activate GRK isoforms, thus leading to differences in receptor phosphorylation and functionally distinct pools of β-arrestin. Differential kinase activation is of fundamental interest to the field of functional selectivity and could perhaps explain the disproportionate affects on agonist signaling in RSK2 KO MEFs. However, considerable evidence argues against this mechanism. First, 5-HT2A receptors are known to be regulated by a GRK-independent mechanism in some cell types (Gray and Roth, 2001; Gray et al., 2001). Second, despite numerous attempts by our laboratory and others, agonist-mediated phosphorylation of 5-HT2A receptors has never been detected (Sheffler et al., 2006; Strachan et al., 2009; B.L. Roth, unpublished observations). Instead, 5-HT2A receptors seem to be constitutively phosphorylated (Vouret-Craviari et al., 1995) and desensitized (i.e., the “tonic brake”) (Sheffler et al., 2006), presumably through growth factor-mediated activation of RSK2 (Strachan et al., 2009). Therefore, the most likely hypothesis remains that RSK2-mediated receptor phosphorylation differentially affects agonist signaling.
To our knowledge, this is the first study to demonstrate that deletion of a single kinase leads to differential patterns of functional selectivity at a GPCR. Because it is well known that different cell types express distinct sets of GPCR-interacting proteins and kinases, this study exposes the potential for minor changes in the kinome to elicit large alterations in effector readouts, with obvious implications for drug actions in vitro and in vivo.
Acknowledgments
We thank Terry P. Kenakin for discussions concerning functional selectivity, Niels H. Jensen for assistance with the CellProfiler image analysis software, and Douglas J. Sheffler and Vincent Setola for help with receptor density measurements.
This work was supported by the National Institutes of Health National Institute of Mental Health Psychoactive Drug Screening Program [Grants R01-MH61887, U19-MH82441]; the Michael Hooker Chair for Therapeutics and Translational Proteomics; and the Michael Hooker Program in Translational Proteomics and Therapeutics.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061440
- GPCR
- G protein-coupled receptor
- RSK2
- p90 ribosomal S6 kinase 2
- 5-HT
- serotonin, 5-hydroxytryptamine
- WT
- wild type
- KO
- knockout
- SB242084
- 6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride hydrate
- IP
- inositol phosphate
- AA
- arachidonic acid
- ERK
- extracellular signal-regulated kinase
- DOI
- (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride
- quipazine
- 2-(1-piperazinyl)-quinoline maleate
- 5-methoxy-DMT
- 5-methoxy-N,N-dimethyltryptamine
- m-CPP
- 1-(m-chlorophenyl)-piperazine
- SCH-23390
- R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- α-Me-5-HT
- α-methyl serotonin
- MK212
- 6-chloro-2-(1-piperazinyl)pyrazine hydrochloride
- MDL-100907
- R-(+)-)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- FLIPR
- Fluorometric Imaging Plate Reader
- RFU
- relative fluorescence unit
- PBS
- phosphate-buffered saline
- ANOVA
- analysis of variance
- MEF
- mouse embryonic fibroblast
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate.
References
- Abbas and Roth, 2008.Abbas A, Roth BL. (2008) Arresting serotonin. Proc Natl Acad Sci U S A 105:831–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen et al., 2008.Allen JA, Yadav PN, Roth BL. (2008) Insights into the regulation of 5-HT2A serotonin receptors by scaffolding proteins and kinases. Neuropharmacology 55:961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg et al., 1998a.Berg KA, Maayani S, Goldfarb J, Clarke WP. (1998a) Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann N Y Acad Sci 861:104–110 [DOI] [PubMed] [Google Scholar]
- Berg et al., 1998b.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. (1998b) Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54:94–104 [PubMed] [Google Scholar]
- Berger et al., 2009.Berger M, Gray JA, Roth BL. (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry et al., 1996.Berry SA, Shah MC, Khan N, Roth BL. (1996) Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol Pharmacol 50:306–313 [PubMed] [Google Scholar]
- Bhatnagar et al., 2001.Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. (2001) The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem 276:8269–877 [DOI] [PubMed] [Google Scholar]
- Bockaert et al., 2004.Bockaert J, Fagni L, Dumuis A, Marin P. (2004) GPCR interacting proteins (GIP). Pharmacol Ther 103:203–221 [DOI] [PubMed] [Google Scholar]
- Bourdon et al., 2006.Bourdon DM, Wing MR, Edwards EB, Sondek J, Harden TK. (2006) Quantification of isozyme-specific activation of phospholipase C-beta2 by Rac GTPases and phospholipase C-epsilon by Rho GTPases in an intact cell assay system. Methods Enzymol 406:489–499 [DOI] [PubMed] [Google Scholar]
- Christopoulos et al., 2003.Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM. (2003) Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem 278:3293–3297 [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère et al., 2004.De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U. (2004) Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24:3235–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi and Duvoisin, 2000.Francesconi A, Duvoisin RM. (2000) Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc Natl Acad Sci U S A 97:6185–6190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frödin and Gammeltoft, 1999.Frödin M, Gammeltoft S. (1999) Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol 151:65–77 [DOI] [PubMed] [Google Scholar]
- Galandrin and Bouvier, 2006.Galandrin S, Bouvier M. (2006) Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol 70:1575–1584 [DOI] [PubMed] [Google Scholar]
- Ghosh et al., 1996.Ghosh D, Snyder SE, Watts VJ, Mailman RB, Nichols DE. (1996) 9-Dihydroxy-2,3,7,11b-tetrahydro-1H-naph[1,2,3-de]isoquinoline: a potent full dopamine D1 agonist containing a rigid-beta-phenyldopamine pharmacophore. J Med Chem 39:549–555 [DOI] [PubMed] [Google Scholar]
- Gray and Roth, 2001.Gray JA, Roth BL. (2001) Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull 56:441–451 [DOI] [PubMed] [Google Scholar]
- Gray et al., 2001.Gray JA, Sheffler DJ, Bhatnagar A, Woods JA, Hufeisen SJ, Benovic JL, Roth BL. (2001) Cell-type specific effects of endocytosis inhibitors on 5-hydroxytryptamine(2A) receptor desensitization and resensitization reveal an arrestin-, GRK2-, and GRK5-independent mode of regulation in human embryonic kidney 293 cells. Mol Pharmacol 60:1020–1030 [DOI] [PubMed] [Google Scholar]
- Kenakin, 1995.Kenakin T. (1995) Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci 16:232–238 [DOI] [PubMed] [Google Scholar]
- Kenakin, 2002.Kenakin T. (2002) Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov 1:103–110 [DOI] [PubMed] [Google Scholar]
- Kenakin, 2007.Kenakin T. (2007) Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol 72:1393–401 [DOI] [PubMed] [Google Scholar]
- Kenakin, 2009.Kenakin T. (2009) A Pharmacology Primer: Theory, Application, and Methods Elsevier, New York: [Google Scholar]
- Kroeze and Roth, 1998.Kroeze WK, Roth BL. (1998) The molecular biology of serotonin receptors: therapeutic implications for the interface of mood and psychosis. Biol Psychiatry 44:1128–1142 [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh et al., 2003.Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. (2003) Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther 304:229–237 [DOI] [PubMed] [Google Scholar]
- Lefkowitz and Shenoy, 2005.Lefkowitz RJ, Shenoy SK. (2005) Transduction of receptor signals by beta-arrestins. Science 308:512–517 [DOI] [PubMed] [Google Scholar]
- Li et al., 2003.Li JG, Zhang F, Jin XL, Liu-Chen LY. (2003) Differential regulation of the human kappa opioid receptor by agonists: etorphine and levorphanol reduced dynorphin A- and U50,488H-induced internalization and phosphorylation. J Pharmacol Exp Ther 305:531–540 [DOI] [PubMed] [Google Scholar]
- Mailman, 2007.Mailman RB. (2007) GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya et al., 2007.Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. (2007) Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther 321:1054–1061 [DOI] [PubMed] [Google Scholar]
- Palanche et al., 2001.Palanche T, Ilien B, Zoffmann S, Reck MP, Bucher B, Edelstein SJ, Galzi JL. (2001) The neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J Biol Chem 276:34853–34861 [DOI] [PubMed] [Google Scholar]
- Roth and Chuang, 1987.Roth BL, Chuang DM. (1987) Multiple mechanisms of serotonergic signal transduction. Life Sci 41:1051–1064 [DOI] [PubMed] [Google Scholar]
- Roush et al., 1999.Roush ED, Warabi K, Kwatra MM. (1999) Characterization of differences between rapid agonist-dependent phosphorylation and phorbol ester-mediated phosphorylation of human substance P receptor in intact cells. Mol Pharmacol 55:855–862 [PubMed] [Google Scholar]
- Schmid et al., 2008.Schmid CL, Raehal KM, Bohn LM. (2008) Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A 105:1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler et al., 2006.Sheffler DJ, Kroeze WK, Garcia BG, Deutch AY, Hufeisen SJ, Leahy P, Brüning JC, Roth BL. (2006) p90 ribosomal S6 kinase 2 exerts a tonic brake on G protein-coupled receptor signaling. Proc Natl Acad Sci U S A 103:4717–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson, 1956.Stephenson RP. (1956) A modification of receptor theory. Br J Pharmacol Chemother 11:379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan et al., 2009.Strachan RT, Sheffler DJ, Willard B, Kinter M, Kiselar JG, Roth BL. (2009) Ribosomal S6 kinase 2 directly phosphorylates the 5-hydroxytryptamine 2A (5-HT2A) serotonin receptor, thereby modulating 5-HT2A signaling. J Biol Chem 284:5557–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminath et al., 2005.Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. (2005) Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem 280:22165–22171 [DOI] [PubMed] [Google Scholar]
- Swaminath et al., 2004.Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. (2004) Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem 279:686–691 [DOI] [PubMed] [Google Scholar]
- Thomas et al., 2000.Thomas WG, Qian H, Chang CS, Karnik S. (2000) Agonist-induced phosphorylation of the angiotensin II (AT(1A)) receptor requires generation of a conformation that is distinct from the inositol phosphate-signaling state. J Biol Chem 275:2893–900 [DOI] [PubMed] [Google Scholar]
- Tobin, 2008.Tobin AB.(2008) G-protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol 153 (Suppl 1):S167–S176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trester-Zedlitz et al., 2005.Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. (2005) Mass spectrometric analysis of agonist effects on posttranslational modifications of the beta-2 adrenoceptor in mammalian cells. Biochemistry 44:6133–6143 [DOI] [PubMed] [Google Scholar]
- Urban et al., 2007.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari et al., 1995.Vouret-Craviari V, Auberger P, Pouysségur J, Van Obberghen-Schilling E. (1995) Distinct mechanisms regulate 5-HT2 and thrombin receptor desensitization. J Biol Chem 270:4813–4821 [DOI] [PubMed] [Google Scholar]
- Willins et al., 1999.Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, Roth BL. (1999) Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience 91:599–606 [DOI] [PubMed] [Google Scholar]
- Wilson et al., 2009.Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ., 2nd (2009) Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther 122:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 1998.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A 95:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 1999.Zhang JH, Chung TD, Oldenburg KR. (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73 [DOI] [PubMed] [Google Scholar]
- Zidar et al., 2009.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. (2009) Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci U S A 106:9649–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]