Abstract
bTREK-1 K+ channels set the resting membrane potential of bovine adrenal zona fasciculata (AZF) cells and function pivotally in the physiology of cortisol secretion. Adrenocorticotropic hormone controls the function and expression of bTREK-1 channels through signaling mechanisms that may involve cAMP and downstream effectors including protein kinase A (PKA) and exchange protein 2 directly activated by cAMP (Epac2). Using patch-clamp and Northern blot analysis, we explored the regulation of bTREK-1 mRNA and K+ current expression by cAMP analogs and several of their putative metabolites in bovine AZF cells. At concentrations sufficient to activate both PKA and Epac2, 8-bromoadenosine-cAMP enhanced the expression of both bTREK-1 mRNA and K+ current. N6-Benzoyladenosine-cAMP, which activates PKA but not Epac, also enhanced the expression of bTREK-1 mRNA and K+ current measured at times from 24 to 96 h. An Epac-selective cAMP analog, 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8CPT-2′-OMe-cAMP), potently stimulated bTREK-1 mRNA and K+ current expression, whereas the nonhydrolyzable Epac activator 8-(4-chlorophenylthio)-2′-O-methyl-cAMP, Sp-isomer was ineffective. Metabolites of 8CPT-2′-OMe-cAMP, including 8-(4-chlorophenylthio)-2′-O-methyladenosine-5′-O-monophosphate and 8CPT-2′-OMe-adenosine, promoted the expression of bTREK-1 transcripts and ion current with a temporal pattern, potency, and effectiveness resembling that of the parent compound. Likewise, at low concentrations, 8-(4-chlorophenylthio)-cAMP (8CPT-cAMP; 30 μM) but not its nonhydrolyzable analog 8-(4-chlorophenylthio)-cAMP, Sp-isomer, enhanced the expression of bTREK-1 mRNA and current. 8CPT-cAMP metabolites, including 8CPT-adenosine and 8CPT-adenine, also increased bTREK-1 expression. These results indicate that cAMP increases the expression of bTREK-1 mRNA and K+ current through a cAMP-dependent but Epac2-independent mechanism. They further demonstrate that one or more metabolites of 8-(4-chlorophenylthio)-cAMP analogs potently stimulate bTREK-1 expression by activation of a novel cAMP-independent mechanism. These findings raise significant questions regarding the specificity of 8-(4-chlorophenylthio)-cAMP analogs as cAMP mimetics.
Bovine adrenal zona fasciculata (AZF) cells express bTREK-1 (or KCNK2) K+ leak-type channels that set the resting membrane potential and function pivotally in the physiology of cortisol secretion (Enyeart et al., 1993, 2002; Mlinar et al., 1993). Cortisol synthesis is stimulated by the pituitary peptide adrenocorticotropin (Simpson and Waterman, 1988). Adrenocorticotropin exerts rapid and long-term control over the electrical and secretory properties of AZF cells by regulating both the activity of pre-existing ion channels and the expression of genes coding for these same channel proteins (Mlinar et al., 1993; Enyeart et al., 1996, 2000, 2003; Liu et al., 2008). In particular, in whole-cell recordings, adrenocorticotropin rapidly (within seconds to minutes) inhibits the activity of bTREK-1 K+ channels by a cAMP-dependent mechanism (Mlinar et al., 1993; Enyeart et al., 1996, 2000, 2003). Adrenocorticotropin also induces, with a delay of several hours, an increase in bTREK-1 mRNA and maintains the expression of the associated K+ current (Enyeart et al., 2003).
The signaling pathways by which adrenocorticotropin regulates the expression of bTREK-1 K+ channel mRNA and current are only partially understood. Early studies of cortisol synthesis established cAMP as the principal intracellular messenger for adrenocorticotropin in AZF cells (Haynes and Berthet, 1957; Grahame-Smith et al., 1967; Richardson and Schulster, 1973; Sala et al., 1979). Accordingly, bovine AZF cells express a high-affinity MC2R melanocortin receptor coupled to adenylate cyclase through Gs (Penhoat et al., 1989; Raikhinstein et al., 1994). Until recently, all of the cAMP-dependent actions of adrenocorticotropin were believed to be mediated by PKA. However, alternative signaling pathways for cAMP-mediated responses are present in these cells. Specifically, two cAMP-activated guanine nucleotide exchange factors Epac1 and Epac2 (also known as cAMP-GEFI and cAMP-GEFII) have been identified and implicated in the regulation of cellular processes, including gene expression (Kawasaki et al., 1998; de Rooij et al., 1998; Holz et al., 2006). Although Epac1 is expressed in many tissues, Epac2 is robustly expressed in selected areas of the brain and the adrenal glands of rats and humans (de Rooij et al., 1998). We discovered recently that Epac2 is strongly expressed in bovine AZF cells, raising the possibility that cAMP could produce responses through this protein and PKA (Liu et al., 2008).
Differentiating between PKA- and Epac-dependent signaling pathways in cells has been hampered by the absence of specific agents that selectively activate each of these two proteins. Exploiting differences in the cAMP-binding domains of these proteins, rational drug design has been used to synthesize cAMP derivatives that, at appropriate concentrations, specifically activate Epacs or PKA (Enserink et al., 2002; Christensen et al., 2003; Holz et al., 2008). In a patch-clamp study, we found that in addition to the well documented PKA-dependent inhibition of bTREK-1 channel activity, the Epac-selective cAMP analog (ESCA) 8CPT-2′-OMe-cAMP potently inhibited these channels (Liu et al., 2008). Thus, cAMP seems to inhibit bTREK-1 channel function by the activation of both PKA and Epac2.
In a more recent study, we found that 8CPT-2′-OMe-cAMP stimulated a delayed increase in cortisol synthesis by inducing the expression of genes coding for steroidogenic proteins, including several steroid hydroxylases (Enyeart and Enyeart, 2009). However, the 8CPT-2′-OMe-cAMP-stimulated increases in cortisol synthesis were not mediated through the activation of Epac2. Rather, the effect was produced by one or more metabolites of this 8-(4-chlorophenylthio)-cAMP derivative.
With this knowledge in hand, the present study was done to characterize the signaling pathways by which adrenocorticotropin and cAMP regulate the expression of bTREK-1 mRNA and corresponding ion current. It was discovered that several cAMP analogs, including those that selectively activate PKA or Epac2, and those that activate both of these proteins, enhanced the expression of bTREK-1 mRNA and membrane current. As a consequence, treatment of AZF cells with any of these cAMP analogs suppresses the time-dependent disappearance of bTREK-1 that typically occurs in culture. However, the increases in bTREK-1 transcripts and current induced by low concentrations of 8CPT-2′-OMe-cAMP and 8CPT-cAMP were mediated indirectly through one or more metabolites of these compounds by activation of an unknown signaling pathway.
Materials and Methods
Materials.
Tissue culture media, antibiotics, fibronectin, and fetal bovine sera (FBS) were obtained from Invitrogen (Carlsbad, CA). Phosphate-buffered saline, BAPTA, MgATP, collagenase, DNase, H-89, and adrenocorticotropin (1–24) were obtained from Sigma-Aldrich (St. Louis. MO). 8-Bromoadenosine-cAMP (8-Br-cAMP), N6-benzoyladenosine-cAMP (6-Bnz-cAMP), 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8CPT-2′-OMe-cAMP), hydrolysis-resistant 8-(4-chlorophenylthio)-2′-O-methyl-cAMP, Sp-isomer (Sp-8CPT-2′-OMe-cAMP), 8-(4-chlorophenylthio)-2′-O-methyladenosine-5′-O-monophosphate (8CPT-2′-OMe-5′AMP), 8-(4-chlorophenylthio)-2′-O-methyladenosine (8CPT-2′-OMe-Ado), 8-(4-chlorophenylthio)-cAMP (8CPT-cAMP), hydrolysis-resistant 8-(4-chlorophenylthio)-cAMP, Sp-isomer (Sp-8CPT-cAMP), 8-(4-chlorophenylthio)-adenosine (8CPT-Ado), and 8-(4-chlorophenylthio)adenine (8CPT-Ade) were purchased from Biolog (distributed by Axxora, LLC, San Diego, CA). [α-32P]dCTP was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Ultrahyb was purchased from Ambion (Austin, TX). RNeasy columns for total RNA isolation were obtained from QIAGEN (Valencia, CA). bTREK-1 probe was labeled with [32P]dCTP by random priming (Prime-It II kit; Stratagene, La Jolla, CA). Full-length bTREK-1 cDNA (1414 base pairs) was obtained as described previously (Enyeart et al., 2002).
Isolation and Culture of AZF Cells.
Bovine adrenal glands were obtained from steers (aged 2–3 years) at a local slaughterhouse. Isolated AZF cells were obtained and prepared as described previously (Enyeart et al., 1997). After isolation, cells were either resuspended in DMEM/Ham's F12 (1:1) with 10% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and the antioxidants α-tocopherol (1 μM), 20 nM selenite, and 100 μM ascorbic acid (DMEM/Ham's F12) and plated for immediate use, or resuspended in FBS/5% dimethyl sulfoxide, divided into 1-ml aliquots, and stored in liquid nitrogen for future use. To ensure cell attachment, dishes were treated with fibronectin (10 μg/ml) at 37°C for 30 min and then rinsed with warm, sterile phosphate-buffered saline immediately before adding cells. For patch-clamp experiments, cells were plated in DMEM/Ham's F12 in 35-mm dishes containing 9-mm2 glass coverslips (Bellco, Vineland NJ). Coverslips were treated with fibronectin (10 μg/ml) as described above. Cells were maintained at 37°C in a humidified atmosphere of 95% air/5% CO2.
Measurement of bTREK-1 mRNA.
RNeasy columns treated with RNase-free DNase (both from Qiagen) to remove genomic contamination were used to extract total RNA from AZF cells. Total mRNA (10 μg/lane) was separated on denaturing 8% formaldehyde, 1.0% agarose gels, and transferred to nylon membranes (GeneScreen Plus; PerkinElmer Life and Analytical Sciences). RNA was fixed to the membrane by UV cross-linking (Stratalinker; Stratagene), prehybridized for 2 h at 42°C in ULTRAhyb (Ambion), and then hybridized with a [α-32P]dCTP-labeled bTREK-1 full-length cDNA as described previously (Enyeart et al., 2003). Northern autoradiograms were imaged using a Typhoon 9200 variable-mode PhosphorImager and quantitated using ImageQuant TL v2003.3 software (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). mRNA values are presented as mean ± S.E.M. of at least three independent determinations. For the figures, a representative Northern blot of at least three independent experiments is shown. Statistically significant differences were determined by unpaired t test analysis (GraphPad Software, Inc., San Diego, CA). P values <0.05 were considered statistically significant.
Patch-Clamp Experiments.
Patch-clamp recordings of K+ channel currents were made in the whole-cell configuration from bovine AZF cells. The standard external solution consisted of 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, and 5 mM glucose, with pH adjusted to 7.3 using NaOH. The standard pipette solution consisted of 120 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 11 mM BAPTA, 10 mM HEPES, 5 mM ATP, and 200 μM GTP, with pH titrated to 6.8 using KOH.
Recording Conditions and Electronics.
AZF cells were used for patch-clamp experiments 2 to 12 h after plating. In general, cells with diameters <15 μm and capacitances of 10 to 15 pF were selected. Coverslips were transferred from 35-mm culture dishes to the recording chamber (volume, 1.5 ml) that was continuously perfused by gravity at a rate of 3 to 5 ml/min. For whole-cell recordings, patch electrodes with resistances of 1.0 to 2.0 MΩ were fabricated from Corning 0010 glass (World Precision Instruments, Sarasota, FL). These electrodes routinely yielded access resistances of 1.5 to 4.0 MΩ, and voltage-clamp time constants of <100 μs. K+ currents were recorded at room temperature (22–25°C) according to the procedure of Hamill et al. (1981) using a List EPC-7 patch-clamp amplifier.
Pulse generation and data acquisition were done using a personal computer and pCLAMP software with a Digidata 1200 interface (Molecular Devices, Sunnyvale, CA). Currents were digitized at 2 to 10 KHz after filtering with an 8-pole Bessel filter (Frequency Devices, Haverhill, MA). Linear leak and capacity currents were subtracted from current records using summed scaled hyperpolarizing steps of 1/2 to 1/4 pulse amplitude. Data were analyzed using Clampfit 9.2 (Molecular Devices) and SigmaPlot (version 10.0; Systat Software, San Jose, CA) software. Drugs were applied by bath perfusion, controlled manually by a six-way rotary valve.
Results
Adrenocorticotropin and 8-Br-cAMP Promote the Expression of bTREK-1 K+ Current.
Bovine AZF cells express two types of K+ channels: a voltage-gated, rapidly inactivating Kv1.4 (or KCNA4) channel; and a two-pore domain, four transmembrane-spanning segment bTREK-1 (or KNCK2) background K+ channel (Mlinar and Enyeart, 1993; Mlinar et al., 1993; Enyeart et al., 2002). In whole-cell patch-clamp recordings, bTREK-1 amplitude spontaneously increases over a period of 10 to 20 min to a stable maximum. The absence of time- and voltage-dependent inactivation allows bTREK-1 K+ currents to be isolated in whole-cell recordings using either of two voltage-clamp protocols. When voltage steps of several hundred milliseconds' duration are applied from a holding potential of −80 mV, bTREK-1 current can be measured near the end of a voltage step when the Kv1.4 current has completely inactivated (Fig. 1, A–D, left traces). Alternatively, bTREK-1 current can be selectively activated by an identical voltage step, applied immediately after a 10-s prepulse to −20 mV has fully inactivated Kv1.4 channels (Fig. 1A-D, right traces).
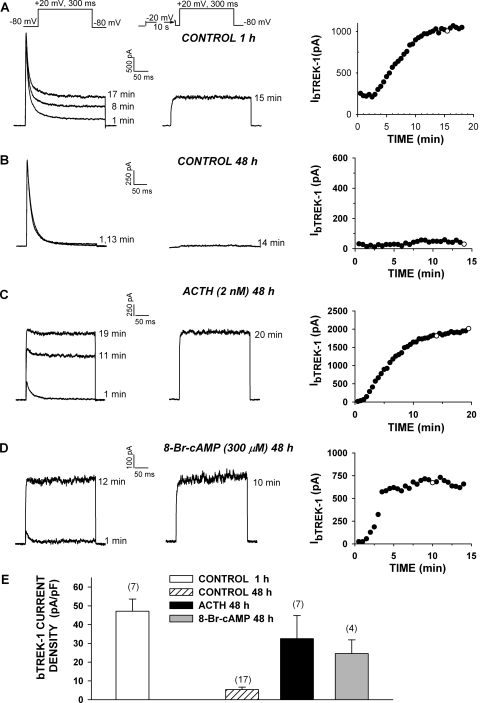
Fig. 1.
Long-term effect of adrenocorticotropin and 8-Br-cAMP on the expression of bTREK-1 current. AZF cells were used for patch-clamp experiments 1 to 48 h after plating. AZF cells were plated in media containing no further addition (control; A and B), 2 nM adrenocorticotropin (ACTH) (C), or 8-Br-cAMP (D). Whole-cell K+ currents were recorded in response to voltage steps to +20 mV applied from −80 mV at 30-s intervals with or without depolarizing prepulses to −20 mV. Pipettes contained standard solution (see Materials and Methods). A to D, representative K+ current traces recorded with (right traces) and without (left traces) depolarizing prepulses, and corresponding plot of bTREK-1 amplitudes with (○) and without (●) depolarizing pulses. Times indicated on traces correspond to those on the graph at right. E, summary of experiments as in A to D. Bars represent bTREK-1 current density expressed as mean ± S.E.M. of indicated number of determinations at 1 and 48 h in control media and after 48-h exposure to adrenocorticotropin (2 nM) or 8-Br-cAMP (300 μM), as indicated.
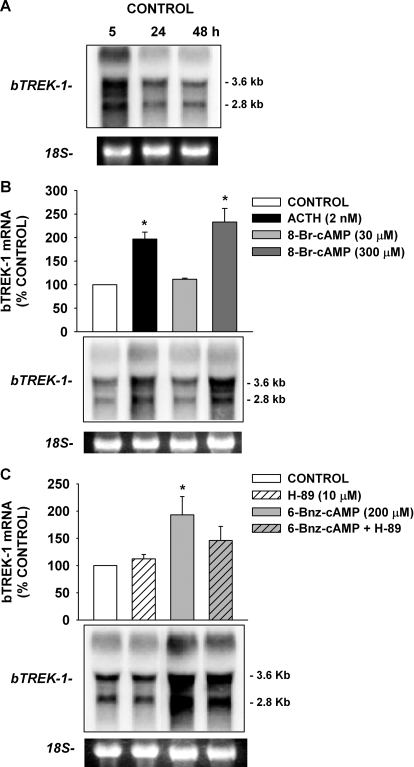
When bovine AZF cells were cultured in serum-supplemented media for periods of 24 h or more, the bTREK-1 K+ current was markedly diminished compared with currents recorded 1 to 3 h after plating (Fig. 1, A, B, and E). In contrast, when these cells were continuously exposed to adrenocorticotropin (2 nM) for 48 h after plating, bTREK-1 current was well maintained, compared with time-matched controls (Fig. 1, C and E). Overall, when AZF cells were cultured in serum-supplemented (control) media for 48 h, bTREK-1 current density decreased from its original density of 47.1 ± 6.5 (n = 7) to 5.37 ± 1.35 pA/pF (n = 17). In contrast, bTREK-1 current density in adrenocorticotropin-treated cells decreased to only 32.5 ± 12.3 pA/pF (n = 7) (Fig. 1D).
cAMP analogs substituted at the 8-position of the adenine ring activate both PKA and Epac2 (Christensen et al., 2003; Poppe et al., 2008). Although 8-substituted cAMP analogs bind to PKA and Epac proteins with Kd values in the low micromolar range, limited membrane permeability dictates that higher concentrations are required to produce effects in intact cells (Christensen et al., 2003; Liu et al., 2008). 8-Br-cAMP stimulates large increases in cortisol secretion by bovine AZF cells only when applied at concentrations greater than 100 μM (Supplementary Fig. 1A).
We tested the effect of 8-Br-cAMP (300 μM) on the expression of bTREK-1 in AZF cells. As illustrated in Fig. 1, D and E, 8-Br-cAMP mimicked adrenocorticotropin in promoting the expression of bTREK-1 current. After a 48-h exposure to 8-Br-cAMP, bTREK-1 current density was 4.6 times greater than the time-matched control. As with adrenocorticotropin, exposing cells to 8-Br-cAMP for prolonged periods suppressed the expression of Kv1.4 (Fig. 1, C and D). This response was not further explored in the present study.
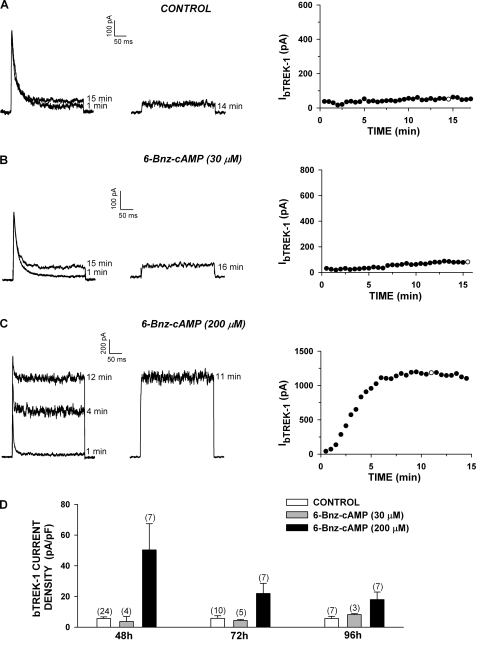
6-Bnz-cAMP Enhances bTREK-1 Expression.
The increases in bTREK-1 current density induced by adrenocorticotropin and 8-Br-cAMP could have occurred through activation of Epac2, PKA, or perhaps an unidentified signaling pathway. cAMP derivatives with substitutions at the 6-position of the adenine ring selectively activate PKA over Epac proteins (Christensen et al., 2003). As with 8-Br-cAMP, limited membrane permeability is likely to diminish the potency of 6-Bnz-cAMP when it is applied to intact cells (Liu et al., 2009). 6-Bnz-cAMP stimulates large increases in cortisol secretion from AZF cells only at concentrations greater than 100 μM (Supplemental Fig. 1B). Accordingly, we found that prolonged incubation of AZF cells with 6-Bnz-cAMP (200 μM), but not 30 μM, markedly enhanced the expression of bTREK-1 current measured at times from 48 to 96 h compared with time-matched controls (Fig. 2, C and D). After 48 h in the presence of 6-Bnz-cAMP (200 μM), bTREK-1 current density was 9-fold greater than that of untreated cells (Fig. 2D). These results suggest that cAMP can enhance the expression of bTREK-1 by activating PKA alone.
Fig. 2.
6-Bnz-cAMP induces the expression of bTREK-1 current. AZF cells were cultured in media containing no further addition (control) (A), 30 μM 6-Bnz-cAMP (B), or 200 μM 6-Bnz-cAMP (C). Whole-cell K+ currents were recorded from AZF cells in response to voltage steps to +20 mV applied from −80 mV at 30-s intervals with or without depolarizing prepulses to −20 mV. Pipettes contained standard solution (see Materials and Methods). A to C, representative K+ current traces recorded with (right traces) and without (left traces) depolarizing prepulses, and corresponding plot of bTREK-1 amplitudes with (○) and without (●) depolarizing pulses. Times indicated on traces correspond to those on the graph at right. Cells were either untreated (A) or were treated with 30 μM (B) or 200 μM (C) 6-Bnz-cAMP for 48 h before recording. D, summary of experiments as in A to C. Bars represent bTREK-1 current density expressed as mean ± S.E.M. of indicated number of determinations after 48 to 96 h of exposure to 6-Bnz-cAMP (30 or 300 μM), as indicated.
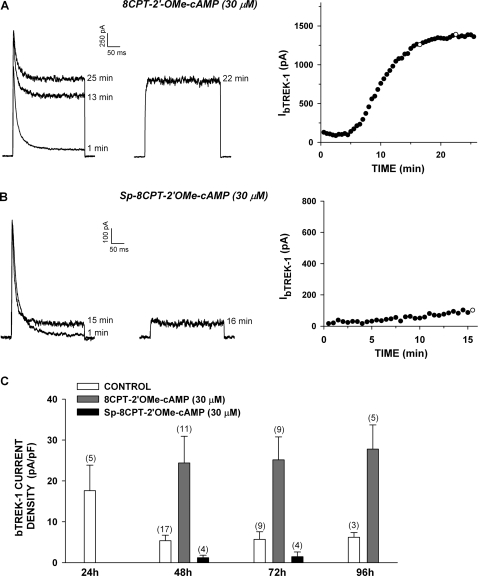
Epac-Selective cAMP Analogs and Metabolites Promote bTREK-1 Expression.
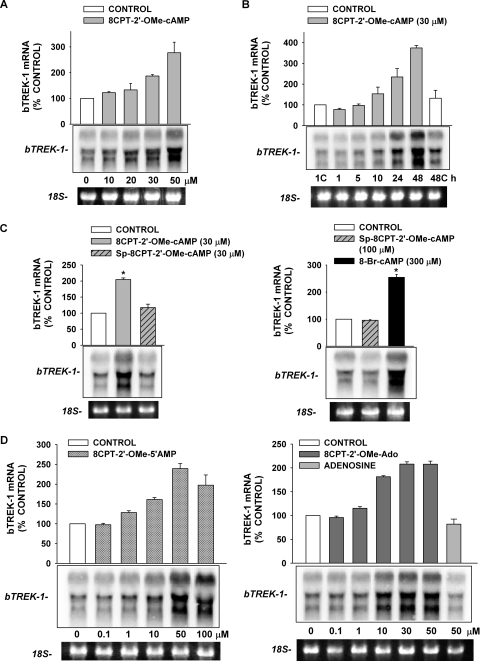
Experiments with 6-Bnz-cAMP indicated that activation of PKA was sufficient to stimulate bTREK-1 current expression. Additional studies were done to determine the role of Epac2 in the expression of this current. The ESCA 8CPT-2′-OMe-cAMP has been shown to activate Epac proteins when applied to intact cells at concentrations from 10 to 100 μM (Enserink et al., 2002; Holz et al., 2008). We found that 8CPT-2′-OMe-cAMP (30 μM) effectively stimulated bTREK-1 current expression measured at times from 48 to 96 h (Fig. 3, A and C). As illustrated in Fig. 3C, in control media, bTREK-1 current density decreased spontaneously with time, reaching an apparent minimum of 5.27 ± 1.35 pA/pF (n = 17) by 48 h, after which no further decay occurred. However, in the presence of 8CPT-2′-OMe-cAMP, bTREK-1 current density was significantly increased at each time point. By 48 h, bTREK-1 current density reached a nearly constant value that was approximately 4.5-fold greater than that of the time-matched controls at 48, 72, and 96 h (Fig. 3C).
Fig. 3.
Effect of Epac2-selective cAMP analogs on bTREK-1 current expression. Whole-cell K+ currents were recorded from AZF cells in response to voltage steps to +20 mV applied from −80 mV at 30-s intervals with or without depolarizing prepulses to −20 mV. Pipettes contained standard solution (see Materials and Methods). A and B, representative K+ current traces recorded with (right traces) and without (left traces) depolarizing prepulses, and corresponding plot of bTREK-1 amplitudes with (○) and without (●) depolarizing pulses. Time indicated on traces corresponds to those plotted on graph at right. AZF cells with treated with 30 μM 8CPT-2′-OMe-cAMP (A) or 30 μM Sp-8CPT-2′-OMe-cAMP (B) for 48 h before recording. C, summary of experiments as in A and B: bars specify bTREK-1 current density expressed as mean ± S.E.M. of an indicated number of determinations after 24 to 96 h of exposure to 8CPT-2′-OMe-cAMP (30 μM) or Sp-8CPT-2′-OMe-cAMP (30 μM) as indicated.
The marked stimulation of bTREK-1 expression by 8CPT-2′-OMe-cAMP suggested that cAMP could induce the expression of bTREK-1 channels by activation of Epac2. However, at the same concentration, Sp-8CPT-2′-OMe-cAMP, a membrane-permeable ESCA that is resistant to hydrolysis by cyclic nucleotide phosphodiesterase, failed to stimulate the expression of bTREK-1 current at 48 and 72 h. In fact, in the presence of this ESCA, bTREK-1 current was significantly reduced compared with controls (Fig. 3, B and C).
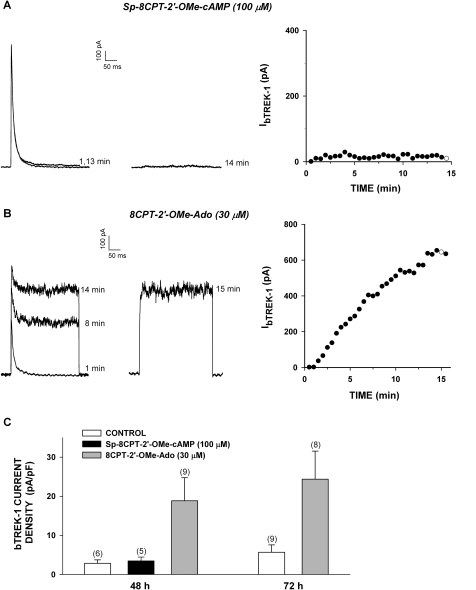
The effective stimulation of bTREK-1 current expression by 8CPT-2′-OMe-cAMP combined with the failure of its nonhydrolyzable analog to mimic this action indicated that, at low concentrations, 8CPT-2′-OMe-cAMP acts independently of Epac2. In this regard, it has become apparent that, in some cells, significantly higher concentrations are required for effective activation of Epac2 (Holz et al., 2008; Chepurny et al., 2009). We have shown previously that in AZF cells, Sp-8CPT-2′-OMe-cAMP (100 μM) activated Rap1, a downstream effector of Epac2 (Enyeart and Enyeart, 2009). However, we now report that treating AZF cells for 48 h with Sp-8CPT-2′-OMe-cAMP (100 μM) did not enhance the expression of bTREK-1 currents (Fig. 4, A and C).
Fig. 4.
Long-term effect of Sp-8CPT-2′-OMe-cAMP and 8CPT-2′-OMe-Ado on expression of bTREK-1 current. AZF cells were cultured for 48 h in media containing no further addition (control), Sp-8CPT-2′-OMe-cAMP (100 μM) (A), or 8CPT-2′-OMe-Ado (30 μM) (B). Whole-cell K+ currents were recorded from AZF cells in response to voltage steps to +20 mV applied from −80 mV at 30-s intervals with or without depolarizing prepulses to −20 mV. Pipettes contained standard solution (see Materials and Methods). A and B, representative K+ current traces recorded with (right traces) and without (left traces) depolarizing prepulses, and corresponding plot of bTREK-1 amplitudes with (○) and without (●) depolarizing pulses. Times indicated on traces correspond to those plotted on the graph at right. C, summary of experiments as in A and B: bars represent bTREK-1 current density expressed as mean ± S.E.M. of indicated number of determinations after 48 or 72 h of exposure to Sp-8CPT-2′-OMe-cAMP (100 μM) or 8CPT-2′-OMe-Ado (30 μM) as indicated.
The results of experiments with the ESCAs indicated that activation of Epac2 does not induce the expression of bTREK-1 current. They further suggested that 8CPT-2′-OMe-cAMP increased bTREK-1 K+ current expression indirectly by the generation of one or more active metabolites.
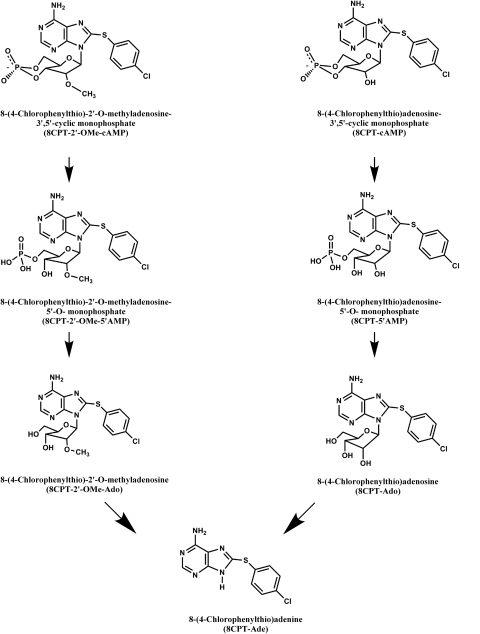
8CPT-2′-OMe-cAMP can be sequentially converted to 8CPT-2′-OMe-5′AMP and 8CPT-2′-OMe-Ado by cyclic nucleotide phosphodiesterase and 5′ nucleotidases, respectively (Price and Stevens, 1999; Holz et al., 2008) (Fig. 5). We discovered that 8CPT-2′-OMe-Ado potently and effectively stimulated the expression of bTREK-1 current. After exposing cells for 48 h to this metabolite at a concentration of 30 μM, bTREK-1 current density reached a value six times that of its time-matched control (Fig. 4C). This large increase in bTREK-1 current persisted after 72 h.
Fig. 5.
Chemical structures of 8CPT- 2′-OMe-cAMP, 8CPT-cAMP, and their metabolites.
Effect of cAMP Analogs and Metabolites on bTREK-1 mRNA.
We have shown previously that adrenocorticotropin-induced increases in bTREK-1 expression were associated with increases in bTREK-1 mRNA (Enyeart et al., 2003). Experiments were done to determine whether the increases in bTREK-1 current induced by cAMP analogs and their presumptive metabolites were mediated through the enhancement of bTREK-1 gene expression. In Northern blots using RNA isolated from AZF cells, a 1414-base pair cDNA probe that included the entire coding sequence of bTREK-1 hybridized to separate mRNA transcripts of ∼4.9, 3.6, and 2.8 kb (Fig. 6A) (Enyeart et al., 2002). When bovine AZF cells were cultured in serum-supplemented media for 5 to 48 h, the quantity of bTREK-1 mRNA decreased over time (Fig. 6A).
Fig. 6.
Effects of adrenocorticotropin, 8-Br-cAMP, and 6-Bnz-cAMP on bTREK-1 gene expression. AZF cells were incubated either without (control) or with adrenocorticotropin, 8-Br-cAMP, 6-Bnz-cAMP, or 6-Bnz-cAMP + H-89, as indicated. Total RNA was isolated as described under Materials and Methods. Membranes were hybridized with specific probe for bTREK-1. 18S rRNA bands from representative gels are shown as evidence of even loading. bTREK-1 mRNA levels are expressed as percent of the 3.6-kb control band value. A, effect of time in culture on bTREK-1 mRNA. AZF cells were plated and total RNA isolated after 5, 24, or 48 h in culture, as indicated. B, effect of adrenocorticotropin (ACTH) and 8-Br-cAMP on bTREK-1 mRNA expression. AZF cells were cultured overnight before either no addition (control, □) or addition of adrenocorticotropin (2 nM, ■) or 8-Br-cAMP (30 μM, light gray bar; 300 μM, dark gray bar) for 48 h before isolating total RNA. *, statistically significant difference between control and treated cells (*, P < 0.02). C, effect of 6-Bnz-cAMP and H-89 on bTREK-1 mRNA. AZF cells were plated and cultured overnight before either no addition (control, open bar) or addition of H-89 (10 μM, striped bar), 6-Bnz-cAMP (200 μM, gray bar), or 6-Bnz-cAMP + H-89 (gray striped bar) for 24 h before isolating total RNA. Cells were preincubated with H-89 (10 μM) for 1 h before the 6-Bnz-cAMP (200 μM) addition (*, P < 0.03).
8-Br-cAMP (300 μM), similar to adrenocorticotropin, markedly increased the expression of bTREK-1 mRNA in AZF cells. In the experiment illustrated in Fig. 6B, AZF cells were cultured overnight in serum-supplemented media before exposing them for 48 h to adrenocorticotropin or 8-Br-cAMP at the indicated concentrations. Adrenocorticotropin (2 nM) and 8-Br-cAMP (300 μM) increased mRNA expression by 2.0 ± 0.1- and 2.3 ± 0.3-fold, respectively. Although each of the three bTREK-1 transcripts were induced by 8-Br-cAMP and adrenocorticotropin, the smaller 3.6- and 2.8-kb transcripts were preferentially increased. At a 10-fold lower concentration, 30 μM 8-Br-cAMP failed to stimulate bTREK-1 mRNA expression (Fig. 6B).
In addition to 8-Br-cAMP, we found that 6-Bnz-cAMP (200 μM) also enhanced the expression of bTREK-1 mRNA (Fig. 6C). However, the PKA antagonist H-89 (10 μM) only partially inhibited 6-Bnz-cAMP-stimulated increases in bTREK-1 expression. In three similar independent experiments, H-89 reduced 6-Bnz-cAMP-stimulated bTREK-1 expression by 56 ± 2%. These results indicate that 6-Bnz-cAMP-stimulated increases in bTREK-1 expression are mediated only in part by PKA.
Patch-clamp experiments indicated that the ESCA 8CPT-2′-OMe-cAMP induced bTREK-1 current indirectly after conversion to one or more active metabolites (Figs. 3 and 4). Accordingly, we found that 8CPT-2′-OMe-cAMP potently and effectively induced the expression of bTREK-1 mRNA, but this effect was again mediated through hydrolysis products of this cAMP analog, rather than by activation of Epac2.
8CPT-2′-OMe-cAMP stimulated the expression of bTREK-1 mRNA at the same concentrations that enhanced the expression of bTREK-1 current. In the experiment illustrated in Fig. 7A, bTREK-1 mRNA was measured after exposing AZF cells to 8CPT-2′-OMe-cAMP at concentrations ranging from 10 to 50 μM for 48 h. A significant increase was observed at 20 μM, whereas 50 μM 8CPT-2′-OMe-cAMP induced a maximum 2.8 ± 0.4-fold increase in this mRNA. The 8CPT-2′-OMe-cAMP-induced increases in bTREK-1 transcripts could be observed after a delay of 5 to 10 h, and bTREK-1 mRNA continued to increase for at least 48 h (Fig. 7B). The temporal pattern of these increases in mRNA was therefore well correlated with the corresponding enhancement in bTREK-1 current.
Fig. 7.
Effects of 8CPT-2′-OMe-cAMP and its metabolites on bTREK-1 gene
expression. AZF cells were cultured overnight and then incubated either
without (control) or with 8CPT-2′-OMe-cAMP,
Sp-8CPT-2′-OMe-cAMP, 8CPT-2′-OMe-5′AMP,
or 8CPT-2′-OMe-Ado as indicated. Total RNA was isolated as
described under Materials and Methods. Membranes were
hybridized with a specific probe for bTREK-1; bTREK-1 mRNA levels are
expressed as a percentage of the 3.6-kb control band value. 18S rRNA bands
from representative gels are shown as evidence of even loading. A,
concentration-dependent effect of 8CPT-2′-OMe-cAMP on bTREK-1
mRNA expression. AZF cells were untreated (control, □) or
treated with 8CPT-2′-OMe-cAMP (1–50 μM,
 ) for 48 h before isolating
total RNA. B, time-dependent effect of 8CPT-2′-OMe-cAMP on
bTREK-1 mRNA expression. AZF cells were either untreated (control,
□) or treated with 30 μM
8CPT-2′-OMe-cAMP (
) for 48 h before isolating
total RNA. B, time-dependent effect of 8CPT-2′-OMe-cAMP on
bTREK-1 mRNA expression. AZF cells were either untreated (control,
□) or treated with 30 μM
8CPT-2′-OMe-cAMP ( )
for 1 to 48 h before isolating total RNA. C, effect of
8CPT-2′-OMe-cAMP, Sp-8CPT-2′-OMe-cAMP, and 8-Br-cAMP
on bTREK-1 mRNA expression. AZF cells were either untreated (control, open
bar) or treated with 30 μM 8CPT-2′-OMe-cAMP (gray
bar, left), 30 μM Sp-8CPT-2′-OMe-cAMP (gray striped
bar, left), 100 μM Sp-8CPT-2′-OMe-cAMP (gray striped
bar, right), or 300 μM 8-Br-cAMP (black bar, right) for 48 h
before isolating total RNA (*, P < 0.005). D,
effect of metabolites of 8CPT-2′-OMe-cAMP on induction of
bTREK-1 mRNA. AZF cells were treated with either
8CPT-2′-OMe-5′AMP (0.1–100
μM, gray dotted bars), 8CPT-2′-OMe-Ado
(0.1–50 μM, dark gray bars). or adenosine (50
μM, light gray bar) for 48 h before isolating total RNA.
)
for 1 to 48 h before isolating total RNA. C, effect of
8CPT-2′-OMe-cAMP, Sp-8CPT-2′-OMe-cAMP, and 8-Br-cAMP
on bTREK-1 mRNA expression. AZF cells were either untreated (control, open
bar) or treated with 30 μM 8CPT-2′-OMe-cAMP (gray
bar, left), 30 μM Sp-8CPT-2′-OMe-cAMP (gray striped
bar, left), 100 μM Sp-8CPT-2′-OMe-cAMP (gray striped
bar, right), or 300 μM 8-Br-cAMP (black bar, right) for 48 h
before isolating total RNA (*, P < 0.005). D,
effect of metabolites of 8CPT-2′-OMe-cAMP on induction of
bTREK-1 mRNA. AZF cells were treated with either
8CPT-2′-OMe-5′AMP (0.1–100
μM, gray dotted bars), 8CPT-2′-OMe-Ado
(0.1–50 μM, dark gray bars). or adenosine (50
μM, light gray bar) for 48 h before isolating total RNA.
In contrast to 8CPT-2′-OMe-cAMP, Sp-8CPT-2′-OMe-cAMP failed to promote the expression of bTREK-1 mRNA when applied to cells at 30 μM or at higher concentrations at which this nonhydrolyzable ESCA produces significant activation of Rap1 (Fig. 7C, left and right) (Enyeart and Enyeart, 2009). Accordingly, we found that the two putative metabolites 8CPT-2′-OMe-5′AMP and 8CPT-2′-OMe-Ado increased the expression of bTREK-1 mRNA with potency similar to that of the parent compound (50 μM 8CPT-2′-OMe-5′ AMP, 2.4 ± 0.1-fold; 50 μM 8CPT-2′-OMe-Ado, 2.1 ± 0.6-fold; Fig. 7D). In contrast, adenosine (50 μM) failed to stimulate any increase in bTREK-1 expression (0.9 ± 0.1-fold, Fig. 7D, right).
Overall, these results indicate that cAMP induces bTREK-1 mRNA and K+ current through activation of PKA but not Epac2. Remarkably, increases in bTREK-1 mRNA and K+ current induced by the ESCA 8CPT-2′-OMe-cAMP seem to be produced indirectly by one or more metabolites of this compound.
Effect of 8CPT-cAMP and Metabolites on bTREK-1 Expression.
In a previous study, we showed that 8CPT-cAMP (250 μM) increased the expression of bTREK-1 mRNA (Enyeart et al., 2003). Although it probably activates both PKA and Epac2 at this concentration, this 8-(4-chlorophenylthio)-derivative of cAMP can be metabolized by the same enzymes that hydrolyze 8CPT-2′-OMe-cAMP (Price and Stevens, 1999; Holz et al., 2008). This raised the possibility that metabolites of 8CPT-cAMP contributed to the observed increases in bTREK-1 mRNA.
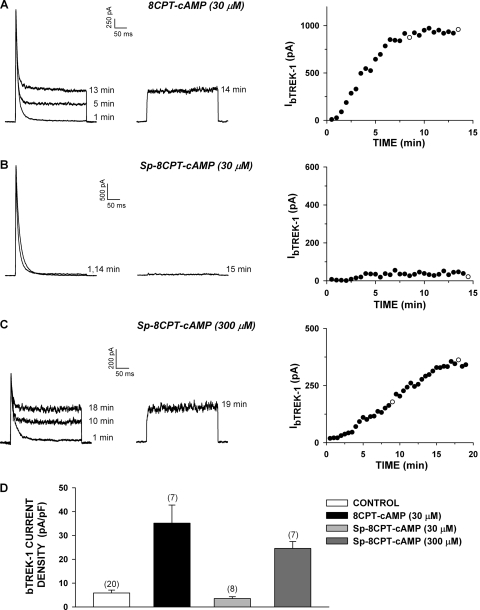
To answer this question, we compared 8CPT-cAMP and its membrane-permeable nonhydrolyzable analog Sp-8CPT-cAMP with respect to their potency as enhancers of bTREK-1 current and mRNA (Dostmann et al., 1990). Exposing cells for 48 h to 30 μM 8CPT-cAMP markedly increased bTREK-1 current density from 5.85 ± 1.22 pA/pF (n = 20) to 35.2 ± 7.6 (n = 7) (Fig. 8A). In contrast, treating cells with Sp-8CPT-cAMP (30 μM) failed to increase bTREK-1 expression (Fig. 8, B and D). These results indicated that, at this low concentration, 8CPT-cAMP increased bTREK-1 expression by a mechanism independent of cAMP. At a 10-fold higher concentration, the poorly hydrolyzable Sp-8CPT-cAMP (300 μM) increased bTREK-1 current density 4.2-fold over the time-matched control to 24.5 ± 2.9 pA/pF (n = 7) (Fig. 8, C and D).
Fig. 8.
Long-term effect of 8CPT-cAMP and Sp-8CPT-cAMP on the expression of bTREK-1 current. AZF cells were cultured overnight and then incubated either without (control) or with 8CPT-cAMP (30 μM) or Sp-8CPT-cAMP (30 or 300 μM), as indicated. Whole-cell K+ currents were recorded from AZF cells in response to voltage steps to +20 mV applied from −80 mV at 30-s intervals with or without depolarizing prepulses to −20 mV. Pipettes contained standard solution as described under Materials and Methods. A to C, representative K+ current traces recorded with (right traces) and without (left traces) depolarizing prepulses and corresponding plot of bTREK-1 amplitudes with (○) and without (●) depolarizing pulses. Time indicated on traces corresponds to those plotted on the graph at right. Cells were treated with 30 μM 8CPT-cAMP (A), 30 μM Sp-8CPT-2′-OMe-cAMP (B), or 300 μM Sp-8CPT-cAMP (C) for 48 h before recording. D, summary of experiments as in A to C. Bars represent bTREK-1 current density expressed as mean ± S.E.M. of indicated number of determinations after 48-h exposure to 8CPT-cAMP (30 μM) or Sp-8CPT-2′-OMe-cAMP (30 or 300 μM) as indicated.
These results suggest that, at low concentrations at which 8CPT-cAMP does not effectively activate PKA, it stimulates bTREK-1 current expression only after it is converted to one or more active metabolites. In contrast, at higher concentrations, the nonhydrolyzable Sp-8CPT-cAMP increases bTREK-1 current expression by activation of PKA.
8CPT-cAMP can be metabolized to 8CPT-5′AMP and 8CPT-Ado by cyclic nucleotide phosphodiesterase and 5′nucleotidase, respectively (Price and Stevens, 1999). 8CPT-Ado can then be converted to 8CPT-Ade by purine nucleoside phosphorylase (Price and Stevens, 1999). It is interesting that 8CPT-Ade may be synthesized from both 8CPT-2′-OMe-cAMP and 8CPT-cAMP in AZF cells (Fig. 5). Therefore, we examined the effects of 8CPT-Ado and 8CPT-Ade on bTREK-1 expression.
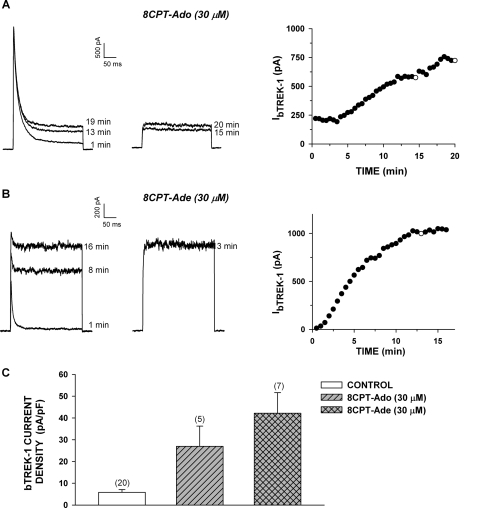
bTREK-1 current was markedly enhanced in response to a 48-h incubation with both 8CPT-Ado (30 μM) and 8CPT-Ade (30 μM) (Fig. 9, A–C). The 4.6- and 7.2-fold increases in current density induced by these metabolites compare with the 6.0-fold increase produced by 8CPT-cAMP at the same concentration. Thus, patch-clamp experiments indicated that, at low concentrations, 8CPT-cAMP enhanced the expression of bTREK-1 K+ current indirectly after conversion to one or more active metabolites.
Fig. 9.
Metabolites of 8CPT-cAMP induce bTREK-1 current. AZF cells were cultured overnight then incubated either without (control) or with 8CPT-Ado (30 μM) or 8CPT-Ade (30 μM) as indicated. Whole-cell K+ currents were recorded from AZF cells in response to voltage steps to +20 mV applied from −80 mV at 30-s intervals with or without depolarizing prepulses to −20 mV. Pipettes contained standard solution as described under Materials and Methods. A and B, representative K+ current traces recorded with (right traces) and without (left traces) depolarizing prepulses and corresponding plot of bTREK-1 amplitudes with (○) and without (●) depolarizing pulses. Time indicated on traces corresponds to those plotted on graph at right. Cells were treated with 30 μM 8CPT-Ado (A) or 30 μM 8CPT-Ade (B) for 48 h before recording. C, summary of experiments as in A and B. Bars represent bTREK-1 current density expressed as mean ± S.E.M. of indicated number of determinations after 48-h exposure to 8CPT-Ado or 8CPT-Ade as indicated.
Effect of 8CPT-cAMP, Sp-8CPT-cAMP, and 8CPT-Adenine on bTREK-1 mRNA Expression.
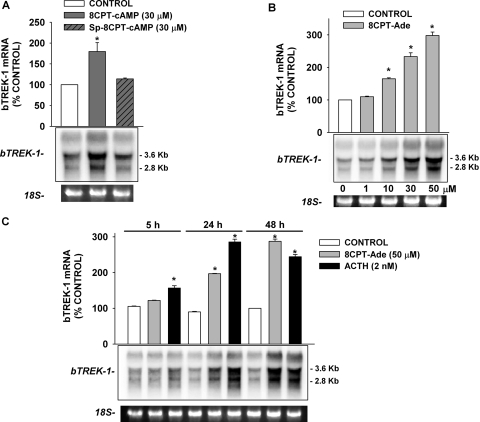
In Northern blot analysis, we found that the effects of 8CPT-cAMP and Sp-8CPT-cAMP on bTREK-1 mRNA expression were consistent with their effects on bTREK-1 K+ current. As in results seen in experiments with 8CPT-2′-OMe-cAMP and Sp-8CPT-2′-OMe-cAMP (Fig. 7C), exposing AZF cells to 30 μM 8CPT-cAMP for 48 h significantly increased bTREK-1 mRNA (1.8 ± 0.2-fold), whereas nonhydrolyzable Sp-8CPT-cAMP was ineffective in this regard (Fig. 10A).
Fig. 10.
Effects of 8CPT-cAMP, Sp-cAMP, and 8CPT-Ade on expression of bTREK-1 mRNA.
AZF cells were cultured overnight then incubated either without (control) or
with 8CPT-cAMP, Sp-cAMP, 8CPT-Ade, or adrenocorticotropin as indicated.
Total RNA was isolated as described under Materials and
Methods. Membranes were hybridized with a specific probe for
bTREK-1; bTREK-1 mRNA levels are expressed as a percentage of the 3.6-kb
control band value.18S rRNA bands from representative gels are shown as
evidence of even loading. A, 8CPT-cAMP but not Sp-8CPT-cAMP induces bTREK-1
mRNA. AZF cells were either untreated (control, open bar) or treated with 30
μM 8CPT-cAMP (dark gray bar) or 30 μM Sp-8CPT-cAMP
(dark gray striped bar) for 48 h before isolating total RNA (*,
P < 0.02). B, concentration-dependent effect of
8CPT-Ade on bTREK-1 mRNA. AZF cells were either untreated (control,
□) or treated with 8CPT-Ade (1–50 μM,
 ) for 48 h before isolating
total RNA (*, P < 0.001). C, time-dependent
effect of 8CPT-Ade and adrenocorticotropin (ACTH) on bTREK-1 mRNA
expression. AZF cells were either untreated (control, □) or
treated with 30 μM 8CPT-cAMP (
) for 48 h before isolating
total RNA (*, P < 0.001). C, time-dependent
effect of 8CPT-Ade and adrenocorticotropin (ACTH) on bTREK-1 mRNA
expression. AZF cells were either untreated (control, □) or
treated with 30 μM 8CPT-cAMP ( ) or 2 nM adrenocorticotropin (■)
for 5, 24, and 48 h before isolating total RNA (*, P
< 0.001).
) or 2 nM adrenocorticotropin (■)
for 5, 24, and 48 h before isolating total RNA (*, P
< 0.001).
8CPT-Ade stimulated a concentration-dependent increase in bTREK-1 mRNA transcripts with a potency, temporal pattern, and effectiveness similar to that of other cAMP metabolites used in this study (Fig. 10B). In the experiment illustrated in Fig. 10B, 8CPT-Ade increased bTREK-1 mRNA at concentrations from 10 to 50 μM. With respect to temporal pattern, adrenocorticotropin induced the expression of bTREK-1 mRNA more rapidly than 8CPT-Ade, but by 48 h, these two agents were similarly effective. In the experiment illustrated in Fig. 10C, at 48 h, 8CPT-Ade and adrenocorticotropin induced 2.9 ± 0.1- and 2.4 ± 0.1-fold increases in bTREK-1 transcript relative to the time-matched control.
Discussion
In this study, we presented evidence indicating that cAMP enhanced the expression of bTREK-1 mRNA and K+ current and prevented their disappearance by activating PKA but not Epac2. Furthermore, hydrolysis products of 8-(4-chlorophenylthio)-cAMP analogs enhanced bTREK-1 mRNA transcripts and corresponding membrane current. The hydrolyzable 8-(4-chlorophenylthio)-cAMP analogs and their putative metabolites were significantly more potent than other cAMP analogs at promoting bTREK-1 expression. These metabolites all induced bTREK-1 with a potency, temporal pattern, and effectiveness similar to that of the parent compound, but by an unknown cAMP-independent pathway.
Overall, we have identified 10 separate cAMP analogs and metabolites that enhanced the expression of bTREK-1 mRNA and associated K+ current (Supplemental Table 1). For each of these, the effects on TREK-1 mRNA and current were well correlated. These results are consistent with the hypothesis that each of these agents increases the rate of transcription of the bTREK-1 gene. However, our results do not exclude the possibility that post-transcriptional mechanisms are involved, including bTREK-1 mRNA stabilization and translational or post-translational control by these compounds. It is unlikely that the cAMP-dependent and -independent actions of these on bTREK-1 are produced by a single common mechanism.
cAMP, PKA, Epac2, and bTREK-1 Expression.
Experiments with 8-Br-cAMP and Sp-8CPT-cAMP clearly established that cAMP stimulates the expression of bTREK-1 mRNA and associated K+ current. These experiments were necessary because our previous study demonstrating that 8CPT-cAMP induced bTREK-1 mRNA was completed without the knowledge that hydrolyzable 8-(4-chlorophenylthio) derivatives of cAMP induce bTREK-1 mRNA indirectly through metabolites (Enyeart et al., 2003). As a consequence, it was important to determine whether cAMP could induce bTREK-1 expression, because adrenocorticotropin seems to produce effects in bovine AZF cells by cAMP-dependent and -independent mechanisms (Moyle et al., 1973; Yamazaki et al., 1998, 2006).
The robust stimulation of bTREK-1 mRNA and K+ current expression by 6-Bnz-cAMP suggests that the expression of this K+ channel is controlled at the pretranslational level by cAMP through the activation of PKA. However, several questions remain regarding the molecular mechanisms and signaling pathways by which 6-Bnz-cAMP functions.
First, with regard to molecular mechanism, adrenocorticotropin and cAMP induce increases in steroid hydroxylase mRNAs by accelerating the rate of gene transcription (John et al., 1986; Waterman, 1994). Our results are consistent with a similar effect of 6-Bnz-cAMP on bTREK-1 gene transcription. However, they do not rule out an effect of this cAMP analog on bTREK-1 mRNA stability.
With respect to signaling mechanism, cAMP analogs substituted in the 6-position of the adenine ring selectively activate PKA over Epac proteins (Christensen et al., 2003). Therefore, our observation that 6-Bnz-cAMP robustly induced the expression of bTREK-1 mRNA and K+ current argue that cAMP-stimulated increases in the expression of this K+ channel were mediated through PKA rather than Epac2. However, several lines of evidence suggest that 6-Bnz-cAMP could function through a third unidentified cAMP receptor. First, when applied at concentrations that suppress PKA activity, H-89 only partially suppressed increases in bTREK-1 mRNA expression (Davies et al., 2000). Furthermore, in patch-clamp experiments, we found that, when included in the patch electrode at very low concentrations (<10 μM), 6-Bnz-cAMP completely blocks this activity of bTREK-1 channels, even in the presence of several PKA antagonists (Liu et al., 2009).
The steroidogenic actions of cAMP in AZF cells also suggest the presence of additional cAMP binding proteins. PKA-stimulated transcription typically occurs within minutes and does not require de novo protein synthesis (Parker and Schimmer, 1995). In contrast, cAMP-induced increases in steroid hydroxylase-specific mRNAs are cycloheximide-sensitive and require periods of up to several hours before they can be observed (Simpson and Waterman, 1988; Waterman and Simpson, 1989; Waterman, 1994). Furthermore, although the steroid hydroxylase genes are all induced by cAMP, they lack consensus cAMP response elements in their 5′-flanking regions and therefore do not bind PKA phosphorylated transcription factors (Simpson and Waterman, 1988; Ahlgren et al., 1990; Kagawa and Waterman, 1990; Lund et al., 1990; Parker and Schimmer, 1995; Payne and Hales, 2004).
In a number of other cells, cAMP synthesized in response to the activation of G protein-coupled receptors produces effects that are independent of PKA or Epac proteins (Buscà et al., 2000; Iacovelli et al., 2001; Fujita et al., 2002; Stork and Schmitt, 2002; Ivins et al., 2004). Overall, these results suggest that 6-Bnz-cAMP may induce the expression of bTREK-1 through a cAMP-dependent pathway in addition to PKA.
Experiments with the ESCAs 8CPT-2′-OMe-cAMP and its nonhydrolyzable analog Sp-8CPT-2′-OMe-cAMP clearly demonstrated that cAMP does not enhance the expression of bTREK-1 K+ channels by activation of Epac2. Specifically, 8CPT-2′-OMe-cAMP induced bTREK-1 mRNA and K+ current at concentrations that produce little or no activation of the Epac2 effector Rap1, whereas Sp-8CPT-2′-OMe-cAMP failed to increase bTREK-1 expression at higher concentrations shown previously to activate Rap1 in these cells (Enyeart and Enyeart, 2009). Thus, adrenocorticotropin- and cAMP-stimulated expression of bTREK-1 seems to be mediated by in part by PKA but independently of Epac2.
8-(4-Chlorophenylthio)-cAMP Metabolites and bTREK-1 Expression.
Results of experiments with hydrolyzable and nonhydrolyzable 8-(4-chlorophenylthio)-cAMP analogs strongly suggest that the hydrolyzable cAMP analogs stimulated bTREK-1 expression indirectly through metabolites. Accordingly, we found that a total of five potential metabolites of 8CPT-cAMP and 8CPT-2′-OMe-cAMP each induced the expression of bTREK-1 mRNA transcripts and ion current with potency and effectiveness similar to those of the parent compounds. Enzymes that could catalyze the conversion of these two cAMP derivatives to each of these putative metabolites are expressed in mammalian cells. These include cyclic nucleotide phosphodiesterases, 5′-nucleotidases, hypoxanthine phosphoribosyltransferase, and nucleotide phosphorylases (Price and Stevens, 1999).
The active metabolites, associated receptor, and signaling pathways that mediate the increases in bTREK-1 gene expression have not been identified. It is possible that each of the five metabolites stimulates bTREK-1 expression. In this regard, it is interesting to note that both 8CPT-cAMP and the ESCA 8CPT-2′-OMe-cAMP can be converted to the same 8-(4-chlorophenylthio)-adenine derivative (Fig. 5). It will be important to determine whether 8CPT-Ade is the final common active metabolite.
Regardless of the identity of the active metabolite(s), it is clear that it does not function by activating a cAMP-dependent mechanism. First, although the hydrolyzable 8-(4-chlorophenylthio)-cAMP analogs and their metabolites at low concentrations all induced bTREK-1 expression, none of these down-regulated the Kv1.4 current whose expression is inhibited by a PKA-dependent mechanism (Enyeart et al., 2000). In contrast, 8-Br-cAMP and 6-Bnz-cAMP, as well as adrenocorticotropin, at concentrations that induced bTREK-1 all inhibited the expression of Kv1.4 current (Figs. 1, 2, 3, 4, and 9). Furthermore, we showed previously that neither 8CPT-2′-OMe-cAMP, 8CPT-2′-OMe-Ado, nor 8CPT-Ade activates PKA when applied to bovine AZF cells (Enyeart and Enyeart, 2009). Finally, in the current study, we demonstrated that a nonhydrolyzable ESCA failed to induce bTREK-1 expression, even at concentrations that have been shown to activate Epac2.
The findings of this study are consistent with the possibility that cAMP metabolites induce bTREK-1 by activation of a cAMP-independent pathway that is also activated by adrenocorticotropin. An O-nitrophenyl-derivative of adrenocorticotropin, O-nitrophenylsulfenyl-adrenocorticotropin, blocks the activity of bTREK-1 K+ channels, induces increases in [Ca2+]i, and stimulates large increases in cortisol synthesis at concentrations that produce little or no increase in cAMP synthesis (Moyle et al., 1973; Yamazaki et al., 1998; Liu et al., 2008). Although these effects are mediated through the MC2R receptor, the cAMP-independent signaling pathway has not been identified.
We have shown previously that metabolites of 8CPT-2′-OMe-cAMP induced large, delayed increases in the expression of genes coding for steroidogenic proteins, resulting in corresponding increases in cortisol synthesis (Enyeart and Enyeart, 2009). With respect to kinetics and concentration-dependence, the effects of the metabolites on the expression of steroidogenic proteins resemble those on bTREK-1, suggesting a common mechanism.
The range of genes whose expression might be modulated by this novel signaling pathway is yet to be determined. It is clear that the presumptive metabolites did not produce a nonspecific global increase in RNA synthesis. Total RNA synthesis was not increased in metabolite-treated cells. Expression of specific genes, including the transcriptional repressor Dax-1 and Kv1.4, were not increased by the metabolites (J. A. Enyeart and J. J. Enyeart, unpublished observations).
This and other studies demonstrate that adrenocorticotropin and cAMP exert long-term control over the electrical properties of AZF cells by regulating the expression of genes that code for ion channels (Enyeart et al., 2000, 2003). Adrenocorticotropin may function through cAMP-dependent and independent mechanisms. In this regard, it is possible that adrenocorticotropin and metabolites of 8-(4-chlorophenylthio)-cAMP analogs may induce the expression of genes coding for bTREK-1 K+ channels and steroidogenic proteins by activating a common, but yet to be identified, cAMP-independent pathway.
The extent to which metabolites of 8-(4-chlorophenylthio)-cAMP derivatives regulate gene expression and cell function in other tissues and organisms is unknown. Hydrolysis products of selected cAMP analogs transform the protozoa Trypanosoma brucei from slender to stumpy-like forms (Laxman et al., 2006). It is possible that the effect of these metabolites in distantly related eukaryotes might be mediated through a common ancient signaling pathway.
Finally, 8CPT-cAMP analogs, including 8CPT-cAMP and 8CPT-2′-OMe-cAMP, have been used in hundreds of studies to determine the roles of cAMP, PKA, and Epac proteins in cell signaling. The findings of our study indicate that the results of some of these previous studies may require re-evaluation.
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK47875].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061861
- AZF
- adrenal zona fasciculata
- Epac
- exchange protein directly activated by cAMP
- ESCA
- exchange protein directly activated by cAMP-selective cAMP analog
- PKA
- cyclic AMP-dependent protein kinase
- FBS
- fetal bovine serum
- DMEM
- Dulbecco's modified Eagle's medium
- BAPTA
- 1,2 bis-(2-aminophenoxy)ethane-N,N,N′,N″-tetraacetic acid
- kb
- kilobase
- 8-Br-cAMP
- 8-bromoadenosine-cAMP
- 6-Bnz-cAMP
- N6-benzoyladenosine-cAMP
- 8CPT-2′-OMe-cAMP
- 8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- Sp-8CPT-2′-OMe-cAMP
- hydrolysis-resistant 8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- 8CPT-2′-OMe-5′AMP
- 8-(4-chlorophenylthio)-2′-O-methyladenosine-5′-O-monophosphate
- 8CPT-2′-OMe-Ado
- 8-(4-chlorophenylthio)-2′-O-methyladenosine
- 8CPT-cAMP
- 8-(4-chlorophenylthio)-cAMP
- Sp-8CPT-cAMP
- hydrolysis-resistant 8-(4-chlorophenylthio)-cAMP
- 8CPT-Ado
- 8-(4-chlorophenylthio)-adenosine
- 8CPT-Ade
- 8-(4-chlorophenylthio)-adenine
- H-89
- N-[2-(4-bromocinnamylamino)ethyl]-5-isoquinoline.
References
- Ahlgren et al., 1990.Ahlgren R, Simpson ER, Waterman MR, Lund J. (1990) Characterization of the promoter/regulatory region of the bovine CYP11A (P-450scc) gene. Basal and cAMP-dependent expression. J Biol Chem 265:3313–3319 [PubMed] [Google Scholar]
- Buscà et al., 2000.Buscà R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychène A, Ortonne JP, Ballotti R. (2000) Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J 19:2900–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepurny et al., 2009.Chepurny OG, Leech CA, Kelley GG, Dzhura I, Dzhura E, Li X, Rindler MJ, Schwede F, Genieser HG, Holz GG. (2009) Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analogue in rat INS-1 cells: studies with 8-pCPT-2′-O-Me-cAMP-AM. J Biol Chem 284:10728–10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen et al., 2003.Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, et al. (2003) cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278:35394–35402 [DOI] [PubMed] [Google Scholar]
- Davies et al., 2000.Davies SP, Reddy H, Caivano M, Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij et al., 1998.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477 [DOI] [PubMed] [Google Scholar]
- Dostmann et al., 1990.Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Døskeland SO, Ogreid D. (1990) Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem 265:10484–10491 [PubMed] [Google Scholar]
- Enserink et al., 2002.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nature Cell Biol 4:901–906 [DOI] [PubMed] [Google Scholar]
- Enyeart et al., 2003.Enyeart JA, Danthi S, Enyeart JJ. (2003) Corticotropin induces the expression of TREK-1 mRNA and K+ current in adrenocortical cells. Mol Pharmacol 64:132–142 [DOI] [PubMed] [Google Scholar]
- Enyeart and Enyeart, 2009.Enyeart JA, Enyeart JJ. (2009) Metabolites of an Epac-selective cAMP analog induce cortisol synthesis by adrenocortical cells through a cAMP-independent pathway. PLoS One 4:e6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart et al., 2000.Enyeart JA, Xu L, Enyeart JJ. (2000) A bovine adrenocortical Kv1.4 K+ channel whose expression is potently inhibited by ACTH. J Biol Chem 275:34640–34649 [DOI] [PubMed] [Google Scholar]
- Enyeart et al., 1997.Enyeart JJ, Gomora JC, Xu L, Enyeart JA. (1997) Adenosine triphosphate activates a noninactivating K+ current in adrenal cortical cells through nonhydrolytic binding. J Gen Physiol 110:679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart et al., 1993.Enyeart JJ, Mlinar B, Enyeart JA. (1993) T-type Ca2+ channels are required for adrenocorticotropin-stimulated cortisol production by bovine adrenal zona fasciculata cells. Mol Endocrinol 7:1031–1040 [DOI] [PubMed] [Google Scholar]
- Enyeart et al., 1996.Enyeart JJ, Mlinar B, Enyeart JA. (1996) Adrenocorticotropic hormone and cAMP inhibit noninactivating K+ current in adrenocortical cells by an A-kinase-independent mechanism requiring ATP hydrolysis. J Gen Physiol 108:251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart et al., 2002.Enyeart JJ, Xu L, Danthi S, Enyeart JA. (2002) An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem 277:49186–49199 [DOI] [PubMed] [Google Scholar]
- Fujita et al., 2002.Fujita T, Meguro T, Fukuyama R, Nakamuta H, Koida M. (2002) New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells. Checkpoint of modulation by cyclic AMP. J Biol Chem 277:22191–22200 [DOI] [PubMed] [Google Scholar]
- Grahame-Smith et al., 1967.Grahame-Smith DG, Butcher RW, Ney RL, Sutherland EW. (1967) Adenosine 3′,5′-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem 242:5535–5541 [PubMed] [Google Scholar]
- Hamill et al., 1981.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100 [DOI] [PubMed] [Google Scholar]
- Haynes and Berthet, 1957.Haynes RC, Jr, Berthet L. (1957) Studies on the mechanism of action of the adrenocorticotropic hormone. J Biol Chem 225:115–124 [PubMed] [Google Scholar]
- Holz et al., 2008.Holz GG, Chepurny OG, Schwede F. (2008) Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal 20:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz et al., 2006.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. (2006) Cell physiology of cAMP sensor Epac. J Physiol 577:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli et al., 2001.Iacovelli L, Capobianco L, Salvatore L, Sallese M, D'Ancona GM, De Blasi A. (2001) Thyrotropin activates mitogen-activated protein kinase pathway in FRTL-5 by a cAMP-dependent protein kinase A-independent mechanism. Mol Pharmacol 60:924–933 [DOI] [PubMed] [Google Scholar]
- Ivins et al., 2004.Ivins JK, Parry MK, Long DA. (2004) A novel cAMP-dependent pathway activates neuronal integrin function in retinal neurons. J Neurosci 24:1212–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John et al., 1986.John ME, John MC, Boggaram V, Simpson ER, Waterman MR. (1986) Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc Natl Acad Sci U S A 83:4715–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa and Waterman, 1990.Kagawa N, Waterman MR. (1990) cAMP-dependent transcription of the human CYP21B (P-450C21) gene requires a cis-regulatory element distinct from the consensus cAMP-regulatory element. J Biol Chem 265:11299–11305 [PubMed] [Google Scholar]
- Kawasaki et al., 1998.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279 [DOI] [PubMed] [Google Scholar]
- Laxman et al., 2006.Laxman S, Riechers A, Sadilek M, Schwede F, Beavo JA. (2006) Hydrolysis products of cAMP analogs cause transformation of Trypanosoma brucei from slender to stumpy-like forms. Proc Natl Acad Sci U S A 103:19194–19199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2008.Liu H, Enyeart JA, Enyeart JJ. (2008) ACTH inhibits bTREK-1 K+ channels through multiple cAMP-dependent signaling pathways. J Gen Physiol 132:279–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2009.Liu H, Enyeart JA, Enyeart JJ. (2009) N6-substituted cAMP analogs inhibit bTREK-1 K+ channels and stimulate cortisol secretion by a protein kinase A-independent mechanism. Mol Pharmacol 76:1290–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund et al., 1990.Lund J, Ahlgren R, Wu DH, Kagimoto M, Simpson ER, Waterman MR. (1990) Transcriptional regulation of the bovine CYP17 (P-450(17)alpha) gene. Identification of two cAMP regulatory regions lacking the consensus cAMP-responsive element (CRE). J Biol Chem 265:3304–3312 [PubMed] [Google Scholar]
- Mlinar et al., 1993.Mlinar B, Biagi BA, Enyeart JJ. (1993) A novel K+ current inhibited by adrenocorticotropic and angiotensin II in adrenal cortical cells. J Biol Chem 268:8640–8644 [PubMed] [Google Scholar]
- Mlinar and Enyeart, 1993.Mlinar B, Enyeart JJ. (1993) Voltage-gated transient currents in bovine adrenal fasciculata cells II: A-type K+ current. J Gen Physiol 102:239–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle et al., 1973.Moyle WR, Kong YC, Ramachandran J. (1973) Steroidogenesis and cyclic adenosine 3′,5′-monophosphate accumulation in rat adrenal cells. Divergent effects of adrenocorticotropin and its o-nitrophenyl sulfenyl derivative. J Biol Chem 248:2409–2417 [PubMed] [Google Scholar]
- Parker and Schimmer, 1995.Parker KL, Schimmer BP. (1995) Transcriptional regulation of the genes encoding the cytochrome P-450 steroid hydroxylases. Vitam Horm 51:339–370 [DOI] [PubMed] [Google Scholar]
- Payne and Hales, 2004.Payne AH, Hales DB. (2004) Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25:947–970 [DOI] [PubMed] [Google Scholar]
- Penhoat et al., 1989.Penhoat A, Jaillard C, Saez JM. (1989) Corticotropin positively regulates its own receptors and cAMP response in cultured bovine adrenal cells. Proc Natl Acad Sci U S A 86:4978–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe et al., 2008.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, et al. (2008) Cyclic nucleotide analogs as probes of signaling pathways. Nature Methods 5:277–278 [DOI] [PubMed] [Google Scholar]
- Price and Stevens, 1999.Price NC, Stevens L. (1999) Fundamentals of Enzymology: The Cell and Molecular Biology of Catalytic Proteins Oxford University Press, New York: [Google Scholar]
- Raikhinstein et al., 1994.Raikhinstein M, Zohar M, Hanukoglu I. (1994) cDNA cloning and sequence analysis of the bovine adrenocorticotropic hormone (ACTH) receptor. Biochim Biophys Acta 1220:329–332 [DOI] [PubMed] [Google Scholar]
- Richardson and Schulster, 1973.Richardson MC, Schulster D. (1973) The role of protein kinase activation in the control of steroidogenesis by adrenocorticotrophic hormone in the adrenal cortex. Biochem J 136:993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala et al., 1979.Sala GB, Hayashi K, Catt KJ, Dufau ML. (1979) Adrenocorticotropin action in isolated adrenal cells. The intermediate role of cyclic AMP in stimulation of corticosterone synthesis. J Biol Chem 254:3861–3865 [PubMed] [Google Scholar]
- Simpson and Waterman, 1988.Simpson ER, Waterman MR. (1988) Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol 50:427–440 [DOI] [PubMed] [Google Scholar]
- Stork and Schmitt, 2002.Stork PJ, Schmitt JM. (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266 [DOI] [PubMed] [Google Scholar]
- Waterman, 1994.Waterman MR. (1994) Biochemical diversity of cAMP-dependent transcription of steroid hydroxylase genes in the adrenal cortex. J Biol Chem 269:27783–27786 [PubMed] [Google Scholar]
- Waterman and Simpson, 1989.Waterman MR, Simpson ER. (1989) Regulation of steroid hydroxylase gene expression is multifactorial in nature. Recent Prog Horm Res 45:533–566 [DOI] [PubMed] [Google Scholar]
- Yamazaki et al., 2006.Yamazaki T, Kawasaki H, Takamasa A, Yoshitomi T, Kominami S. (2006) Ca2+ signal stimulates the expression of steroidogenic acute regulatory protein and steroidogenesis in bovine adrenal fasciculata-reticularis cells. Life Sci 78:2923–2930 [DOI] [PubMed] [Google Scholar]
- Yamazaki et al., 1998.Yamazaki T, Kimoto T, Higuchi K, Ohta Y, Kawato S, Kominami S. (1998) Calcium ion as a second messenger for o-nitrophenylsulfenyl-adrenocorticotropin (NPS-ACTH) and ACTH in bovine adrenal steroidogenesis. Endocrinology 139:4765–4771 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.