Abstract
Acquired apoptosis resistance plays an important role in acquired chemoresistance in cancer cells during chemotherapy. Our previous observations demonstrated that acquired tumor necrosis factor-related apoptosis-inducing ligand resistance in lung cancer cells was associated with Akt-mediated stabilization of cellular FLICE-like inhibitory protein (c-FLIP) and Mcl-1. In this report, we determined that these cells also have acquired resistance to apoptosis induced by chemotherapeutics such as cisplatin and doxorubicin (Adriamycin), which was detected in vitro in cell cultures and in vivo in xenografted tumors. We further found that cyclooxygenase-2 (COX-2) is dramatically overexpressed in cells with acquired apoptosis resistance. COX-2 seems to be a crucial mediator in acquired apoptosis resistance because suppressing COX-2 activity with a chemical inhibitor or reducing COX-2 protein expression level with COX-2 small interfering RNA dramatically alleviated resistance to therapeutic-induced apoptosis. Inhibiting Akt markedly suppressed COX-2 expression, suggesting COX-2 is a downstream effector of this cell survival kinase-mediated apoptosis resistance. Furthermore, the expression of Mcl-1 but not c-FLIP was significantly reduced when COX-2 was suppressed, and knockdown of Mcl-1 substantially sensitized the cells to apoptosis. Our results establish a novel pathway that consists of Akt, COX-2, and Mcl-1 for acquired apoptosis resistance, which could be a molecular target for circumventing acquired chemoresistance in lung cancer.
Apoptosis is an evolutionarily conserved cell suicide procedure that multicellular animals use to eliminate damaged, infected, and unwanted cells. Because it is the most effective way in limiting the expansion of genome-damaged or gene-mutated cells, it is believed that apoptosis plays a critical role in deterring cancer development (Fulda, 2009). However, cancer cells readily escape the body's natural defense mechanism. Cancer cells gain apoptosis resistance (primary) through dysfunctional apoptosis pathways and/or by elevating survival signals stemming from the acquisition of genetic and epigenetic aberrations acquired during transformation (Fulda, 2009). In addition, cancer cells acquire apoptosis resistance during chemotherapy, the mechanism of which is not well understood (Wajant et al., 2005; Wilson et al., 2009). It is noteworthy that the chemotherapy-induced apoptosis resistance (secondary or acquired apoptosis resistance) has a severely detrimental impact on chemotherapy because it not only dampens the anticancer activity of the drugs, but it also promotes cancer progression. For example, when TNF-related apoptosis-inducing ligand (TRAIL) loses its cell-killing capacity, it promotes proliferation and metastasis in apoptosis-resistant cancer cells (Malhi and Gores, 2006). Therefore, it is crucial to understand the mechanism of acquired apoptosis resistance to retain the cancer-killing activity while circumventing the cancer-promoting potential of chemotherapeutics.
Apoptosis plays a major role in preventing normal cellular integrity and is strictly regulated. Two main distinct apoptosis pathways have been developed, namely the intrinsic and extrinsic pathways (Heath-Engel et al., 2008; Papenfuss et al., 2008). Initiating signals in the intrinsic pathway are generated by developmental cues or cellular damage that cause the loss of mitochondrial potential and release of proapoptotic factors such as cytochrome c and Smac from the mitochondria to the cytosol. A protein complex called apoptosome consisting of cytochrome c and Apaf1 subsequently is formed to activate the initiator caspase-9, which activates effector caspases 3 and 7 that execute apoptosis. This pathway involves the physical and functional interplay between the prosurvival Bcl2 family members, including Bcl2, Bcl-XL, and Mcl-1, and the proapoptosis members Bax, Bak, and Bok. The extrinsic pathway is activated by stimulation from outside of the cell through the ligation of the TNF family of cytokines to their cognate receptors located on the cell membrane. The TNF family of receptors are also called death receptors and include TNF's TNF receptor 1 and TRAIL's death receptors 4 and 5 (DR4 and DR5). This pathway is initiated by the formation of the death-inducing signaling complex consisting of the receptor, receptor-interacting protein, and Fas-associated death domain that activates initiator caspase-8, which leads to activation of effector caspases 3 and 7 to execute apoptosis. The caspase-8 competitor cellular FLICE-like inhibitory protein (c-FLIP) can be recruited to the death-inducing signaling complex to inhibit the recruitment and activation of caspase-8 (Ashkenazi, 2008). It is noteworthy that cross-talks between the two apoptosis pathways occur to accelerate cell death. For example, the extrinsic pathway-activated caspase-8 cleaves Bid, a BH3-only member of the Bcl-2 family, to generate the proapoptotic tBid that migrates to mitochondria and activates the mitochondrial apoptosis pathway (Papenfuss et al., 2008; Wang and Lin, 2008). In addition, there is a positive feedback loop that leads to further activation of the initiator caspases by effector caspases (Pop and Salvesen, 2009). Regarding anticancer agent-induced cytotoxicity, TRAIL mainly activates the extrinsic pathway, whereas the DNA-damaging drugs doxorubicin [Adriamycin (Adr); Bedford Laboratories, Bedford, OH] and cisplatin (CDDP) mainly induce the intrinsic pathway (Wilson et al., 2009).
We have recently established acquired resistance to TRAIL-induced apoptosis in lung cancer cell lines by continuously exposing the TRAIL-sensitive lung cancer cells to a nontoxic dose and gradually increasing the concentrations of TRAIL. We found that the Akt-dependent overexpression of c-FLIPL and Mcl-1L is associated with acquired TRAIL resistance (Wang et al., 2008). In this study, we further determined that TRAIL-resistant cancer cells are also refractory to apoptosis caused by other anticancer therapeutic drugs, which is associated with an Akt-mediated increase of cyclooxygenase-2 (COX-2) expression. Pharmacological or genetic suppression of COX-2 drastically alleviated apoptosis resistance accompanied by reduction in Mcl-1 expression. These results established a novel pathway composed of Akt, COX-2, and Mcl-1 that confers lung cancer cells' acquired resistance to apoptosis. This pathway could provide a molecular target for preventing and alleviating acquired apoptosis resistance during anticancer chemotherapy.
Materials and Methods
Reagents.
Glutathione transferase TRAIL was prepared as described previously (Lin et al., 2000; Chen et al., 2007b). Adr and CDDP were from Sigma-Aldrich (St. Louis, MO). IKK inhibitor II, COX-2 inhibitor II, and LY294002 were purchased from Calbiochem (San Diego, CA). Small interfering RNA (siRNA; SMATpool) for COX-2, Mcl-1, Akt (pooled siRNAs that targeting the Akt isoforms Akt1, Akt2, and Akt3) and negative control were purchased from Dharmacon (Lafayette, CO). The following antibodies were used for Western blot: anti-caspase-8 and anti-caspase-3 (BD Biosciences Pharmingen, San Diego, CA); anti-poly(ADP-ribose) polymerase (PARP) (BioSource International, Camarillo, CA); anti-β-tubulin, anti-β-actin (Sigma-Aldrich); anti-Akt and -phospho-Akt(Ser473) and -phospho-GSK3β (Cell Signaling Technology, Danvers, MA); anti-COX-2 (Cayman Chemical, Ann Arbor, MI); and anti-c-FLIP and anti-Mcl-1 (Alexis Laboratories, San Diego, CA).
Cell Culture, Transfection, and Establishing the TRAIL-Resistant Cells.
The human lung cancer cell lines H460 and H1568 were obtained from American Type Culture Collection (Manassas, VA) and grown in RPMI 1640 supplemented with 10% fetal bovine serum, 1 mM glutamate, 100 U/ml penicillin, and 100 μg/ml streptomycin. For transfection of siRNA, cells were grown in 12-well plates and were transfected with siRNA with INTERFERin siRNA transfection reagent according to manufacturer's instructions (Polyplus-transfection, Inc. New York, NY). The knockdown of protein expression was determined by Western blot. H460-TRAIL-resistant (TR) and H1568-TR cells were established by continuously exposing the TRAIL-sensitive H460 and H1568 cells to increasing doses of TRAIL as described previously (Wang et al., 2008). The parental cell (PC) lines were designated as H460-PC and H1568-PC, respectively.
Cytotoxicity Assay.
Cytotoxicity was determined using a lactate dehydrogenase (LDH) release-base cytotoxicity detection kit (Promega, Madison, WI). Cells were seeded in 48-well plates at 70 to 80% confluence, cultured overnight, and then treated as indicated in the figure legends. LDH release was determined, and cell death was calculated as described previously (Wang et al., 2006b; Ju et al., 2007). The experiments were repeated no less than three times, and representative results are shown in the figures.
Western Blotting.
Total protein lysates were prepared by suspending cells in M2 buffer (20 mM Tris-HCl, pH 7.6, 0.5% Nonidet P-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM β-glycerophosphate, 1 mM sodium vanadate, and 1 μg/ml leupeptin). Equal amounts of cell lysate were resolved by 10, 12, or 15% SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. The proteins were visualized by enhanced chemiluminescence according to the manufacturer's instructions (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Reverse Transcription-Polymerase Chain Reaction.
Total RNA was extracted with the RNAeasy kit (QIAGEN, Valencia, CA). One microgram of RNA from each sample was used as a template for cDNA synthesis with a reverse transcription kit (Promega). An equal volume of cDNA product was used in the PCR. The primers used were the following: COX-2, CGCTCAGCCATACAGCAAATCCTT and GTGCACTGTGTTTGGAGTGGGTTT; and β-actin, CCAGCCTTCCTTCCTGGGCAT and AGGAGCAATGATCTTGATCTTCATT. The reaction condition was 94°C for 45 s, 55°C for 40 s, and 72°C for 45 s. For COX-2 and β-actin, the cycles for PCR were 27 and 21, respectively. PCR products were run on 2% agarose gel with 0.5 μg/ml ethidium bromide, visualized, and photographed.
Tumorigenicity Assay in Nude Mice.
H460-PC and H460-TR cells were harvested and washed twice with phosphate-buffered saline. Aliquots of 1 million cells were suspended in 50 μl of phosphate-buffered saline mixed with 50 μl of Matrigel (BD Biosciences, San Jose, CA) and injected subcutaneously at the right and left flanks of athymic nude mice (BALB/cnu/nu, 4–6 weeks old). When the average tumor volume reached approximately 100 mm3, the mice injected with H460-PC or H460-TR cells were randomized into three groups of three mice per group. Group 1 was the untreated control, group 2 was treated with TRAIL (15 mg/kg, i.p.), and group 3 was treated with CDDP (6 mg/kg, i.p.) on the indicated days. Tumors were measured using a caliper, and tumors were calculated using the following formula: tumor volume = length × width2/2.
Statistics.
Data are expressed as mean ± S.D. Statistical significance was examined by one-way analysis of variance. In all analyses, P < 0.05 was considered statistically significant.
Results
Acquired Apoptosis-Resistance Established in H460 and H1568 Cells by Long-Term Exposure to TRAIL.
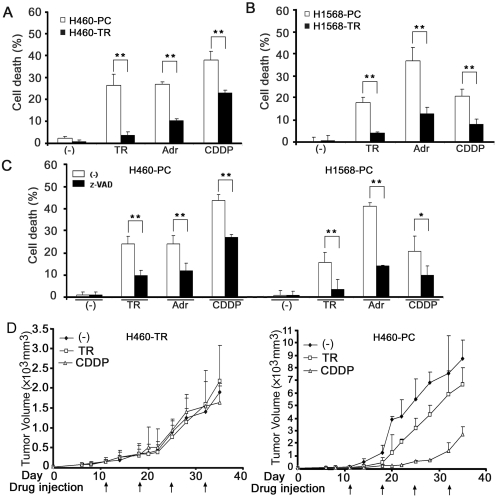
We generated acquired TRAIL-resistant cells by continuously exposing the ensured TRAIL-sensitive H460 and H1568 cells to gradually increasing concentrations of TRAIL, which was started with a nontoxic low dose and gradually increased to a high dose that completely killed the parental cells (Wang et al., 2008). The resulting cell lines, designated as H460-TR and H1568-TR, respectively, acquired resistance to TRAIL-induced apoptosis (Fig. 1, A and B) (Wang et al., 2008). The TRAIL-resistant cells also acquired cross-resistance to the anticancer drugs Adr- and CDDP-induced cytotoxicity (Fig. 1, A and B). The cytotoxicity induced by TRAIL, Adr, and CDDP was confirmed to be mainly apoptotic because it was associated with caspase activation and cytochrome c release and could be effectively inhibited with the pan-caspase inhibitor z-VAD (Fig. 1C and data not shown). The resistance to TRAIL- or CDDP-induced cancer cell death was further confirmed in vivo with the H460 parental cells (H460-PC) and H460-TR cells and a xenografted tumor nude mouse model (Fig. 1D). TRAIL or CDDP treatment caused substantial growth retardation in tumors derived from H460-PC cells (Fig. 1D, right). However, the H460-TR cells were complete refractory to these drugs (Fig. 1D, left). Tumors formed by H460-TR cells grew slower than those formed by H460-PC cells, which is consistent with previous reports showing that chemoresistant cancer cells grow more slowly than sensitive ones (Kusumoto et al., 1996; Gatenby et al., 2009). Taken together, these in vitro and in vivo results suggest that the continuously TRAIL-exposed cells have acquired resistance against apoptosis mediated by both the extrinsic and intrinsic pathways, mainly induced by TRAIL, and Adr and CDDP, respectively.
Fig. 1.
Acquired apoptosis resistance established in H460 and H1568 cells by long-term TRAIL exposure. A, H460-PC and H460-TR cells were treated with TRAIL (50 ng/ml), Adr (0.25 μg/ml), or CDDP (50 μM) for 30 h. Cell death was measured by LDH leakage assay. Data shown are the mean ± S.D.; *, p < 0.05; **, p < 0.01. B, H1568-PC and H1568-TR cells were treated with TRAIL (100 ng/ml), Adr (0.5 μg/ml), or CDDP (100 μM) for 30 h. Cell death was detected as described in A. C, H460-PC and H1568-PC cells were pretreated with z-VAD (10 μM) or were left untreated for 1 h followed by TRAIL (50 ng/ml for H460-PC, 100 ng/ml for H1568-PC), Adr (0.25 μg/ml for H460-PC, 0.5 μg/ml for H1568-PC), or CDDP (50 μM for H460, 100 μM for H1568-PC) for 30 h. Cell death was detected as described in A. D, H460-PC or H460-TR cells (106 in 50 μl) were injected subcutaneously at the right and left flanks of athymic nude mice to form tumors. The mice were treated with TRAIL (15 mg/kg) or CDDP (6 mg/kg) at the indicated times or were left untreated. Tumor volume was detected and expressed as mean ± S.D.
COX-2 Expression Is Dramatically Increased in H460-TR and H1568-TR Cells.
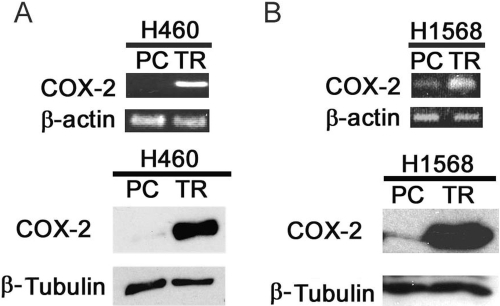
Gene expression microarray was used to search for potential factors contributing to the acquired apoptosis resistance in H460-TR cells, and COX-2 was one of the genes most significantly increased in these cells. To verify this result, reverse transcription-polymerase chain reaction (RT-PCR) and Western blot were used to detect COX-2 mRNA and COX-2 protein, respectively. COX-2 was barely expressed in H460 cells. However, both the COX-2 mRNA and protein expression levels were significantly higher in H460-TR cells (Fig. 2A). A similar observation was made in H1568 parental cells (H1568-PC) and H1568-TR cells (Fig. 2B). Because COX-2 was reported to be involved in cancer cells' insensitivity to apoptosis (Brown and DuBois, 2004), these results prompted us to investigate whether COX-2 plays a role in acquired apoptosis resistance generated by long-term TRAIL exposure.
Fig. 2.
COX-2 expression is increased in H460TR and H1568-TR cells. A, top, equal amounts of total RNA from H460-PC and H460-TR cells were detected for COX-2 mRNA expression by RT-PCR. β-Actin was detected as an input control. Bottom, equal amounts of cell extracts from H460-PC and H460-TR cells were detected for COX-2 protein expression by Western blot. B, top, equal amounts of total RNA from H1568-PC and H1568-TR cells were detected for COX-2 mRNA expression by RT-PCR. β-Actin was detected as an input control. Bottom, equal amounts of cell extracts from H1568-PC and H1568-TR cells were detected for COX-2 protein expression by Western blot. β-Tubulin was detected as an input control.
Suppressing COX-2 Substantially Sensitizes H460-TR and H1568-TR cells to TRAIL-, Adr-, and CDDP-Induced Apoptosis.
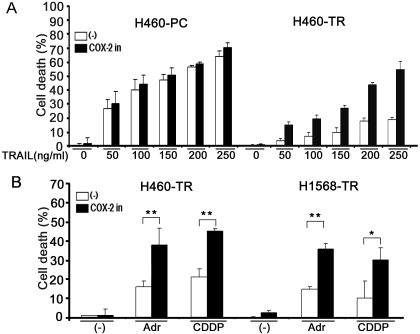
A COX-2 inhibitor was used first to examine the effect of suppressing COX-2 activity on TRAIL-induced cytotoxicity. The results showed that the COX-2 inhibitor substantially sensitized H460-TR cells to TRAIL-induced cytotoxicity (Fig. 3A). It is interesting that this COX-2 inhibitor had a marginal effect on TRAIL-induced cytotoxicity in H460-PC cells expressing very low levels of COX-2 protein (Fig. 3A). Likewise, the COX-2 inhibitor significantly sensitized H1568-TR but not H1568-PC cells to TRAIL-induced cytotoxicity (data not shown). These results strongly suggest that the potentiation of cytotoxicity by the COX-2 inhibitor is COX-2-specific, which differs from reports that some COX-2 inhibitors sensitized cancer cell apoptosis in a COX-2-independent manner (Chen et al., 2007a; Chuang et al., 2008). Likewise, the COX-2 inhibitor also markedly potentiated Adr- or CDDP-induced cytotoxicity (Fig. 3B).
Fig. 3.
COX-2 inhibitor sensitizes H460-TR and H1568-TR cells to TRAIL-, Adr-, and CDDP-induced apoptosis in vitro. A, H460-PC and H460-TR cells were pretreated with COX-2 inhibitor II (100 nM) for 1 h or were left untreated followed by the indicated concentrations of TRAIL for 30 h. Cell death was measured by LDH leakage assay. Data shown are the mean ± S.D. B, H460-TR or H1568-TR cells were pretreated with COX-2 inhibitor II (100 nM) for 1 h or were left untreated followed by exposure to Adr (0.25 μg/ml for H460-TR, 0.5 μg/ml for H1568-TR) or CDDP (25 μM for H460-TR, 50 μM for H1568-TR) for 30 h. Cell death was detected as described in A; *, p < 0.05; **, p < 0.01.
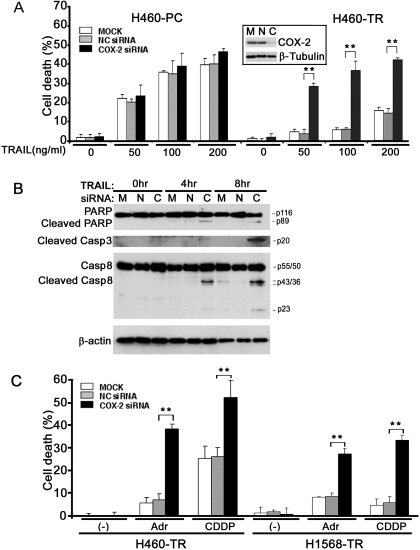
To validate and substantiate the results from the COX-2 inhibitor, COX-2 siRNA was used to deplete COX-2 protein from the cells. COX-2 siRNA significantly potentiated TRAIL-induced apoptosis in H460-TR but not H460-PC cells (Fig. 4A). COX-2 expression was effectively suppressed by the COX-2 siRNA (Fig. 4A, inset). The effect was specific to COX-2 depletion because the transfection of control negative siRNA, which did not alter the expression of COX-2, had no effect on TRAIL-induced cell death (Fig. 4A). The sensitized cytotoxicity was mainly apoptotic because it was associated with enhanced activation of caspases 8 and 3 and cleavage of the caspase 3 substrate PARP (Fig. 4B). Similar effects were seen in H1568-PC and H1568-TR cells (data not shown). In addition, Adr- or CDDP-induced cytotoxicity was also substantially increased in both H460-TR and H1568-TR cells when COX-2 siRNA, but not the control negative siRNA, was transfected (Fig. 4C). These results imply that COX-2 overexpression plays a substantial role in acquired apoptosis resistance.
Fig. 4.
Knockdown of COX-2 potentiated TRAIL-, Adr-, and CDDP-induced apoptosis. A, H460-PC and H460-TR cells were mock-transfected or were transfected with 5 nM COX-2-siRNA or negative control siRNA. Forty-eight hours after transfection, the cells were treated with the indicated concentrations of TRAIL for 30 h or were left untreated. Cell death was measured by LDH leakage assay. Data shown are mean ± S.D.; **, p < 0.01. Inset, knockdown of COX-2 was confirmed by Western blot in H460-TR cells. B, H1568-TR cells were mock-transfected or were transfected with 5 nM COX-2-siRNA or negative control siRNA. Forty-eight hours after transfection, the cells were treated with TRAIL (100 ng/ml) for 4 or 8 h or were left untreated (0 h). PARP and caspases 3 and 8 were detected by Western blot. β-Actin was detected as an input control. C, H460-TR or H1568-TR cells were mock-transfected or were transfected with 5 nM COX-2-siRNA or NC siRNA. Forty-eight hours after transfection, the cells were treated with Adr (0.25 μg/ml for H460-TR, 0.5 μg/ml for H1568-TR) or CDDP (50 μM for H460-TR and 100 μM for H1568-TR) for 30 h or were left untreated. Cell death was detected as described in A. **, p < 0.01. C, COX-2 siRNA; M, mock transfection; N, negative control siRNA.
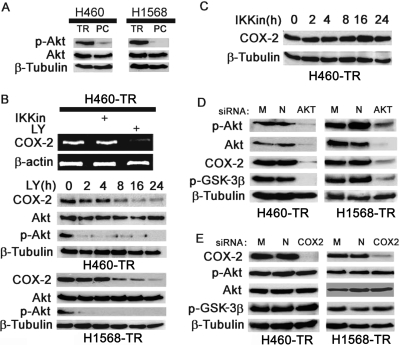
COX-2 Expression in H460TR and H1568 TR Cells Is Regulated by Akt.
Our previous studies found that Akt-mediated overexpression of c-FLIPL and Mcl-1L plays an important role in acquired TRAIL-resistance (Wang et al., 2008). Akt was constitutively activated in H460-TR and H1568-TR cells (Fig. 5A). Previous reports suggested that under some circumstances, the PI3K/Akt pathway regulates COX-2 expression (Ladu et al., 2008). Thus, we examined whether the COX-2 overexpression in H460-TR and H1568-TR cells is due to constitutive Akt activation. When Akt activity was suppressed with the PI3K/Akt inhibitor LY294002, COX-2 mRNA and protein expression was dramatically inhibited in H460-TR cells (Fig. 5B). In contrast, the IKK inhibitor II, which is potent in suppressing NF-κB (Bai et al., 2009), had no detectable effect on COX-2 expression (Fig. 5, B and C). These results were validated with Akt siRNAs that target all of the Akt isoforms (Akt1, Akt2, and Akt3), which effectively reduced the expression and activity of Akt, that suppressed COX-2 expression (Fig. 5D). As expected, the activity of GSK3β was also suppressed by the Akt siRNA (Fig. 5D). In contrast, suppressing COX-2 with COX-2 siRNA had no detectable effect on the activity of Akt and GSK3β (Fig. 5E). These results demonstrate that COX-2 functions downstream of Akt, and the elevated Akt activity is pivotal for COX-2 overexpression in the apoptosis-resistant cells.
Fig. 5.
Suppressing Akt reduces COX-2 expression in H460-TR and H1568-TR cells. A, equal amounts of cell extract from the indicated cells were Western-blotted for p-Akt and total Akt. β-Tubulin was detected as an input control. B, top, H460-TR cells were treated with IKK inhibitor II (10 nM) or LY294002 (10 μM) overnight. Expression of COX-2 mRNA was detected by RT-PCR. β-Actin was detected as an input control. Center and bottom, H460-TR and H1568-TR cells were treated with LY294002 for the indicated times. COX-2, Akt, and p-Akt were detected by Western blot. C, H460-TR cells were treated with IKK inhibitor II for the indicated times. COX-2 was detected by Western blot. D, H460-TR and H1568-TR cells were mock-transfected or were transfected with 5 nM Akt- or negative control siRNA. Forty-eight hours after transfection, COX-2, Akt, and p-Akt were detected by Western blot. β-Tubulin was detected as an input control. E, H460-TR and H1568-TR cells were mock-transfected or transfected with 5 nM COX-2- or negative control siRNA. Forty-eight hours after transfection, COX-2, p-Akt, and p-GSK3β were detected by Western blot. β-Tubulin was detected as an input control. M, mock-transfected; N, negative control.
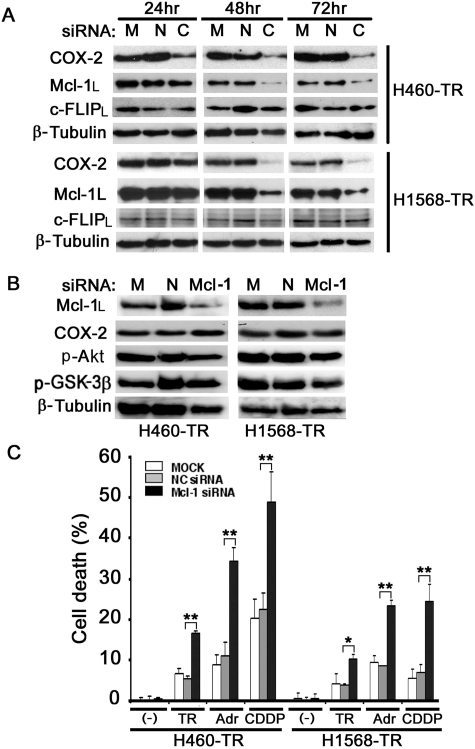
COX-2 Regulates Mcl-1 Expression and Mcl-1-Mediated Apoptosis Resistance in H460-TR and H1568-TR Cells.
Our previous studies found that Akt-mediated overexpression of c-FLIPL and Mcl-1L plays an important role in acquired TRAIL resistance (Wang et al., 2008). Because in some cell contexts COX-2 expression regulates the expression and function of c-FLIP or Mcl-1 (Lin et al., 2001; Xu et al., 2004), we examined whether the up-regulation of c-FLIPL or Mcl-1L expression underlies the mechanism of COX-2-mediated apoptosis resistance. The COX-2 expression level was significantly reduced in H460-TR cells 24 h after transfection with COX-2 siRNA, whereas the expression of Mcl-1L was dramatically decreased starting at 48 h. The reduction of Mcl-1L expression was specific to COX-2 siRNA transfection because the transfection of negative siRNA did not affect Mcl-1L expression (Fig. 6A, top). Similar effects were seen in the H1568-TR cells (Fig. 6A, bottom). The slower kinetics of Mcl-1L reduction compared with those of COX-2 further suggest that the decrease in Mcl-1L was a secondary effect of COX-2 suppression. In contrast, the expression of c-FLIPL was not affected by COX-2 siRNA in either H460-TR or H1568-TR cells (Fig. 6A), suggesting that c-FLIPL is not involved in COX-2-mediated apoptosis resistance. The knockdown of Mcl-1L with siRNA had no detectable effect on expression of COX-2, p-Akt, or p-GSK3β, further indicating that Mcl-1L is downstream of Akt and COX-2 (Fig. 6B). In both H460-TR and H1568-TR cells, Mcl-1 siRNA, but not the negative control siRNA, dramatically sensitized TRAIL-, Adr-, and CDDP-induced cytotoxicity, suggesting that Mcl-1L is one of the main downstream effectors of COX-2 in mediating the acquired apoptosis resistance (Fig. 6C).
Fig. 6.
COX-2 regulates Mcl-1 expression and Mcl-1-mediated apoptosis resistance in H460-TR and H1568-TR cells. A, H460-TR and H1568-TR cells were mock-transfected or were transfected with 5 nM COX-2- or negative control siRNA. COX-2, Mcl-1L, and c-FLIPL were detected by Western blot 24, 48, or 72 h after transfection. β-Tubulin was detected as an input control. B, H460-TR and H1568-TR cells were mock-transfected or were transfected with 5 nM Mcl-1- or negative control siRNA. Mcl-1L, COX-2, p-Akt, and p-GSK3β were detected by Western blot 48 h after transfection. β-Tubulin was detected as an input control. C, H460-TR and H1568-TR cells were mock-transfected (□) or were transfected with 5 nM Mcl-1-siRNA (■) or negative control siRNA ( ). Forty-eight hours after transfection, H460-TR cells were treated with TRAIL (50 ng/ml), Adr (0.25 μg/ml), or CDDP (50 μM), and H1568-TR cells were treated with TRAIL (100 ng/ml), Adr (0.5 μg/ml), or CDDP (100 μM) for 30 h. Cell death was measured by LDH leakage assay. Data shown are the mean ± S.D. *, p < 0.05; **, p < 0.01. C, COX-2; N, negative control; M, mock-transfected.
). Forty-eight hours after transfection, H460-TR cells were treated with TRAIL (50 ng/ml), Adr (0.25 μg/ml), or CDDP (50 μM), and H1568-TR cells were treated with TRAIL (100 ng/ml), Adr (0.5 μg/ml), or CDDP (100 μM) for 30 h. Cell death was measured by LDH leakage assay. Data shown are the mean ± S.D. *, p < 0.05; **, p < 0.01. C, COX-2; N, negative control; M, mock-transfected.
Discussion
This study establishes a new cellular signaling pathway consisting of Akt, COX-2, and Mcl-1L that confers acquired apoptosis resistance to lung cancer cells. Having discovered that Akt and Mcl-1L play an important role in acquired resistance against TRAIL-induced apoptosis (Wang et al., 2008), we now have identified that a dramatic overexpression of COX-2 in lung cancer cells is integral to acquired apoptosis resistance that can be abrogated through inhibiting Akt. Suppressing COX-2 activity with either a chemical inhibitor or COX-2 siRNA drastically alleviated resistance to therapeutic-induced apoptosis. Alleviating this apoptosis resistance was associated with a reduction in Mcl-1L expression. Thus, our data suggest that Akt, COX-2, and Mcl-1L form a novel signaling pathway conferring lung cancer cells' acquired apoptosis resistance, and this pathway could be a molecular target for preventing and circumventing acquired chemoresistance in lung cancer.
As a potent mechanism for chemoresistance, acquired apoptosis resistance contributes to therapy failure and tumor recurrence (Wilson et al., 2009). The mechanism of acquired apoptosis resistance is complex and not well elucidated (Fulda, 2009; Wilson et al., 2009). Activating the drug inactivation or clearance mechanisms can directly remove apoptosis-inducing drugs from cancer cells. For example, chemotherapy induces the expression of the ATP-binding cassette multidrug transporter proteins that trigger efflux of the drugs. Directly suppressing the apoptosis pathways or reducing the activity of the apoptosis machinery is also involved (Fulda, 2009). In addition, activating antiapoptotic cell survival signals such as NF-κB and Akt, which have been commonly found in a variety of cancer cells, could be another mechanism in acquired apoptosis resistance. Our results show a constitutive Akt activation in acquired apoptosis resistance, adding more evidence supporting the involvement of cell survival signaling, although other mechanisms are not excluded.
As a master kinase, Akt regulates apoptosis through directly phosphorylating the apoptotic machinery proteins such as caspase-9 and apoptosis regulator BAD and indirectly suppressing apoptosis through modulating other pathways such as NF-κB, c-Jun NH2-terminal kinase, and p53 (Hanada et al., 2004; Wang et al., 2007). In this report, we show that Akt activates a COX-2-mediated antiapoptotic pathway that involves the antiapoptotic Bcl2 family member Mcl-1. The expression of COX-2 at both the messenger RNA and protein levels was effectively blocked when Akt was suppressed with either siRNA or a chemical inhibitor, suggesting that COX-2 is a downstream factor of Akt, and the up-regulation of COX-2 is probably due to increased transcription rate or COX-2 mRNA stability. This is consistent with previous reports showing that PI3K/Akt regulates COX-2 expression (Ladu et al., 2008; Lee et al., 2008b). Numerous cell signaling pathways, including NF-κB, Ap1, and PI3K/Akt, have been reported to be involved in inducing COX-2 expression (Telliez et al., 2006). Under some circumstances NF-κB also is downstream of Akt (Ozes et al., 1999; Wang et al., 2007). However, NF-κB is unlikely to be involved in up-regulation of COX-2 by Akt in the apoptosis-resistant cells established in this study because blocking NF-κB had no detectable effect on this action of Akt. Because we clearly detected a reduction of GSK3β activity when Akt was blocked and GSK3β was implicated in regulating COX-2 expression (Rao et al., 2004; Wang et al., 2006a), it remains to be determined whether the Akt downstream kinase GSK3β is involved in regulating COX-2 expression in the context of acquired apoptosis resistance.
COX-2 is inducible by inflammatory cytokines, tumor promoters, and growth factors, and thus could be involved in inflammation-associated cancer development (Greenhough et al., 2009). Indeed, increased expression of COX-2 is found in a variety of cancer cells that are resistant to apoptosis (Greenhough et al., 2009). Therefore, COX-2 is implicated as a target for alleviating apoptosis resistance in cancer cells. COX-2 inhibitors were tested for cancer therapy, and substantial anticancer activity was achieved. However, because of cellular effects that are unrelated to COX-2 activity, such as inducing DR5 or triggering endoplasmic reticulum stress that directly contributes to COX-2-inhibitor-induced apoptosis (Kern et al., 2006; Chen et al., 2007a), some questions have been raised regarding the role of COX-2 in apoptosis regulation. Here we show that suppressing COX-2 activity with chemical inhibitors and eliminating COX-2 protein with siRNA were equally effective in sensitizing the cells to apoptosis induced by different anticancer agents and was not accompanied by increases in DR5 (data not shown) as reported previously (Chen et al., 2007a). The difference in non-COX-2-related activity between our findings and those of others may be due to the use of different COX-2 inhibitors (Hardy et al., 2003; Malhotra et al., 2004). Nevertheless, our data clearly show that COX-2 has an antiapoptosis role in cancer cells with acquired apoptosis resistance.
How COX-2 converts cells to be apoptosis resistance is not well elucidated. In this study, we found that COX-2 is mainly involved in Mcl-1L- but not c-FLIPL-mediated antiapoptotic signaling. Because c-FLIPL is clearly involved in Akt-mediated resistance to TRAIL-induced apoptosis (Wang et al., 2008), this finding suggests that up-regulation of COX-2 underlies one of the mechanisms of Akt-mediated apoptosis resistance. Because Mcl-1 messenger RNA is not increased in H460-TR and H1568-TR cells, the regulation of Mcl-1 expression by COX-2 is probably through the stability of Mcl-1L protein (Wang et al., 2008). It remains to be determined how COX-2 stabilizes Mcl-1L to ensue apoptosis resistance. It is unlikely that the proapoptosis Bcl-2 family member Noxa is involved in COX-2-mediated stabilization of Mcl-1, because knockdown of COX-2 had no effect on Noxa expression (data not shown). In addition, it was not clear why suppressing COX-2 enhanced TRAIL-induced activation caspase-8, the initiator caspase for the extrinsic apoptosis pathway (Fig. 4B), but had no effect on c-FLIPL expression (Fig. 6A). It is possibly due to the cross-talk between the intrinsic and extrinsic pathways that is regulated by Mcl-1(Pop and Salvesen, 2009). Indeed, Mcl-1 was able to block caspase-8 activation through the intrinsic pathway (Keuling et al., 2009).
It is noteworthy that although COX-2 was overexpressed in ∼70% of human lung tumors, heterogeneity (positive and negative) of COX-2 expression was commonly seen in these tumors (Hida et al., 1998; Lee et al., 2008a). Thus, it is interesting to determine whether the Akt/COX-2/Mcl-1 pathway is involved in constitutive COX-2 expression and response to chemotherapy in COX-2-expressing tumor cells. It is also warranted to determine whether this pathway is activated in COX-2-negative tumor cells and whether the activation of this pathway is associated with tumor relapse during chemotherapy. Several clinical trials combining the COX-2 inhibitor celecoxib and therapeutics such as paclitaxel, etoposide, and cisplatin showed moderate potentiated effects (Arúajo et al., 2009; Mutter et al., 2009; Suzuki et al., 2009). However, it was noted that the responders had either low COX-2 activity or higher efficacy of COX-2 inhibition by the treatment, suggesting that to decrease the COX-2 expression or activity to lower than the responding threshold could be an important determinant for cancer patients' response (Mutter et al., 2009). It would be interesting to determine whether acquired apoptosis associated with activation of the Akt/COX-2/Mcl-1 pathway is induced in the patients with poor response. Nevertheless, developing more effective and cancer cell-specific COX-2-inhibiting approaches is warranted for cancer therapy.
In summary, results from this study demonstrate a new cellular signaling pathway consisting of Akt, COX-2, and Mcl-1, established during long-term TRAIL exposure that renders lung cancer cells resistant to therapeutic-induced apoptosis. The key components of this pathway could be molecular targets for circumventing acquired chemoresistance in lung cancer. Further study is warranted to understand how this pathway is constitutively activated during the development of acquired apoptosis resistance.
Acknowledgments
We thank Donna Klinge for assistance in animal work and Vicki Fisher for help in preparing the manuscript.
This study is supported in part by the National Institutes of Health National Cancer Institute [Grant R03-CA125796] and the Department of Energy Low Dose Radiation Research Program [Grant DE-SC0001173].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.061226
- TRAIL
- tumor necrosis factor-related apoptosis-inducing ligand
- Adr
- doxorubicin
- CDDP
- cisplatin
- COX-2
- cyclooxygenase-2
- c-FLIP
- cellular FLICE-like inhibitory protein
- DR
- death receptor
- PC
- parental cell
- TR
- tumor necrosis factor-related apoptosis-inducing ligand-resistant
- TNF
- tumor necrosis factor
- siRNA
- small interfering RNA
- IKK
- IκB kinase complex
- PARP
- poly(ADP-ribose) polymerase
- LDH
- lactate dehydrogenase
- PCR
- polymerase chain reaction
- RT-PCR
- reverse transcription-polymerase chain reaction
- PI3K
- phosphatidylinositol 3-kinase
- GSK3β
- glycogen synthase kinase 3β
- z-VAD
- N-benzyloxycarbonyl-Val-Ala-Asp-
- LY294002
- 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride.
References
- Arúajo et al., 2009.Arúajo AM, Mendez JC, Coelho AL, Sousa B, Barata F, Figueiredo A, Amaro T, Azevedo I, Soares M. (2009) Phase II study of celecoxib with cisplatin plus etoposide in extensive-stage small cell lung cancer. Cancer Invest 27:391–396 [DOI] [PubMed] [Google Scholar]
- Ashkenazi, 2008.Ashkenazi A. (2008) Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev 19:325–331 [DOI] [PubMed] [Google Scholar]
- Bai et al., 2009.Bai L, Chen W, Chen W, Wang X, Tang H, Lin Y. (2009) IKKbeta-mediated nuclear factor-kappaB activation attenuates smac mimetic-induced apoptosis in cancer cells. Mol Cancer Ther 8:1636–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown and DuBois, 2004.Brown JR, DuBois RN. (2004) Cyclooxygenase as a target in lung cancer. Clin Cancer Res 10:4266s–4269s [DOI] [PubMed] [Google Scholar]
- Chen et al., 2007a.Chen S, Liu X, Yue P, Schönthal AH, Khuri FR, Sun SY. (2007a) CCAAT/enhancer binding protein homologous protein-dependent death receptor 5 induction and ubiquitin/proteasome-mediated cellular FLICE-inhibitory protein down-regulation contribute to enhancement of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by dimethyl-celecoxib in human non small-cell lung cancer cells. Mol Pharmacol 72:1269–1279 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2007b.Chen W, Wang X, Zhuang J, Zhang L, Lin Y. (2007b) Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis 28:2114–2121 [DOI] [PubMed] [Google Scholar]
- Chuang et al., 2008.Chuang HC, Kardosh A, Gaffney KJ, Petasis NA, Schönthal AH. (2008) COX-2 inhibition is neither necessary nor sufficient for celecoxib to suppress tumor cell proliferation and focus formation in vitro. Mol Cancer 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda, 2009.Fulda S. (2009) Tumor resistance to apoptosis. Int J Cancer 124:511–515 [DOI] [PubMed] [Google Scholar]
- Gatenby et al., 2009.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. (2009) Adaptive therapy. Cancer Res 69:4894–4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhough et al., 2009.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. (2009) The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30:377–386 [DOI] [PubMed] [Google Scholar]
- Hanada et al., 2004.Hanada M, Feng J, Hemmings BA. (2004) Structure, regulation and function of PKB/AKT–a major therapeutic target. Biochim Biophys Acta 1697:3–16 [DOI] [PubMed] [Google Scholar]
- Hardy et al., 2003.Hardy MM, Blomme EA, Lisowski A, Chinn KS, Jones A, Harmon JM, Opsahl A, Ornberg RL, Tripp CS. (2003) Selective cyclooxygenase-2 inhibition does not alter keratinocyte wound responses in the mouse epidermis after abrasion. J Pharmacol Exp Ther 304:959–967 [DOI] [PubMed] [Google Scholar]
- Heath-Engel et al., 2008.Heath-Engel HM, Chang NC, Shore GC. (2008) The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene 27:6419–6433 [DOI] [PubMed] [Google Scholar]
- Hida et al., 1998.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. (1998) Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res 58:3761–3764 [PubMed] [Google Scholar]
- Ju et al., 2007.Ju W, Wang X, Shi H, Chen W, Belinsky SA, Lin Y. (2007) A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol 71:1381–1388 [DOI] [PubMed] [Google Scholar]
- Kern et al., 2006.Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H, Fleischer B, Trautwein C, Michalski C, Schulze-Bergkamen H, et al. (2006) Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res 66:7059–7066 [DOI] [PubMed] [Google Scholar]
- Keuling et al., 2009.Keuling AM, Felton KE, Parker AA, Akbari M, Andrew SE, Tron VA. (2009) RNA silencing of Mcl-1 enhances ABT-737-mediated apoptosis in melanoma: role for a caspase-8-dependent pathway. PLoS One 4:e6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto et al., 1996.Kusumoto H, Rodgers QE, Boege F, Raimondi SC, Beck WT. (1996) Characterization of novel human leukemic cell lines selected for resistance to merbarone, a catalytic inhibitor of DNA topoisomerase II. Cancer Res 56:2573–2583 [PubMed] [Google Scholar]
- Ladu et al., 2008.Ladu S, Calvisi DF, Conner EA, Farina M, Factor VM, Thorgeirsson SS. (2008) E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer. Gastroenterology 135:1322–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2008a.Lee JM, Yanagawa J, Peebles KA, Sharma S, Mao JT, Dubinett SM. (2008a) Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol 66:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2008b.Lee YH, Suzuki YJ, Griffin AJ, Day RM. (2008b) Hepatocyte growth factor regulates cyclooxygenase-2 expression via beta-catenin, Akt, and p42/p44 MAPK in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 294:L778–L786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al., 2001.Lin MT, Lee RC, Yang PC, Ho FM, Kuo ML. (2001) Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells. Involvement of phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem 276:48997–49002 [DOI] [PubMed] [Google Scholar]
- Lin et al., 2000.Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, Liu ZG. (2000) The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol Cell Biol 20:6638–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi and Gores, 2006.Malhi H, Gores GJ. (2006) TRAIL resistance results in cancer progression: a TRAIL to perdition? Oncogene 25:7333–7335 [DOI] [PubMed] [Google Scholar]
- Malhotra et al., 2004.Malhotra S, Shafiq N, Pandhi P. (2004) COX-2 inhibitors: a CLASS act or Just VIGORously promoted. MedGenMed 6:6. [PMC free article] [PubMed] [Google Scholar]
- Mutter et al., 2009.Mutter R, Lu B, Carbone DP, Csiki I, Moretti L, Johnson DH, Morrow JD, Sandler AB, Shyr Y, Ye F, et al. (2009) A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res 15:2158–2165 [DOI] [PubMed] [Google Scholar]
- Ozes et al., 1999.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82–85 [DOI] [PubMed] [Google Scholar]
- Papenfuss et al., 2008.Papenfuss K, Cordier SM, Walczak H. (2008) Death receptors as targets for anti-cancer therapy. J Cell Mol Med 12:2566–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop and Salvesen, 2009.Pop C, Salvesen GS. (2009) Human caspases: activation, specificity, and regulation. J Biol Chem 284:21777–21781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao et al., 2004.Rao R, Hao CM, Breyer MD. (2004) Hypertonic stress activates glycogen synthase kinase 3beta-mediated apoptosis of renal medullary interstitial cells, suppressing an NFkappaB-driven cyclooxygenase-2-dependent survival pathway. J Biol Chem 279:3949–3955 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 2009.Suzuki R, Yamamoto M, Saka H, Taniguchi H, Shindoh J, Tanikawa Y, Nomura F, Gonda H, Imaizumi K, Hasegawa Y, et al. (2009) A phase II study of carboplatin and paclitacel with meloxicam. Lung Cancer 63:72–76 [DOI] [PubMed] [Google Scholar]
- Telliez et al., 2006.Telliez A, Furman C, Pommery N, Hénichart JP. (2006) Mechanisms leading to COX-2 expression and COX-2 induced tumorigenesis: topical therapeutic strategies targeting COX-2 expression and activity. Anticancer Agents Med Chem 6:187–208 [DOI] [PubMed] [Google Scholar]
- Wajant et al., 2005.Wajant H, Gerspach J, Pfizenmaier K. (2005) Tumor therapeutics by design: targeting and activation of death receptors. Cytokine Growth Factor Rev 16:55–76 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2006a.Wang Q, Zhou Y, Wang X, Evers BM. (2006a) Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene 25:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2007.Wang X, Chen W, Lin Y. (2007) Sensitization of TNF-induced cytotoxicity in lung cancer cells by concurrent suppression of the NF-kappaB and Akt pathways. Biochem Biophys Res Commun 355:807–812 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2008.Wang X, Chen W, Zeng W, Bai L, Tesfaigzi Y, Belinsky SA, Lin Y. (2008) Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Mol Cancer Ther 7:1156–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2006b.Wang X, Ju W, Renouard J, Aden J, Belinsky SA, Lin Y. (2006b) 17-allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Res 66:1089–1095 [DOI] [PubMed] [Google Scholar]
- Wang and Lin, 2008.Wang X, Lin Y. (2008) Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin 29:1275–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al., 2009.Wilson TR, Johnston PG, Longley DB. (2009) Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer Drug Targets 9:307–319 [DOI] [PubMed] [Google Scholar]
- Xu et al., 2004.Xu L, Zhang L, Yi Y, Kang HK, Datta SK. (2004) Human lupus T cells resist inactivation and escape death by up-regulating COX-2. Nat Med 10:411–415 [DOI] [PubMed] [Google Scholar]