Abstract
GABAA receptor (R) positive allosteric modulators that selectively modulate GABAARs containing β2- and/or β3- over β1-subunits have been reported across diverse chemotypes. Examples include loreclezole, mefenamic acid, tracazolate, and etifoxine. In general,“β2/3-selective” GABAAR positive allosteric modulators are nonbenzodiazepines (nonBZs), do not show α-subunit isoform selectivity, yet have anxiolytic efficacy with reduced ataxic/sedative effects in animal models and humans. Here, we report on an enantiomeric pair of nonBZ GABAAR positive allosteric modulators that demonstrate differential β-subunit isoform selectivity. We have tested this enantiomeric pair along with a series of other β2/3-subunit selective, α-subunit isoform-selective, BZ and nonBZ GABAA positive allosteric modulators using electrophysiological, pharmacokinetic, and behavioral assays to test the hypothesis that ataxia may be correlated with the extent of modulation at β1-subunit-containing GABAARs. Our findings provide an alternative strategy for designing anxioselective allosteric modulators of the GABAAR with BZ-like anxiolytic efficacy by reducing or eliminating activity at β1-subunit-containing GABAARs.
Positive allosteric modulators of the GABAA receptor (R) such as the benzodiazepines (BZs) continue to be used to treat anxiety, despite the well-known side effect of sedation. Diverse drug discovery efforts over two decades have focused on generating “anxioselective” (i.e., reducing anxiety without sedation) GABAAR positive allosteric modulators. Medicinal chemistry efforts have focused primarily on modifications of the BZ template with limited success in reducing sedative liability (Whiting, 2006).
One strategy to generate anxioselective positive allosteric modulators involves creation of positive allosteric modulators that selectively modulate individual GABAAR subtypes involved in anxiety, while avoiding those mediating sedation. Several laboratories have focused on α-subunit isoform-selective BZ site agonists that evoke positive modulation of α2- and α3- but not α1-subunit-containing GABAARs. This “α2/3-selective” approach is based on pharmacological and genetic data suggesting that α2- and α3-subunit-containing GABAARs mediate the anxiolytic actions of BZs, whereas those with α1-subunits, especially the α1β2γ2 subtype, are thought to mediate their sedative effects (Rudolph et al., 1999; McKernan et al., 2000). Consistent with this general theory, L-838,417 is a α2,3-subunit-selective partial agonist BZ receptor ligand reported to be anxioselective in animal models (McKernan et al., 2000). However, recent clinical studies designed to determine whether α2,3-subunit selectivity imparts reduced sedative liability has resulted in equivocal results where BZ-like side effects were observed (de Haas et al., 2008, 2009). Moreover, the α3-subunit-selective BZ site partial agonist adipiplon has potent sedative activity and was in clinical development as a sedative-hypnotic (Sprenger et al., 2007).
Selective activity at different α-subunit isoforms does not fully explain the sedative effects of all BZ site agonists. A prominent example that argues against the theory is the BZ site ligand ocinaplon. It has robust full agonist-like activity at α1-subunit-containing GABAARs in vitro, yet it has reduced sedative liability in preclinical and clinical studies (Basile et al., 2004; Lippa et al., 2005; Popik et al., 2006). Furthermore, there have been no reports of compounds with α2,3-subunit selectivity achieving clinical proof-of-concept (i.e., anxioselectivity), despite the passage of almost a decade since the initial proposal of the α2,3-subunit selectivity hypothesis.
Apart from the intense focus on GABAAR subtype-selective BZs, other ways to elicit anxioselectivity via the GABAAR are relatively unexplored, especially as it relates to nonBZ site positive allosteric modulators with receptor subtype selectivity (Whiting, 2006). One interesting approach focuses on GABAAR positive allosteric modulators that are selective modulators of β2- or β3- over β1-subunit-containing GABAARs. This type of selective modulation has been reported for compounds across diverse chemotypes (e.g., loreclezole, mefenamic acid, tracazolate, and etifoxine) (Halliwell et al., 1999; Thompson et al., 2002; Hamon et al., 2003; Groves et al., 2006). Empirical observations of these positive allosteric modulators in both animals and humans provide anecdotal evidence that suggests that the degree of activation of β1-subunit-containing GABAARs may contribute to their sedative/ataxic potential.
Based on this correlative evidence, we propose the hypothesis that among nonBZ site positive allosteric modulators the continuum of sedative, ataxic and hypnotic effects elicited by GABAAR activation may also depend on β-subunit isoform selectivity. Thus, limiting or eliminating activity at β1-subunit-containing GABAARs will reduce or abolish these effects, respectively. We recently identified a series of nonBZ site enaminone positive allosteric modulators with a range of potencies and efficacies, thus providing us with a template to create tools to test our hypothesis (Hogenkamp et al., 2007). Here, we report the characterization of an enantiomeric pair of enaminones, 2-261 and 2-262, that have differential β-subunit isoform selectivity. We have tested this pair and 15 other β-subunit isoform-selective and -nonselective positive allosteric modulators for GABAAR potency/efficacy, pharmacokinetic profiles, and behavior in anxiety models. Our studies provide correlative pharmacological evidence that selective modulation of β-subunit-containing GABAAR subtypes may be another mechanism to separate anxiolytic from ataxic effects among nonBZ site GABAAR positive allosteric modulators.
Materials and Methods
Drugs.
Loreclezole (Heeres, 1985; Astleford et al., 1989), tracazolate (Bare et al., 1989), etifoxine (Putman et al., 2007), and ocinaplon (Skolnick and Epstein, 2005) were synthesized in our lab using methods reported in the literature. The enaminones were synthesized as described previously (Hogenkamp et al., 2007; Table 1). Diazepam, bretazenil, etomidate, and mefenamic acid were purchased from Sigma-Aldrich (St. Louis, MO), whereas L-838,417 (McKernan et al., 2000) was from Tocris Biosciences (Ellisville, MO). For electrophysiology experiments, drugs were first dissolved in dimethyl sulfoxide (DMSO) to 10 mM and diluted in Ringer's salt solution (0.1% total DMSO final solution). Drugs for per os administration were dissolved in polyethylene glycol-400 and administered at ≤2 ml/kg. Drugs administered by intraperitoneal injection were dissolved in DMSO and administered at ≤1 ml/kg.
TABLE 1.
Potency and maximal efficacy of test compounds at α1β1γ2 or α1β2γ2 GABAARs in rank order of ascending efficacy at α1β1γ2
The mouse RR AD50 value is provided for each compound if determinable over the range of doses tested. The peak brain levels associated with the AD50 or the highest doses tested, when an AD50 could not be attained, are expressed in micromolar. In vitro potency is expressed as the EC50 (micromolar) followed by its 95% confidence interval in parentheses, and maximal efficacy is expressed as the percentage of modulation of an EC10 GABA effect. Brain levels are expressed as the mean ± S.E.M. (n = 3–9). All test compounds were administered by intraperitoneal injection except for 2-261, 2-262, and mefenamic acid, which were administered orally.
| Compound | Maximal Efficacy at α1β1γ2 | EC50 at α1β2γ2 | Maximal Efficacy at α1β2γ2 | EC50 at α1β2γ2 | RR AD50 | Brain Level |
|---|---|---|---|---|---|---|
| % | μM | % | μM | mg/kg | μM | |
| L-838,417 a | 0 | N.D. | 0 | N.D. | 30 | 8 ± 1 |
| 2-325 | 16 | N.D. | 1330 | 0.22 (0.1–4.6) | >30 | 2.1 ± 0.4 |
| Mefenamic acid | 18 | N.D. | 315 | 3.6 (2.5–5.1) | >500 | 3.1 ± 0.7 |
| 2-313 | 33 | N.D. | 727 | 0.14 (0.09–0.2) | >50 | 1.2 ± 0.1 |
| 2-261 | 34 | N.D. | 1101 | 0.3 (0.1–0.6) | >100 | 3.7 ± 0.5 |
| 2-301 | 47 | N.D. | 686 | 0.22 (0.1–0.7) | >100 | 4.0 ± 1.5 |
| Ocinaplon | 81 | 2.8 (0.7–11) | 72 | 2.1 (0.3–19) | 54 | 12.5 ± 1.5 |
| Bretazenil | 95 | 0.05 (0.01–0.42) | 58 | 0.004 (0.001–0.008) | 25 | 11.7 ± 3.0 |
| Diazepam | 109 | 0.11 (0.03–0.5) | 166 | 0.05 (0.02–0.1) | 2 | 0.64 ± 0.3 |
| 2-128 | 113 | 2.4 (1.2–4.9) | 552 | 0.9 (0.6–1.3) | >120 (40% failure at 120) | 5.8 ± 2.0 |
| Loreclezole | 125 | 40 (31–52) | 498 | 4.8 (3.1–7.5) | 83 | 11.7 ± 3.2 |
| 2-262 | 181 | 1.4 (0.7–2.7) | 1102 | 0.04 (0.02–0.09) | 43 | 0.8 ± 0.3 |
| 2-249 | 249 | 3.6 (1.4–9.5) | 905 | 0.03 (0.01–0.1) | 3 | 0.6 ± 0.1 |
| 2-148 | 369 | 6.9 (3–12) | 817 | 0.2 (0.1–0.4) | 5 | 1.1 ± 0.1 |
| Tracazolate | 375 | 4.7 (2–12) | 821 | 0.6 (0.3–1.3) | 42 | 7.6 ± 1.7 |
| 2-314 | 655 | 0.2 (0.1–0.4) | 814 | 0.11 (0.03–0.4) | 2.5 | 0.30 ± 0.06 |
| Etomidate | 819 | 18 (12–27) | 1307 | 2 (1–8) | N.D. | N.D. |
N.D., not determined.
L-838,417 is inactive at both α1-containing subtypes because of selectivity for α2/3- over α1-subunits.
Animals.
Mice were male NSA (Harlan Labs, Los Angeles, CA) and weighed 24 to 28 g. Rats were male Sprague-Dawley (Harlan Labs) and weighed 250 to 350 g. Animals were housed under a 12:12-h light/dark cycle starting at 6:30 AM and tested according to University of California, Irvine Institutional Animal Care and Use Committee-approved protocols. Oocytes were obtained from Xenopus laevis frogs using procedures approved and monitored by the Institutional Animal Care and Use Committee.
Two-Electrode Voltage-Clamp Oocyte Electrophysiology.
cDNA clones were provided as kind gifts from the following sources: human α7, α4, and β2 nicotinic acetylcholine receptors (nAChRs) were from Dr. Jon Lindstrom (The University of Pennsylvania, Philadelphia, PA); human GABAA receptor subunits (α1, α2, α3, β1, β2, β3, γ2, and δ) were from CoCensys Inc. (Irvine, CA); and rat δ and ε nAChRs were from Dr. James Boulter (University of California, Los Angeles, CA). 5-HT3A was purchased from the American Type Culture Collection (Manassas, VA).
Preparation, microinjection, and maintenance of oocytes was as described previously (Ng et al., 2007). Individual oocytes were injected with 0.005 to 50 ng of each subunit mRNA as follows (ratio of subunits in parentheses): GABAA receptor subunit combinations (α1,2, or 3; β1,2, or 3γ2L; or δ): (5:1:1). Stage IV to V oocytes were plucked from ovary membranes and defolliculized with collagenase type IA (Worthington Biochemicals, Freehold, NJ) for 45 min, rinsed 10 times with Ringer's salt solution, and then cRNA was injected at 50 nl. Oocytes were tested 3 to 28 days after injection (n = 3–7/compound) in Ringer's salt solution by linear drug application method using electrodes with 1- to 2-ω tip resistance. Changes in membrane current were passed through a preamplifier and then through a T200 patch amplifier (Axon Instruments, Sunnyvale, CA), with a bandpass filter of 2 kHz. pCLAMP software (Molecular Devices) was used to monitor, record, and analyze data. All compounds were tested with a 30-s pretreatment before coapplication, with EC10 (concentration of GABA that evokes 10% of the maximal response) GABA for the control response. The GABA EC10 was determined in each individual oocyte expressing the receptor subtype of interest. For example, the EC10 value ranges from 3 × 10−7 to 10−6 M at α1β1γ2 and from 3 × 10−6 to 10−5 M at α1β2γ2 isoforms. The EC100 value was 10−3 M. Responses in presence of test compound were calculated as percentage of modulation above control. Concentration-response curves were fit to nonlinear regression analysis on Prism 4.0 (GraphPad Software Inc., San Diego, CA) for percentage of maximal stimulation, EC50 values, and their 95% confidence limits. In cases where 0% modulation was not defined, the bottom of the concentration-response curve was constrained to zero. However, when several concentrations tested resulted in a well defined 0% response then constraining to zero was unnecessary. Percentage of stimulation corresponding to brain levels of the compounds tested was extrapolated from these concentration-response curves.
Pharmacokinetic Studies.
Blood was removed at various time points after drug administration via cardiac puncture under halothane anesthesia and centrifuged at 1000g for 6 min to separate the plasma. After euthanization, brains were perfused with saline and removed, stored at −20°C until processed for extraction and high-performance liquid chromatography (HPLC) analysis. Plasma and brain extracts were run through HPLC in a 30 to 70% acetonitrile/phosphate buffer mobile phase through a C18 column, detecting the maximal absorption wavelength via UV-spectrometry as described previously (Ng et al., 2007). Extraction methods were repeated for liquid chromatography/mass spectrometry determination. Approximately 0.5 g (brain) or 250 μl (plasma) containing the analyte was precipitated with acetonitrile, and the supernatant was evaporated and reconstituted in acetonitrile. The samples were analyzed by reversed phase high-performance liquid chromatography in a 2790 HPLC (Waters, Milford, MA) using a Nova-Pak C18 column (Waters) and were detected using a QuatroUltima triple quad mass spectrometer (Waters) by collision-induced dissociation/tandem mass spectrometry.
Mouse Light-Dark Transition.
Naive mice were acclimated (1 h) to a darkened room before administration of test compounds. Testing occurred during peak brain levels of compounds, in automated light-dark transition (LD) boxes (Coulbourn Instruments, Allentown, PA), tracked by infrared beam-breaking collar and TruScan software (Coulbourn Instruments). Light bulbs were placed 60 cm above the floor of the test box where light intensity was 400 lux and centered on the lit half of the box. The time spent in the dark was recorded. Data were analyzed with Prism 4.0 (GraphPad Software Inc.) for statistical significance by one-way ANOVA with Dunnett's multiple comparison post-hoc test.
Rat Elevated Plus Maze.
Rats were group housed and handled daily for 3 days before testing in the elevated plus maze (EPM; Coulbourn Instruments). Testing was conducted in a dimly lit (2-lux) room, with two 60-W bulbs pointed at the ceiling near the open arms (122 cm above the maze, 400 lux at the surface of the maze). The maze was cleaned between each run. All compounds were tested at times that correspond to peak brain concentrations. Automated counting of time spent in the open arms of the maze was achieved by using the MedPC-IV program (MED Associates, St. Albans, VT). Data were analyzed with Prism 4.0 (GraphPad Software Inc.) for statistical significance by one-way ANOVA with Dunnett's multiple comparison post-hoc test.
Mouse Rotarod.
Naive mice were trained on a Rotarod (RR; Columbus Instruments, Columbus, OH) in four sessions (6–15 rpm) over 2 days to successfully complete the 2-min trial before final testing (6 rpm). On day 3, the mice were administered compound and tested over a period of 360 min at various intervals. The percentage of animals remaining on the RR throughout each 2-min trial was recorded. The results that coincided with the time of peak effect were analyzed by the method of Litchfield and Wilcoxon (1949) to determine the ataxogenic half-maximal dose where half of the mice fail the RR assay (AD50).
Effect of 2-261 versus Diazepam on Ethanol-Induced RR Deficit.
Naive mice were trained on an RR (Columbus Instruments) in four sessions (6–15 rpm) over 2 days to successfully complete the 2-min trial before final testing (6 rpm). On the third day, the mice (12 mice/condition) were administered an AD25 dose (1 g/kg i.p.) of EtOH followed 10 min later by 2-261 (30 mg/kg p.o.), diazepam (1 mg/kg i.p.), or test drug vehicle, and then they were tested for RR performance at various time intervals. The percentage of animals remaining on the RR throughout the 2-min trial was recorded and analyzed. Among the EtOH-treated animals, the statistical difference between test drug- and vehicle-treated animals was determined by the Fischer-Yates exact probability test.
Results
2-261 and 2-262 Evoke β- but Not α-Subunit Isoform-Selective Positive Allosteric Modulation of GABAARs in X. laevis Oocytes.
We previously synthesized a series of nonBZ site GABAAR positive allosteric modulators, which included the racemic enaminone designated 2-247 (Hogenkamp et al., 2007). The individual enantiomers of 2-247 (2-261 and 2-262) differ only in the chirality of the sec-butyl amide side chain (Fig. 1). We chose to test 2-247, 2-261, 2-262, and subsequently other enaminones from our compound library for subunit-selective modulation of GABA-evoked currents in oocytes expressing different α- and β-subunit isoform combinations. At GABAARs containing β2- and β3-subunits, the enantiomers 2-261 and 2-262 both evoked high-efficacy positive modulation of GABA-evoked control currents (control currents were “EC10” or 10% of maximal current), with maximal modulation to ∼1000% at α1β2γ2 (Fig. 2A) and α1β3γ2 (data not shown) GABAARs for both compounds. This efficacy far exceeds those of the typical full agonist BZ (∼166% maximum for diazepam; Table 1) tested at α1β2γ2 in the same assay. GABA-dependent modulation by 2-261 and 2-262 at β2- and β3-subunit-containing GABAARs did not appear to be strongly dependent on α-subunits, with equivalent maximal modulation observed at α1-, α2-, and α3-subunits (Fig. 3).
Fig. 1.
Structural diversity of GABAAR allosteric modulators in the present study.
Fig. 2.
Modulation of submaximal (EC10) GABA-evoked currents by 2-261 and 2-262. Top, representative current tracings from two-electrode voltage-clamp recordings in X. laevis oocytes expressing human α1β2γ2 (A) or α1β1γ2 (B) GABAARs. Substitution of the β2- with the β3-subunit results in a similar response. Control currents for modulation were 10% of maximal current (EC100). Drug exposures are indicated by the bars. Bottom, concentration-response relationship for modulation of EC10 currents by 2-261 and 2-262. Data represents mean ± S.E.M. (n = 3–8).
Fig. 3.
α-Subunit selectivity of 2-261 and 2-262. Concentration-response curves for 2-261 and 2-262 across α1-, α2-, or α3-subunit-containing GABAAR subtypes. The curves depicted (left) show the effect of 2-261 on α1β1γ2 versus α1β2γ2 (A), α2β1γ2 versus α2β2γ2 (B), and α3β1γ2 versus α3β2γ2 (C) GABAARs, with the filled and open symbols denoting the presence of β2- or β1-subunits, respectively. 2-261 elicits high-efficacy modulation GABA currents at α1β2γ2, α2β2γ2, and α3β2γ2 but not at α1β1γ2, α2β1γ2, or α3β1γ2. 2-262 (right) elicits a similar pattern of responses at α1β1γ2 versus α1β2γ2 (D), α2β1γ2 versus α2β2γ2 (E), and α3β1γ2 versus α3β2γ2 (F), except that greater responses are observed at β1-subunit-containing GABAARs when concentrations exceed 10−7 M.
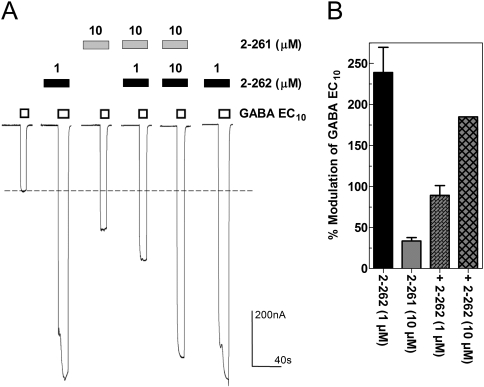
In contrast, maximal modulation by 2-261 and 2-262 at β1-subunit-containing GABAARs was significantly lower than observed at those with β2- and β3-subunits. For example, at α1β1γ2, maximal modulation was 181% for 2-262 and only 34% for 2-261 (Fig. 2B), consistent with β-subunit isoform selectivity. The ratio of maximal modulation at α1β2γ2 versus α1β1γ2 is 32-fold for 2-261 and 6-fold for 2-262.
To further characterize the β-subunit selectivity of 2-261 and 2-262, we obtained GABA concentration-response data in the presence and absence of 2-261 and 2-262 at α1β1γ2 and α1β2γ2 subtypes. Both enantiomers induced a significant leftward shift of the GABA concentration-response curve at α1β2γ2, with little or no shift at α1β1γ2 (Fig. 4).
Fig. 4.
Electrophysiological shift of GABA by 2-261 and 2-262. GABA concentration-response curves derived from α1β2γ2 (A) versus α1β1γ2 (B) GABAARs ± 0.7 μM 2-261. Likewise, the effect of GABA on α1β2γ2 (C) versus α1β1γ2 (D) GABAARs ± 3 μM 2-262 is depicted in the bottom panels. GABA responses are expressed as I/Imax, with each data point representing the mean ± S.E.M. (n = 3–4). The dashed line represents the GABAmax current with GABA only.
Positive Allosteric Modulation by 2-261 and 2-262 Occurs at the Same Site.
The interaction of the two enantiomers was examined at α1β1γ2 GABAARs to establish whether they share the same site. We hypothesized that the low-efficacy modulator (2-261) should block the effects of the high-efficacy modulator (2-262) if these compounds have a common site of action. Modulation evoked by 2-262 was inhibited by 2-261, and this blockade was overcome by increasing concentrations of 2-262 consistent with a shared site of action (Fig. 5).
Fig. 5.
Electrophysiological evidence that 2-261 blocks modulation by 2-262. A, representative voltage-clamp recording illustrating surmountable blockade of 2-262-induced modulation by 2-261 in oocytes expressing human GABAA α1β1γ2 GABAARs. Drugs were applied as indicated by the bars. Dashed line indicates EC10 control current magnitude used for calculating percentage of modulation. B, quantitative analysis of percentage of modulation (mean ± S.E.M.; n = 3).
Anxiolytic Activity of 2-261 and 2-262 in Mice and Rats.
In the mouse LD paradigm, both enantiomers reduced the time spent in the dark chamber. The minimal effective dose (MED) for 2-261 in this assay was 1 mg/kg p.o., with activity observed up to 30 mg/kg p.o. (Fig. 6A). 2-262 was more potent, with an MED value of 0.1 mg/kg p.o. and activity up to 1 mg/kg p.o. (Fig. 6B). For 2-262, anxiolytic activity was lost at 3 mg/kg p.o., possibly due to the onset of apparent sedative/ataxic effects of 2-262 (see below). The BZ receptor agonist alprazolam (0.1 mg/kg i.p.) was also active in this anxiety model.
Fig. 6.
Anxiolytic activity of 2-261 and 2-262 in mice. Dose-dependent effects of 2-261 (A) and 2-262 (B) versus alprazolam (0.1 mg/kg i.p.) on time (seconds) spent in the dark in the mouse LD paradigm after per os administration of each drug. The effects are compared with vehicle control. Each bar represents the mean ± S.E.M. time spent in the dark during a 5-min interval (n = 5–42 animals). Statistically significant differences from vehicle at ∗, P < 0.05 and ∗∗, P < 0.01 after ANOVA and post-hoc Dunnett's test.
We measured brain levels of 2-261 and 2-262 in a parallel group to correlate brain concentrations of drug at anxiolytic doses with GABAAR modulation in vitro. 2-261 and 2-262 were administered at their MEDs as determined in the LD paradigm described above. At their anxiolytic MEDs, the peak brain levels of 2-261 and 2-262 were 4.0 ± 0.6 and 2.7 ± 0.4 nM, respectively. These levels correspond to an ∼19 and ∼80% modulation of EC10 GABA-induced currents (in oocytes) at the α1β2γ2 GABAAR subtype by 2-261 and 2-262, respectively (Fig. 2A). Using similar analyses, these levels would have no measurable activity at α1β1γ2 GABAARs. Similar anxiolytic activity was observed in the rat EPM paradigm (Fig. 7).
Fig. 7.
Anxiolytic activity of 2-261 and 2-262 in rats. Dose-dependent effects of 2-261 (A) versus 2-262 (B) on time spent in the open arms in the rat EPM paradigm measured 30 min after intraperitoneal administration of each enantiomer. The effects are compared with vehicle control and diazepam (1.0 mg/kg i.p.). Each bar represents the mean ± S.E.M. of time spent in the open arms during a 5-min interval (n = 8–35 animals). Statistically significant differences from vehicle control at ∗, P < 0.05 and ∗∗, P < 0.01 after ANOVA and post-hoc Dunnett's test.
Effects of 2-261 and 2-262 in the RR Assay and the Pharmacokinetic Profile of Both Enantiomers at Ataxic Doses.
We tested 2-261 and 2-262 in the RR assay in mice to obtain AD50 values to quantitate ataxia. No RR failures were observed over a period of 360 min in 2-261-treated mice at doses up to 60 mg/kg p.o. (Fig. 8A). Only 10% of animals failed the RR assay at 120 mg/kg p.o. so that the AD50 value could not be determined. In contrast, 2-262 has an AD50 value of 43 mg/kg p.o. (Fig. 8B).
Fig. 8.
Effect on Rotarod performance by 2-261 and 2-262 in mice. Time course of RR performance in mice after per os administration of various doses of 2-261 (A) versus 2-262 (B). The AD50 values for 2-261 and 2-262 at the time of peak effect were >120 and 43 mg/kg, respectively, as calculated by the method of Litchfield and Wilcoxon (1949) (n = 8–10 mice/time point).
We measured the peak brain and plasma concentrations of 2-261 at the maximal dose tested (120 mg/kg p.o.) and 2-262 at its AD50 (43 mg/kg p.o.) to determine whether limited brain bioavailability could account for the limited ability of 2-261 to induce RR deficit. The maximal brain levels observed for 2-261 and 2-262 were 3.7 ± 0.5 and 0.8 ± 0.3 μM, respectively. At 10 mg/kg p.o., 2-261 and 2-262 had plasma half-lives of 1.5 and 1.6 h and peak brain levels of 0.7 ± 0.2 and 0.26 ± 0.11 μM, respectively (Fig. 9). Therefore, the differences in RR effects cannot be ascribed to limitations in bioavailability of 2-261 relative to 2-262.
Fig. 9.
Pharmacokinetics of 2-261 and 2-262 in mice. The pharmacokinetic profile of 2-261 (A) and 2-262 (B) in mice where plasma and brain levels (micromolar) are shown at various time (minutes) points after 10 mg/kg p.o. Each data point represents the mean ± S.E.M. levels (n = 4 animals/time point).
Estimation of Anxioselectivity by Dose and Anxioselective Index.
To estimate the dose separation between anxiolysis and ataxia, we compared the RR AD50 value with the MED value for anxiolytic activity. For 2-262, the separation is 430× (RR AD50 ÷ LD MED). For 2-261, RR failures never exceeded 10%; thus, the separation could only be estimated as >120×.
To examine the influence of bioavailability on anxioselectivity, we compared brain levels at ataxic and anxiolytic doses. The ratio of peak brain concentration at the ataxic AD50 value divided by the peak brain concentration at the anxiolytic MED value yields an anxioselective index (AI) for comparisons between compounds. For 2-262 in the LD paradigm, the AI corresponding to the MED (0.1 mg/kg p.o.) is ∼296 (800 nM at AD50 ÷ 2.7 nM at MED). In contrast, for 2-261, the AI corresponding to the MED (1 mg/kg p.o.) is >925 (3700 nM at 120 mg/kg ÷ 4 nM at MED), because the AD50 value could not be calculated for 2-261. Based on the PK/pharmacodynamic (PD) relationship, 2-261 has a significantly greater AI than 2-262.
Does the Extent of Modulation at β1-Subunit-Containing GABAARs Determine the AI?
To address this question, we tested additional enaminones and other reference positive allosteric modulators for 1) β-subunit isoform selectivity in vitro to determine the β-subunit isoform selectivity SAR in the enaminone series, 2) the ability to cause RR deficit, and 3) the PK profile to determine whether a PK/PD correlation exists. The data from the enaminones tested on α1β2γ2 and α1β1γ2 GABAARs are summarized in Table 1 (SAR details can be found in Supplemental Table 1). These enaminones potentiated EC10 GABA-evoked currents with varying degrees of β-subunit isoform selectivity and efficacy. As observed for 2-261 and 2-262, the enaminones do not appear to be α-subunit isoform-selective (Hogenkamp et al., 2007). Moreover, these enaminones had a generally small effect when tested for receptor selectivity at selected Cys-loop LGICs (i.e., α7, α4β2, and α1β1δε nicotinic acetylcholine; 5-HT3A; and N-methyl-d-aspartate receptors; Table 2).
TABLE 2.
Effect of 2-261, 2-262, and other GABAAR active enaminones on related LGICs
| Compound | % Modulation of Selected
LGICs |
||||
|---|---|---|---|---|---|
| α7 nAChR | α4β2 nAChR | α1β1δε nAChR | 5-HT3A | N-Methyl-d-aspartate | |
| 2-128 | 272 ± 81 | 9.2 ± 8 | 0 | 0 | 0 |
| 2-148 | 0 | 0 | 0 | −37 ± 8 | 0 |
| 2-261 | 166 ± 46 | 23 ± 8 | 0 | 0 | 33 ± 23 |
| 2-262 | 175 ± 116 | 26 ± 15 | 0 | 0 | 0 |
| 2-313 | 535 ± 55 | 17 ± 12 | 13 ± 7 | 0 | 16 ± 4 |
| 2-314 | 111 ± 52 | 0 | 0 | 0 | 21 ± 12 |
| 2-325 | 0 | 0 | 0 | 0 | −19 ± 1 |
The effect is expressed as percentage of modulation ± S.E.M. (n = 3–4) of an EC5 concentration of agonist at the respective LGICs in the presence of 10 μM test compound.
Like 2-261, 2-313 showed β-subunit isoform selectivity, with maximal modulation of 727% at β2-subunit-containing GABAARs and only 33% at those containing β1-subunits. The isopropyl amide 2-249 was found to resemble 2-262, with maximal modulation of β1- and β2-subunit-containing GABAARs in vitro of 249 and 905%, respectively. 2-301 was highly selective for β2-subunit-containing GABAARs, but the maximal efficacy was reduced compared with 2-261 (maximal modulation of β2 = 686%). 2-325 has almost absolute selectivity, with a maximal modulation of β1-subunit-containing GABAARs of 16%, while modulating β2-subunit-containing GABAARs to over the GABAmax current (>1300%).
Correlation between Activity at β1-Subunit-Containing GABAARs and RR Deficit.
Selected enaminones and nonenaminone reference compounds (Fig. 1) were tested in RR assays to determine AD50 values. In parallel, PK assays were performed to determine brain concentrations corresponding to RR deficit to allow determination of whether a PK/PD correlation exists among peak brain concentrations, RR response, and activation of β1-subunit-containing GABAARs.
An apparent threshold effect is observed when comparing activity at β1-containing GABAARs and RR failure (Fig. 10A). In contrast, there is no clear correlation between activity at β2-containing GABAARs and RR failure (Fig. 10B). Specifically, compounds with high efficacy at β1-containing GABAARs induce RR failures, whereas those with low activity at β1-containing GABAARs do not. This same general phenomenon was observed for the enantiomeric pair 2-261 and 2-262 described above.
Fig. 10.
Activity at α1β1γ2 GABAARs is predictive of ataxia. Rank order of apparent maximal percentage of modulation of EC10 GABA-evoked currents by various test compounds in oocytes expressing the α1β1γ2 (A) or α1β2γ2 (B) GABAAR subtypes. Maximal modulation was defined by that calculated from the concentration-response curves generated for each compound in the oocyte assays or that observed at ≥10 μM when limited by compound solubility. All compounds with efficacy below the threshold (dashed line) at the α1β1γ2-subtype receptor pass the RR test, whereas those with efficacy above the threshold fail the RR test as defined by a calculable AD50. Filled columns, passing RR test; open columns, failing RR test. ∗, L-838,417 causes RR failure, with AD50 = 30 mg/kg i.p. but has no activity at α1β1γ2 or α1β2γ2 GABAAR subtypes.
Compound 2-325 has almost no efficacy (∼16%) at the α1β1γ2 receptor subtype when tested at 10 μM. It fails to induce RR deficit at 30 mg/kg i.p., despite a maximal efficacy of >1300% at the α1β2γ2 GABAAR subtype. The brain level of 2-325 at 30 mg/kg i.p. is 2.1 μM, which is ∼9.5× its EC50 value at α1β2γ2 GABAARs. The AD50 value for 2-301 is >100 mg/kg i.p. At this dose, the peak brain 2-301 level is 4.0 μM, which is ∼18× the EC50 value (0.22 μM) for stimulation of the α1β2γ2 GABAAR subtype; yet, no RR deficit is observed, despite brain levels that correspond to maximal stimulation (maximum, 686%) in the oocyte electrophysiological assays.
Mefenamic acid is a clinically used nonsteroidal anti-inflammatory drug that is structurally different from the enaminones. It has no appreciable activity at the α1β1γ2 receptor subtype and is also incapable of eliciting RR deficit, despite brain concentrations equal to the EC50 value for activity at the α1β2γ2 receptor subtype (Table 1). A similar lack of RR deficit is observed with other compounds (e.g., 2-313), despite brain levels in excess of levels required for maximal stimulation of β2-subunit-containing GABAARs.
The anxioselective BZ receptor ligand ocinaplon has maximal stimulation of α1β1γ2 GABAARs subtypes of ∼81%. The AD50 value for ocinaplon is 54 mg/kg i.p., which corresponds to a brain level (12.5 μM) that is ∼4.5× the EC50 value for stimulation of the α1β1γ2 GABAAR subtype and induces 76% stimulation. This is consistent with the observation that when compounds reach brain levels associated with activity exceeding the 47% stimulation of the α1β1γ2 receptor subtype observed with 2-301, RR deficit will occur regardless of compound potency as reflected by their AD50 values. Based on these studies, a PK/PD correlation emerges where activity exceeding a threshold efficacy at the α1β1γ2 GABAAR subtype is required for eliciting RR deficit.
A threshold level of activation is required to observe RR deficit, and it lies in between the efficacy for 2-301 and ocinaplon (Fig. 10A). In contrast, a threshold could not be identified when a similar bar graph analysis was performed based on efficacy at the α1β2γ2 GABAAR subtype (Fig. 10B) or at α1-, α2-, or α3-subunit (data not shown)-containing GABAARs. There also was no correlation with γ- or δ-subunit-containing receptors (data not shown). This β-subunit isoform selectivity does not appear to be predictive of anxiolytic activity as compounds with great variations in efficacy, such as 2-148 and 2-313, can still show robust anxiolytic activity (Fig. 11).
Fig. 11.
Anxiolytic activity of 2-313 and 2-148 in mice. Effects of 2-313 (A) and 2-148 (B) versus alprazolam (0.1 mg/kg) on time (seconds) spent dark in the mouse LD paradigm after intraperitoneal administration of each compound. The effects of the test compounds are compared with vehicle control. Each bar represents the mean ± S.E.M. time spent in the dark chamber during a 5-min interval (n = 6–30 animals). Significantly different from vehicle control at ∗, P < 0.05 after ANOVA and post-hoc Dunnett's test.
Efficacy Required for Achieving an AD50 in the RR Assay.
The degree of stimulation of α1β1γ2 GABAARs necessary to achieve an RR AD50 can be deduced by measuring the peak brain levels of each of the compounds at the AD50 dose and extrapolating the degree of stimulation by this concentration of compound observed in the oocyte assays. Ten compounds of diverse chemotypes reveal that a range of 52 to 270% potentiation at α1β1γ2 GABAARs is necessary to observe an AD50 for RR deficit (Table 3). In contrast, the range is 58 to 1060% for α1β2γ2 GABAARs. A similar analysis using activity at α1-, α2-, or α3-subunit-containing GABAAR subtypes does not result in a comparably narrow range of stimulation observed with the α1β1γ2 GABAAR. Despite robust efficacy at the α1β2γ2 subtype, compounds such as 2-261 do not show potentiation of ethanol-induced RR failure commonly displayed by BZs (Fig. 12).
TABLE 3.
Degree of α1β1γ2 or α1β2γ2 GABAAR subtype stimulation by the peak brain levels attained at the AD50 dose of various test compounds
Brain levels and AD50 values of compound are from Table 1. The degree of activation associated with these levels of compound is derived from the concentration-response curves for each compound and defined as a percentage of stimulation of EC10 GABA in oocytes expressing the α1β1γ2 or α1β2γ2 GABAAR subtypes. All test compounds were administered by intraperitoneal injection except for 2-262 which was administered orally.
| Compound | AD50 | Brain Levels at AD50 | Stimulation at α1β1γ2 | Stimulation at α1β2γ2 |
|---|---|---|---|---|
| mg/kg | μM | % | ||
| Ocinaplon | 54 | 12.5 | 76 | 58 |
| Bretazenil | 25 | 11.7 | 65 | 69 |
| Diazepam | 2 | 0.64 | 90 | 130 |
| 2-128 a | >120 | 5.8 | 76 | 518 |
| Loreclezole | 83 | 11.7 | 99 | 382 |
| 2-262 | 43 | 0.8 | 71 | 1060 |
| 2-249 | 3 | 0.6 | 52 | 857 |
| 2-148 | 5 | 1.1 | 71 | 739 |
| Tracazolate | 42 | 7.6 | 270 | 803 |
| 2-314 | 2.5 | 0.3 | 230 | 583 |
2-128 has poor solubility and bioavailability so that an accurate AD50 could not be determined; therefore, the brain levels of the compound were determined at the highest dose (120 mg/kg) tested where 40% of the animals failed the RR test.
Fig. 12.
Lack of ethanol potentiation of RR failure by 2-261. The effect of 2-261(30 mg/kg p.o.) versus diazepam (1 mg/kg i.p.) on EtOH (1 g/kg i.p.)-induced RR deficit in mice. The effect is expressed as a percentage of mice passing the RR assay, with 12 mice tested under each condition. Significantly different from diazepam vehicle + EtOH at ∗, P < 0.01 by the Fischer-Yates exact probability test.
Discussion
We provide clear evidence in support of the hypothesis that a correlation exists between the efficacy of compounds at β1-subunit-containing GABAARs and ataxia. 2-261 and 2-262, a pair of enantiomers that share a common site of action, have differential ataxic liability that is related to the degree of α1β1γ2 GABAAR subtype activation. Strong evidence for this relationship results from the use of a chemically diverse set of positive allosteric modulators as tools. This correlation becomes evident when PK/PD studies on these GABAAR positive allosteric modulators revealed that a threshold of activity at β1-subunit-containing GABAARs must be exceeded to observe performance deficit in the RR assay. We have unequivocally demonstrated that RR deficit can be reliably predicted based on activity at β1-subunit-containing GABAARs. No such correlation could be shown for the other major subunits (i.e., β2, β3, α1, α2, or α3). There also was no dependence upon γ- or δ-subunit-containing receptors. Moreover, limited ataxia due to reduced potency and/or efficacy are not confounds because many of our compounds meet or greatly exceed the potency/efficacy of the commonly used full agonist BZs. It is interesting to note that the existence of an association between β1-subunit-containing GABAARs and sedation/ataxia can be deduced from the literature. Collectively, these reports are prescient in view of our current hypothesis.
Clinically Tested/Used Compounds Support the β1-Subunit Hypothesis.
The most compelling anecdotal evidence relating receptor subtype selectivity and sedation is based on clinically used compounds. For example, mefenamic acid, a nonsteriodal anti-inflammatory drug that has been used for several decades, is a selective modulator of β2-subunit-containing GABAARs that has virtually no activity at β1-subunit-containing GABAARs (Halliwell et al., 1999). This drug has no sedative activity at clinically relevant (i.e., inflammation) serum levels (∼83 μM) that would supersaturate α1β2γ2 GABAARs (Cryer and Feldman, 1998). We have shown that the maximal stimulation of β2-subunit-containing GABAARs by mefenamic acid exceeds that of diazepam. Therefore, a lack of agonist BZ-like efficacy is not the explanation for lack of ataxia. Likewise, the anticonvulsant loreclezole has β2/3-subunit selectivity, diazepam-like efficacy, and anxiolytic/anticonvulsant activity in animal models with reduced sedative effects (Smith et al., 2004; Groves et al., 2006) and is a nonsedating antiepileptic in human clinical trials (Fisher and Blum, 1995). Etifoxine, with β2/3-subunit selectivity, also has diazepam-like efficacy at GABAARs (Smith et al., 2004) and is used clinically as a nonsedating anxiolytic (Nguyen et al., 2006). We firmly believe that these clinical observations along with our own data provide a cogent argument that designing compounds (see Supplemental Data for SAR on β-subunit isoform selectivity) with reduced activity at β1-subunit-containing GABAARs will ultimately result in anxioselective drugs.
Neuroanatomical Correlates of β-Subunit Selectivity Support the β1-Subunit Hypothesis.
The neuroanatomical localization of the β1-subunit explains, in part, why limited efficacy at β1-subunit-containing GABAARs may reduce sedative/ataxic liability. The β1-subunits are reported to be associated with extrasynaptic GABAARs and are expressed in brainstem and arousal-related areas of the rat brain (Pirker et al., 2000; Sun et al., 2004; Harrison, 2007). Several regions in the brain responsible for either the induction or maintenance of sleep have β1-subunit-containing GABAAR subtypes. For example, an important area for modulating sleep and facilitating EEG synchronization is the reticular thalamic nucleus that has primarily β1-subunit-containing GABAARs and receives input from the basal forebrain, substantia nigra, and globus pallidus and generally enhances arousal/attention (Paré et al., 1990; Hazrati and Parent, 1991; Asanuma, 1994). The reticular thalamic nucleus projects to the thalamic relay nuclei to promote sleep, whereas the intralaminar thalamic nuclei receive innervation from the ascending reticular activating system and facilitate awareness (McCormick and Bal, 1997; Hartings et al., 2000). The positive modulation of β1-subunit-containing GABAARs in these nuclei normally serves to induce sleep such as with agonist BZs; thus, compounds devoid of activity at β1-subunit-containing GABAARs would be expected have reduced sedative potential (Pirker et al., 2000; Van der Werf et al., 2002; Huntsman and Huguenard, 2006). The distribution of the β1-subunit in human brain has not been fully characterized. It is assumed that its distribution in rodent brain is representative of that in humans. Nevertheless, human clinical evidence on the sedative potential of β1-subunit-selective compounds (e.g., etifoxine, loreclezole, and mefenamic acid) is consistent with this brain structure-function relationship.
A PK/PD Correlation Provides Support of an Activity Threshold for Ataxia.
The strongest argument that ataxic liability depends on β1-subunit-containing GABAARs is provided by the correlative evidence derived from the PK/PD relationship of our test compounds. The ability to identify a threshold of activity at α1β1γ2 GABAARs, which must be exceeded to observe RR deficit (Fig. 10A), that is not observed with the other subunits evaluated strongly supports our hypothesis. Furthermore, this β-subunit isoform selectivity does not appear to be predictive of anxiolytic activity as compounds with extreme differences in efficacy, such as 2-148 and 2-313, still show robust anxiolytic activity.
Based on the compounds evaluated, the empirically derived threshold for ataxia lies in between 47% (2-301) and 81% (ocinaplon) stimulation of EC10 GABA at the α1β1γ2 GABAAR subtype. Yet, upon cursory analysis, arguments can be made that inconsistencies with our β1-subunit hypothesis occur. For example, etomidate, which is β2/3-subunit-selective, can cause sedation and anesthesia (Hill-Venning et al., 1997). However, this anomaly can be readily explained by a scenario where increasing doses of etomidate will result in brain levels of drug that are associated with activity that exceed the threshold at β1-subunit-containing GABAARs necessary for sedation. Etomidate also has direct channel-activating properties that can account for its anesthetic action. In contrast, 2-325 neither causes ataxia nor anesthesia; yet, it has a similar maximal efficacy at the α1β2γ2 GABAAR subtype as etomidate. The most parsimonious explanation for this difference is that 2-325, unlike etomidate, has virtually no activity at α1β1γ2 GABAARs or direct channel effects.
A Range of Efficacy at β1-Subunit-Containing GABAARs Can Be Defined for RR Activity.
A well-defined range (52–270%) of activity at β1-subunit-containing GABAARs appears necessary to achieve an RR AD50. It is noteworthy that among the compounds tested, none with measurable AD50 values are associated with brain levels that are below the threshold level required for RR deficit. This is extraordinary when there is the possibility of confounding variables such as off-target effects or active metabolites that can cause RR deficit.
The ∼5× difference (52–270%) in the maximal and minimal efficacy measured may be a result of two compounds. First, the steep concentration-response curve of 2-314 is a potential confound. A single log unit change in the concentration (10−7–10−6 M) results in a just-detectable (<15%) response to maximal modulation of ∼635% (i.e., Hill slope >2) at α1β1γ2 GABAARs, resulting in the magnification of the experimental error (Supplemental Fig. 1). Second, tracazolate levels at its AD50 value are associated with 270% enhancement of α1β1γ2 GABAARs, which may result from its opposing action mediated by adenosine A1 and A2 receptors (Daly et al., 1988). Tracazolate is an antagonist of adenosine A1 and A2 receptors, with micromolar potency where it is predicted to promote arousal and wakefulness (Van Dort et al., 2009). Therefore, greater stimulation of α1β1γ2 GABAARs by tracazolate would be required for physiological antagonism of its actions at these adenosine receptor subtypes. It is interesting to note that when tracazolate and 2-314 are excluded from determining the range of activity necessary to achieve an AD50, it results in a much narrower ∼1.9× range (52–99%) of modulation. In contrast, the activity at the α1β2γ2 GABAAR subtype is much broader, even upon exclusion of tracazolate and 2-314, with an ∼18× range (58–1060%) of efficacy associated with RR deficit and is thus unlikely to be the key receptor subtype in mediating ataxia. Regardless, the existence of a narrow range of activity that is chemotype-independent and discernible, despite the influence of multiple confounds lends strong support for the broad applicability of our hypothesis.
The β1-Subunit Hypothesis also Explains the Ataxic Actions of BZ Site Agonists.
The ataxic actions of the BZ site agonists can be explained by our hypothesis. Both diazepam and bretazenil have efficacies that exceed the threshold activity required at α1β1γ2 GABAARs for sedation, whereas L-838,417 does not. The observation of an AD50 value (30 mg/kg i.p.) for L-838,417 at a brain level of 8 μM is probably the result of an off-target(s)-mediated effect because it has <1 nM affinity for its site of action (McKernan et al., 2000). In contrast, the anxioselectivity profile of our nonBZ site positive allosteric modulators cannot be explained by the α2/3-subunit isoform selectivity hypothesis alone. For example, 2-325, 2-313, and 2-301 have maximal efficacies mediated by the α1β2γ2 subtype that are 4- to 8-fold greater than that of diazepam. Yet, they do not cause RR deficit at brain levels expected to saturate the α1β2γ2 GABAAR, a subtype that according to the α2/3-subunit selectivity hypothesis is thought to mediate the sedative/ataxic effects of the agonist BZs. The two hypotheses are not mutually exclusive but both may be necessary to provide a full explanation for the anxioselectivity of certain GABAAR positive allosteric modulators.
Summary.
The reduction of activity at β1-subunit-containing GABAARs reduces ataxia among the chemically diverse compounds that we have tested. It is highly unlikely that this correlation between activity at β1-subunit-containing GABAAR subtypes and ataxia as measured by RR deficit is purely coincidental. The doses of the agonist BZs that induce RR deficit and a reduction in spontaneous locomotor activity are overlapping (Bourin et al., 1992; Crabbe et al., 1998). Because the latter measure is believed to be a reflection of sedative activity and the former measure indicates motor ataxia, it renders the ability to discriminate the two adverse effects by dose-separation difficult, if not impossible. If the β1-subunit-selective enaminones in the present study are like the agonist BZs in this regard, it is conceivable that the lack of Rotarod deficit may reflect their inability to induce both effects. Nevertheless further testing for the robustness of this general hypothesis is still necessary. For example, phenotype characterization of β1-subunit “knockout” or “knockdown” mice would be corroborative where full agonist BZ receptor ligands such as diazepam would be predicted to have reduced sedative/ataxic activity. The characterization of a pair of enantiomers and related enaminones has enabled us to design GABAAR positive allosteric modulators with reduced activity at β1-subunit-containing GABAARs. These compounds have predictably reduced ataxic effects but retain robust anxiolytic activity. Our approach of enhancing activity at β2/3-subunit and reducing/eliminating activity at β1-subunit-containing GABAARs may be an effective strategy to create anxioselective positive allosteric modulators if its validity is confirmed by clinical testing.
Supplementary Material
Acknowledgments
We thank Dr. Jose Aguilar, Dr. David Putman, Wen-Yen Li, and Chuck Foster for technical assistance and Drs. Jon Lindstrom and Jim Boulter for generous gifts.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH082241]; and the University of California Discovery [Grant bio06-10577] (to K.W.G.). Parts of this work were previously presented by Bagnera RE, Johnstone TBC, Tran MB, Whittemore ER, Hogenkamp DJ, and Gee KW (2007) Characterization of a series of novel allosteric modulators of GABAA receptors with differential beta subunit selectivity. The Society for Neuroscience Conference; 2007 Oct 3–Nov 7; San Diego, CA. Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161885.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- R
- receptor
- BZ
- benzodiazepine
- nonBZ
- nonbenzodiazepine
- DMSO
- dimethyl sulfoxide
- 5-HT
- 5-hydroxytryptamine
- nAChR
- nicotinic acetylcholine receptor
- HPLC
- high-performance liquid chromatography
- LD
- light-dark transition
- ANOVA
- analysis of variance
- RR
- Rotarod
- AD50
- ataxogenic half-maximal dose where half of the mice fail the RR assay
- EtOH
- ethanol
- MED
- minimal effective dose
- EPM
- elevated plus maze
- PK
- pharmacokinetic
- PD
- pharmacodynamic
- AI
- anxioselective index
- SAR
- structure-activity relationship
- LGIC
- ligand-gated ion channel.
References
- Asanuma C. (1994) GABAergic and pallidal terminals in the thalamic reticular nucleus of squirrel monkeys. Exp Brain Res 101:439–451 [DOI] [PubMed] [Google Scholar]
- Astleford BA, Goe GL, Keay JG, Scriven EFV. (1989) Synthesis of 1-alkyl-1,2,4-triazoles: a new one-pot regiospecific procedure. J Org Chem 54:731–732 [Google Scholar]
- Bare TM, McLaren CD, Campbell JB, Firor JW, Resch JF, Walters CP, Salama AI, Meiners BA, Patel JB. (1989) Synthesis and structure-activity relationships of a series of anxioselective pyrazolopyridine ester and amide anxiolytic agents. J Med Chem 32:2561–2573 [DOI] [PubMed] [Google Scholar]
- Basile AS, Lippa AS, Skolnick P. (2004) Anxioselective anxiolytics: can less be more? Eur J Pharmacol 500:441–451 [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M, Mansouri B, Colombel MC, Bradwejn J. (1992) Comparison of behavioral effects after single and repeated administrations of four benzodiazepines in three mice behavioral models. J Psychiatry Neurosci 17:72–77 [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Gallaher EJ, Cross SJ, Belknap JK. (1998) Genetic determinants of sensitivity to diazepam in inbred mice. Behav Neurosci 112:668–677 [DOI] [PubMed] [Google Scholar]
- Cryer B, Feldman M. (1998) Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med 104:413–421 [DOI] [PubMed] [Google Scholar]
- Daly JW, Hong O, Padgett WL, Shamim MT, Jacobson KA, Ukena D. (1988) Non-xanthine heterocycles: activity as antagonists of A1- and A2-adenosine receptors. Biochem Pharmacol 37:655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas SL, Franson KL, Schmitt JA, Cohen AF, Fau JB, Dubruc C, van Gerven JM. (2009) The pharmacokinetic and pharmacodynamic effects of SL65.1498, a GABA-A alpha2,3 selective agonist, in comparison with lorazepam in healthy volunteers. J Psychopharmacol 23:625–632 [DOI] [PubMed] [Google Scholar]
- de Haas SL, de Visser SJ, van der Post JP, Schoemaker RC, van Dyck K, Murphy MG, de Smet M, Vessey LK, Ramakrishnan R, Xue L, et al. (2008) Pharmacodynamic and pharmacokinetic effects of MK-0343, a GABA(A) alpha2,3 subtype selective agonist, compared to lorazepam and placebo in healthy male volunteers. J Psychopharmacol 22:24–32 [DOI] [PubMed] [Google Scholar]
- Fisher R, Blum D. (1995) Clobazam, oxcarbazepine, tiagabine, topiramate, and other new antiepileptic drugs. Epilepsia 36:S105–S114 [DOI] [PubMed] [Google Scholar]
- Groves JO, Guscott MR, Hallett DJ, Rosahl TW, Pike A, Davies A, Wafford KA, Reynolds DS. (2006) The role of GABAbeta2 subunit-containing receptors in mediating the anticonvulsant and sedative effects of loreclezole. Eur J Neurosci 24:167–174 [DOI] [PubMed] [Google Scholar]
- Halliwell RF, Thomas P, Patten D, James CH, Martinez-Torres A, Miledi R, Smart TG. (1999) Subunit-selective modulation of GABAA receptors by the non-steroidal anti-inflammatory agent, mefenamic acid. Eur J Neurosci 11:2897–2905 [DOI] [PubMed] [Google Scholar]
- Hamon A, Morel A, Hue B, Verleye M, Gillardin JM. (2003) The modulatory effects of the anxiolytic etifoxine on GABA(A) receptors are mediated by the beta subunit. Neuropharmacology 45:293–303 [DOI] [PubMed] [Google Scholar]
- Harrison NL. (2007) Mechanisms of sleep induction by GABA(A) receptor agonists. J Clin Psychiatry 68 (Suppl 5):6–12 [PubMed] [Google Scholar]
- Hartings JA, Temereanca S, Simons DJ. (2000) High responsiveness and direction sensitivity of neurons in the rat thalamic reticular nucleus to vibrissa deflections. J Neurophysiol 83:2791–2801 [DOI] [PubMed] [Google Scholar]
- Hazrati LN, Parent A. (1991) Projection from the external pallidum to the reticular thalamic nucleus in the squirrel monkey. Brain Res 550:142–146 [DOI] [PubMed] [Google Scholar]
- Heeres J. (1985) inventor; Janssen Pharmaceutica N.V., assignee 1-(2-Aryl-2-halo-1-ethenyl)-1H-azoles, and anticonvulsant use thereof. U.S. patent 4,539,325.1985September3
- Hill-Venning C, Belelli D, Peters JA, Lambert JJ. (1997) Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. Br J Pharmacol 120:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenkamp DJ, Johnstone TB, Huang JC, Li WY, Tran M, Whittemore ER, Bagnera RE, Gee KW. (2007) Enaminone amides as novel orally active GABAA receptor modulators. J Med Chem 50:3369–3379 [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. (2006) Fast IPSCs in rat thalamic reticular nucleus require the GABAA receptor beta1 subunit. J Physiol 572:459–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield JT, Jr, Wilcoxon F. (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113 [PubMed] [Google Scholar]
- Lippa A, Czobor P, Stark J, Beer B, Kostakis E, Gravielle M, Bandyopadhyay S, Russek SJ, Gibbs TT, Farb DH, et al. (2005) Selective anxiolysis produced by ocinaplon, a GABA(A) receptor modulator. Proc Natl Acad Sci U S A 102:7380–7385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. (1997) Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 20:185–215 [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et al. (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci 3:587–592 [DOI] [PubMed] [Google Scholar]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW. (2007) Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci U S A 104:8059–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Fakra E, Pradel V, Jouve E, Alquier C, Le Guern ME, Micallef J, Blin O. (2006) Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: a double-blind controlled study in general practice. Hum Psychopharmacol 21:139–149 [DOI] [PubMed] [Google Scholar]
- Paré D, Hazrati LN, Parent A, Steriade M. (1990) Substantia nigra pars reticulata projects to the reticular thalamic nucleus of the cat: a morphological and electrophysiological study. Brain Res 535:139–146 [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. (2000) GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101:815–850 [DOI] [PubMed] [Google Scholar]
- Popik P, Kostakis E, Krawczyk M, Nowak G, Szewczyk B, Krieter P, Chen Z, Russek SJ, Gibbs TT, Farb DH, et al. (2006) The anxioselective agent 7-(2-chloropyridin-4-yl)pyrazolo-[1,5-a]-pyrimidin-3-yl](pyridin-2-yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA-gated currents at alpha1 subunit-containing GABAA receptors. J Pharmacol Exp Ther 319:1244–1252 [DOI] [PubMed] [Google Scholar]
- Putman DG, Hogenkamp DJ, Dasse OA, Whittemore ER, Jensen MS. (2007) inventors; Xytis Inc., assignee Enantiomerically pure R-etifoxine, pharmaceutical compositions thereof and methods of their use. World patent, WO109288.2007September27
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. (1999) Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature 401:796–800 [DOI] [PubMed] [Google Scholar]
- Skolnick P, Epstein JW. (2005) inventors 2-Pyridinyl[7-(substituted-pyridin-4-yl)pyrazolo[1,5-a]pyrimidin-3-yl]methanones. U.S. patent application 20050245517.2005November3
- Smith AJ, Oxley B, Malpas S, Pillai GV, Simpson PB. (2004) Compounds exhibiting selective efficacy for different beta subunits of human recombinant gamma-aminobutyric acid A receptors. J Pharmacol Exp Ther 311:601–609 [DOI] [PubMed] [Google Scholar]
- Sprenger KJ, Aneiro L, Fund L, Liu Y, Changchit A, Rajachandran L, Kehne JH, Xie L. (2007) Clinical trial data demonstrating sedative-hypnotic efficacy of the α3-subunit preferring GABAA receptor partial allosteric activator, NG2-73: translational validity of pharmacokinetic/pharmacodynamic (PK/PD) relationships derived from preclinical studies, in Proceedings of the Society for Neuroscience, 2007 Oct–Nov 7; San Diego, CA Program no. 632.2/AAA17 Society for Neuroscience, Washington, DC: [Google Scholar]
- Sun C, Sieghart W, Kapur J. (2004) Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res 1029:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Wingrove PB, Connelly L, Whiting PJ, Wafford KA. (2002) Tracazolate reveals a novel type of allosteric interaction with recombinant gamma-aminobutyric acid(A) receptors. Mol Pharmacol 61:861–869 [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev 39:107–140 [DOI] [PubMed] [Google Scholar]
- Van Dort CJ, Baghdoyan HA, Lydic R. (2009) Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci 29:871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ. (2006) GABA-A receptors: a viable target for novel anxiolytics? Curr Opin Pharmacol 6:24–29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.