Abstract
Milk thistle (Silybum marianum) is a popular herbal product used for hepatoprotection and chemoprevention. Two commercially available formulations are the crude extract, silymarin, and the semipurified product, silibinin. Silymarin consists of at least seven flavonolignans, of which the most prevalent are the diastereoisomers silybin A and silybin B; silibinin consists only of silybin A and silybin B. Based on a recent clinical study showing an interaction between a silymarin product and the CYP2C9 substrate losartan, the CYP2C9 inhibition properties of silybin A and silybin B and corresponding regioisomers, isosilybin A and isosilybin B, were evaluated using human liver microsomes (HLMs), recombinant CYP2C9 (rCYP2C9) enzymes, and the clinically relevant probe, (S)-warfarin. Silybin B was the most potent inhibitor in HLMs, followed by silybin A, isosilybin B, and isosilybin A (IC50 of 8.2, 18, 74, and >100 μM, respectively). Next, silybin A and silybin B were selected for further characterization. As with HLMs, silybin B was more potent than silybin A toward rCYP2C9*1 (6.7 versus 12 μM), rCYP2C9*2 (9.3 versus 19 μM), and rCYP2C9*3 (2.4 versus 9.3 μM). Using a matrix of five substrate (1–15 μM) and six inhibitor (1–80 μM) concentrations and HLMs, both diastereoisomers inhibited (S)-warfarin 7-hydroxylation in a manner described best by a mixed-type inhibition model (Ki values of 4.8 and 10 μM for silybin B and silybin A, respectively). These observations, combined with the high systemic silibinin concentrations (>5–75 μM) achieved in a phase I study involving prostate cancer patients, prompt clinical evaluation of a potential warfarin-milk thistle interaction.
Milk thistle [Silybum marianum (L.) Gaertn.] is a resilient and sometimes noxious plant that has been valued for its medicinal qualities for more than 2000 years (Kroll et al., 2007; Post-White et al., 2007). In modern herbal compendia, milk thistle is used to self-treat hepatic disorders, including hepatitis C and cirrhosis, and as a hepatoprotectant, particularly for mushroom poisoning. Milk thistle is available mainly as an extract prepared from the seeds of the plant. The two most common commercial preparations are termed silymarin and silibinin. Silymarin, a crude extract, is a complex mixture of at least seven flavonolignans and one flavonoid (taxifolin) (Davis-Searles et al., 2005; Kroll et al., 2007). The most abundant flavonolignans are the diastereoisomers silybin A and silybin B (Fig. 1). The diastereoisomers isosilybin A and isosilybin B (Fig. 1) also are present and are regioisomers of silybin A and silybin B. The remaining three flavonolignans are silychristin, isosilychristin, and silydianin, all of which are constitutional isomers of the aforementioned compounds (Davis-Searles et al., 2005). Silibinin is a semipurified extract, representing approximately a 1:1 mixture of silybin A and silybin B. Because silymarin and silibinin are mixtures of compounds derived from a natural source, batch-to-batch variation in bioactive ingredient composition occurs, which can confound the interpretation of study results (Kroll et al., 2007). Fortuitously, methods have been developed to isolate and purify gram quantities of each flavonolignan from milk thistle extract, permitting delineation of the disposition and action of single constituents (Kim et al., 2001; Graf et al., 2007).
Fig. 1.
Structures of the four selected flavonolignans from milk thistle.
Milk thistle has garnered attention since the 1990s for its chemopreventive properties, particularly for prostate cancer (Agarwal et al., 2006; Gazák et al., 2007; Kroll et al., 2007). With the advent of single components from silymarin, the antiproliferative effects of each flavonolignan, as well as those of silymarin and silibinin, were compared using human prostate cancer cell lines; at concentrations ranging from 15 to 90 μM, isosilybin B was consistently the most potent of all the individual components and mixtures examined (Davis-Searles et al., 2005). In rodent models of prostate cancer, dietary feeding of silymarin and silibinin has been shown, respectively, to decrease the incidence of 3,2′-dimethyl-4-aminobiphenyl-induced prostatic adenocarcinoma (Kohno et al., 2005) and to inhibit prostate tumor growth (Ramasamy and Agarwal, 2008; Singh et al., 2008). The latter studies set the target systemic concentration of silibinin at 10 to 15 μM.
Clinical studies have indicated that both silymarin and silibinin have poor oral bioavailability because of extensive first-pass conjugation via UDP-glucuronosyl transferases and sulfotransferases (Flaig et al., 2007; Schrieber et al., 2008; Wen et al., 2008). As such, at typical “doses,” systemic concentrations of unconjugated flavonolignans >1 μM are rarely achieved. The first study to demonstrate systemic concentrations of unconjugated silibinin in the purported therapeutic range was a phase I dose escalation study involving prostate cancer patients (Flaig et al., 2007). These patients were administered 2.5 to 20 g/day of a silibinin-phosphatidylcholine complex (Siliphos), which has improved absorption characteristics and presumably improved bioavailability compared with noncomplexed silibinin (Flaig et al., 2007). Average peak plasma concentrations of unconjugated silibinin ranged from 5 to 75 μM. Moreover, these high doses were well tolerated, and several patients experienced prolonged stable disease, prompting a phase II study that is currently underway.
Herbal products often are taken concomitantly with medications, which could potentially lead to dangerous interactions (Hu et al., 2005; Wu et al., 2009). Clinical studies involving milk thistle extracts to date have shown minimal to no drug interaction liability, at least with drugs that are considered probe substrates for CYP1A2, CYP2D6, CYP2E1, and CYP3A and the efflux transporter P-glycoprotein (Gurley et al., 2004, 2006a,b, 2008). A caveat to these studies is that a low total daily “dose” (<1 g) of extract (silymarin) was given, which is much less than that postulated to have clinical benefit, at least for prostate cancer. However, a recent healthy volunteer study demonstrated an interaction between the antihypertensive agent and CYP2C9/CYP3A substrate losartan and silymarin, the latter of which was given as a low total daily dose (420 mg) (Han et al., 2009). Relative to placebo, in CYP2C9*1 carriers, silymarin decreased the area under the curve ratio of the CYP2C9-mediated active metabolite, E-3174, to that of losartan, by ∼50% (p < 0.05), suggesting inhibition of hepatic CYP2C9 by one or more components of silymarin. These observations, coupled with the high systemic concentrations observed in the dose escalation study, prompted a systematic evaluation of the inhibitory effects of individual components from silymarin on the CYP2C9-mediated metabolism of another clinically relevant substrate, (S)-warfarin, the more pharmacologically active enantiomer of the widely prescribed oral anticoagulant warfarin. In particular, the inhibitory potencies of four key flavonolignans (silybin A, silybin B, isosilybin A, and isosilybin B) toward (S)-warfarin 7-hydroxylation were compared using human liver microsomes and recombinant CYP2C9 enzymes. To the authors' knowledge, this work represents the first evaluation of the drug interaction liability of single, purified constituents from milk thistle.
Materials and Methods
Chemicals and Reagents
Human liver microsomes (HLMs) (pooled from 50 donors, mixed gender) were purchased from XenoTech, LLC (Lenexa, KS). Baculovirus insect cell-expressed CYP2C9*1, CYP2C9*2, CYP2C9*3 (supplemented with cDNA-expressed reductase but not cytochrome b5), tienilic acid, and 7-hydroxywarfarin were purchased from BD Biosciences (San Jose, CA). (S)-Warfarin, chlorowarfarin, sulfaphenazole, NADPH, and high-performance liquid chromatography-grade water, methanol, ammonium acetate, and 1-propanol were purchased from Sigma-Aldrich (St. Louis, MO). Silybin A, silybin B, isosilybin A, and isosilybin B were isolated from milk thistle extract as described previously (Graf et al., 2007); all flavonolignans were >97% pure as determined by high-performance liquid chromatography.
Evaluation of Silymarin Flavonolignans as Inhibitors of CYP2C9 Activity
The inhibitory effects of each flavonolignan on (S)-warfarin 7-hydroxylation were evaluated using pooled HLMs and recombinant CYP2C9 (rCYP2C9). (S)-Warfarin and sulfaphenazole were dissolved in methanol to yield working concentrations of 10 and 1 mM, respectively. Each flavonolignan was dissolved in methanol to yield a working concentration of 50 mM. NADPH was dissolved fresh in potassium phosphate buffer (0.1 M, pH 7.4) to yield a working concentration of 4 mM. Incubation mixtures were prepared in 96-well plates. Under all experimental conditions, the amount of 7-hydroxywarfarin formed was linear with respect to incubation time and mass/amount of microsomal protein or rCYP2C9 (data not shown).
Initial Testing.
Incubation mixtures consisted of HLMs (0.1 mg/ml microsomal protein), (S)-warfarin (4 μM), flavonolignan (1, 10, and 100 μM), and potassium phosphate buffer (100 mM, pH 7.4). As a positive control for CYP2C9 inhibition, incubation mixtures contained sulfaphenazole (1 μM) in place of flavonolignan. Control incubation mixtures contained 0.75% methanol (v/v) in place of flavonolignan/sulfaphenazole. The plates were placed on a dry heat block, and the mixtures were equilibrated for 5 min at 37°C before initiation of the reactions with NADPH (1 mM final concentration) to yield a final volume of 200 μl. After 30 min, the reactions were quenched with 400 μl of cold methanol containing 3.33 nM chlorowarfarin as the internal standard. After centrifugation (1350g for 10 min at 4°C), the supernatant (8 μl) was analyzed for 7-hydroxywarfarin by LC-MS/MS (described below).
Ki Determination for Silybin A and Silybin B Using HLMs.
Incubation mixtures were prepared in a manner similar to that described above using a 5 × 6 matrix of substrate (1–15 μM) and flavonolignan (1–80 μM) concentrations. The reaction mixtures were processed further and analyzed for 7-hydroxywarfarin as described above.
IC50 Determination for Silybin A and Silybin B Using rCYP2C9 Enzymes.
Incubation mixtures consisting of rCYP2C9 (12.5 pmol/ml), (S)-warfarin (4 μM), flavonolignan (0.5–100 μM), and potassium phosphate buffer were equilibrated for 5 min at 37°C before initiation of the reactions with NADPH. After 30 (rCYP2C9*1 and rCYP2C9*2) or 60 (rCYP2C9*3) min, the reactions were quenched and processed as described above.
Testing Of Silybin A and Silybin B as Mechanism-Based Inhibitors of CYP2C9 Using HLMs.
IC50 shift experiments (Obach et al., 2007) were used to determine whether silybin A and silybin B are mechanism-based inhibitors of hepatic CYP2C9 activity. Primary incubation mixtures consisting of HLMs (1 mg/ml), flavonolignan (0.1–1000 μM), and potassium phosphate buffer were equilibrated for 5 min at 37°C before initiation of the reactions with NADPH. Control primary reaction mixtures were identical except that NADPH was absent. As a positive control for mechanism-based inhibition, incubation mixtures contained tienilic acid (1.0–500 μM) in place of flavonolignan. After 30 min, an aliquot (20 μl) was removed and diluted 10-fold into a secondary incubation mixture containing (S)-warfarin (4 μM) and NADPH (1 mM). After an additional 30 min, the secondary reactions were quenched and processed as described above.
Analysis of Microsomal Incubations for 7-Hydroxywarfarin
7-Hydroxywarfarin was quantified by LC-MS/MS using a solvent delivery system (Shimadzu, Columbia, MD) and an HTC Pal thermostated autosampler (LEAP Technologies, Carrboro, NC) connected to an API 4000 triple quadruple mass spectrometer equipped with a TurboSpray ion source (Applied Biosystems, Foster City, CA). Tuning, operation, integration, and data analyses were carried out in negative mode using multiple reaction monitoring (Analyst software version 1.4.1; Applied Biosystems). Analytes (7-hydroxywarfarin and chlorowarfarin) and other metabolites were separated using a Gemini C18 column (30 × 2.0 mm, 5 μm particle size; Phenomenex, Torrance, CA) and a solvent flow rate of 0.75 ml/min. The initial gradient condition was 100% 10 mM ammonium acetate, which was held for 0.7 min, and the eluent was directed to waste. From 0.7 to 4.5 min, the mobile phase composition increased linearly to 60% methanol, and the eluent was directed to the mass spectrometer. At 5.0 min, the eluent was directed again to waste, and the column was flushed with 80% methanol for 0.4 min. From 6.0 to 6.5 min, the system was equilibrated with 100% 10 mM ammonium acetate. Total run time, including equilibration, was 6.5 min/injection. Duplicate 10-point calibration curves for 7-hydroxywarfarin (0.2–100 nM) were constructed using the peak area ratio of 7-hydroxywarfarin (323.06 → 176.8; retention time 3.34 min) to chlorowarfarin (341.2 → 160.9; retention time 4.24 min). Interday accuracy and precision ranged from 99 to 110% and from 9.0 to 12%, respectively, for all quality controls (0.23, 1.5, and 15 pmol).
Data Analysis
Apparent IC50 Determination.
Initial estimates of apparent IC50 values were derived from linear regression of the velocity versus natural logarithm of flavonolignan concentration data. Apparent IC50 values were determined by fitting eq. 1 with untransformed data using WinNonlin (version 5.0.1; Pharsight, Mountain View, CA):
 |
where S denotes the concentration of (S)-warfarin, and v0 and v denote the velocity of 7-hydroxywarfarin formation in the absence and presence of flavonolignan, respectively.
Apparent Ki Determination.
Initial estimates of apparent Km and Vmax were derived from Eadie-Hofstee plots of the velocity versus velocity/[substrate] data in the absence of flavonolignan. Initial estimates of apparent Ki values were derived from Dixon plots of velocity−1 versus flavonolignan concentration data. Kinetic parameters (Km, Vmax, and Ki) were obtained by fitting eqs. 2 to 4 for a unienzyme system with untransformed data:
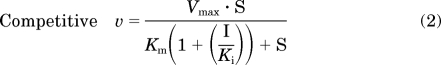 |
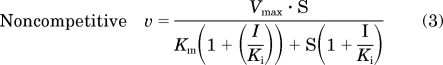 |
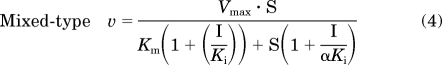 |
where S again denotes the concentration of (S)-warfarin, I denotes the concentration of inhibitor, and α (eq. 4) denotes a parameter to describe the affinity change of the enzyme-substrate and enzyme-inhibitor complexes (Geng, 2003). When α = 1, eq. 4 simplifies to the pure noncompetitive inhibition model (eq. 3); when α is very large (approaches infinity), eq. 4 simplifies to the pure competitive inhibition model (eq. 2). The best-fit equation was assessed from visual inspection of the observed versus predicted data, randomness of the residuals, Akaike information criteria, and S.E.s of the parameter estimates. Apparent intrinsic clearance (Clint) was calculated as the ratio of Vmax to Km.
Statistical Analysis
All statistical analyses were carried out using SigmaStat (version 3.5; Systat Software, Inc., San Jose, CA). Data are presented as means ± S.D. of triplicate determinations, unless indicated otherwise. Concentration-dependent inhibition of each flavonolignan in HLMs was evaluated by one-way analysis of variance (ANOVA); post hoc comparisons were made using Tukey's test when an overall difference resulted (p < 0.05). Enzyme kinetic parameters are presented as estimates ± S.E. Statistical differences between the calculated IC50 for silybin A and silybin B within an enzyme source was evaluated by a Student's t test of two independent samples; p < 0.05 was considered significant. Statistical differences between the calculated IC50 for a given inhibitor among enzyme sources was evaluated by one-way ANOVA; post hoc comparisons were made using Tukey's test when an overall difference resulted (p < 0.05).
Results
Selected Flavonolignans Differentially Inhibit CYP2C9-Mediated Warfarin Metabolism.
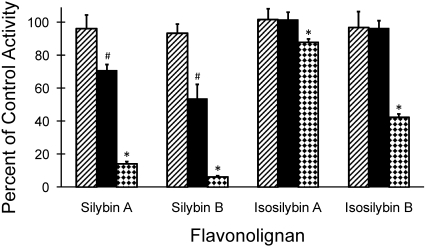
Each flavonolignan inhibited the 7-hydroxylation of (S)-warfarin in a concentration-dependent manner, with the diastereoisomer pair silybin A and silybin B showing greater potency than their regioisomer counterparts isosilybin A and isosilybin B (Fig. 2). Within each diastereoisomer pair, the B forms were slightly more potent than the A forms. As reflected by the IC50 values, silybin B was the most potent of the flavonolignans tested, followed by silybin A, isosilybin B, and isosilybin A (Table 1). Isosilybin A and isosilybin B were not evaluated further because of the relative lack of inhibitory potency.
Fig. 2.
Inhibitory effects of selected flavonolignans on (S)-warfarin 7-hydroxylation activity in human liver microsomes. Human liver microsomes (0.1 mg/ml) were incubated with (S)-warfarin (4 μM) and flavonolignan (1, 10, or 100 μM; hatched, solid, and checkered bars, respectively) for 30 min. Reactions were initiated by the addition of NADPH (1 mM). (S)-Warfarin 7-hydroxylation activity in the presence of vehicle control (0.75% methanol, v/v) was 5.8 ± 0.1 pmol/min/mg microsomal protein. Bars and error bars denote means and S.D., respectively, of triplicate incubations. *, p < 0.05 versus flavonolignan at 10 μM; #, p < 0.05 versus flavonolignan at 1 μM (two-way ANOVA, followed by Tukey's test).
TABLE 1.
Comparison of IC50 values for four key flavonolignans from milk thistle using (S)-warfarin 7-hydroxylation as an index of CYP2C9 activity
Values represent the estimate ± S.E.
| Enzyme Source | IC50 |
|||
|---|---|---|---|---|
| Silybin A | Silybin B | Isosilybin A | Isosilybin B | |
| μM | ||||
| Reversible inhibition experimental design | ||||
| HLMs | 18 ± 2.2 | 8.2 ± 1.4a | >100 | 74 ± 11 |
| CYP2C9*1 | 12 ± 0.9 | 6.7 ± 0.5a | N.D. | N.D. |
| CYP2C9*2 | 19 ± 1.7 | 9.3 ± 0.9a | N.D. | N.D. |
| CYP2C9*3 | 9.3 ± 2.0b | 2.4 ± 0.6a,b | N.D. | N.D. |
| IC50 shift experimental design | ||||
| HLMs (−NADPH)c | 18 ± 0.7 | 7.8 ± 0.5 | N.D. | N.D. |
| HLMs (+NADPH)d | 17 ± 0.7 | 7.9 ± 0.9 | N.D. | N.D. |
N.D., not determined.
Significantly different from the IC50 for silybin A (p < 0.05, Student's t test of two independent samples).
Significantly different from the IC50 obtained with HLMs, CYP2C9*1, and CYP2C9*2 (p < 0.05, one-way ANOVA, followed by Tukey's test) for a given inhibitor.
NADPH was absent during the primary incubation.
NADPH was present during the primary incubation.
Silybin B Is a More Potent Inhibitor of CYP2C9-Mediated Warfarin Metabolism than Silybin A.
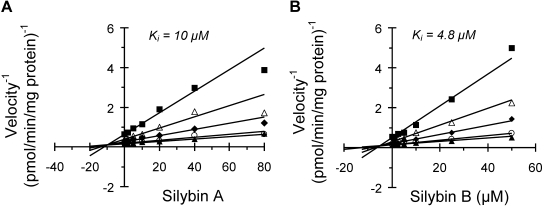
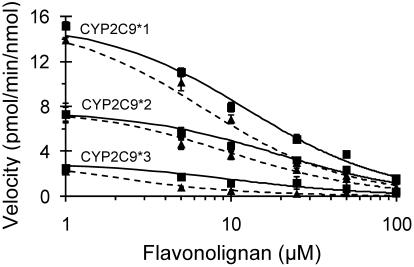
The apparent Ki for silybin A and silybin B toward (S)-warfarin 7-hydroxylation was determined using a 5 × 6 matrix of substrate-inhibitor concentrations and HLMs (Fig. 3). The simple linear mixed-type inhibition model (eq. 4) best described the data for both silybin A and silybin B. In the absence of inhibitor, 7-hydroxywarfarin formation was consistent with classic Michaelis-Menten unienzyme kinetics, as evidenced by linear Eadie-Hofstee plots (data not shown). The Km and Vmax of 7-hydroxywarfarin formation were, respectively, 4.0 ± 0.5 μM and 7.7 ± 0.5 pmol/min/mg protein (silybin A) or 3.4 ± 0.4 μM and 9.1 ± 0.6 pmol/min/mg protein (silybin B). Clint values were 1.9 and 2.7 μl/min/mg, respectively. The Ki for silybin A was twice that for silybin B (Fig. 3). The α values for silybin A and silybin B were 5 and 8, respectively. As observed with HLMs, silybin B was more potent than silybin A with all recombinant enzyme variants (Fig. 4). At the lowest flavonolignan concentration tested, the velocity for CYP2C9*1 was approximately twice that of CYP2C9*2, which was approximately twice that of CYP2C9*3. For a given inhibitor, the calculated IC50 value for CYP2C9*3 was significantly lower than that for HLM, CYP2C9*1, and CYP2C9*2 (Table 1).
Fig. 3.
Dixon plots showing the inhibition of (S)-warfarin 7-hydroxylation by silybin A (A) and silybin B (B) in HLMs. HLMs (0.1 mg/ml) were incubated with (S)-warfarin (1–15 μM) and flavonolignan (1–80 μM silybin A or 1–50 μM silybin B) for 30 min. Reactions were initiated by the addition of NADPH (1 mM). Symbols denote means of duplicate incubations. Solid lines denote regression lines through values generated from a simple mixed-type inhibition model using WinNonlin (version 5.0.1).
Fig. 4.
Inhibitory effects of silybin A and silybin B on (S)-warfarin 7-hydroxylation activity in recombinant CYP2C9 enzymes. Recombinant enzymes (12.5 pmol/ml) were incubated with (S)-warfarin (4 μM) and a range of concentrations (1–100 μM) of silybin A (■) or silybin B (▴) for 30 (CYP2C9*1 and CYP2C9*2) or 60 (CYP2C9*3) min. Reactions were initiated by the addition of NADPH (1 mM). (S)-Warfarin 7-hydroxylation activity in the presence of vehicle control (0.75% methanol, v/v) was, respectively, 15 ± 1.3, 8.0 ± 0.4, and 3.6 ± 0.3 pmol/min/nmol of recombinant enzyme. Symbols and error bars denote means and S.D., respectively, of triplicate incubations. Solid (silybin A) and dashed (silybin B) curves denote nonlinear least-squares regression of observed values using WinNonlin (version 5.0.1).
Silybin A and Silybin B Do Not Appear to Be Mechanism-Based Inhibitors of (S)-Warfarin 7-Hydroxylation.
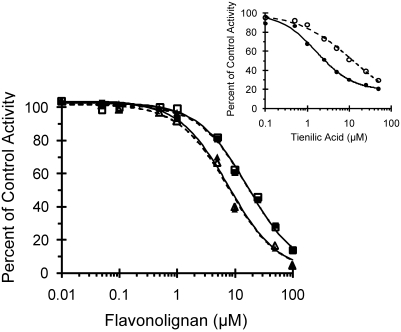
IC50 shift experiments with HLMs were carried out to determine whether silybin A and silybin B are mechanism-based inhibitors of hepatic CYP2C9. When NADPH was absent from the primary incubation mixture, the calculated IC50 values for each flavonolignan agreed with those from initial experiments (Table 1). When NADPH was present in the primary reaction mixture, IC50 values remained unchanged (Fig. 5). The calculated IC50 value for the positive control, tienilic acid, shifted from 12 ± 0.9 μM in the absence of NADPH to 1.7 ± 0.1 μM in the presence of NADPH (Fig. 5, inset).
Fig. 5.
IC50 shift plot for silybin A and silybin B. Human liver microsomes (0.1 mg/ml) were incubated first with silybin A (squares) or silybin B (triangles) (0.1–1000 μM) in the presence (closed symbols) or absence (open symbols) of NADPH (1 mM). The primary reaction mixture was diluted 10-fold to initiate the secondary reaction, which contained NADPH (1 mM) and (S)-warfarin (4 μM). (S)-Warfarin 7-hydroxylation activity in the presence of vehicle control (0.75% methanol, v/v) was 1.5 and 1.6 pmol/min/mg microsomal protein in the absence and presence, respectively, of NADPH. The inset depicts the effects of the known CYP2C9 mechanism-based inhibitor, tienilic acid (circles) (1–500 μM). Symbols denote means of duplicate incubations. Open symbols denote observed values when NADPH was absent from the primary reaction mixture; solid symbols denote observed values when NADPH was present in the primary reaction mixture. Curves denote nonlinear least-squares regression of observed values using WinNonlin (version 5.0.1).
Discussion
Warfarin is a widely prescribed oral anticoagulant used to treat thromboembolic disorders (Rettie and Tai, 2006). Despite more than 50 years of clinical experience, optimal warfarin therapy remains challenging because of a narrow therapeutic window and large interpatient differences in anticoagulant response. Thus, the daily therapeutic warfarin dose varies by more than an order of magnitude in a given population (Rettie and Tai, 2006). The clinically available formulation of warfarin is a racemic mixture, with the S-enantiomer having an estimated 5-fold greater pharmacologic potency than the R-enantiomer (Jonas and McLeod, 2009). (S)-Warfarin is eliminated from the body almost exclusively via hepatic metabolism by CYP2C9, with 7-hydroxylation representing the major metabolic pathway (Jonas and McLeod, 2009). Accordingly, any process that significantly impairs CYP2C9 activity would be expected to decrease the clearance of (S)-warfarin and increase anticoagulant response. Indeed, a number of clinically used CYP2C9 inhibitors, e.g., fluconazole, amiodarone, and trimethoprim/sulfamethoxazole, have been associated with serious bleeding events in patients receiving warfarin (Thi et al., 2009).
Whereas specific drugs have been identified as CYP2C9 inhibitors, such is not the case for herbal products, which continue to increase in popularity as complementary and alternative medicines for health maintenance, disease prevention, and even disease treatment (Ulbricht et al., 2008). The fact that herbal products are derived from natural sources has led to the widespread notion that they are safe. Hence, these products often are taken with conventional medications, raising the potential for dangerous drug-herb interactions. Moreover, unlike most drug products, herbal products typically contain multiple bioactive ingredients that vary in composition between batches and manufacturers, precluding between-study comparisons, as well as accurate predictions of drug interaction liability (Paine and Oberlies, 2007). A recent dose escalation study involving prostate cancer patients showed that oral gram doses of a crude/semipurified extract of milk thistle, silibinin (2.5–20 g/day), were well tolerated with minimal side effects; based on these encouraging results, a phase II study is underway (Flaig et al., 2007; Kroll et al., 2007; Post-White et al., 2007). Taken together, the goal of the current work was to characterize, systematically, the CYP2C9 inhibition properties of single constituents from milk thistle. Based on previous studies that compared the antiproliferative effects of individual flavonolignans in prostate cancer cell lines and to discern whether changes in stereo- and regiochemistry alter metabolic inhibition, four key flavonolignans (silybin A, silybin B, isosilybin A, and isosilybin B) were selected for evaluation.
Similar to the differential effects of individual flavonolignans observed in prostate cancer cell models (Davis-Searles et al., 2005), individual flavonolignans differentially inhibited the 7-hydroxylation of (S)-warfarin. Whereas isosilybin A and isosilybin B were the most potent antiproliferative compounds, silybin A and silybin B were the most potent CYP2C9 inhibitors. Based on the greater than 4-fold difference in IC50 values between silybin A/silybin B and isosilybin A/isosilybin B (<20 μM versus >70 μM), the latter two flavonolignans were not evaluated further. To determine the mode of CYP2C9 inhibition by silybin A and silybin B, reversible inhibition design experiments that involved a range of substrate and flavonolignan concentrations were undertaken. The mixed-type model best described the data for both compounds, producing Ki values of 4.8 and 10 μM, respectively. The lack of mechanism-based inhibition, as evidenced from the IC50 shift experiments, is inconsistent with results obtained by Sridar et al. (2004), who reported a Ki of 5 μM and a kinact of 0.14 min−1. These investigators used the semipurified mixture, silibinin (termed silybin), a reconstituted enzyme system, and a fluorescent probe substrate, any or all of which could account for this between-laboratory discrepancy.
Xenobiotics, including drugs, herbal products, and dietary substances, are considered to have a high drug interaction liability when the ratio of in vivo inhibitor concentration to Ki is greater than unity (Bachmann and Lewis, 2005). The average plasma and presumably total (i.e., bound + unbound) concentrations of unconjugated silybin A/silybin B achieved in the prostate cancer patient dose-escalation study (Flaig et al., 2007) were at least 5-fold greater than the apparent Ki values measured in the current work. It should be noted that the systemic concentrations reported for these patients represented the sum of silybin A and silybin B. Although dosed as a ∼1:1 mixture (i.e., silibinin), systemic concentrations cannot be considered 1:1 due to differential clearance (Kroll et al., 2007; Wen et al., 2008). Despite this limitation, the low Ki values for both compounds compared with the high concentration of the mixture in plasma would necessitate caution when milk thistle products are taken with warfarin. The potential interproduct variation in milk thistle constituents in commercial products, along with the recent clinical study involving losartan and a silymarin product further support this contention.
Two common single nucleotide polymorphisms in the coding region of the CYP2C9 gene, CYP2C9*2 (R144C) and CYP2C9*3 (I359L), are known to influence warfarin anticoagulant response. Both of the corresponding proteins have lower catalytic activity (approximately 50% and less than 10%, respectively) compared with the protein encoded by the reference allele, CYP2C9*1, and carriers of these variants often require a lower daily dose of warfarin (Jonas and McLeod, 2009). CYP2C9*2 and CYP2C9*3 carriers also may require a longer period of time to become stabilized on warfarin therapy (Rettie and Tai, 2006). Consistent with previous reports, in the absence of inhibitor, the velocities of 7-hydroxywarfarin formation with rCYP2C9*2 and rCYP2C9*3 were roughly 50 and 25%, respectively, of that of rCYP2C9*1 (8.0, 3.6, and 15 pmol/min/nmol, respectively). Among the different recombinant enzyme preparations, rCYP2C9*3 was significantly more sensitive to inhibition by both silybin A and silybin B. These observations, coupled with the increased time to stabilize warfarin therapy and the increased bleeding risk that is associated with this variant, may suggest that coadministration of warfarin and silymarin could further complicate therapy in CYP2C9*3 carriers. Despite the increased sensitivity of rCYP2C9*3, the relatively potent inhibition potential of these compounds toward rCYP2C9*1 and rCYP2C9*2 would not exclude CYP2C9*1 and CYP2C9*2 carriers from being at risk for a potential warfarin-milk thistle interaction.
In summary, unlike drug products, dietary/natural products are not consistently regulated and, accordingly, strict preclinical or clinical testing is not required before marketing. Patients often take these products with their medications, sometimes unbeknownst to their physicians and/or pharmacists, which can lead to potentially dangerous adverse events. The current observations, combined with the reported interaction between silymarin and losartan (Han et al., 2009) and the interproduct variation in milk thistle composition, prompts a clinical study with a standardized milk thistle product and warfarin. Finally, the current work provides an impetus to revisit cytochrome P450-mediated drug-milk thistle interactions using purified single constituents.
Acknowledgments
We thank Dr. Arlene Bridges (University of North Carolina-Chapel Hill ADME Mass Spectrometry Center) for assistance with the development of the LC/MS-MS analytical method for 7-hydroxywarfarin. M.F.P. dedicates this article to Dr. David P. Paine.
This work was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM077482]; and the National Institutes of Health National Cancer Institute [Grant R01-CA104286].
This work was presented in part as a poster: Brantley SJ, Oberlies NH, Kroll DJ, and Paine MF (2008) The flavonolignans silybin A and silybin B from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations. The 15th Annual Meeting of the North American International Society for the Study of Xenobiotics; 2008 Oct 12–16; San Diego, CA. International Society for the Study of Xenobiotics, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161927.
- HLMs
- human liver microsomes
- r
- recombinant
- LC-MS/MS
- liquid chromatography-mass spectrometry
- Clint
- intrinsic clearance
- ANOVA
- analysis of variance.
References
- Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. (2006) Anticancer potential of silymarin: from bench to bed side. Anticancer Res 26:4457–4498 [PubMed] [Google Scholar]
- Bachmann KA, Lewis JD. (2005) Predicting inhibitory drug-drug interactions and evaluating drug interaction reports using inhibition constants. Ann Pharmacother 39:1064–1072 [DOI] [PubMed] [Google Scholar]
- Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ. (2005) Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res 65:4448–4457 [DOI] [PubMed] [Google Scholar]
- Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. (2007) A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs 25:139–146 [DOI] [PubMed] [Google Scholar]
- Gazák R, Walterová D, Kren V. (2007) Silybin and silymarin—new and emerging applications in medicine. Curr Med Chem 14:315–338 [DOI] [PubMed] [Google Scholar]
- Geng W. (2003) A method for identification of inhibition mechanism and estimation of Ki in in vitro enzyme inhibition study. Drug Metab Dispos 31:1456–1457 [DOI] [PubMed] [Google Scholar]
- Graf TN, Wani MC, Agarwal R, Kroll DJ, Oberlies NH. (2007) Gram-scale purification of flavonolignan diastereoisomers from Silybum marianum (milk thistle) extract in support of preclinical in vivo studies for prostate cancer chemoprevention. Planta Med 73:1495–1501 [DOI] [PubMed] [Google Scholar]
- Gurley B, Hubbard MA, Williams DK, Thaden J, Tong Y, Gentry WB, Breen P, Carrier DJ, Cheboyina S. (2006a) Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J Clin Pharmacol 46:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Barone GW, Williams DK, Carrier J, Breen P, Yates CR, Song PF, Hubbard MA, Tong Y, Cheboyina S. (2006b) Effect of milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa) supplementation on digoxin pharmacokinetics in humans. Drug Metab Dispos 34:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Carrier J, Khan IA, Edwards DJ, Shah A. (2004) In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Ther 76:428–440 [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Swain A, Hubbard MA, Williams DK, Barone G, Hartsfield F, Tong Y, Carrier DJ, Cheboyina S, Battu SK. (2008) Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St. John's wort, and Echinacea. Mol Nutr Food Res 52:755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Guo D, Chen Y, Chen Y, Tan ZR, Zhou HH. (2009) Effect of silymarin on the pharmacokinetics of losartan and its active metabolite E-3174 in healthy Chinese volunteers. Eur J Clin Pharmacol 65:585–591 [DOI] [PubMed] [Google Scholar]
- Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S. (2005) Herb-drug interactions: a literature review. Drugs 65:1239–1282 [DOI] [PubMed] [Google Scholar]
- Jonas DE, McLeod HL. (2009) Genetic and clinical factors relating to warfarin dosing. Trends Pharmacol Sci 30:375–386 [DOI] [PubMed] [Google Scholar]
- Kim NC, Oberlies NH, Brine DR, Handy RW, Wani MC, Wall ME. (2001) Isolation of symlandine from the roots of common comfrey (Symphytum officinale) using countercurrent chromatography. J Nat Prod 64:251–253 [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Sugie S, Tsuda H, Tanaka T. (2005) Dietary supplementation with silymarin inhibits 3,2′-dimethyl-4-aminobiphenyl-induced prostate carcinogenesis in male F344 rats. Clin Cancer Res 11:4962–4967 [DOI] [PubMed] [Google Scholar]
- Kroll DJ, Shaw HS, Oberlies NH. (2007) Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther 6:110–119 [DOI] [PubMed] [Google Scholar]
- Obach RS, Walsky RL, Venkatakrishnan K. (2007) Mechanism-based inactivation of human cytochrome P450 enzymes and the prediction of drug-drug interactions. Drug Metab Dispos 35:246–255 [DOI] [PubMed] [Google Scholar]
- Paine MF, Oberlies NH. (2007) Clinical relevance of the small intestine as an organ of drug elimination: drug-fruit juice interactions. Expert Opin Drug Metab Toxicol 3:67–80 [DOI] [PubMed] [Google Scholar]
- Post-White J, Ladas EJ, Kelly KM. (2007) Advances in the use of milk thistle (Silybum marianum). Integr Cancer Ther 6:104–109 [DOI] [PubMed] [Google Scholar]
- Ramasamy K, Agarwal R. (2008) Multitargeted therapy of cancer by silymarin. Cancer Lett 269:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettie AE, Tai G. (2006) The pharmocogenomics of warfarin: closing in on personalized medicine. Mol Interv 6:223–227 [DOI] [PubMed] [Google Scholar]
- Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, Hawke RL. (2008) The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos 36:1909–1916 [DOI] [PubMed] [Google Scholar]
- Singh RP, Raina K, Sharma G, Agarwal R. (2008) Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res 14:7773–7780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridar C, Goosen TC, Kent UM, Williams JA, Hollenberg PF. (2004) Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metab Dispos 32:587–594 [DOI] [PubMed] [Google Scholar]
- Thi L, Shaw D, Bird J. (2009) Warfarin potentiation: a review of the “FAB-4” significant drug interactions. Consult Pharm 24:227–230 [DOI] [PubMed] [Google Scholar]
- Ulbricht C, Chao W, Costa D, Rusie-Seamon E, Weissner W, Woods J. (2008) Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr Drug Metab 9:1063–1120 [DOI] [PubMed] [Google Scholar]
- Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. (2008) Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos 36:65–72 [DOI] [PubMed] [Google Scholar]
- Wu JW, Lin LC, Tsai TH. (2009) Drug-drug interactions of silymarin on the perspective of pharmacokinetics. J Ethnopharmacol 121:185–193 [DOI] [PubMed] [Google Scholar]