Abstract
The cannabinoid CB1 (CB1) and dopamine D2 (D2) receptors are coexpressed in the basal ganglia, an area of the brain involved in such processes as cognition, motor function, and emotional control. Several lines of evidence suggest that CB1 and D2 receptors may oligomerize, providing a unique pharmacology in vitro and in vivo. However, limited information exists on the regulation of CB1 and D2 receptor dimers. We used a novel technique, multicolor bimolecular fluorescence complementation (MBiFC) to examine the subcellular localization of CB1-D2L heterodimers as well as D2L-D2L homodimers in a neuronal cell model, Cath. a differentiated cells. MBiFC was then used to explore the effects of persistent ligand treatment on receptor dimerization at the plasma membrane and intracellularly. Persistent (20-h) agonist treatment resulted in increased formation of CB1-D2L heterodimers relative to the D2L-D2L homodimers. The effects of the D2 agonist quinpirole were restricted to the intracellular compartment and may reflect increased D2L receptor expression. In contrast, treatment with the CB1 receptor agonist (2)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl) cyclohexanol (CP55, 940) produced increases in both membrane and intracellular CB1-D2L heterodimers independently of alterations in CB1 receptor expression. The effects of CB1 receptor activation were attenuated by the CB1 antagonist 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide (AM281) and were both time- and dose-dependent. The effects of CB1 activation were examined further by combining MBiFC with a constitutively active CB1 receptor mutant, CB1T210I. These studies demonstrated that the expression of CB1T210I increased intracellular CB1-D2L heterodimer formation. In summary, agonist-induced modulation of CB1-D2L oligomerization may have physiological implications in diseases such as Parkinson's disease and drug abuse.
Increasing evidence suggests that G protein-coupled receptors (GPCRs) may function in receptor dimeric or higher order oligomeric complexes (for review, see Milligan, 2008). One set of receptors that has received significant attention relevant to oligomerization is the CB1 cannabinoid (CB1) receptor and dopamine D2 (D2) receptor (for review, see Fuxe et al., 2008). It is thought that the cannabinoid system negatively modulates dopamine circuits as activation of the CB1 receptor leads to an attenuation of dopamine signaling (Laviolette and Grace, 2006). The CB1 receptor is widely expressed in the central nervous system, with great abundance in the basal ganglia (Herkenham et al., 1991). CB1 receptors are located on striatal GABAergic neurons (Herkenham et al., 1991), and they are also found on dendrites in both the dorsal striatum and the nucleus accumbens (Pickel et al., 2006). The D2 receptor exists as two splice variants, D2S (short) and D2L (long). The D2S variant is highly expressed on presynaptic dopaminergic neurons, whereas the D2L variant is found postsynaptically on dopaminergic neurons throughout the striatum (Khan et al., 1998; Usiello et al., 2000). 1 These observations reveal that CB1 and D2L receptors have overlapping expression patterns in the striatum and also suggest that they are colocalized in neurons in the nucleus accumbens (see references within Kearn et al., 2005; Pickel et al., 2006).
It has been reported that CB1 and D2 receptors oligomerize, providing unique pharmacology in vitro and in vivo (Glass and Felder, 1997; Jarrahian et al., 2004; Kearn et al., 2005; Marcellino et al., 2008). For example, it was demonstrated in primary rat striatal neurons that concurrent activation of Gαi/o-coupled CB1 and D2 receptors resulted in stimulation of cAMP accumulation (Glass and Felder, 1997). Subsequent experiments using recombinant CB1 and D2L receptors suggested that D2L receptor activation promoted a switch in CB1 receptor coupling from Gαi/o to Gαs (Glass and Felder, 1997). One proposed mechanism for D2 receptor modulation of CB1-G protein coupling may involve receptor oligomerization. This hypothesis was examined by demonstrating a physical interaction between CB1 and D2L receptors using coimmunoprecipitation (Kearn et al., 2005). The same investigators also revealed that the CB1-D2L receptor complex can be dynamically modulated by receptor agonists. More recent studies have examined CB1-D2L heteromers using FRET techniques (Marcellino et al., 2008). Using human embryonic kidney cells transiently transfected with fluorescently tagged CB1 and D2L receptors, a FRET interaction was detected. However, no significant changes in the FRET signal were detected after short-term exposure to CB1 or D2L receptor agonists (Marcellino et al., 2008). The ability of CB1 and D2L receptors to interact is consistent with suggestion of a CB1-D2L heterodimer. Additional behavior and biochemical data support further the physiological relevance of CB1 and D2 receptors heterodimers (Fuxe et al., 2008). However, limited information exists on the cellular localization and regulation of CB1-D2L receptor heterodimers. Despite the therapeutic potential of drugs targeting these receptors, the effect of persistent receptor activation on the dynamics of receptor oligomerization has not been explored.
The most common techniques currently being used to study the physical association of GPCRs include coimmunoprecipitation and traditional resonance energy transfer (FRET and BRET) techniques (Vidi and Watts, 2009). These techniques are typically limited to the study of a single protein-protein complex. In addition, coimmunoprecipitation does not allow for detection of an interacting protein complex within a living cell. To gain further insight into GPCR dimerization in live cells, we recently established the use of multicolor bimolecular fluorescence complementation (MBiFC) (Hu et al., 2002; Shyu et al., 2006) as a tool to investigate GPCR homo- and heteromer oligomerization (Vidi et al., 2008a,b). MBiFC allows for the detection of two separate protein-protein complexes in living cells by visualizing the fluorescence complementation of two distinct spectral variants of green fluorescent protein (Hu and Kerppola, 2003). Moreover, this technique can be used to measure the relative amounts of homodimer versus heterodimer formation in a cell region-specific manner (Vidi et al., 2008b).
The present study uses MBiFC to examine CB1-D2L heterodimers and D2L-D2L homodimers in Cath. a differentiated (CAD) cells. CAD cells are a neuronal cell model that express GAP-43, synaptotagmin, and synaptosome-associated protein of 25 kDa and upon differentiation, form neurite-like processes (Qi et al., 1997). The present results provide additional evidence for the existence of CB1 and D2L receptor oligomers. We also revealed that persistent agonist (i.e., dopaminergic or cannabinergic) treatment favors the formation of the CB1-D2L heterodimer relative to the formation of the D2L-D2L homodimer. The D2 agonist-mediated effects were accompanied by an increase in D2L receptor expression, whereas the CB1 agonist-mediated changes in heterodimer formation appeared to involve primarily CB1 receptor activation. These results provide further insight into the dynamic nature of CB1-D2L oligomerization.
Materials and Methods
Materials.
Human CB1 and D2L cDNAs were obtained from the Missouri S&T cDNA Resource Center (Rolla, MO). Growth media (Dulbecco's modified Eagle's medium), quinpirole, and sulpiride were obtained from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum and bovine calf serum were purchased from Thermo Fisher Scientific (Waltham, MA). Penicillin/streptomycin/amphotericin B antibiotic/antimycotic was purchased from Invitrogen (Carlsbad, CA). Forskolin was purchased from Tocris Bioscience (Ellisville, MO). CP55,940 was a generous gift from Pfizer Pharmaceuticals (New York, NY). [3H]cAMP (25 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). [3H]Spiperone (91 Ci/mmol) and [3H]SR141716A (42 Ci/mmol) were obtained from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). Specific cellular compartment markers (mCherry-mem, YFP-ER, YFP-Endo, and YFP-Golgi) were gifts from Dr. Catherine Berlot (Weis Center for Research, Danville, PA).
Expression Vectors.
Full-length human CB1 and D2L cDNAs were amplified by polymerase chain reaction (PCR) using oligonucleotides with EcoRI, XbaI, or XhoI restriction sites and omitting the stop codons. The PCR products were digested with either EcoRI/XbaI or EcoRI/XhoI and ligated into the corresponding pBiFC vectors. These expression vectors contain nonfluorescent fragments of the N and C termini of the enhanced yellow fluorescent protein (Venus) and the enhanced cyan fluorescent protein (Cerulean). The N-terminal fragments (VN or CN) include residues 1 to 172, whereas the C-terminal fragment of Cerulean (CC) includes residues 155 to 238. This cloning strategy places the fragment on the C terminus of the receptors. In addition, the CB1 and D2L receptor PCR products were digested with either EcoRI/XbaI or EcoRI/XhoI and ligated into expression vectors containing the full-length Venus or Cerulean proteins resulting in the CB1 and D2L receptors tagged at the C terminus with either Venus or Cerulean. The CB1 receptor mutant (CB1T210I) was generated using the QuikChange kit according to the supplier's protocol (Stratagene, La Jolla, CA) in pcDNA3-CB1 and then subcloned into the pBiFC vectors using EcoRI and XbaI restriction enzyme sites. All constructs were verified by DNA sequencing.
Cell Culture and Transient Transfections.
CAD cells were maintained as described previously (Vortherms and Watts, 2004). For microscopic evaluation of BiFC, CAD cells were grown to approximately 70% confluence in four-well LabTek chambered coverslips (Nalge Nunc International, Rochester, NY). Cells were transfected with 1 μl of Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations. In MBiFC experiments, CB1-VN (500 ng), D2L-CC (300 ng), and D2L-CN (300 ng) were transiently cotransfected with 20 ng of either mCherry-Mem, YFP-Endo, YFP-ER, or YFP-Golgi depending on the experiment. Twenty-four hours after transfection, the growth media and transfection reagent were replaced with 400 μl of warm phosphate-buffered saline (PBS), and images were taken using a charge-coupled device camera mounted on a TE2000-U inverted fluorescence microscope (Nikon, Melville, NY) equipped with a 100-W mercury lamp and band-pass filters (Chroma Technology Corp., Rockingham, VT) for Venus (excitation at 500/20 nm; emission at 535/30 nm), Cerulean (excitation, 430/25 nm; emission, 470/30 nm), or mCherry (Texas Red, excitation, 572/23 nm; emission, 625/25 nm). Fluorescent images were acquired using MetaMorph software (Molecular Devices, Sunnyvale, CA). For MBiFC experiments investigating the effects of receptor ligands on receptor dimer population, the cells were transfected as described above and 4 h after transfection, the appropriate drug treatment was added to the growth medium for an additional 20 h before image acquisition.
Quantitative Image Analysis.
Quantification of fluorescent signals was performed as described previously using ImageJ software (http://rsb.info.nih.gov/ij/; Hu et al., 2002; Supplemental Fig. 1). In each experiment, approximately 40 to 50 individual cells were quantified. Ten microscopic fields at 60× magnification were acquired as stacks of images from the YFP, CFP, and Texas Red channels corresponding to the fluorescent signals from Venus, Cerulean, and mCherry proteins, respectively. Background fluorescence intensity was measured in each channel in an area devoid of cells and subtracted from the fluorescent signals. The signals corrected for background fluorescence were then scaled to a factor equal to that of the inverse of the exposure time for each pixel intensity measurement. The images of the mCherry-Mem membrane marker signal were used to select cells for image analysis and to normalize BiFC signals (Supplemental Fig. 1). Cellular analysis of BiFC signals was performed in two parts. First, the fluorescent signal intensity maximum at the membrane was determined by drawing a perpendicular line through the membrane using the mCherry-Mem image. The maximum signal intensity was determined in all three channels, YFP, CFP, and Texas Red to estimate the BiFC signals at the membrane. The BiFC signal intensity in the intracellular space was determined by outlining the intracellular compartment (excluding the plasma membrane) and determining the average pixel intensity in all three channels, YFP, CFP, and Texas Red, to estimate the intracellular BiFC signals. Cells with saturated signals as well as cells with signals that were 1.2 times lower than background were not used for quantification. BiFC experiments assessing bleed-through/overflow of Cerulean or Venus in the opposite channels (i.e., YFP or CFP) revealed minimal cross-talk. Specifically, complemented Cerulean contributed less than 2% of the YFP signal and complemented Venus contributed less than 3% of the CFP signal (data not shown). Venus/Cerulean fluorescence ratios exhibit a non-Gaussian distribution; therefore, median values were calculated and averaged between experiments.
Cyclic AMP Accumulation Assays.
CAD cells were grown to 70% confluence in 24-well plates and were transiently transfected as described previously (Vidi et al., 2008a). CAD cells were either transfected with 300 ng/well D2L constructs or 500 ng/well CB1 constructs. All drugs were diluted in Earle's balanced salt solution assay buffer (Earle's balanced salt solution containing 2% bovine calf serum, 0.025% ascorbic acid, and 15 mM HEPES, pH 7.4) and added to the cells on ice. Determination of cAMP accumulation was performed by incubating the transfected CAD cells with forskolin (10 μM) in the absence and presence of either CP55,940 (10 μM) or quinpirole (10 μM) for 15 min at 37°C. All assays were performed in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (500 μM) and terminated with ice-cold 3% trichloroacetic acid. Quantification of cAMP accumulation was determined using a competitive binding assay as described previously (Vortherms and Watts, 2004).
Radioligand Binding Assays.
Single point radioligand binding assays were used to estimate CB1 and D2L receptor densities after drug treatments as described previously (Vidi et al., 2008a). CAD cells were plated in a 12-well plate and were grown to 70% confluence before being transiently transfected with CB1-VN, D2L-CN, and D2L-CC using 2 μl/well of Lipofectamine 2000. Four hours after transfection, the appropriate drug treatment was added in triplicate to the growth medium and transfection reagent. The cells were incubated for an additional 20 h before single point radioligand binding assays. Cells were washed three times with 500 μl of receptor binding buffer (50 mM Tris and 4 mM MgCl2, pH 7.4). The cells were lysed with 500 μl of ice-cold lysis buffer (1 mM HEPES and 2 mM EDTA, pH 7.4) for 10 min on ice. The cells were removed from each well by trituration, and crude cell membranes were collected by centrifugation (30,000g for 15 min at 4°C). Membrane pellets were resuspended by mechanical homogenization in 1 ml of receptor binding buffer. For CB1 receptor binding, the addition of 0.5% bovine serum albumin to the receptor binding buffer was used to decrease nonspecific binding. Crude cell membranes (approximately 30 μg in 150 μl) were added in duplicate to the assay tubes to determine both nonspecific and total binding. For CB1 binding, nonspecific binding was defined by 10 μM nonradioactive SR141716A (essentially identical levels of nonspecific binding were obtained using 10 μM AM281; data not shown). All tubes contained a near-saturating amount of [3H]SR141716A (50 μl; final concentration, ∼5.0 nM) in a total volume of 500 μl. Likewise, for D2 binding, nonspecific binding was defined with 5 μM (+)-butaclamol, with all reaction conditions containing a near-saturating amount of [3H]spiperone (50 μl; final concentration, ∼1.5 nM) in a total volume of 500 μl. The reaction was terminated by filtration onto FB glass fiber plates with ice-cold wash buffer (10 mM Tris and 0.9% NaCl) using a cell harvester (FilterMate; PerkinElmer Life and Analytical Sciences). Radioactivity was determined a TopCount scintillation counter (PerkinElmer Life and Analytical Sciences). Specific binding was determined as the difference between the average of the nonspecific and total binding conditions. The specific binding amount was normalized to the amount of protein using the bicinchoninic acid protein assay (Pierce Chemical, Rockford, IL) following the supplier's protocol. Under the transfection conditions used to explore the effects of drug treatments on BiFC, the following estimated Kd and Bmax values were obtained via radioligand saturation binding experiments: [3H]SR141716A, Kd = 0.74 ± 0.18 nM and Bmax = 204 ± 28 fmol/mg; and [3H]spiperone, Kd = 0.051 ± 0.02 nM and Bmax = 3550 ± 200 fmol/mg.
Fluorescence Energy Transfer.
CAD cells were grown to 70% confluence in 12-well plates before transfection. Cells were transiently transfected with three general conditions depending on the receptor dimer species to be studied including: cells only expressing the FRET donor (Cerulean), cells only expressing the FRET acceptor (Venus), and cells expressing both the donor and acceptor. To normalize for protein expression in cells only expressing either the donor or acceptor, the total amount of DNA transfected was normalized with the untagged receptor. In each FRET assay, 750 ng/well of the donor (CB1-Cerulean or D2L-Cerulean) and 750 ng/well of the acceptor (CB1-Venus or D2L-Venus) were transiently transfected either alone or in combination 24 h before the experiment. Cells were washed with 500 μl of warm PBS and resuspended in 300 μl of warm PBS. Protein concentration was determined on the cell suspension using the bicinchoninic acid assay method (Pierce Chemical) and normalized to 200 ng/μl with PBS. CAD cells suspensions (40 μg) were transferred into a 96-well black plate (Nalge Nunc International), and fluorescence measurements were evaluated on the FUSION plate reader (PerkinElmer Life and Analytical Sciences). Determination of FRET signals was performed as described previously (Vidi et al., 2008b). In brief, FRET signals were measured using the sensitized acceptor method. Mock-transfected cells were used for background fluorescence. For each sample, Cerulean (C) and Venus (V) was measured using 430/25 nm and 500/20 nm excitation and 470/30 nm and 535/30 nm emission filters. FRET signals (F) were measured using excitation at 430/25 nm and emission at 535/30 nm. Bleed-through coefficients were calculated for the acceptor (a = F/V) and for the donor (d = F/C) in cells only expressing either Cerulean (donor) or Venus (acceptor) fusion proteins. The FRET signals were corrected (cFRET) for acceptor and donor bleed-through using the equation cFRET = F − aV − dC. The signals were then normalized to donor (C) and acceptor (Y) intensities as follows: nFRET = cFRET/√C × V.
Data and Statistical Analysis.
Data and statistical analyses were performed using Prism (GraphPad Software Inc., San Diego, CA). A p value <0.05 was considered significant.
Results
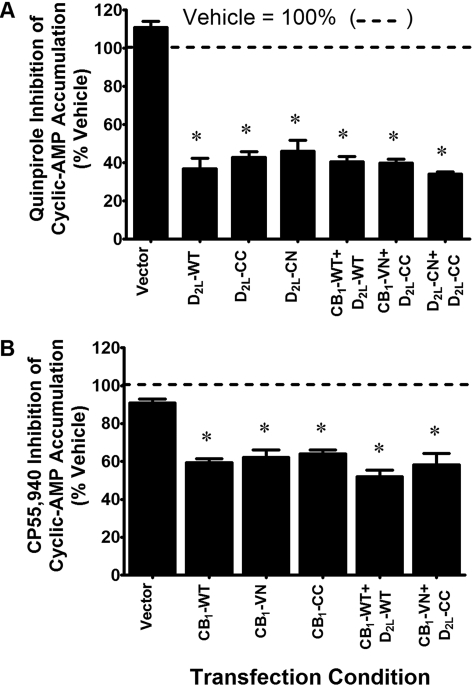
Functional cAMP accumulation assays were performed to verify the signaling properties of the BiFC-tagged CB1 and D2L receptors (Fig. 1). Because CB1 and D2L receptors couple to inhibitory G proteins (i.e., Gαi/o), agonist-induced inhibition of forskolin-stimulated cAMP accumulation was used to evaluate receptor function. The BiFC-tagged D2L receptors D2L-CN and D2L-CC were functional after stimulation with the D2 agonist quinpirole (10 μM), revealing approximately 60% inhibition of forskolin-stimulated cAMP accumulation (Fig. 1A). Additional experiments confirming the functionality of the BiFC-tagged CB1 receptors, CB1-VN and CB1-CC, were performed. Both constructs were functional after stimulation with the CB1 receptor agonist CP55,940 (10 μM), yielding more than 35% inhibition of forskolin-stimulated cAMP accumulation (Fig. 1B). A modest but insignificant degree (approximately 10%) of inhibition was also observed in vector-transfected CAD cells. Receptor signaling in cells coexpressing the wild-type or BiFC-tagged receptors was also examined (Fig. 1, A and B). The D2 agonist quinpirole robustly inhibited forskolin-stimulated cAMP accumulation in cells coexpressing CB1 and D2L receptors (CB1 + D2L or CB1-VN + D2L-CC) as well as cells cotransfected with D2L-CN and D2L-CC. Similar experiments revealed that CP55,940, inhibited forskolin-stimulated cAMP accumulation in cells coexpressing CB1 and D2L receptors (CB1 + D2L and CB1-VN + D2L-CC). These data suggest that the addition of a C-terminal tag (-VN, -CN, or -CC) and fluorescence complementation (see below) do not adversely affect agonist-mediated inhibition of cAMP accumulation.
Fig. 1.
Functional characterization of receptor-BiFC fragment fusion proteins by measurement of acute inhibition of forskolin-stimulated cAMP accumulation. CAD cells were transiently transfected as indicated. Cyclic AMP accumulation was measured after a 15-min incubation with forskolin (10 μM) in the presence of quinpirole (10 μM) (A) or CP55,940 (10 μM; B) as shown. All data are normalized to the percentage of forskolin-stimulated cAMP accumulation under matched transfection conditions. Each bar represents the mean ± S.E.M. of three to four independent experiments assayed in duplicate. *, p < 0.05 compared with forskolin-stimulated cAMP accumulation under vehicle conditions (one-sample t test).
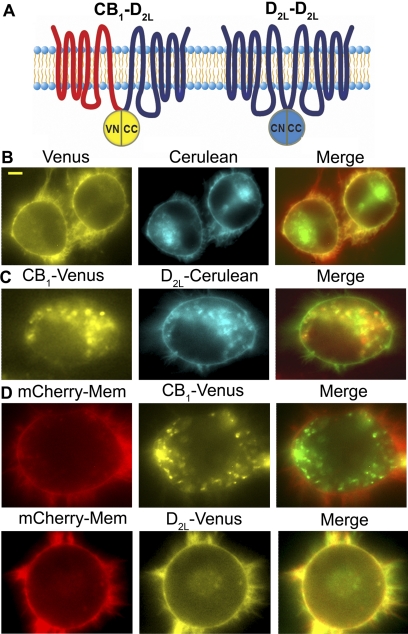
MBiFC is novel technique that allows for the simultaneous study of two receptor dimer species within living cells (Fig. 2A; Vidi and Watts, 2009). Initial single color BiFC experiments used the fusion receptors to confirm interactions between CB1 and D2L receptors. Coexpression of either combination of BiFC constructs (CB1-VN + D2L-CC or D2L-VN + CB1-CC) in CAD cells produced a robust Venus signal (Supplemental Fig. 2A). Additional BiFC studies compared the CB1-D2L fluorescent signal with CB1 or D2L receptors in combination with the M4 muscarinic receptor BiFC constructs (i.e., CB1-VN + M4-CC or D2L-CC + M4-VN). The CB1-D2L heterodimer displayed an enhanced fluorescent signal compared with the M4-containing heterodimers (Supplemental Fig. 2B). The formation of CB1-D2L heterodimers supports previous studies demonstrating interactions between CB1 and D2L receptors (Kearn et al., 2005; Marcellino et al., 2008).
Fig. 2.
CB1-D2L and D2L-D2L dimers detected by MBiFC. A, schematic representing the MBiFC approach used in these studies. CB1-D2L dimerization reconstitutes the Venus fluorescent protein (yellow) and D2L-D2L dimerization reconstitutes the Cerulean fluorescent protein (cyan). B, representative images of the fluorescent signals observed in an MBiFC study as described in the schematic in A. CAD cells were transiently transfected and imaged as described under Materials and Methods. The merged image (overlapping signal in yellow) represents an overlap of the Venus signal (depicted in red) and the Cerulean signal (depicted in green). Scale bar, 5 μm. C, representative images of the expression patterns of CB1-Venus and D2L-Cerulean receptors after cotransfection. The merged image (overlapping signal in yellow) represents an overlap of CB1-Venus (depicted in red) and D2L-Cerulean (depicted in green). D, representative images of the expression patterns of CB1-Venus (top) and D2L-Cerulean (bottom) after individual transfections in the presence of mCherry-mem. The merge image (overlapping signal in yellow) represents the overlap of either CB1-Venus or D2L-Venus (green signal) with mCherry-mem (red signal).
One goal of the present study was to assess the dynamic nature of the CB1-D2L heterodimer in response to persistent drug treatment. This required the establishment of MBiFC as described previously for A2A adenosine and D2L dopamine receptors (Vidi et al., 2008a). Using this approach, CAD cells were transiently transfected with CB1-VN, D2L-CC, and D2L-CN to simultaneously visualize CB1-D2L and D2L-D2L receptor dimers using fluorescence microscopy (Fig. 2A). The presence of a Venus signal is indicative of the CB1-D2L heterodimer, whereas a Cerulean signal corresponds to the D2L-D2L homodimer (Fig. 2B). CAD cells transfected with CB1-VN, D2L-CC, and D2L-CN expressed both Venus and Cerulean signals consistent with the coexistence of CB1-D2L heterodimers and D2L-D2L homodimers (Fig. 2B). Fluorescent signals corresponding to the receptor dimers showed a similar pattern of distribution and were found at the plasma membrane as well as intracellularly. For comparison with the BiFC signals, the localization patterns of CB1-Venus and D2L-Cerulean were evaluated after coexpression (Fig. 2C). The CB1-Venus signal showed significant intracellular localization, whereas the D2L-Cerulean displayed localization at both the plasma membrane and intracellular compartments. Moderate overlap between the CB1 and D2L signals was also observed. For additional comparison, the individual expression patterns of CB1-Venus and D2L-Venus were examined (Fig. 2D). When expressed alone, the CB1-Venus signal was primarily localized intracellularly demonstrated by the lack of overlap with the membrane marker (merge panel). Conversely, the D2L-Venus expression was found primarily at the membrane and extensive overlap with the membrane marker was displayed (Fig. 2D).
We also attempted to perform MBiFC experiments to simultaneously examine D2L-CB1 and CB1-CB1 dimers. Unfortunately, the fluorescent signal of the CB1-CB1 dimer under MBiFC conditions was too low to reliably measure, restricting our MBiFC experiments to CB1-D2L and D2L-D2L receptor dimers. The lack of a CB1-CB1 dimer BiFC signal may reflect one of the disadvantages of BiFC. Specifically, the intensity of the fluorescence complementation signal is considerably weaker (2.5–5.5-fold) than the signal from the corresponding full-length fluorescent protein under similar transfection conditions (Vidi and Watts, 2009).
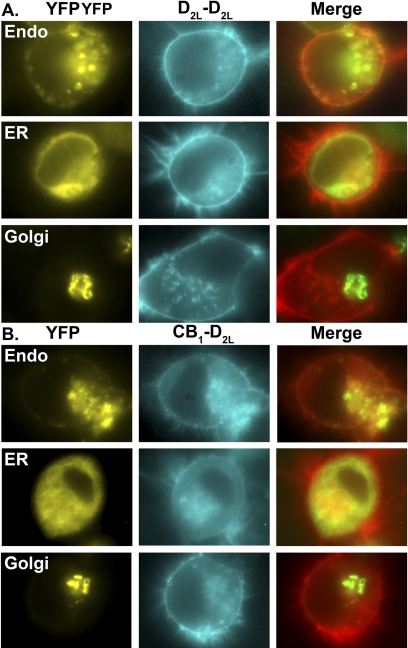
One advantage of BiFC is the ability to investigate the localization of the receptor dimers using epifluorescence. With the use of fluorescently tagged intracellular makers, the patterns of intracellular expression of the CB1-D2L and D2L-D2L receptor dimers were investigated using fluorescent microscopy (Fig. 3). CAD cells were transiently transfected with BiFC constructs that reconstitute Cerulean to either express the CB1-D2L heterodimer (CB1-CN + D2L-CC) or the D2L-D2L homodimer (D2L-CN + D2L-CC). In addition, these cells were transfected with the indicated YFP-tagged intracellular marker proteins (YFP-Endo, YFP-ER, or YFP-Golgi; Fig. 3). The endosome marker (YFP-Endo) is a fusion protein with RhoB, a known endosomal protein fused to YFP. The ER marker (YFP-ER) consists of YFP fused to the ER targeting sequence of calreticulin and the KEDL ER retrieval sequence. The Golgi marker (YFP-Golgi) is a YFP fusion protein with residues 1 to 81 of the β1,4-galactosyltransferase protein. Overall, both receptor dimers, D2L-D2L and CB1-D2L displayed moderate to extensive overlap with endosome and ER structures (Fig. 3, A and B). However, CB1-D2L and D2L-D2L receptor dimers demonstrated minimal to no overlap with the Golgi apparatus. These expression patterns are consistent with receptor dimer assembly at the ER (Herrick-Davis et al., 1997) and proper trafficking into endosomes (Leterrier et al., 2004). However, the additional possibility that receptors dimerize at the plasma membrane cannot be excluded in the absence of additional studies.
Fig. 3.
Intracellular localization patterns of the D2L-D2L homomers and CB1-D2L heteromers. CAD cells were transiently transfected with both D2L-CC and D2L-CN (cyan signal in A) or CB1-VN and D2L-CC (cyan signal in B) along with the indicated YFP fluorescent marker proteins (yellow signal). The merged image (overlapping signal in yellow) represents an overlap of the BiFC signal (depicted in red) and the fluorescent marker signal (depicted in green). Images are representative of three independent transfections.
The results demonstrating MBiFC in neuronal cells were further validated by examining dimerization of these receptors using FRET, which has been used previously to investigate interactions of CB1 and D2L receptors (Marcellino et al., 2008). CAD cells were transiently transfected with either CB1-Venus + CB1-Cerulean, CB1-Venus + D2L-Cerulean, or D2L-Venus + D2L-Cerulean (Fig. 4). A significant FRET signal was detected with all three receptor pairs compared with the mix control sample in which suspensions of cells only expressing the donor or acceptor was mixed in the FRET sample plate. These results provide further confirmation of our BiFC studies, supporting the hypothesis that CB1 and D2 form both homo- and heteromeric receptor oligomers in a neuronal-like cell model.
Fig. 4.
CB1 and D2L receptor form homo- and heteromeric receptor oligomers as measured by FRET. CAD cells were transiently transfected with 750 ng/well of either CB1-Venus and CB1-Cerulean, D2L-Venus and D2L-Cerulean, or CB1-Venus and D2L-Cerulean. Mix samples represent a mixture of CAD cell suspensions individually expressing the respective fluorescently tagged receptors of interest. Data represent the mean ± S.E.M. of three independent experiments assayed in triplicate. **, p < 0.01 compared with mixed samples (one-way analysis of variance followed by Dunnett's post hoc test).
Using MBiFC and FRET techniques, we have provided evidence that CB1 and D2 receptors participate in receptor dimer complexes. We next sought to investigate the effects of persistent ligand treatment on the formation of CB1 and D2L heterodimer and D2L homodimers using MBiFC as a tool to monitor changes in relative receptor dimer population. CAD cells were transiently transfected with CB1-VN, D2L-CC, and D2L-CN, and the presence of the CB1-D2L heterodimer (Venus) and D2L-D2L homodimer (Cerulean) was simultaneously measured. The fluorescent intensity ratio of Venus to Cerulean in both the plasma membrane and intracellular compartments was determined after drug treatment. Under the conditions used, an increase in the Venus-to-Cerulean ratio would be indicative of an increase in the formation of the CB1-D2L receptor dimer relative to the D2L-D2L receptor dimer compared with vehicle-treated cells.
Our previous work with D2 and A2A receptor ligands suggested that a 20-h drug treatment provided a robust BiFC signal in which drug-induced changes in A2A-D2L, D2L-D2L, and A2A-A2A dimers could be observed (Vidi et al., 2008a). In the present study, we completed MBiFC time course experiments with the CB1 receptor ligand CP55,940 to verify that a similar treatment duration produced robust responses in the absence of a ceiling effect. The results of the time course study revealed that CP55,940 treatments shorter than 10 h (i.e., 5 h) had very low fluorescent signals and did not allow us to quantify an adequate number of cells for analysis (data not shown). However, robust YFP and CFP signals were evident after 10 h and the drug effects were time-dependent showing the greatest response at 30 h (Fig. 5). The time course study also suggested that the 20-h time point is on the dynamic portion of the temporal scale potentially allowing us to observe ratiometric changes in both directions as shown previously (Vidi et al., 2008a). Examination of the overall YFP and CFP intensities at 20 h indicated that the CP55,940-induced increase in the YFP/CFP ratio reflected a combined increase in the YFP signal (CB1-D2L) and a decrease in the CFP signal (D2L-D2L) compared with vehicle-treated cells. Specifically, the membrane showed an 11% increase in YFP and a 27% decrease in CFP intensity. Intracellularly, there was 33% increase in the YFP signal and a 15% decrease in the CFP signal (n = 4).
Fig. 5.
Time course examining the effects of persistent CP55,940 treatment on heteromer (D2L-CB1-Venus) and homomer (D2L-D2L-Cerulean) formation. CAD cells were transiently transfected with CB1-VN, D2L-CC, and D2L-CN followed by quantitative image analyses of the Venus/Cerulean ratios for the membrane and intracellular compartments. A, cells were incubated with 10 μM CP55,940 for 10, 20, or 30 h before image analysis. Data represents the average median Venus-to-Cerulean ratio values normalized to percentage of vehicle treatment (±S.E.M.) in four independent experiments. B, images from 20-h time point depicting the effects of CP55,940 on Venus and Cerulean signals.
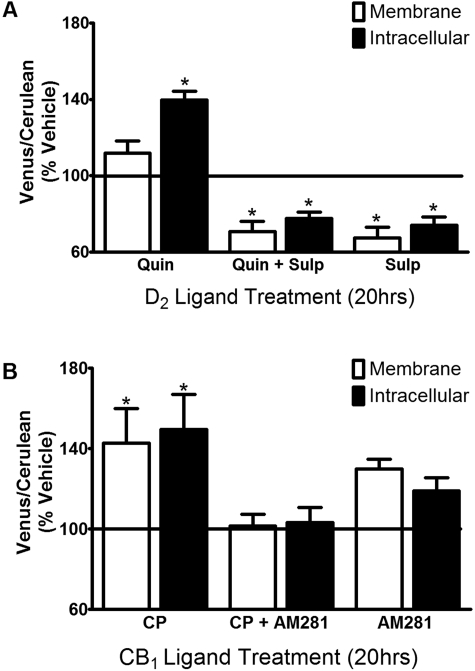
Drug-induced changes in the relative receptor dimer population were measured after treatment (20 h) with either D2 (Fig. 6A) or CB1 (Fig. 6B) receptor ligands. Persistent activation of the D2L receptor with quinpirole (10 μM) resulted in a significant increase in the Venus-to-Cerulean ratio consistent with an increase in CB1-D2L heterodimers relative to D2L-D2L homodimers. However, this effect was only significant in the intracellular compartment. The effect of quinpirole was prevented by coapplication of the selective D2 receptor antagonist sulpiride (1 μM). Treatment with sulpiride alone or in combination with quinpirole resulted in a significant decrease in the Venus to Cerulean ratio in both the membrane and intracellular compartments. Because the observed alterations in receptor dimer population may involve changes in receptor expression, single point radioligand binding experiments were used to estimate relative receptor densities after drug treatment. The results of these studies revealed that persistent treatment with quinpirole (10 μM), sulpiride (1 μM), or quinpirole + sulpiride significantly increased D2L receptor density (118 ± 6, 149 ± 17, or 129 ± 9%; n = 5) compared with vehicle treatment (100%). These ligand-induced increases in D2L receptor expression are consistent with our previous report (Vidi et al., 2008a) and work from others (Sibley and Neve, 1997). No significant changes in CB1 receptor density were observed upon persistent treatment with either of the D2 receptor ligands alone or the combination (data not shown).
Fig. 6.
Effects of persistent ligand treatment on heteromer (D2L-CB1-Venus) and homomer (D2L-D2L-Cerulean) formation. CAD cells were transiently transfected and imaged as described for Fig. 5. A, cells were incubated with 10 μM quinpirole (Quin), 1 μM sulpiride (Sulp), or quinpirole + sulpiride (Quin + Sulp) for 20 h. B, cells were incubated with 10 μM CP55,940 (CP), 10 μM AM281, or CP55,940 + AM281 (CP + AM281) for 20 h. Data represent the average median Venus-to-Cerulean ratio values normalized to percentage of vehicle treatment (± S.E.M.). *, p < 0.05 (compared with vehicle, one-sample t test, n = 5–8).
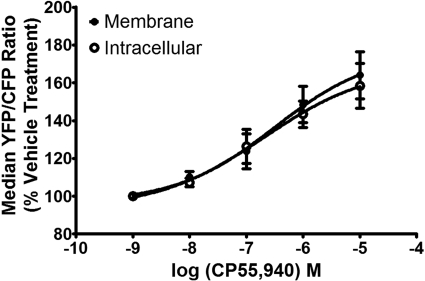
MBiFC experiments were also performed using CB1 ligands. Persistent treatment with the CB1 receptor agonist CP55,940 (10 μM) led to a significant increase in the Venus-to-Cerulean ratio in both the plasma membrane and the intracellular regions compared with vehicle-treated cells (Fig. 6B). The addition of the CB1 receptor antagonist AM281 (10 μM) attenuated the CP55,940-induced increase in the Venus-to-Cerulean ratio. Dose-response experiments revealed that the average EC50 values for CP55,940 increasing the YFP/CFP ratio were 320 and 210 nM for membrane and intracellular signals, respectively (Fig. 7). Subsequent single point radioligand binding experiments revealed that 20-h treatment with CP55,940 had no effect on CB1 receptor density (106 ± 12%; n = 5); however, a modest decrease in D2L receptor density (82 ± 3%; n = 5) was observed.
Fig. 7.
Dose-response analysis for CP55,940 modulation of the Venus/Cerulean ratio. CAD cells were transiently transfected and imaged as described for Fig. 5. Cells were incubated with increasing concentrations of CP55,940 for 20 h. Data represent the average median Venus-to-Cerulean ratio values normalized to percentage of vehicle treatment (±S.E.M.) in three independent experiments.
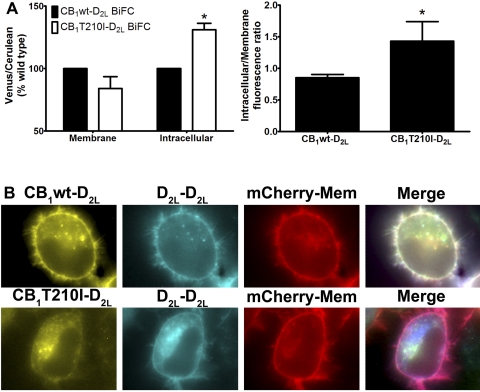
The observations described above suggest that persistent activation of the CB1 receptor favors the formation of CB1-D2L heterodimers without alterations in CB1 receptor expression. To investigate further the role of persistent activation on receptor dimerization, a constitutively active CB1 receptor mutant was constructed for use in the MBiFC experiments. Threonine 210 of the CB1 receptor was mutated to an isoleucine (CB1T210I) to create a constitutively active receptor (D'Antona et al., 2006). The presence of an isoleucine at amino acid 210 disrupts the salt bridge in the DRY motif mimicking receptor activation, leading to enhanced agonist affinity and increased intracellular localization (D'Antona et al., 2006). We examined and compared the relative receptor heterodimer (CB1-D2L) and homodimer (D2L-D2L) populations in cells expressing either the wild-type (CB1wt) or the constitutively active CB1 receptor (CB1T210I) using MBiFC (Fig. 8). The Venus- (CB1-D2L) to-Cerulean (D2L-D2L) ratios at the plasma membrane were similar in cells expressing the wild-type or constitutively active CB1 (Fig. 8A). In contrast, expression of CB1T210I resulted in a significant increase in the intracellular Venus-to-Cerulean ratio compared with the wild type CB1 (Fig. 8A). The intracellular-to-membrane ratio of the Venus signal (i.e., CB1wt-D2L or T210I-D2L dimer) in cells expressing the CB1T210I mutant was also significantly increased (approximately 150%) compared with cells expressing CB1wt (Fig. 8, A and B). The overlapping expression patterns of CB1-D2L and D2L-D2L dimers were markedly reduced in cells coexpressing CB1T210I as indicated by a loss of white signal on the membrane in the merged images. Subsequent localization studies with the CB1T210I-D2L heterodimer revealed significant signal overlap with the endosomes and limited overlap in the ER consistent with enhanced endocytosis of the CB1T210I mutant (D'Antona et al., 2006; Supplemental Fig. 3).
Fig. 8.
Effect of constitutively active CB1 receptor (T210I) on relative dimer population at the plasma membrane or intracellular compartment. CAD cells were transiently transfected with either CB1wt-VN or CB1T210I-VN with D2L-CC and D2L-CN. A, left, quantitative image analyses of the Venus/Cerulean ratios were determined for the membrane and intracellular compartments as described under Materials and Methods. The Venus-to-Cerulean ratio induced by CB1T210I was normalized to the Venus-to-Cerulean ratio measured in cells expressing CB1wt receptor; *, p < 0.05 (compared with wild type, one-sample t test). Right, quantitative image analyses of the intracellular/membrane ratios were determined for the CB1-D2L-Venus signal in cells either expressing CB1wt or CB1T210I. *, p < 0.05 (compared with wild type, t test). Data for both analyses were generated from the same experiments and represent the average median ± S.E.M. from three independent experiments. B, representative images of CAD cells expressing either CB1wt (top) or CB1T210I (bottom) to reconstitute the BiFC signals CB1(wt or T210I)-D2L-Venus and D2L-D2L-Cerulean and the membrane marker (mCherry-mem). The merge panel represents overlap of the three channels and overlapping pixel intensity is presented in white.
Discussion
Evidence for the existence and functional significance of CB1 and D2L heterodimers has continued to evolve over the past 10 to 15 years. However, investigations examining the regulation of these heterodimers and their homodimer counterparts are just beginning as new technological advances for studying protein-protein interactions are developed (Vidi and Watts, 2009). In the present study, we have applied MBiFC as a novel technique to study the dimerization of CB1 and D2L receptors, and we reveal for the first time the localization patterns of these receptor heterodimers in a neuronal cell model.
Early studies of CB1 and D2L function were central to the development of the concept of CB1-D2L heterodimer (for review, see Glass et al., 1997). Several studies suggest that the CB1-D2L dimer possesses stimulatory properties toward adenylyl cyclase via the CB1 receptor engaged in the heterodimer (Glass and Felder, 1997; Jarrahian et al., 2004; Kearn et al., 2005). However, conflicting conclusions from studies examining the regulation of CB1 and D2L receptor dimerization remain. One potential mechanism for regulating the CB1-D2 dimer is based on observations that the physical association of CB1 and D2L increases in the presence of acute coactivation of both receptors (Kearn et al., 2005). Activation of either CB1 or D2L receptor individually did not significantly increase the physical association, suggesting that coactivation of both receptors is necessary for enhanced receptor dimerization. It was also reported that expression (and not activation) of the D2L receptor was sufficient to induce a switch in CB1-G protein coupling to a stimulatory pathway, however, measurements of the CB1-D2L receptor dimer were not performed (Jarrahian et al., 2004). In addition, another study reported a lack of agonist-mediated increase in the FRET interaction between CB1 and D2 receptors under conditions of both single and concurrent receptor activation (Marcellino et al., 2008). The lack of consistency between the reports described above may reflect differences in the choice of receptor ligands, the model systems, technical approaches, or the complex pharmacology of the CB1-D2 dimer.
In the present study, we used MBiFC to show that persistent activation of either the CB1 or D2L receptor leads to the formation of more CB1-D2L heterodimers relative to the D2L-D2L homodimers. There are several differences between our study of CB1-D2L interactions and the previous work described above (e.g., cell type, methods to measure receptors, drug treatment); however, the drug treatment conditions and technology used to assess the receptor dimers probably have significant influence. Each of the drug treatments reported here represents an extended drug exposure (i.e., 10–30 h). Drugs were added 4 h after transfection and were present during the time of ongoing receptor biosynthesis and subsequent oligomerization. Therefore, the dimers observed in our studies probably involve mechanisms not reflected in shorter drug treatments or acute studies (Kearn et al., 2005; Marcellino et al., 2008). The present study used BiFC technology, which differs from FRET in that the complementation of fluorescent signal is essentially irreversible (Vidi and Watts, 2009). This property of MBiFC allows investigators to “capture” and subsequently measure drug-induced changes in receptor dimers over an extended period in which a sufficient signal can be collected.
Persistent D2 agonist treatment with quinpirole favored the formation of CB1-D2L heterodimers versus D2L-D2L homodimers. This effect was accompanied by an increase in D2L receptor expression and was prevented by the D2 antagonist sulpiride. The increase in D2L receptor expression may suggest a pharmacological chaperone effect on receptor dimer formation where D2 ligands stabilize the receptor, somehow promoting CB1-D2L receptor interactions (Vidi et al., 2008b). However, treatment with the D2 antagonist sulpiride also increased D2L receptor density, but instead favored the formation of D2L-D2L homodimers. These opposing effects of D2 agonists and antagonists on D2L-D2L versus CB1-D2L dimer formation argues against a simple role of increased D2L receptor expression. One explanation for these differential effects may involve ligand-specific changes in receptor dimerization patterns (Vidi et al., 2008b). In addition to ligands, these dimerization patterns also appear to be influenced by the receptors under investigation. In a previous study of D2L and A2A receptor dimerization, quinpirole increased D2L-D2L homodimers relative to A2A-D2L heterodimers (Vidi et al., 2008b). The potential scenario gets increasingly complicated when considering a recent BiFC-BRET study providing evidence for a CB1-D2-A2A receptor oligomer (Navarro et al., 2008). Linking the observations described above and the present results suggests a scenario where striatal neurons expressing D2L, A2A, and CB1 receptors would be subject to a very complicated receptor regulation scheme. For example, persistent D2 agonist treatment would increase overall D2L receptor expression levels and perhaps promote the following pattern of receptor oligomers D2L-CB1 > D2L-D2L > A2A-D2L. The potential physiological and functional significance of these ligand-induced changes in heterodimers are intriguing and await biochemical and behavioral analysis (Marcellino et al., 2008). In addition to in vivo studies, new molecular tools to study these complex systems are becoming increasingly available as methods to study interactions of higher ordered GPCR oligomers (e.g., trimers and tetramers) such as BiLC-FRET, BiFC-FRET, and BiFC-BRET are developed (Vidi and Watts, 2009).
The ability of quinpirole to alter the formation of receptor oligomers involving D2L receptors may provide insight into the disease states associated with persistent D2 receptor activation, as in the treatment of Parkinson's disease with l-DOPA and D2 dopamine receptor agonists (Hurley and Jenner, 2006). For example, persistent quinpirole treatment increases A2A-A2A homodimer formation and A2A signaling (Vortherms and Watts, 2004; Vidi et al., 2008a). These observations may provide a molecular explanation for the beneficial clinical effects of A2A antagonists in treating l-DOPA-induced dyskinesias (Morelli et al., 2007; Fuxe et al., 2008). The current results suggest that persistent treatment with D2 receptor agonist drugs may promote the formation of CB1-D2L heterodimers. The increase in CB1-D2L dimer formation may allow the CB1 receptor to have enhanced antagonistic effects over the D2 receptor signaling (Marcellino et al., 2008). This scenario would provide for increased CB1 signaling after a dopamine receptor-dependent increase in endocannabinoid release (Giuffrida et al., 1999; Piomelli, 2003). In addition, evidence linking the CB1-D2L heterodimer to a stimulatory pathway (Glass and Felder, 1997; Kearn et al., 2005) may provide a mechanism for CB1 antagonism of D2 signaling at the intracellular level (i.e., cAMP). Further in vivo investigations of CB1 receptor and CB1-D2L heterodimer signaling after persistent D2 receptor activation are warranted; however, studies suggest that the CB1 receptor antagonists/inverse agonists may have beneficial effects in the management of Parkinson's disease. For example, the CB1 antagonist 1-[7-(2-chlorophenyl)-8-(4-chlorophenyl)-2- methylpyrazolo[1,5-a]-[1,3,5]triazin-4-yl]-3-ethylaminoazetidine-3-carboxylic acid amide benzenesulfonate dose-dependently enhances the anti-Parkinson's activity of l-DOPA (Cao et al., 2007). Another study revealed that rimonabant, a CB1 receptor inverse agonist, had beneficial effects in managing l-DOPA-induced dyskinesias (van der Stelt et al., 2005).
Similar to the D2L receptors, the precise mechanism by which persistent activation of the CB1 receptor favors the formation of CB1-D2L heterodimers relative to D2L-D2L homodimers remains largely unknown. Our observations suggest that the formation of the heterodimer is mediated by receptor activation and not alterations in CB1 receptor expression. It is possible that the activated conformational state of the CB1 receptor possesses enhanced affinity for the D2L receptor and that persistent activation promotes CB1-D2L heterodimerization. This hypothesis is supported by the report that the CB1 receptor increases the association with the D2L receptor in a dose-dependent manner (Kearn et al., 2005). Furthermore, the present study demonstrated that expression of a constitutively active CB1 mutant, CB1T210I, promoted more CB1-D2L heterodimerization. Although the identification of a molecular mechanism awaits further study, it is tempting to consider that CB1-D2L interactions will represent a new CB1 receptor signaling pathway that may be subject to functional selectivity (Glass and Northup, 1999; Mukhopadhyay and Howlett, 2005; Urban et al., 2007).
The physiological significance and functional consequences of CB1 receptor-induced CB1-D2L dimers may have implications in the use of clinical cannabinoids to treat chronic pain as well as chronic marijuana use. Such conditions would involve persistent CB1 receptor activation, providing an impetus to understand the molecular adaptations that occur in the nervous system (Cooper and Haney, 2008). Although we were able to study drug-induced changes of the CB1-D2L and D2L-D2L receptor dimers, a low BiFC signal between CB1 receptors prevented us from examining the ratios of CB1-CB1 homodimers to CB1-D2L heterodimers. In the absence of CB1-CB1 studies, the CP55,940-induced increase in the CB1-D2L heterodimer may reflect a relative decrease in D2L-D2L homodimers and perhaps D2L function. Consistent with this possibility we observed a modest CP55,940-induced decrease (approximately 15–25%) in D2L receptor expression and D2L-D2L homodimers. These observations may suggest that persistent CB1 receptor activation and subsequent CB1-D2L heterodimer formation could reduce D2L receptor expression. In partial support of this hypothesis, it has been shown in rats and humans that chronic prenatal exposure to marijuana decreases the expression of dopamine D2 receptors in the brain (Walters and Carr, 1986; Wang et al., 2004).
In the present report, we have visualized simultaneously the localization patterns of CB1-D2L heterodimers and D2L-D2L homodimers in living cells and provided evidence for agonist-regulated effects on receptor dimerization patterns. Recent studies propose that an increasing number of GPCRs may participate in higher order receptor oligomers or “receptor mosaics” and that these structures may mediate many signaling events (for review, see Fuxe et al., 2008). The present work and other recent studies are consistent with this concept (Carriba et al., 2008; Navarro et al., 2008). We anticipate the continued development of new technologies will allow investigators to examine these receptor mosaics in greater detail. Finally, the use of MBiFC provides a new tool to study drug-induced changes in receptor oligomerization and may offer an important asset relevant to the future of drug discovery in the area of receptor heterodimers.
Supplementary Material
Acknowledgments
We thank Dr. Karin Ejendal, Jason Conley, and Benjamin Chemel for careful reading of the manuscript and David Przybyla for assistance in preparing the figures.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH060397]; and the Purdue Research Foundation and the Department of Medicinal Chemistry and Molecular Pharmacology, Purdue University.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.162701.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
Personal communications confirmed that the D2L receptor was used in Glass and Felder (1997) (per Dr. David Sibley, who supplied the CHO-D2 cells) as well as in Marcellino et al. (2008) (per Dr. Kjell Fuxe).
- GPCR
- G protein-coupled receptor
- CB1
- cannabinoid 1 receptor
- CC
- Cerulean C-terminal fragment
- D2
- dopamine D2 receptor
- D2S
- short form of D2 receptor
- D2L
- long form of D2 receptor
- FRET
- fluorescence resonance energy transfer
- BRET
- bioluminescence resonance energy transfer
- BiFC
- bimolecular fluorescence complementation
- BRET
- bioluminescence resonance energy transfer
- MBiFC
- multicolor bimolecular fluorescence complementation
- CAD
- Cath. a differentiated
- CP55,940
- (2)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl) cyclohexanol
- SR141716A
- N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboximide hydrochloride
- YFP
- yellow fluorescent protein
- PCR
- polymerase chain reaction
- PBS
- phosphate-buffered saline
- CFP
- cyan fluorescent protein
- VN
- Venus N-terminal fragment
- CN
- Cerulean N-terminal fragment
- CC
- C-terminal fragment of Cerulean
- cFRET
- corrected fluorescence resonance energy transfer
- M
- muscarinic receptor
- A2A
- adenosine2A receptor
- AM281
- 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide
- ER
- endoplasmic reticulum
- wt
- wild type.
References
- Cao X, Liang L, Hadcock JR, Iredale PA, Griffith DA, Menniti FS, Factor S, Greenamyre JT, Papa SM. (2007) Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther 323:318–326 [DOI] [PubMed] [Google Scholar]
- Carriba P, Navarro G, Ciruela F, Ferré S, Casadó V, Agnati L, Cortés A, Mallol J, Fuxe K, Canela EI, et al. (2008) Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods 5:727–733 [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. (2008) Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol 13:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona AM, Ahn KH, Kendall DA. (2006) Mutations of CB1 T210 produce active and inactive receptor forms: correlations with ligand affinity, receptor stability, and cellular localization. Biochemistry 45:5606–5617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Rivera A, Diaz-Cabiale Z, Filip M, Gago B, Roberts DC, Langel U, Genedani S, Ferraro L, et al. (2008) Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res Rev 58:415–452 [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. (1999) Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2:358–363 [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. (1997) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci 17:5327–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Northup JK. (1999) Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol 56:1362–1369 [DOI] [PubMed] [Google Scholar]
- Glass M, Brotchie JM, Maneuf YP. (1997) Modulation of neurotransmission by cannabinoids in the basal ganglia. Eur J Neurosci 9:199–203 [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. (1991) Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res 547:267–274 [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Egan C, Teitler M. (1997) Activating mutations of the serotonin 5-HT2C receptor. J Neurochem 69:1138–1144 [DOI] [PubMed] [Google Scholar]
- Hu CD, Chinenov Y, Kerppola TK. (2002) Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell 9:789–798 [DOI] [PubMed] [Google Scholar]
- Hu CD, Kerppola TK. (2003) Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat Biotechnol 21:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MJ, Jenner P. (2006) What has been learnt from study of dopamine receptors in Parkinson's disease? Pharmacol Ther 111:715–728 [DOI] [PubMed] [Google Scholar]
- Jarrahian A, Watts VJ, Barker EL. (2004) D2 dopamine receptors modulate Gα-subunit coupling of the CB1 cannabinoid receptor. J Pharmacol Exp Ther 308:880–886 [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. (2005) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol 67:1697–1704 [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. (1998) Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A 95:7731–7736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. (2006) The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci 63:1597–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. (2004) Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem 279:36013–36021 [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, Tanganelli S, Müller CE, Fisone G, Lluis C, et al. (2008) Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology 54:815–823 [DOI] [PubMed] [Google Scholar]
- Milligan G. (2008) A day in the life of a G protein-coupled receptor: the contribution to function of G protein-coupled receptor dimerization. Br J Pharmacol 153 (Suppl 1):S216–S229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzschild MA. (2007) Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog Neurobiol 83:293–309 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. (2005) Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol Pharmacol 67:2016–2024 [DOI] [PubMed] [Google Scholar]
- Navarro G, Carriba P, Gandía J, Ciruela F, Casadó V, Cortés A, Mallol J, Canela EI, Lluis C, Franco R. (2008) Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. ScientificWorldJournal 8:1088–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. (2006) Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol 495:299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884 [DOI] [PubMed] [Google Scholar]
- Qi Y, Wang JK, McMillian M, Chikaraishi DM. (1997) Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci 17:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu YJ, Liu H, Deng X, Hu CD. (2006) Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques 40:61–66 [DOI] [PubMed] [Google Scholar]
- Sibley DR, Neve KA. (1997) Regulation of dopamine receptor function and expression, in The Dopamine Receptors (Neve KA, Neve RL. eds) pp 383–424, Humana Press, Totowa, NJ: [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408:199–203 [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, Brotchie JM. (2005) A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson's disease. FASEB J 19:1140–1142 [DOI] [PubMed] [Google Scholar]
- Vidi PA, Watts VJ. (2009) Fluorescent and bioluminescent protein-fragment complementation assays in the study of G protein-coupled receptor oligomerization and signaling. Mol Pharmacol 75:733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidi PA, Chemel BR, Hu CD, Watts VJ. (2008a) Ligand-dependent oligomerization of dopamine D(2) and adenosine A(2A) receptors in living neuronal cells. Mol Pharmacol 74:544–551 [DOI] [PubMed] [Google Scholar]
- Vidi PA, Chen J, Irudayaraj JM, Watts VJ. (2008b) Adenosine A(2A) receptors assemble into higher-order oligomers at the plasma membrane. FEBS Lett 582:3985–3990 [DOI] [PubMed] [Google Scholar]
- Vortherms TA, Watts VJ. (2004) Sensitization of neuronal A2A adenosine receptors after persistent D2 dopamine receptor activation. J Pharmacol Exp Ther 308:221–227 [DOI] [PubMed] [Google Scholar]
- Walters DE, Carr LA. (1986) Changes in brain catecholamine mechanisms following perinatal exposure to marihuana. Pharmacol Biochem Behav 25:763–768 [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Anderson V, Minkoff H, Hurd YL. (2004) In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry 56:909–915 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.