Abstract
In the present study, we evaluated the disposition of inorganic mercury (Hg2+) in sham-operated and 75% nephrectomized (NPX) Wistar and transport-deficient (TR−) rats treated with saline or the chelating agent meso-2,3-dimercaptosuccinic acid (DMSA). Based on previous studies, DMSA and TR− rats were used as tools to examine the potential role of multidrug-resistance protein 2 (MRP2) in the disposition of Hg2+ during renal insufficiency. All animals were treated with a low dose (0.5 μmol/kg i.v.) of mercuric chloride (HgCl2). At 24 and 28 h after exposure to HgCl2, matched groups of Wistar and TR− rats received normal saline or DMSA (intraperitoneally). Forty-eight hours after exposure to HgCl2, the disposition of Hg2+ was examined. A particularly notable effect of 75% nephrectomy in both strains of rats was enhanced renal accumulation of Hg2+, specifically in the outer stripe of the outer medulla. In addition, hepatic accumulation, fecal excretion, and blood levels of Hg2+ were enhanced in rats after 75% nephrectomy, especially in the TR− rats. Treatment with DMSA increased both the renal tubular elimination and urinary excretion of Hg2+ in all rats. DMSA did not, however, affect hepatic content of Hg2+, even in the 75% NPX TR− rats. We also show with real-time polymerase chain reaction that after 75% nephrectomy and compensatory renal growth, expression of MRP2 (only in Wistar rats) and organic anion transporter 1 is enhanced in the remaining functional proximal tubules. We conclude that MRP2 plays a significant role in the renal and corporal disposition of Hg2+ after a 75% reduction of renal mass.
Under homeostatic conditions, renal proximal tubular epithelial cells (in rats) do not, or cannot, secrete efficiently mercuric ions into the tubular lumen after exposure to a non-nephrotoxic dose of inorganic mercury (Hg2+). The low level of secretion of Hg2+ under these circumstances probably relates to significant binding of mercuric ions to protein-thiols in the intracellular milieu. However, after treatment with the dithiol-chelating agent 2,3-dimercaptopropane-1-sulfonic acid (DMPS) or meso-2,3-dimercaptosuccinic acid (DMSA), mobilization and secretory elimination of Hg2+ along the proximal tubule has been shown to be great enough to reduce the renal burden of Hg2+ in normal and uninephrectomized [50% nephrectomized (NPX)] rats by as much as 80 to 85% within a 24-h period (Zalups, 1993). In a series of recent studies, we demonstrated that the ATP-binding cassette (ABC) protein, multidrug-resistance protein 2 (MRP2), plays a significant role in the secretion of Hg2+ at the luminal plasma membrane of proximal tubular epithelial cells, especially after treatment with DMPS or DMSA (Bridges et al., 2008a,b; Zalups and Bridges, 2008).
Interestingly, when 50% of the functional renal mass is removed surgically in rats, and then the rats are exposed to Hg2+ or methylmercury (CH3Hg+), enhanced uptake and retention of mercuric ions occurs along the pars recta of renal proximal tubules, especially in the portion present in the outer stripe of the outer medulla (Zalups et al., 1987, 1992; Zalups and Lash, 1990; Zalups, 1991a,b, 1993; Zalups and Cherian, 1992a,b). This enhanced uptake seems to be causally linked to profound structural and functional adaptive changes associated with compensatory renal growth, which occurs after renal mass is reduced significantly.
Moreover, the enhanced accumulation of mercuric ions that occurs in the pars recta after a 50% reduction of renal mass also appears to increase the severity of the nephropathy induced by lower nephrotoxic doses of Hg2+ (Zalups and Diamond, 1987; Zalups et al., 1987; Houser and Berndt, 1988). At present, the mechanisms responsible for the enhanced net accumulation of mercuric ions along the pars recta of proximal tubules after a 50% reduction of renal mass remain not fully defined, although an amplification of transport mechanisms and increases in the intracellular contents of the thiols glutathione and metallothionein have been suggested (Zalups and Lash, 1990; Zalups et al., 1995).
Experimental evidence indicates that both luminal and basolateral mechanisms participate in the accumulation of mercury after uninephrectomy (Zalups, 1997) and a para-aminohippuric acid (PAH)-dependent mechanism is active. PAH has a particularly high affinity for organic anion transporter 1 (OAT1), which in the kidneys is present almost exclusively in the basolateral membrane of proximal tubular epithelial cells. We have recently demonstrated in vitro that OAT1, which has a broad substrate specificity, is capable of transporting the most likely mercuric conjugates of nonprotein thiols (such as cysteine, homocysteine, and N-acetylcysteine) believed to be present in blood (Aslamkhan et al., 2003; Zalups and Ahmad, 2004, 2005).
When functional renal mass is reduced by approximately 75 to 80% of normal (as a result of disease, trauma, or surgery), the hypertrophic changes that occur along the remaining functional proximal tubules are greater than those engendered by a 50% reduction of renal mass. However, the diminished capacity for ultrafiltration combined with the imposed increased workload on the hypertrophied remaining nephrons becomes too great for them to maintain normal fluid and electrolyte homeostasis, and chronic renal failure ensues.
We postulate that increased expression of certain membrane transporters constitutes a significant part of the adaptive structural and functional responses that occur in hypertrophied proximal tubular epithelial cells after renal mass has been reduced by 75% nephrectomy. In particular, we hypothesize that expression of transporters involved in tubular secretion (such as OAT1 in the basolateral membrane and MRP2 in luminal membrane) is enhanced.
In the present investigation, we studied the disposition of an intravenously administered low dose (0.5 μmol/kg) of mercuric chloride (HgCl2) in 75% NPX and sham-operated (SO) Wistar and transport-deficient (TR−) rats treated with normal saline or DMSA (a Food and Drug Administration-approved chelating agent that mobilizes Hg2+ in renal proximal tubular epithelial cells). Treatment with DMSA was used as a tool to assess the potential role of MRP2 in the renal disposition of Hg2+ in rats that had undergone a sham operation or a 75% reduction of renal mass. The primary aims of the present study were to determine whether 75% nephrectomy: 1) affects the expression of OAT1 and MRP2 in the remaining functional proximal tubular epithelial cells and 2) affects the role of MRP2 in the renal and corporal disposition of Hg2+.
Materials and Methods
Animals.
Male TR− and normal (control) Wistar rats weighing 175 to 200 g were purchased from Harlan Laboratories (Indianapolis, IN). All animals were provided a commercial laboratory diet (Tekland 6% rat diet; Harlan Laboratories) and water ad libitum throughout all aspects of animal experimentation.
In the first phenotypic characterization of TR− rats, Jansen et al. (1985) demonstrated that the rats had hereditary conjugated hyperbilirubinemia, which was subsequently attributed to a mutation in the mrp2 gene (Mayer et al., 1995; Paulusma et al., 1996). As a result of this mutation, the presence of Mrp2 mRNA is low, and the presence of Mrp2 protein is absent in the tissues of these rats (Mayer et al., 1995). TR− rats represent a reliable model for studying the hepatic and renal secretion of various substrates of MRP2 (de Vries et al., 1989; Masereeuw et al., 2003; Smeets et al., 2004).
Groups.
Four groups of four TR− rats and four groups of four Wistar rats were selected at random from the pool of purchased TR− and Wistar rats. Two groups of TR− rats and two groups of Wistar rats underwent 75% nephrectomy, whereas the remaining four corresponding groups of rats underwent a sham operation.
Experimental Design.
Fourteen days after surgery, all rats were injected intravenously with a nontoxic 0.5 μmol/kg dose of mercuric chloride (HgCl2; in 2 ml/kg normal saline), containing 203HgCl2 (1 μCi/rat). The nephrotoxic nature of the 0.5 μmol/kg dose of HgCl2 in the 75% NPX rats was determined previously with histopathological analyses of renal slices (unpublished findings).
Twenty-four and 48 h after exposure to HgCl2, one group of SO rats and one group of 75% NPX rats of both strains (Wistar and TR−) received an 100 mg/kg i.p. dose of DMSA (in 2 ml/kg normal saline). At the same time, one group of SO rats and one group of 75% NPX rats of both strains received an injection of normal saline (2 ml/kg i.p.).
Forty-eight hours after the injection of HgCl2, all groups of rats were sacrificed, and samples of blood, liver, kidneys, urine, and feces were collected for analysis of Hg2+ content.
Surgery.
After anesthesia was induced with 70 mg/kg ketamine and 6 mg/kg xylazine (intramuscularly), a midline incision was made through the skin and musculature of the abdomen with a scalpel. Subsequently, the right and left kidneys of each animal were isolated from the perinephric fascia and fat, without damaging the liver or corresponding adrenal glands.
For the groups that underwent 75% nephrectomy, the right renal artery and vein and right ureter were ligated with a single sterile 1-0 silk suture. Then, the right kidney was excised distal to the ligature, after which the left kidney was exteriorized from the body through the midline incision. Subsequently, it was placed in a Lucite cup in a manner that exposed the posterior surface of the organ. With use of a dissecting microscope, a sterile 4-0 silk suture was threaded between the renal vein and posterior branch of the renal artery. When the ligature was in place, it was tied tightly. Approximately half of the left kidney would generally turn to a darker color, indicating a cessation or great reduction of blood flow to that portion of the kidney. SO animals were treated similarly, except that their right kidneys were not excised and the left renal arteries were not ligated.
Based on numerous findings from our laboratory, the removal of the right kidney and the tying off of the posterior branch of the left renal artery are sufficient to induce early systemic changes associated with chronic renal failure (Zalups, 1989, 1995; Zalups and Henderson, 1992). With the ligature tied, the left kidney was removed from the Lucite cup and placed back into its normal retroperitoneal position. The abdominal muscles were sewn together with sterile 4-0 silk suture, and the opposite ends of the incised skin were approximated by using sterile 9-mm stainless steel wound clips.
Recovery from Surgery.
A period of 14 days was allowed for recovery from surgery and the completion of the rapid phase of compensatory renal growth in the 75% NPX rats.
Injection of Hg2+.
Animals were first anesthetized lightly with ether. Then, a small incision was made through the skin in the midventral region of the thigh to expose the femoral vein and artery. A non-nephrotoxic 0.5 μmol/kg dose of HgCl2 (in 2 ml of normal saline) (containing 1 μCi of 203Hg2+) was administered into the vein. The wound was closed with sterile, 9-mm, stainless-steel wound clips. Animals were then placed individually in plastic metabolic cages, in which water and food were provided ad libitum.
Injections of DMSA.
At both 24 and 28 h after exposure to HgCl2, corresponding groups of Wistar and TR− rats received an intraperitoneal injection of either normal saline (2 ml/kg) or a 100-mg/kg dose of DMSA (in 2 ml/kg normal saline). Forty-eight hours after receiving the non-nephrotoxic dose of HgCl2, the rats were anesthetized deeply (with 70 mg/kg ketamine and 6 mg/kg xylazine), and blood, liver, kidneys, urine and feces were collected for analysis of Hg2+ content.
Collection of Tissues, Organs, Urine, and Feces.
Forty-eight hours after the injection of Hg2+, rats were anesthetized with ketamine (70 mg/kg) and xylazine (6 mg/kg). Once anesthetized, two 1-ml samples of blood were obtained from the inferior vena cava. One of the samples was placed in a polystyrene tube for determination of 203Hg2+ content, and the other sample was placed in a Microtainer tube (BD Biosciences, Franklin Lakes, NJ), which was centrifuged at 21,000g for 90 s. Subsequently, the cellular and plasma fractions were removed and placed in separate polystyrene tubes for estimation of Hg content.
The kidney was also removed from each animal. After each kidney was trimmed of fascia and fat, it was weighed and cut in half along the midtransverse plain. From half of the left kidney, a 3-mm transverse slice was used for separation of cortex, outer stripe of outer medulla, and inner stripe of outer medulla and inner medulla. Each zone of the kidney was weighed and placed in a polystyrene tube for estimation of 203Hg2+ content. After removal of the kidneys, the liver was excised carefully and weighed, and a 1-g section was removed for determination of 203Hg2+ content.
Urine and feces were collected at 24-h intervals throughout the duration of the study. Each 24-h collection was mixed by vortexing, and a 1-ml sample was weighed and placed in a polystyrene tube for estimation of 203Hg2+ content. All of the feces excreted by each animal during the final 24-h period were counted to determine the content of 203Hg2+ excreted in the feces.
Determination of Hg2+ Content in Samples of Tissue, Organs, Urine, and Feces.
All samples were placed in 12 × 75-mm polystyrene tubes, which were sealed immediately to prevent evaporation or desiccation. The content of 203Hg2+ in each sample was determined by counting the samples in a Wallac Wizard 3 automatic gamma counter (PerkinElmer Life and Analytical Sciences, Waltham, MA). The total content of Hg2+ in the entire kidney, liver, and blood volume is expressed as a percentage of administered dose. Concentrations of Hg2+ in the renal samples are expressed as a percentage of dose per gram of tissue. Total blood volume was estimated to be 6% of body weight. Urinary and fecal excretion of Hg2+ is expressed as a percentage of administered dose per the last 24 h of study.
Real-Time PCR.
Cortex and outer stripe were obtained from each set of animals. Tissues were frozen immediately in liquid nitrogen. Frozen tissues were then ground up, and total RNA was isolated by using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Reverse transcription of 1 μg of RNA was carried out by using reverse transcriptase and random hexamers (Applied Biosystems, Foster City, CA). Real-time polymerase chain reaction (PCR) analyses of Mrp2 and Oat1 expression were performed by using an ABI Prism 7000 sequence detection system and commercially available gene expression assays (Mrp2, Rn_01411039; Oat1, Rn_01450741; Applied Biosystems). Analyses were designed according to the manufacturer's recommendation. Glyceraldehyde-3-phosphate dehydrogenase was used as a reference gene.
Generation of 203Hg2+.
203Hg2+ was generated by neutron activation of a target of mercuric oxide (HgO) at the Missouri University Research Reactor and subsequent chemical handling and analyses by the method described previously (Belanger et al., 2001; Bridges and Zalups, 2004; Bridges et al., 2004). The specific activities of the 203Hg2+ ranged from 6 to12 mCi/mg.
Data Analyses.
Each set of data was analyzed first with the Kolmogorov-Smirnov to test for normality and then with Levene's test for homogeneity of variances. Differences among means were then analyzed by two-way analysis of variance. When statistically significant F values were obtained, the data were analyzed further by using Tukey's post hoc multiple comparison test. A p value < 0.05 was considered statistically significant.
Results
Renal Expression of Mrp2 and Oat1.
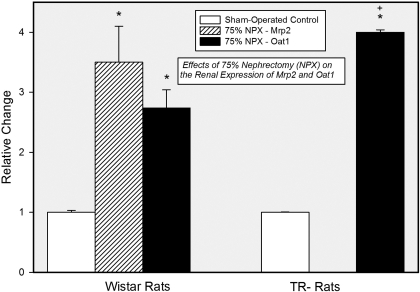
Real-time PCR analyses were performed on renal tissue 14 days after surgery. In the Wistar rats, expression of Mrp2 was enhanced approximately 3.5-fold, whereas expression of Oat1 was increased approximately 2.5-fold (Fig. 1). Transcripts encoding Mrp 2 were not detected in SO or 75% NPX TR− rats. A similar finding has also been reported recently (Oswald et al., 2006). In addition, the expression of Oat1 in the 75% NPX TR− rats was amplified almost 4-fold more than that in corresponding SO rats.
Fig. 1.
Results from real-time PCR analyses of the expression of Mrp2 and Oat1 in samples of combined renal cortex and outer stripe of the outer medulla from SO and 75% NPX Wistar and TR− rats 14 days after surgery. ∗ indicates significantly different (p < 0.05) from corresponding control. + indicates significantly different (p < 0.05) from the corresponding mean obtained from Wistar rats.
Renal Concentration of Hg2+.
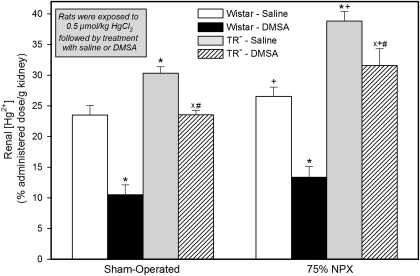
In the group of SO Wistar rats exposed to mercuric chloride and then treated with normal saline, the renal concentration of Hg2+ was approximately 23% of the dose per gram of tissue 48 h after exposure to Hg2+ (Fig. 2). In the corresponding group of Wistar rats treated with DMSA, the renal concentration of Hg2+ was approximately 55% less than that in the saline-treated group (Table 1). Interestingly, the renal concentration of Hg2+ in the SO TR− rats treated with saline was approximately 28% higher than that in the corresponding group of saline-treated Wistar rats. However, the renal concentration of Hg2+ in the group of TR− rats treated with DMSA was, on average, only approximately 22% less than that in the corresponding group of saline-treated TR− rats.
Fig. 2.
Renal concentration of Hg2+ in SO and 75% NPX Wistar and TR− rats, treated with an intraperitoneal 100 mg/kg dose of DMSA or saline 24 and 28 h after being injected intravenously with a 0.5 μmol/kg dose of mercuric chloride. Animals were sacrificed 48 h after being injected with Hg2+. Each value represents the mean ± S.E. from four animals. ∗ indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with saline. X indicates significantly different (p < 0.05) from the corresponding group of TR− rats treated with saline. # indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with DMSA. + indicates significantly different (p < 0.05) from the corresponding group of SO rats treated in the same manner.
TABLE 1.
Relative differences in mean values for renal [Hg2+]
For NaCl rats received 0.9% normal saline (2 ml · kg−1); for DMSA rats received 100 mg · kg−1 DMSA 24 and 28 h after surgery. Each directional arrow indicates the relative change of a group identified by a row label with respect to a group identified by a column label.
| Group | SO + Wistar + NaCl | SO + Wistar + DMSA | SO + TR− + NaCl | SO + TR− + DMSA | 75% NPX + Wistar + NaCl | 75% NPX + Wistar + DMSA | 75% NPX + TR− + NaCl | 75% NPX + TR− + DMSA |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| SO + Wistar + NaCl | N.A. | 55 ↓ | 28 ↑ | N.A. | 13 ↑ | N.A. | N.A. | N.A. |
| SO + Wistar + DMSA | 55 ↓ | N.A. | N.A. | 124 ↑ | N.A. | N.S.D. | N.A. | N.A. |
| SO + TR− + NaCl | 28 ↑ | N.A. | N.A. | 22 ↓ | N.A. | N.A. | 29 ↑ | N.A. |
| SO + TR− + DMSA | N.A. | 124 ↑ | 22 ↓ | N.A. | N.A. | N.A. | N.A. | 34 ↑ |
| 75% NPX + Wistar + NaCl | 13 ↑ | N.A. | N.A. | N.A. | N.A. | 50 ↓ | 46 ↑ | N.A. |
| 75% NPX + Wistar + DMSA | N.A. | N.S.D. | N.A. | N.A. | 50 ↓ | N.A. | N.A. | 136 ↑ |
| 75% NPX + TR−+ NaCl | N.A. | N.A. | 29 ↑ | N.A. | 46 ↑ | N.A. | N.A. | 19 ↓ |
| 75% NPX + TR−+ DMSA | N.A. | N.A. | N.A. | 34 ↑ | N.A. | 136 ↑ | 19 ↓ | N.A. |
N.A., not applicable; N.S.D., not significantly (P > 0.05) different.
The overall renal concentration of Hg2+ in the group of 75% NPX Wistar rats treated with normal saline was approximately 13% higher than that in the corresponding group of SO Wistar rats treated with normal saline (Fig. 2 and Table 1). By contrast, the renal concentration of Hg2+ in the 75% NPX Wistar rats treated with DMSA was nearly 51% less than that in 75% NPX Wistar rats treated with normal saline. In the 75% NPX TR− rats treated with saline the renal concentration of Hg2+ was approximately 46% higher than that in the group of 75% NPX Wistar rats treated with saline. In addition, the renal concentration of Hg2+ in the 75% NPX TR− rats treated with saline was approximately 28% higher than that in the corresponding SO group treated with saline. In the group of 75% NPX TR− rats treated with DMPS, the renal concentration of Hg2+ was approximately only 19% less than that in the 75% NPX rats treated with saline.
Concentration of Hg2+ in the Renal Cortex.
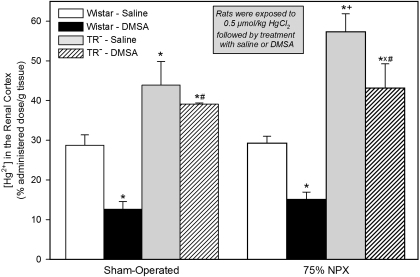
Among the SO rats, the renal cortical concentration of Hg2+ in the DMSA-treated group was approximately 56% less than that in the SO rats treated with saline (Fig. 3 and Table 2). By contrast, there was no significant difference in the renal cortical concentration of Hg2+ between the two groups of SO TR− rats. The renal cortical concentration of Hg2+ in the two groups of SO TR− rats was significantly higher than that in the corresponding SO group of Wistar rats, with the greatest difference detected between the two groups of DMSA-treated rats.
Fig. 3.
Concentration of Hg2+ in the renal cortex of SO and 75% NPX Wistar and TR− rats, treated with an intraperitoneal 100 mg/kg dose of DMSA or saline 24 and 28 h after being injected intravenously with a 0.5 μmol/kg dose of mercuric chloride. Animals were sacrificed 48 h after being injected with Hg2+. Each value represents the mean ± S.E. from four animals. ∗ indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with saline. X indicates significantly different (p < 0.05) from the corresponding group of TR− rats treated with saline. # indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with DMSA. + indicates significantly different (p < 0.05) from the corresponding group of SO rats treated in the same manner.
TABLE 2.
Relative differences in mean values for [Hg2+] in the renal cortex
For NaCl rats received 0.9% normal saline (2 ml · kg−1); for DMSA rats received 100 mg · kg−1 DMSA 24 and 28 h after surgery. Each directional arrow indicates the relative change of a group identified by a row label with respect to a group identified by a column label.
| Group | SO + Wistar + NaCl | SO + Wistar + DMSA | SO + TR− + NaCl | SO + TR− + DMSA | 75% NPX + Wistar + NaCl | 75% NPX + Wistar + DMSA | 75% NPX + TR− + NaCl | 75% NPX + TR−+ DMSA |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| SO + Wistar + NaCl | N.A. | 56 ↓ | 52 ↑ | N.A. | N.S.D. | N.A. | N.A. | N.A. |
| SO + Wistar + DMSA | 56 ↓ | N.A. | N.A. | 210 ↑ | N.A. | N.S.D. | N.A. | N.A. |
| SO + TR− + NaCl | 52 ↑ | N.A. | N.A. | N.S.D. | N.A. | N.A. | 31 ↑ | N.A. |
| SO + TR− + DMSA | N.A. | 210 ↑ | N.S.D. | N.A. | N.A. | N.A. | N.A. | N.S.D. |
| 75% NPX + Wistar + NaCl | N.S.D. | N.A. | N.A. | N.A. | N.A. | 49 ↓ | 96 ↑ | N.A. |
| 75% NPX + Wistar + DMSA | N.A. | N.S.D. | N.A. | N.A. | 49 ↓ | N.A. | N.A. | 187 ↑ |
| 75% NPX + TR−+ NaCl | N.A. | N.A. | 31 ↑ | N.A. | 96 ↑ | N.A. | N.A. | 25 ↓ |
| 75% NPX + TR−+ DMSA | N.A. | N.A. | N.A. | N.S.D. | N.A. | 187 ↑ | 25 ↓ | N.A. |
N.A., not applicable; N.S.D., not significantly (P > 0.05) different.
The renal cortical concentration of Hg2+ in the 75% NPX Wistar rats treated with DMSA was approximately 49% less than that in the 75% NPX Wistar rats treated with saline. In the 75% TR− rats treated with saline, the renal cortical concentration of Hg2+ was approximately 96% higher than that in the corresponding group of 75% NPX Wistar rats treated with saline. Between the two groups of 75% NPX rats treated with DMSA, the renal cortical concentration of Hg2+ was approximately 187% higher in the 75% NPX TR− rats.
In the two groups of 75% NPX Wistar rats treated with saline or DMSA, the renal concentration of Hg2+ was not significantly different from that in the corresponding group of SO Wistar rats treated in the same manner. Only in the group of 75% TR− rats treated with saline was the renal cortical concentration significantly higher than that in the corresponding group of SO rats treated in the same manner.
Concentration of Hg2+ in the Renal Outer Stripe of the Outer Medulla.
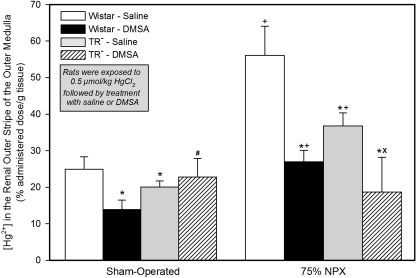
The patterns for the accumulation of inorganic Hg2+ in the outer stripe of the outer medulla were considerably different from those detected in the renal cortex (Fig. 4 and Table 3).
Fig. 4.
Concentration of Hg2+ in the renal outer stripe of the outer medulla of SO and 75% NPX Wistar and TR− rats, treated with an intraperitoneal 100 mg/kg dose of DMSA or saline 24 and 28 h after being injected intravenously with a 0.5 μmol/kg dose of mercuric chloride. Animals were sacrificed 48 h after being injected with Hg2+. Each value represents the mean ± S.E. from four animals. ∗ indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with saline. X indicates significantly different (p < 0.05) from the corresponding group of TR− rats treated with saline. # indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with DMSA. + indicates significantly different (p < 0.05) from the corresponding group of SO rats treated in the same manner.
TABLE 3.
Relative differences in mean values for [Hg2+] in the renal outer stripe of the outer medulla
For NaCl rats received 0.9% normal saline (2 ml · kg−1); for DMSA rats received 100 mg · kg−1 DMSA 24 and 28 h after surgery. Each directional arrow indicates the relative change of a group identified by a row label with respect to a group identified by a column label.
| Group | SO + Wistar + NaCl | SO + Wistar + DMSA | SO + TR− + NaCl | SO + TR− + DMSA | 75% NPX + Wistar + NaCl | 75% NPX + Wistar + DMSA | 75% NPX + TR− + NaCl | 75% NPX + TR−+ DMSA |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| SO + Wistar + NaCl | N.A. | 45 ↓ | 20 ↓ | N.A. | 125 ↑ | N.A. | N.A. | N.A. |
| SO + Wistar + DMSA | 45 ↓ | N.A. | N.A. | 64 ↓ | N.A. | 94 ↑ | N.A. | N.A. |
| SO + TR− + NaCl | 20 ↓ | N.A. | N.A. | N.S.D. | N.A. | N.A. | 83 ↑ | N.A. |
| SO + TR− + DMSA | N.S.D. | 64 ↑ | N.S.D. | N.A. | N.A. | N.A. | N.A. | N.S.D. |
| 75% NPX + Wistar + NaCl | 125 ↑ | N.A. | N.A. | N.A. | N.A. | 52 ↓ | 34 ↓ | N.A. |
| 75% NPX + Wistar + DMSA | N.A. | 94 ↑ | N.A. | N.A. | 52 ↓ | N.A. | N.A. | N.S.D. |
| 75% NPX + TR−+ NaCl | N.A. | N.A. | 83 ↑ | N.A. | 34 ↓ | N.A. | N.A. | 49 ↓ |
| 75% NPX + TR−+ DMSA | N.A. | N.A. | N.A. | N.S.D. | N.A. | N.S.D. | 49 ↓ | N.A. |
N.A., not applicable; N.S.D., not significantly (P > 0.05) different.
Among the four groups of SO rats, the concentration of Hg2+ in the outer stripe of the outer medulla was significantly less in the DMSA-treated Wistar rats and the saline-treated TR− rats relative to that in the Wistar rats treated with saline. The only other significant difference in the outer stripe of the outer medulla among the SO rats was detected between the two groups of rats treated with DMSA. There was no significant difference in the concentration of Hg2+ in the outer stripe of the outer medulla between the two groups of TR− rats.
Significant differences in the concentration of Hg2+ in the outer stripe of the outer medulla were also detected among the four groups of 75% NPX rats. In both groups of 75% NPX rats treated with DMSA, the average concentration of Hg2+ in the outer stripe of the outer medulla was significantly less than that in the corresponding group of 75% NPX rats treated with saline. In addition, the mean concentration of Hg2+ in the outer stripe of the outer medulla of the 75% NPX Wistar rats treated with DMSA was higher than that in the 75% NPX TR− rats.
The most marked differences in the average concentration of Hg2+ in the outer stripe of the outer medulla were detected between corresponding groups of 75% NPX rats and SO rats. The greatest difference in the concentration of Hg2+ in the outer stripe of the outer medulla was detected between the 75% NPX Wistar rats treated with saline and the corresponding SO Wistar rats treated with saline. Values were approximately 125% higher in the corresponding NPX rats. Accumulation of Hg2+ in the outer stripe of the outer medulla was also significantly greater in the 75% NPX rats between the paired groups of Wistar rats treated with DMSA and the TR− rats treated with saline. There was no significant difference in the average concentration of Hg2+ in the outer stripe of the outer medulla between the 75% NPX and SO groups of TR− rats treated with DMSA.
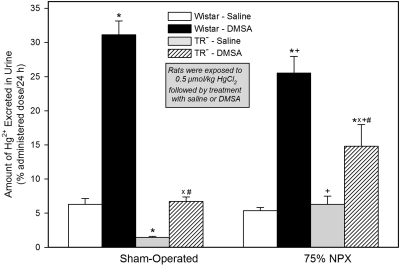
Urinary Excretion of Hg2+.
Profound changes in the urinary excretion of Hg2+ were detected among the groups of SO and 75% NPX Wistar and TR− rats during the second 24-h period of study (Fig. 5 and Table 4).
Fig. 5.
Amount of Hg2+ excreted in the urine in 24 h by SO and 75% NPX Wistar and TR− rats treated with an intraperitoneal 100 mg/kg dose of DMSA or saline 24 and 28 h after being injected intravenously with a 0.5 μmol/kg dose of mercuric chloride. Animals were sacrificed 48 h after being injected with Hg2+. Each value represents the mean ± S.E. from four animals. ∗ indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with saline. X indicates significantly different (p < 0.05) from the corresponding group of TR− rats treated with saline. # indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with DMSA. + indicates significantly different (p < 0.05) from the corresponding group of SO rats treated in the same manner.
TABLE 4.
Relative differences in mean values for Hg2+ excretion in urine
For NaCl rats received 0.9% normal saline (2 ml · kg−1); for DMSA rats received 100 mg · kg−1 DMSA 24 and 28 h after surgery. Each directional arrow indicates the relative change of a group identified by a row label with respect to a group identified by a column label.
| Group | SO + Wistar + NaCl | SO + Wistar + DMSA | SO + TR− + NaCl | SO + TR− + DMSA | 75% NPX + Wistar + NaCl | 75% NPX + Wistar + DMSA | 75% NPX + TR− + NaCl | 75% NPX + TR−+ DMSA |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| SO + Wistar + NaCl | N.A. | 394 ↑ | 76 ↓ | N.A. | N.S.D. | N.A. | N.A. | N.A. |
| SO + Wistar + DMSA | 394 ↑ | N.A. | N.A. | 78 ↓ | N.A. | 18 ↓ | N.A. | N.A. |
| SO + TR− + NaCl | 76 ↓ | N.A. | N.A. | 363 ↑ | N.A. | N.A. | 320 ↑ | N.A. |
| SO + TR− + DMSA | N.A. | 78 ↓ | 363 ↑ | N.A. | N.A. | N.A. | N.A. | 121 ↑ |
| 75% NPX + Wistar + NaCl | N.S.D. | N.A. | N.A. | N.A. | N.A. | 372 ↑ | N.S.D. | N.A. |
| 75% NPX + Wistar + DMSA | N.A. | 18 ↓ | N.A. | N.A. | 372 ↑ | N.A. | N.A. | 42 ↓ |
| 75% NPX + TR−+ NaCl | N.A. | N.A. | 320 ↑ | N.A. | N.S.D. | N.A. | N.A. | 135 ↑ |
| 75% NPX + TR−+ DMSA | N.A. | N.A. | N.A. | 121 ↑ | N.A. | 42 ↓ | 135 ↑ | N.A. |
N.A., not applicable; N.S.D., not significantly (P > 0.05) different.
Among the four groups of SO rats, urinary excretion of Hg2+ was greatest in the Wistar rats treated with DMSA. In fact, the amount of Hg2+ excreted in 24 h by the Wistar rats treated with DMSA was on average 394% more than that in Wistar rats treated with saline. In addition, the average amount of Hg2+ excreted in 24 h in the TR− rats treated with DMSA was approximately 363% more than that in the corresponding saline-treated TR− rats. Urinary excretion of Hg2+ in the TR− rats treated with saline was approximately 76% less than that in the corresponding group of Wistar rats treated with saline. Moreover, urinary excretion of Hg2+ in the TR− rats treated with DMSA was approximately 78% less than that in the Wistar rats treated with DMSA.
Urinary excretion of Hg2+ among the four groups of 75% NPX rats was also highest in the group of Wistar rats treated with DMSA. In that group, the mean urinary excretion of Hg2+ over 24 h was approximately 372% more than that in the corresponding group of Wistar rats treated with saline. In addition, the mean urinary excretion of Hg2+ in the 75% NPX TR− rats treated with DMSA was significantly higher than that in the 75% NPX TR− rats treated with saline. Furthermore, urinary excretion of Hg2+ in the group of 75% NPX TR− rats treated with DMSA was less than that in the 75% NPX Wistar rats treated with DMPS.
Differences in the urinary excretion of Hg2+ were detected between corresponding groups of 75% NPX rats and SO rats, but there was no significant difference between the two groups of saline-treated Wistar rats.
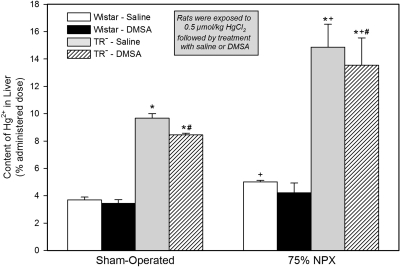
Hepatic Content of Hg2+.
Approximately 4% of the administered dose of Hg2+ was present in the liver of the SO Wistar rats during the last 24 h of study (Fig. 6). In both groups of SO TR− rats, the hepatic content of Hg2+ was approximately 9 to 10% of the administered dose. Among the four groups of SO rats, hepatic content of Hg2+ was more than 2-fold higher in both groups of TR− rats relative to that in the corresponding groups of Wistar rats. A similar pattern in the hepatic disposition of Hg2+ was also detected among the four groups of 75% NPX rats.
Fig. 6.
Hepatic content of Hg2+ in SO and 75% NPX Wistar and TR− rats treated with an intraperitoneal 100 mg/kg dose of DMSA or saline 24 and 28 h after being injected intravenously with a 0.5 μmol/kg dose of mercuric chloride. Animals were sacrificed 48 h after being injected with Hg2+. Each value represents the mean ± S.E. from four animals. ∗ indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with saline. # indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with DMSA. + indicates significantly different (p < 0.05) from the corresponding group of SO rats treated in the same manner.
The hepatic content of Hg2+ in the group of 75% NPX saline-treated Wistar rats was significantly different from that in the corresponding SO saline-treated Wistar rats, [j]although the difference in hepatic content was not profound. Hepatic content of Hg2+ in the two groups of 75% NPX TR− rats was as high as 14 to 15% of the administered dose at the end of 48 h of study, which was the largest hepatic burden of Hg2+ detected among the eight groups of rats studied.
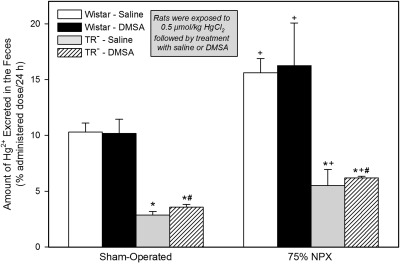
Fecal Excretion of Hg2+.
Among the four groups of SO rats and four groups of 75% NPX rats, the most profound differences in the fecal excretion of Hg2+ during the second 24 h of study were detected in the corresponding groups of TR− rats (Fig. 7).
Fig. 7.
Amount of Hg2+ excreted in the feces in 24 h by SO and 75% NPX Wistar and TR− rats treated with an intraperitoneal 100 mg/kg dose of DMSA or saline 24 and 28 h after being injected intravenously with a 0.5 μmol/kg dose of mercuric chloride. Animals were sacrificed 48 h after being injected with Hg2+. Each value represents the mean ± S.E. from four animals. ∗ indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with saline. # indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with DMSA. + indicates significantly different (p < 0.05) from the corresponding group of SO rats treated in the same manner.
Both groups of SO Wistar rats excreted approximately 10% of the dose in the feces in 24 h. By contrast the two groups of SO TR− rats excreted only between 3 and 4% of the dose of Hg2+ in the feces in 24 h.
Both groups of 75% NPX Wistar rats excreted approximately 16% of the administered dose of Hg2+ in the feces in 24 h. This level of fecal excretion of Hg2+ was significantly different from that in the two corresponding groups of SO rats. In addition, both groups of 75% NPX TR− rats excreted more Hg2+ in the feces than the corresponding groups of SO TR− rats treated in the same manner.
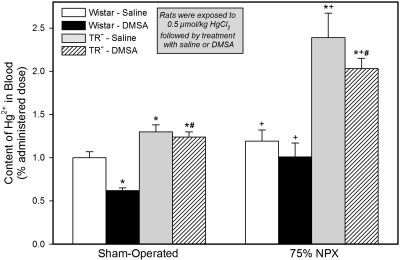
Content of Hg2+ in Blood.
Only approximately 1% of the administered dose was present in the estimated blood volume of the SO saline-treated Wistar rats 48 h after the administration of the 0.5 μmol/kg dose of Hg2+ (Fig. 8). In the SO DMSA-treated Wistar rats the content of Hg2+ in blood was only approximately 0.6% of the administered dose. Approximately 1.2 to 1.3% of the dose of Hg2+ was present in the blood of the two groups of SO TR− rats.
Fig. 8.
Estimated content of Hg2+ in the total blood volume of SO and 75% NPX Wistar and TR− rats treated with an intraperitoneal 100 mg/kg dose of DMSA or saline 24 and 28 h after being injected intravenously with a 0.5 μmol/kg dose of mercuric chloride. Animals were sacrificed 48 h after being injected with Hg2+. Blood volume was estimated to be 6% of body weight. Each value represents the mean ± S.E. from four animals. ∗ indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with saline. # indicates significantly different (p < 0.05) from the corresponding group of Wistar rats treated with DMSA. + indicates significantly different (p < 0.05) from the corresponding group of SO rats treated in the same manner.
The content of Hg2+ in the blood of each of the four groups of 75% NPX rats was higher than that in the corresponding group of SO rats. By far, the largest levels of Hg2+ in blood among all groups were detected in the two groups of TR− rats. In those two groups, the levels of Hg2+ in blood, 48 h after injection of mercuric chloride, averaged between 2.0 and 2.4% of the dose.
Discussion
We along with other investigators have shown that performing a 75% nephrectomy induces hypertrophic changes along the remaining functional nephrons and metabolic changes consistent with early chronic renal failure (Kunau and Whinnery, 1978; Zalups et al., 1985, 1989; Zalups and Henderson, 1992). Most notably, it has been demonstrated that the concentration of creatinine in the plasma and the fractional excretion of potassium increase in response to a diminution in whole animal glomerular filtration rate. Moreover, selective cellular hypertrophy occurs along the remaining functional nephrons, especially along the proximal tubule (Taal et al., 2004). It has been well established that significant reductions in functional renal mass result in increased transcriptional and translational activity, which ultimately results in cellular hypertrophy. One theory for this hypertrophic response purports that putative humoral mediators known as renotropins are important factors underlying the renal hypertrophic changes that occur after renal mass is reduced significantly (Taal et al., 2004).
In addition to the aforementioned changes, our present findings show that 75% nephrectomy induces significant changes in the expression of two important secretory transporters in the remaining hypertrophied functional proximal tubules. In Wistar rats, the expression of Oat1 and Mrp2 increased significantly after 75% nephrectomy. The renal expression of Oat1 was higher in the 75% NPX TR− rats than in the 75% Wistar rats, although the reason for this pattern of expression is not clear at this time.
Enhanced accumulation of Hg2+ in the renal outer stripe of the outer medulla was a prominent finding after 75% nephrectomy. This was especially true in the 75% NPX Wistar rats treated with saline. Increased accumulation and/or retention of Hg2+ in this zone of the kidney is an established response found in uninephrectomized rats treated with nontoxic doses of Hg2+ (Zalups and Lash, 1990; Zalups, 1991a,b, 1993; Zalups and Cherian, 1992a,b). The altered accumulation of mercuric ions in the outer stripe of the outer medulla likely represents the product of a number of biochemical and physiological changes that occur during compensatory cellular hypertrophy along segments of the proximal tubule. As mentioned above, the expression of both Oat1 and Mrp2 was enhanced in the Wistar rats after 75% nephrectomy. We hypothesize that enhanced expression of these two transporters provides a more efficient secretory route for the elimination of Hg2+ and other xenobiotics in hypertrophied proximal tubular epithelial cells, particularly those lining the pars recta where tubular secretion is most prominent. Additional findings from Zhang et al. (2008) support our current molecular findings. They demonstrated that expression of OAT1 and OAT3 proteins was enhanced in proximal tubular cells in an ischemic/reperfusion rat model in which the animals underwent a right-sided uninephrectomy followed by compression of the left renal pedicle for 50 min. Moreover, recent findings demonstrate enhanced expression of P-glycoprotein in the luminal membrane of proximal tubular epithelial cells in Wistar rats with reduced renal mass and diabetes (Amaral et al., 2009).
Previous findings from our laboratories demonstrated that intracellular concentrations of the thiols, glutathione and metallothioneins 1 and 2, increase significantly in the renal cortex and outer stripe of the outer medulla of rats 10 to 14 days after uninephrectomy (Zalups and Lash, 1990; Zalups and Cherian, 1992a,b; Zalups et al., 1995). The enhanced thiol content in hypertrophied proximal tubular epithelial cells, especially those present in the outer stripe of the outer medulla, probably plays an important role in the intracellular accumulation, retention, and luminal elimination of mercuric ions in pars recta segments of hypertrophied renal proximal tubules in 75% NPX rats.
Renal, hepatic, urinary, and fecal data from SO TR− rats and 75% NPX TR− rats provide further support for our hypothesis implicating MRP2 in the luminal secretion and elimination of Hg2+ (Bridges et al., 2008a,b). In summary, renal and hepatic contents of Hg2+ were significantly higher, and the rates of urinary and fecal excretion of Hg2+ were significantly less in the TR− rats than in corresponding control Wistar rats.
Treatment with DMSA decreased the renal concentrations of Hg2+ (specifically in both the cortex and outer stripe of the outer medulla) and enhanced the urinary excretion of Hg2+ in both control and 75% NPX Wistar and TR− rats. No significant differences in the overall renal concentration of Hg2+ were detected between the groups of 75% NPX and SO Wistar rats treated with DMSA. However, at the level of the outer stripe of the outer medulla, treatment with DMSA diminished the concentration of Hg2+ to a much greater degree in 75% NPX Wistar rats than in SO Wistar rats. Treatment with DMSA had no significant effect on the concentration of Hg2+ in the renal outer stripe of the SO TR− rats, although net accumulation of Hg2+ in the renal outer stripe of the medulla of the saline-treated, 75% NPX TR− rats was significantly higher than that in corresponding SO TR− rats.
The fact that the accumulation of Hg2+ in the outer stripe of the outer medulla was enhanced in both strains of 75% NPX rats suggests that the rates of uptake of Hg2+ in the hypertrophied proximal tubular cells are enhanced in this renal zone secondary to enhanced activity of and/or amplification in the number of membrane transporters capable of taking up mercuric species (such as cysteine and homocysteine S-conjugates of Hg2+). An additional factor that may have contributed to the enhanced accumulation of Hg2+ in the outer stripe of the outer medulla of the 75% NPX rats is an increase in thiol-containing proteins and other molecules capable of retaining mercuric ions in the hypertrophied proximal tubular epithelial cells.
Based on our molecular findings, amplification in the number and activity of OAT1 transporters along the pars recta of proximal tubules is a potential factor that contributed to the enhanced accumulation of Hg2+ detected in the renal outer stripe of the outer medulla after 75% nephrectomy. It is well established that there is a specific amplification in surface density of both luminal and basolateral membranes in proximal tubular epithelial cells subsequent to significant reductions in renal mass (Taal et al., 2004). Moreover, as a result of the profound effects of DMSA in the Wistar rats, but not the TR− rats, one is led to suggest that the enhanced elimination of Hg2+ detected in the 75% Wistar rats is caused largely by the export of intracellular species of Hg2+ (in the form of a DMSA conjugate) by MRP2. Both of the aforementioned hypotheses are supported further by the demonstration of an amplification of OAT1 and MRP2 subsequent to 75% nephrectomy.
Cumulative fecal excretion of Hg2+ was increased significantly in all groups of NPX rats relative to that in corresponding SO rats. Enhanced fecal excretion of Hg2+ in NPX rats relative to that in control rats has also been documented in a couple of recent studies (Zalups et al., 1987; Zalups, 1993). Moreover, fecal excretion of Hg2+ was severalfold less in TR− rats than in corresponding Wistar rats. The patterns for fecal excretion of Hg2+ correspond (for the most part) to the patterns for the magnitude of hepatic retention of Hg2+. Because of the inherent genetic defect in the TR− rats, hepatocytes lack the ability to eliminate Hg2+ into the biliary system, which strongly supports a role for MRP2 in the biliary secretion of mercuric species from within hepatocytes.
Estimated contents of Hg2+ in blood were also higher in 75% NPX rats than in corresponding SO rats, with the greatest effects of 75% nephrectomy seen in the TR− rats. Increased levels of Hg2+ in blood probably reflect the effects of diminished renal and hepatic clearance of Hg2+.
In summary, the present findings show for the first time that 75% nephrectomy greatly affects the disposition of Hg2+ in the kidneys, liver, and blood of both Wistar rats and rats lacking a functional form of the export-transporter MRP2. In the kidneys, 75% nephrectomy caused the accumulation of Hg2+ to increase greatly in the outer stripe of the outer medulla, which is probably caused by enhanced retention of Hg2+ in the S3 segment of proximal tubules. This enhanced accumulation of Hg2+ in the outer stripe correlates, in part, to our findings showing that 75% nephrectomy induces enhanced expression of the secretory transporters OAT1 and MRP2. Moreover, the enhanced urinary excretion of Hg2+ in 75% NPX TR− rats treated with either saline or DMSA indicates that the expression of some additional transporter (other than MRP2) in the luminal plasma membrane of proximal tubules in the TR− rats, which is capable of exporting mercuric species, is amplified in a compensatory manner because of factors associated with proximal tubular hypertrophy.
This work was supported in part by the National Institutes of Health, National Institute of Environmental Health Sciences [Grants ES05980, ES015511] (to R.K.Z. and C.C.B., respectively).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.163774.
- Hg2+
- inorganic mercury
- ABC
- ATP-binding cassette
- DMSA
- meso-2,3-dimercaptopropanesunccinc acid
- DMPS
- 2,3-dimercaptopropanesulfonic acid
- HgCl2
- mercuric chloride
- HgO
- mercuric oxide
- MRP2
- multidrug-resistance protein 2
- NPX
- nephrectomized
- OAT1
- organic anion transporter 1
- PAH
- para-aminohippuric acid
- SO
- sham-operated
- TR−
- transport-deficient
- PCR
- polymerase chain reaction.
References
- Amaral JS, Pinho MJ, Soares-da-Silva P. (2009) Regulation of amino acid transporters in the rat remnant kidney. Nephrol Dial Transplant 24:2058–2067 [DOI] [PubMed] [Google Scholar]
- Aslamkhan AG, Han YH, Yang XP, Zalups RK, Pritchard JB. (2003) Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol Pharmacol 63:590–596 [DOI] [PubMed] [Google Scholar]
- Belanger M, Westin A, Barfuss DW. (2001) Some health physics aspects of working with 203Hg in university research. Health Phys 80 (Suppl 1):S29–S30 [PubMed] [Google Scholar]
- Bridges CC, Bauch C, Verrey F, Zalups RK. (2004) Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry. J Am Soc Nephrol 15:663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. (2008a) MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR− and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci 105:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. (2008b) Multidrug-resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther 324:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. (2004) Homocysteine, system b0,+ and the renal epithelial transport and toxicity of inorganic mercury. Am J Pathol 165:1385–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries MH, Redegeld FA, Koster AS, Noordhoek J, de Haan JG, Oude Elferink RP, Jansen PL. (1989) Hepatic, intestinal, and renal transport of 1-naphthol-β-d-glucuronide in mutant rats with hereditary-conjugated hyperbilirubinemia. Naunyn Schmiedebergs Arch Pharmacol 340:588–592 [DOI] [PubMed] [Google Scholar]
- Houser MT, Berndt WO. (1988) Unilateral nephrectomy in the rat: effects on mercury handling and renal cortical subcellular distribution. Toxicol Appl Pharmacol 93:187–194 [DOI] [PubMed] [Google Scholar]
- Jansen PL, Peters WH, Lamers WH. (1985) Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology 5:573–579 [DOI] [PubMed] [Google Scholar]
- Kunau RT, Jr, Whinnery MA. (1978) Potassium transfer in distal tubule of normal and remnant kidneys. Am J Physiol 235:F186–F191 [DOI] [PubMed] [Google Scholar]
- Masereeuw R, Notenboom S, Smeets PH, Wouterse AC, Russel FG. (2003) Impaired renal secretion of substrates for the multidrug-resistance protein 2 in mutant transport-deficient (TR−) rats. J Am Soc Nephrol 14:2741–2749 [DOI] [PubMed] [Google Scholar]
- Mayer R, Kartenbeck J, Buchler M, Jedlitschky G, Leier I, Keppler D. (1995) Expression of the MRP gene-encoded conjugate export pump in liver and its selective absence from the canalicular membrane in transport-deficient mutant hepatocytes. J Cell Biol 131:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald S, Westrup S, Grube M, Kroemer HK, Weitschies W, Siegmund W. (2006) Disposition and sterol-lowering effect of ezetimibe in multidrug resistance-associated protein 2-deficient rats. J Pharmacol Exp Ther 318:1293–1299 [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. (1996) Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271:1126–1128 [DOI] [PubMed] [Google Scholar]
- Smeets PH, van Aubel RA, Wouterse AC, van den Heuvel JJ, Russel FG. (2004) Contribution of multidrug-resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J Am Soc Nephrol 15:2828–2835 [DOI] [PubMed] [Google Scholar]
- Taal MW, Luyckx VA, Brenner BM. (2004) Adaptation to nephron loss, in The Kidney (Brenner BM. ed) pp 1955–1997, Saunders, Philadelphia: [Google Scholar]
- Zalups RK. (1989) Effect of dietary K+ and 75% nephrectomy on the morphology of principal cells in CCDs. Am J Physiol 256:F387–F396 [DOI] [PubMed] [Google Scholar]
- Zalups RK. (1991a) Autometallographic localization of inorganic mercury in the kidneys of rats: effect of unilateral nephrectomy and compensatory renal growth. Exp Mol Pathol 54:10–21 [DOI] [PubMed] [Google Scholar]
- Zalups RK. (1991b) Renal accumulation and intrarenal distribution of inorganic mercury in the rabbit: effect of unilateral nephrectomy and dose of mercuric chloride. J Toxicol Environ Health 33:213–228 [DOI] [PubMed] [Google Scholar]
- Zalups RK. (1993) Influence of 2,3-dimercaptopropane-1-sulfonate (DMPS) and meso-2,3-dimercaptosuccinic acid (DMSA) on the renal disposition of mercury in normal and uninephrectomized rats exposed to inorganic mercury. J Pharmacol Exp Ther 267:791–800 [PubMed] [Google Scholar]
- Zalups RK. (1995) Progressive losses of renal mass and the renal and hepatic disposition of administered inorganic mercury. Toxicol Appl Pharmacol 130:121–131 [DOI] [PubMed] [Google Scholar]
- Zalups RK. (1997) Enhanced renal outer medullary uptake of mercury associated with uninephrectomy: implication of a luminal mechanism. J Toxicol Environ Health 50:173–194 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. (2004) Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1. J Am Soc Nephrol 15:2023–2031 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. (2005) Handling of cysteine S-conjugates of methylmercury in MDCK cells expressing human OAT1. Kidney Int 68:1684–1699 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW, Kostyniak PJ. (1992) Altered intrarenal accumulation of mercury in uninephrectomized rats treated with methylmercury chloride. Toxicol Appl Pharmacol 115:174–182 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Bridges CC. (2008) MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA. Toxicol Appl Pharmacol 235:10–17 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Cherian MG. (1992a) Renal metallothionein metabolism after a reduction of renal mass. I. Effect of unilateral nephrectomy and compensatory renal growth on basal and metal-induced renal metallothionein metabolism. Toxicology 71:83–102 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Cherian MG. (1992b) Renal metallothionein metabolism after a reduction of renal mass. II. Effect of zinc pretreatment on the renal toxicity and intrarenal accumulation of inorganic mercury. Toxicology 71:103–117 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Diamond GL. (1987) Mercuric chloride-induced nephrotoxicity in the rat following unilateral nephrectomy and compensatory renal growth. Virchows Arch B Cell Pathol Incl Mol Pathol 53:336–346 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Fraser J, Koropatnick J. (1995) Enhanced transcription of metallothionein genes in rat kidney: effect of uninephrectomy and compensatory renal growth. Am J Physiol 268:F643–F650 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Henderson DA. (1992) Cellular morphology in outer medullary collecting duct: effect of 75% nephrectomy and K+ depletion. Am J Physiol 263:F1119–F1127 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Klotzbach JM, Diamond GL. (1987) Enhanced accumulation of injected inorganic mercury in renal outer medulla after unilateral nephrectomy. Toxicol Appl Pharmacol 89:226–236 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Lash LH. (1990) Effects of uninephrectomy and mercuric chloride on renal glutathione homeostasis. J Pharmacol Exp Ther 254:962–970 [PubMed] [Google Scholar]
- Zalups RK, Stanton BA, Wade JB, Giebisch G. (1985) Structural adaptation in initial collecting tubule following reduction in renal mass. Kidney Int 27:636–642 [DOI] [PubMed] [Google Scholar]
- Zhang R, Yang X, Li J, Wu J, Peng WX, Dong XQ, Zhou SF, Yu XQ. (2008) Up-regulation of rat renal cortical organic anion transporter (OAT1 and OAT3) expression in response to ischemia/reperfusion injury. Am J Nephrol 28:772–783 [DOI] [PubMed] [Google Scholar]