Abstract
Thrombolysis with recombinant tissue plasminogen activator (rtPA) in ischemic stroke is limited by the increased risk of hemorrhage transformation due to blood-brain barrier breakdown. We determined the interaction of 17β-estradiol (E2) and rtPA on activation of plasminogen system and matrix metalloproteinases (MMPs) in a transient middle cerebral artery occlusion (MCAO) model. Ovariectomized female rats were subjected to 1-h transient focal cerebral ischemia using a suture MCAO model. Ischemic lesion volume was significantly reduced with acute treatment of E2 despite of exogenous administration of rtPA. The expression and activation of urokinase (uPA), MMP2, and MMP9 were significantly increased in ischemic hemisphere after transient cerebral ischemia. Exogenous rtPA administration further enhanced expression and activation of uPA, MMP2, and MMP9, which was blocked by E2 treatment. We further determined the effect of combination therapy of E2 and rtPA in an embolic MCAO model. Although no protection was indicated upon acute treatment of E2 alone, combination treatment of E2 and rtPA provided protective action at 3 h after embolism. Collectively, the present study suggests that estrogen could be a candidate for combination therapy with rtPA to attenuate its side effect and hence expand its short therapeutic window for treatment of ischemic stroke.
Ischemic stroke is the most frequent type of stroke, accounting for 83% of all stroke cases (Gillum, 2002). Therapies for acute ischemic stroke have achieved several important successes during the past decade, primarily related to thrombolysis. The rtPA has been shown to significantly increase the number of stroke patients with no or minimal deficit when treated within 3 h of symptom onset. Unfortunately, few patients can reach emergency services within 3 h after the onset of stroke, and many hospitals are not able to provide the emergency services required to treat stroke patients. Beyond this time window, systemic rtPA does not seem to be as beneficial and actually increases the risk of serious side effects. Delayed administration of rtPA treatment has been associated with significant increase in the frequency of hemorrhage transformation (del Zoppo, 2000). As a result, only 2 to 8% of United States ischemic stroke patients receive rtPA treatment, and rtPA has had a modest impact in the overall burden of ischemic stroke (Bambauer et al., 2006; Goldberg, 2007). Therefore, the clinical problem at hand is how to expand the therapeutic window of rtPA, decrease or eliminate the risks of hemorrhagic transformation and other potential side effects, and ultimately to increase the overall efficacy of rtPA thrombolytic therapy (Wang et al., 2004).
Neuroprotection refers to therapeutic interventions that produce benefits by favorably influencing underlying etiology or pathogenesis and thereby forestalling the onset of neuronal damage caused by stroke or other neurodegenerative diseases. Tremendous efforts have been made to develop neuroprotectants to prevent progression of ischemic cascade and reduce brain damage. However, the failure of clinical trials for neuroprotective drugs has argued against single cell type strategy for treatment of ischemic stroke (Young et al., 2007). It is reasonable that combination therapies that target the whole neurovascular unit and act on multiple pathways of ischemic cascade might have a greater chance of success to reduce ischemic brain damage in stroke.
Estrogens have been intensively studied as a group of neuroprotectant against neurodegenerative diseases, including stroke, in the last decade. There is now abundant evidence for protection by estrogens in different experimental stroke models. Estrogens' protective effects have been shown in both females and males, in both pretreatment and post-treatment paradigms (McCullough and Hurn, 2003; Yang et al., 2005). It is important to note that the protective effects of estrogen on acute treatment paradigm have been mostly demonstrated in a transient middle cerebral artery occlusion (MCAO) model, using a monofilament nylon suture (Hurn and Macrae, 2000; Liu et al., 2005). This transient focal cerebral stroke model simulates clinical rationale that an ischemic stroke patient is treated with rtPA, which enable a successful reperfusion after transient focal cerebral ischemia. However, the protective action of estrogens has not been tested in embolic stroke models. In the present study, we determined the interaction of estradiol and rtPA on activation of endogenous plasminogen activators (PAs) and matrix metalloproteinases (MMPs) using a transient suture MCAO model in rat. Furthermore, we determined the effect of combination treatment of estradiol and rtPA in a rat embolic MCAO model.
Materials and Methods
Experimental Animals.
Female Sprague-Dawley rats (250 g) were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and bilaterally ovariectomized to eliminate endogenous sex steroids. All animal procedures were approved by the University of North Texas Health Science Center Animal Care and Use Committee. Stroke was induced 2 weeks after ovariectomy.
Experimental Middle Cerebral Artery Occlusion Models and Treatments.
Two experimental ischemic stroke models were used in the current study, transient MCAO by suture and embolic MCAO by homologous blood clots. Transient MCAO was induced as described previously (Liu et al., 2005). Animals were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). Rectal temperature was maintained at 37.0 ± 0.5°C during stroke procedure. For suture MCAO model, the left MCA was occluded by a 3-0 monofilament suture introduced via internal carotid artery. After 1 h, the suture was withdrawn for reperfusion. Animals were randomly allocated to three groups: control (n = 9) treated with vehicle (0.2M l-arginine in saline with phosphoric acid, pH 7.4), rtPA (n = 8) treated with rtPA, and 17β-estradiol (E2) plus rtPA group (n = 8) treated with E2 plus rtPA. For E2 treatment, E2 (100 μg/kg body weight) was administered via subcutaneous injection at 2 h before stroke. rtPA (Genentech, South San Francisco, CA) was dissolved in saline and administered intravenously at a dose of 10 mg/kg body weight with a 10% bolus and 90% continuous infusion over a 30-min start at the onset of reperfusion.
Embolic MCAO was induced as described previously (Busch et al., 1997). Fresh arterial blood (0.6 ml) was mixed with 0.15 ml of thrombin (1 mg/ml) and injected into 50-cm-long polyethylene 50 catheter immediately. One hour later, the clot was flushed out of the catheter and rinsed with normal saline. The fibrin-rich clots were selected and cut into small pieces under microscope (1.5 mm). The clots were put into a solution of albumin in phosphate-buffered saline (1 mg/ml) for 4 h. For embolic MCAO, female Sprague-Dawley rats were anesthetized with ketamine (60 mg/kg) in combination with xylazine (10 mg/kg) administered intraperitoneally. The polyethylene 50 catheter with 12 clots was inserted through a small puncture into the isolated external carotid artery, and clots were injected into the internal carotid artery. rtPA treatment was administered as described above. For chronic E2 treatment (n = 9), a 3-cm-long pellet containing 4 mg/ml E2 was implanted subcutaneously 1 week before embolic MCAO. For acute E2 treatment (n = 9), E2 (100 μg/kg) was administered subcutaneously immediately before rtPA administration.
Lesion Volume Determination.
Animals were sacrificed at 24 h after experimental MCAO. Brains were harvested, and seven slices were made at 3, 5, 7, 9, 11, 13, and 15 mm posterior to olfactory bulb. Slices were incubated for 30 min in a 2% solution of 2,3,5-triphenyltetrazolium chloride at 37°C and then fixed in 10% formalin. The stained slices were photographed and subsequently measured for ischemic lesion volume.
Tissue Preparation and Zymography.
Another set of 18 rats (six rats in each group) were randomly allocated to three groups: control, rtPA, and E2 plus rtPA. Transient focal cerebral ischemia was induced by 1-h MCAO followed by reperfusion. All rats were sacrificed at 24 h after reperfusion. Each hemisphere was harvested separately in lysis buffer (50 mM Tris-HCl, pH 7.5, 0.1 M l-arginine, 150 mM NaCl, 1.0% Triton X-100, 0.005% Brij 35, and 0.05% NaN3) and homogenized by a Dounce homogenizer. The homogenate was centrifuged at 4°C for 20 min at 9000 rpm, and protein concentration of supernatant was determined by the Bradford method (Bradford, 1976).
For plasminogen-dependent zymography, equal amount of samples were resolved under nonreducing conditions on 10% SDS-polyacrylamide gels containing 0.01 U/ml plasminogen and 5 mg/ml casein. After electrophoresis, gels were washed with 2.5% Triton X-100 solution for 1 h and then incubated with 50 mM Tris-HCl, pH 7.4, 5 mM CaCl2, 1 μM ZnCl2, and 1% Triton X-100 for 24 h at 37°C. The gels were stained with Coomassie Blue solution (0.25% Coomassie Blue, 45% MeOH, and 10% acetic acid) for 2 h and then destained with a destain solution (30% MeOH and 10% acetic acid).
For gelatin zymography, equal amounts of sample were run at nonreducing conditions on 10% polyacrylamide gel containing 0.5% gelatin. After electrophoresis, SDS was removed by washing 2 h with 2.5% Triton X-100 solution followed by washing for 24 h in 1% Triton X-100 solution at 37°C. The gels were stained with staining solution and destained until bands became clear.
Western Blot Analysis.
Antibodies for tPA, urokinase (uPA), and PAI-1 were purchased from American Diagnostica (Greenwich, CT). Antibodies for MMP2 and MMP9 were purchased from Calbiochem (San Diego, CA). Equal amounts of brain samples were separated under nonreducing conditions on 8% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After blocking with 5% nonfat milk, the blot was incubated with primary antibody at 4°C overnight. Membranes were incubated with a horseradish peroxidase-conjugated secondary antibody for 2 h. The blots were then washed and detected by enhanced chemiluminescent reagents. The images were acquired and analyzed by a Western blot imaging system (UVP, Inc., Upland, CA).
Data Analysis.
All data are expressed as mean ± S.E.M. The ischemic lesion volume was compared by one-way analysis variance followed by Tukey's test. For other biomedical analysis, data were normalized to control level at nonischemic hemisphere, and the difference between each group was compared by one-way analysis variance followed by Tukey's test. The difference for each comparison was considered significant at the p < 0.05 level.
Results
Estrogen Provides Protective Effect against Transient Focal Cerebral Ischemia Despite Exogenous Administration of rtPA.
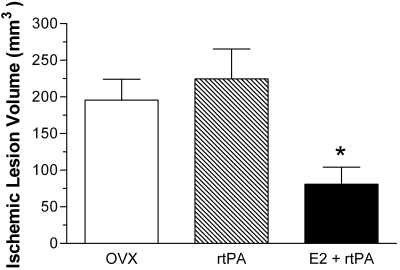
To determine the effects of E2 against transient focal cerebral ischemia in combination with rtPA, transient MCAO by a suture was induced in ovariectomized female rats. At 24 h after ischemic stroke, lesion volume was 195.7 ± 28.3, 224.5 ± 40.95, and 80.99 ± 23.03 mm3 in control, rtPA, and E2 plus rtPA combined treatment group, respectively (Fig. 1). rtPA treatment led to a slight increase of lesion volume compared with control, although no statistically difference was indicated. Combination treatment with E2 and rtPA significantly decreased lesion volume by 58.6 and 64% (p < 0.05), compared with control and rtPA treatment, respectively.
Fig. 1.
Quantitative analysis of lesion volume in control (OVX), rtPA (10 mg/kg), and E2 (100 μg/kg) + rtPA (10 mg/kg) group at 24 h after transient focal MCAO by suture. E2 treatment in combination with rtPA (E2 + rtPA) significantly (p < 0.05) reduced ischemic lesion volume, compared with rtPA treatment alone (rtPA) and control treatment.
Interaction of Estrogen and rtPA on Activation of Endogenous Plasminogen Activators after Transient Focal Cerebral Ischemia.
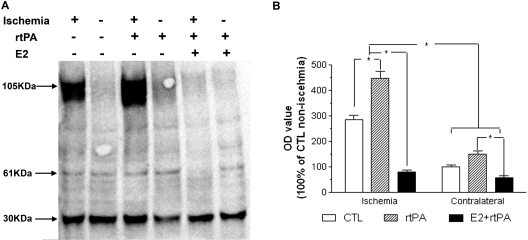
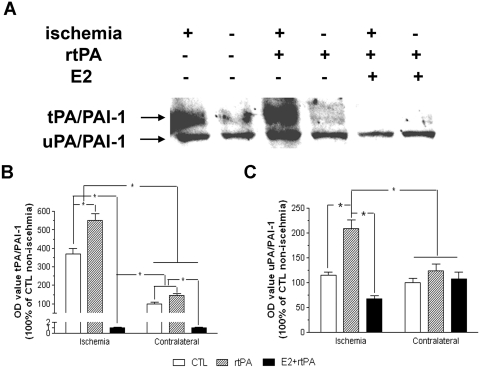
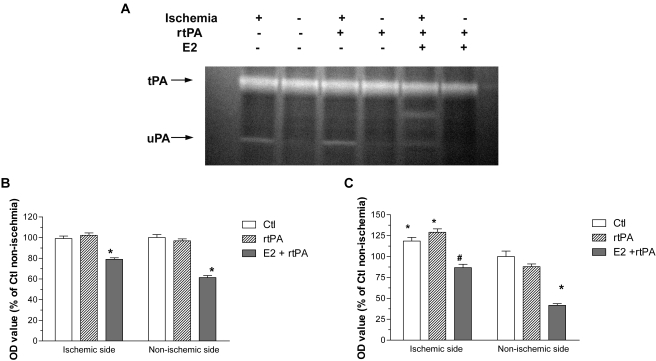
We determined the interaction of estrogen and rtPA on endogenous PAs system. Three forms of tPA were detected by Western blots, depicted as single chain, double chains, and tPA/PAI-1 complex. An increase of expression of tPA, mainly in complex forms, was observed in ischemic hemisphere, which was greatly attenuated by combined estrogen treatment (Fig. 2, A and B). Two forms of PAI-1, PAI-1/uPA complex and PAI-1/tPA complex, were detected by Western blots. A markedly increase of PAI-1 expression, mainly complex with tPA, was indicated in ischemic hemisphere, which was attenuated by estrogen treatment (Fig. 3, A and B). Plasminogen-dependent casein zymography showed no significant difference in tPA activity between ischemic and nonischemic hemisphere at 24 h after transient focal cerebral ischemia in both control and rtPA-treated groups (Fig. 4, A and B). Treatment with E2 significantly decreased tPA activity in both ischemic and nonischemic side, which was 79 ± 1.5 and 61 ± 1.9% of nonischemic side in control group, respectively (Fig. 4, A and B; p < 0.001). In contrast, a significant increase of uPA activity was suggested in ischemic hemisphere, which was 118 ± 4.3% of nonischemic hemisphere in control group (p < 0.05). Administration of rtPA caused further increase of uPA activity to 129 ± 4.1% in ischemic side. Pretreatment with E2 significantly decreased the uPA activity in both ischemic and nonischemic side, which was 86.8 ± 4.0 and 41.7 ± 2.0% of nonischemic hemisphere in control group, respectively (p < 0.05; Fig. 4C).
Fig. 2.
A, representative Western blot of tPA in control, rtPA, and E2 + rtPA group at 24 h after transient focal MCAO by suture. B, densitometric analysis of tPA in tPA/PAI-1 complex in control, rtPA, and E2 + rtPA group. A marked increase of tPA in complex with PAI-1 was indicated in ischemic side, which was attenuated by estradiol treatment. ∗, p < 0.05.
Fig. 3.
A, representative Western blot of PAI-1 in control, rtPA, and E2 + rtPA group at 24 h after transient focal MCAO by suture. B, densitometric analysis of PAI-1 in complex with tPA in control, rtPA, and E2 + rtPA group. A significant increase of PAI-1 in complex with tPA was demonstrated, which was further enhanced by rtPA treatment. E2 treatment attenuated PAI-1 in complex of tPA in both ischemic and contralateral hemisphere. C, densitometric analysis of PAI-1 in complex with uPA in control, rtPA, and E2 + rtPA group. rtPA significantly increased PAI-1 in complex with uPA in the ischemic hemisphere, which was attenuated by E2 treatment. ∗, p < 0.05.
Fig. 4.
A, representative plasminogen-dependent casein zymogram of control, rtPA, and E2 + rtPA group at 24 h after transient focal MCAO by suture. A dramatic increase of uPA activity was shown in the ischemic side, which was attenuated by E2 treatment. The lytic zones of tPA (64 kDa) and uPA (48 kDa) were quantitatively analyzed. Normalized values of tPA (B) and uPA (C) expressed as 100% of nonischemic hemisphere in controls (Ctl). ∗, p < 0.05 versus nonischemic hemisphere in controls. #, p < 0.01 versus rtPA group.
Interaction of Estrogen and rtPA on Activation of MMP2 and MMP9 after Transient Focal Cerebral Ischemia.
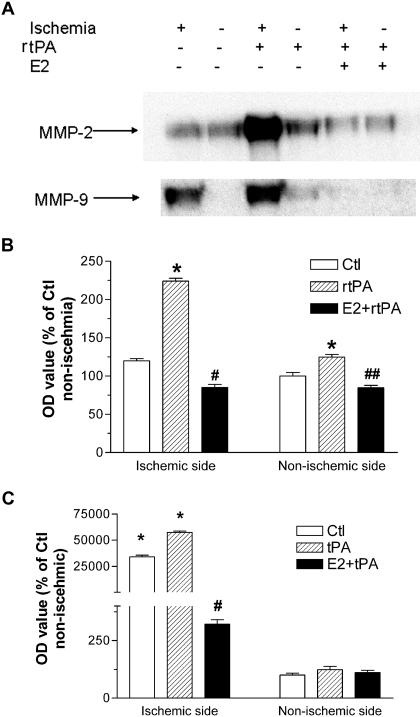
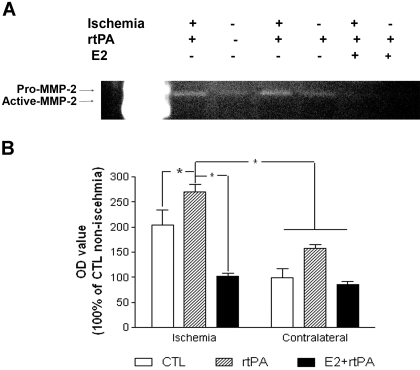
We determined the interaction of estrogen and rtPA on MMP2 and MMP9 activation. Western blots demonstrated that MMP9 expression significantly increased in ischemic hemisphere at 24 h after reperfusion. Exogenous administration of rtPA dramatically enhanced the expression of MMP2 and MMP9 in both ischemic and no-ischemic hemisphere, which was attenuated by estrogen treatment (Fig. 5, A–C). Consistently, MMP2 activity after transient focal cerebral ischemia was enhanced by exogenous administration of rtPA, which was attenuated by estrogen treatment (Fig. 6, A and B).
Fig. 5.
A, representative Western blots of MMP2 and MMP9 in control, rtPA, and E2 + rtPA group at 24 h after transient focal MCAO by suture. Dramatic increase of MMP9 expression was shown in the ischemic side. rtPA treatment marked enhanced expression of both MMP2 and MMP9 in the ischemic and nonischemic side, which were attenuated by combination treatment of E2. B and C, densitometric analysis of expression of MMP2 (B) and MMP9 (C). The ratio was normalized to 100% of nonischemic hemisphere of controls (Ctl). ∗, p < 0.05 versus nonischemic side in controls. # and ##, p < 0.01 versus ischemic side in rtPA group.
Fig. 6.
A, representative gelatin-dependent zymography in control, rtPA, and E2 + rtPA group. Positive medium (C1) containing MMP2 was used as positive control. B, densitometric analysis of MMP2 activity in control, rtPA, and E2 + rtPA group. rtPA significant increased MMP2 activity in the ischemic hemisphere, which was attenuated by E2 treatment. ∗, p < 0.05.
Effect of Combination Treatment of Estrogen and rtPA in Embolic MCAO Model.
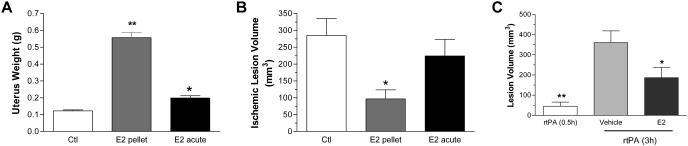
Using a rat embolic MCAO model, we determined the effect of estradiol in embolic stroke. A significant uteri hypertrophy was indicated in ovariectomized female rats with both short-term and acute estrogen treatment (Fig. 7A). No protection was indicated in acute treatment, whereas a significant reduction of lesion volume was observed upon short-term pretreatment (Fig. 7B). We further determined the action of combined estrogen and rtPA treatment in the embolic MCAO model. When rtPA was administered at 0.5 h after embolism, a very small lesion (45.24 ± 20.36 mm3) was found. Whereas a significantly larger lesion volume with 360.4 ± 58.14 mm3 was observed when rtPA was given at 3 h after embolism. The combination treatment of estradiol and rtPA at 3 h after embolism significantly decreased lesion to 186.6 ± 51.67 mm3 (Fig. 7C).
Fig. 7.
A, E2 induces uteri hypertrophy in ovariectomized (Ctl) rats in both short-term chronic (E2 pellet) and acute treatment (E2 acute) paradigms. ∗, p < 0.01 versus Ctl; ∗∗, p < 0.01 versus Ctl and acute estrogen treatment. B, effect of chronic and acute E2 treatments in ischemic lesion volume at 24 h after embolic MCAO. A significant reduction of lesion volume was indicated upon short-term chronic E2 treatment (E2 pellet) but not acute treatment (E2 acute). ∗, p < 0.05 versus control (Ctl). C, effect of combination therapy of estrogen and rtPA in embolic MCAO model. rtPA was administered (10 mg/kg i.v.) at 0.5 h (rtPA 0.5h) or 3 h (rtPA 3h) after embolic MCAO. E2 or vehicle was administered subcutaneously at 3 h immediately before the administration of rtPA. ∗, p < 0.05: E2 versus vehicle; ∗∗, p < 0.05: rtPA (0.5h) versus vehicle.
Discussion
rtPA is currently the only Food and Drug Administration-approved treatment for acute ischemic stroke in United States. If performed in a timely manner, there is no doubt that rtPA-induced reperfusion may rescue brain from ischemic damage. However, whether reperfusion will be beneficial depends on duration of ischemia and degree of cellular alteration. Under some circumstances, thrombolysis-induced reperfusion leads to cerebral edema and hemorrhage. Consistently, hemorrhagic transformation induced by rtPA is related to the duration of ischemia, as more hemorrhagic transformation is seen when rtPA is administered at a later time after stroke (Clark et al., 2000; del Zoppo, 2000). Likewise, in rodent MCAO model, late rtPA treatment rapidly aggravated BBB damage and thereby enhanced hemorrhage transformation (Dijkhuizen et al., 2002).
For over a decade, monotherapies focused on a single pathway have dominated stroke studies, with neuroprotection as one of them. Almost all neuroprotectants that have been studied in clinical trials were targeted at one of cerebral ischemia cascades, and all these trials have failed (Richard Green et al., 2003; Goldberg, 2007). Without thrombolysis derived reperfusion, neuroprotectants may not be effective, simply because not enough neuroprotectants reached ischemic area, or neuroprotectants could not antagonize the extensive damage as a result of prolonged arterial occlusion. Combination therapies of neuroprotectants and rtPA might reduce adverse events of rtPA and extend its therapeutic window. In addition, combination therapies might also decrease dosages for each agent and hence decrease the occurrence of side effects.
The MCAO in rodents has been accepted as the primary model to simulate ischemic stroke in humans (Traystman, 2003). The protective effects of estrogens against ischemic stroke have been well demonstrated in the MCAO models. The protective actions of estrogens have also been demonstrated on glial cells, maintenance of extracellular matrix integrity as well as cerebral vasculature (Liu et al., 2005; Yang et al., 2005; Krause et al., 2006; Miller and Duckles, 2008). In the present study, we determined whether estradiol exerts protection against cerebral ischemia when exogenous rtPA was administered in an experimental ischemic stroke model. Our data suggested that the protective action of estrogens against cerebral ischemia reperfusion injury was not compromised by exogenous rtPA administration.
We determined the interaction of estrogen and rtPA on expression and activation of PAs and MMPs, two critical factors that contribute to hemorrhagic transformation during ischemic stroke. Hemorrhagic transformation is a common accompaniment of ischemic stroke, even in the absence of revascularization therapy (del Zoppo, 2000; Khatri et al., 2007). Up to 30% of all ischemic strokes undergo spontaneous hemorrhagic transformation, and this phenomenon becomes even more prevalent with the use of thrombolytic therapy (Wang and Lo, 2003). The fundamental mechanism leading to fluid and blood extravasation is the disruption of BBB (del Zoppo, 2000), which, in part, is due to generation and secretion of selective MMPs and PAs (Rosenberg et al., 1998). MMP2 and MMP9 are two of the major MMPs expressed in brain (Kaczmarek et al., 2002). Up-regulation of MMP2 and MMP9 after cerebral ischemia has been found in rodents, nonhuman primates, and humans (Sumii and Lo, 2002; Lee et al., 2004; Rosell et al., 2006; del Zoppo et al., 2007). Proteolytic damage involving MMPs have also been implicated in rtPA thrombolytic therapy (Sumii and Lo, 2002). In primary cerebral microvascular endothelial cell culture, rtPA up-regulates MMP9 activation (Wang et al., 2003). Recently, rtPA has been demonstrated to induce MMP2 and MMP9 activation in primary astrocytes (Wang et al., 2006). Increasing evidence indicate that rtPA can increase MMP2 and MMP9 action after stroke, which may contribute to BBB disruption and hemorrhagic transformation (Sumii and Lo, 2002; Lee et al., 2004; Kahles et al., 2005). In rat MCAO model, rtPA promotes MMP9 up-regulation after focal cerebral ischemia (Tsuji et al., 2005). Consistently, MMP inhibitors decreased cerebral hemorrhage and BBB disruption after rtPA treatment (Lapchak et al., 2000; Sumii and Lo, 2002). Our present studies demonstrated that exogenous rtPA administration enhances expression of MMP9 dramatically in ischemic hemisphere. In addition, rtPA also increases activation of MMP2 and uPA. Treatment of estrogen totally blocks action of rtPA on MMPs and uPA in both the ischemic and nonischemic hemisphere. Although total expression of endogenous tPA is up-regulated after ischemia, the activity of tPA does not increase as the majority of tPA is complexed with PAI-1, which is also up-regulated after stroke. Nonetheless, the expression and activation of tPA were reduced by estrogen treatment.
The inhibitory effect of estrogen on interaction of exogenous rtPA with PAs and MMPs could be secondary to its protective action. It has been suggested that up-regulation of MMP9 by rtPA is partly due to over production of free radicals induced by reperfusion injury as the MMP9 promoter contains nuclear factor-κB sites (Wang et al., 2003). Thus, estrogen could potentially modulate MMPs activation through its identified action on nuclear factor-κB. In addition, our results also suggest a direct effect of estrogen on the interaction of rtPA and PAs/MMPs, as the inhibitory action of estrogen could also be seen in the nonischemic hemisphere. The inhibitory effect of estrogen on MMP2 and MMP9 activation during stroke is consistent to its anti-inflammatory action. Estrogen has been shown to inhibit lipopolysaccharide-induced increase of MMP9 activity in both primary astrocyte and microglial cultures (Vegeto et al., 2001; Lewis et al., 2008). Given the multifaceted action of estrogen on free radical production and many cell signal pathways, estrogens might modulate activation of PAs/MMPs induced by ischemia reperfusion damage and constitutive activation of PAs/MMPs in normal brain tissue through different mechanisms.
The protective action of estrogens on stroke has been tested in many clinical trials (Bushnell et al., 2006). Unfortunately, recent analysis suggested that estrogen not only increases the risk of ischemic stroke but also venous thromboembolism (Sare et al., 2008). The discrepancy in the findings between experimental studies and clinical trials could be due to the limitation of stroke models used in translational research, such as differences in tolerance to cerebral edema; differences in size and region of ischemia between human and stroke models; and important molecular differences in thrombotic, inflammatory, and DNA repair cascades between rodents and primates (Carmichael, 2005). In addition, different treatment paradigms used in experimental stroke and clinical trials could contribute to the unsuccessful translation of basic research to clinical trial. Acute treatments have been almost exclusively used in experimental stroke, whereas all clinical trails have been set up to test the effect of long-term estrogen replacement therapy (Gibson et al., 2006; Sare et al., 2008). In the current study, the protective action of estradiol was tested in an embolic ischemic stroke model, which more closely simulates ischemic stroke in the human model compared with the monofilament MCAO model. The protective effect of estrogen was found upon chronic treatment. However, no protection was afforded for acute treatment, which contradicts previous studies using the monofilament permanent MCAO model (Yang et al., 2000). Although the precise reason contributing to different actions of estrogen between these two models is unclear, the lack of protection upon acute treatment in the embolic stroke model argues against single treatment of estrogen for ischemic stroke in future clinical trial. In the embolic MCAO model, early administration of rtPA at 0.5 h after embolism provided significant protection compared with treatment at 3 h after embolism, suggesting that this embolic stroke model could simulate human ischemic stroke for investigation of thrombolytic therapy. Our study demonstrated that combination therapy of rtPA and estrogen at 100 μg/kg provided protection against ischemic stroke when administered at 3 h after embolism compared with rtPA treatment alone. Given the dose-dependent therapeutic window of estrogen in transient focal cerebral ischemia model (Liu et al., 2007), higher dosages might potentially provide optimal protection and hence maximize the therapeutic window of rtPA in the embolic stroke model. It is interesting to note that arterial occlusion lesions are more likely to recanalize in women than in men after rtPA treatment for acute stroke (Savitz et al., 2005). In addition, increased estrogen status has been associated with increased fibrinolytic potential (Gebara et al., 1995). Given the consistent protective effect of acute estrogens treatment in experimental ischemic stroke studies and the protective action of combination therapy in embolic ischemic stroke model, further studies are warranted to dissect the underlying cell signaling mechanisms of combination therapy, to determine the optimum dosage, to test whether protective action of combination therapy of estrogen and rtPA could be afforded in different gender and whether combined estrogen therapy could extend the short therapeutic window of rtPA, and to validate the possibility of a combined therapy for future clinical application.
Collectively, the present study demonstrated that estrogen could provide protection against transient focal cerebral ischemia despite administration of exogenous rtPA. Our results in the experimental ischemic stroke models suggest that estrogens could attenuate the detrimental effect of rtPA on activation of PAs and MMPs during ischemic stroke. In addition, a combination therapy of estrogens and rtPA provided protection against ischemic stroke when administered at 3 h after embolization in an embolic MCAO model. Therefore, estrogen could be a candidate for combined therapy with rtPA to attenuate side effects of rtPA and hence expand its short therapeutic window for treatment of ischemic stroke.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01-NS054687, R01-NS054651] (to S.Y.); and the National Institutes of Health National Institute on Aging [Grants P01-AG22550, P01-AG10485] (to J.W.S.); and the American Heart Association, Inc. Texas Affiliate (to S.Y.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160937.
- rtPA
- recombinant tissue plasminogen activator
- MCAO
- middle cerebral artery occlusion
- PA
- plasminogen activator
- MMP
- matrix metalloproteinase
- E2
- 17β-estradiol
- uPA
- urokinase
- PAI-1
- plasminogen activator inhibitor-1
- BBB
- blood-brain barrier
- tPA
- tissue plasminogen activator.
References
- Bambauer KZ, Johnston SC, Bambauer DE, Zivin JA. (2006) Reasons why few patients with acute stroke receive tissue plasminogen activator. Arch Neurol 63:661–664 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Busch E, Krüger K, Hossmann KA. (1997) Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res 778:16–24 [DOI] [PubMed] [Google Scholar]
- Bushnell CD, Hurn P, Colton C, Miller VM, del Zoppo G, Elkind MS, Stern B, Herrington D, Ford-Lynch G, Gorelick P, et al. (2006) Advancing the study of stroke in women: summary and recommendations for future research from an NINDS-Sponsored Multidisciplinary Working Group. Stroke 37:2387–2399 [DOI] [PubMed] [Google Scholar]
- Carmichael ST. (2005) Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx 2:396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WM, Albers GW, Madden KP, Hamilton S. (2000) The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Thrombolytic therapy in acute ischemic stroke study investigators. Stroke 31:811–816 [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. (2000) Antithrombotic treatments in acute ischemic stroke. Curr Opin Hematol 7:309–315 [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. (2007) Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke 38:646–651 [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH. (2002) Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke 33:2100–2104 [DOI] [PubMed] [Google Scholar]
- Gebara OC, Mittleman MA, Sutherland P, Lipinska I, Matheney T, Xu P, Welty FK, Wilson PW, Levy D, Muller JE. (1995) Association between increased estrogen status and increased fibrinolytic potential in the Framingham Offspring Study. Circulation 91:1952–1958 [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Murphy SP, Bath PM. (2006) Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab 26:1103–1113 [DOI] [PubMed] [Google Scholar]
- Gillum RF. (2002) New considerations in analyzing stroke and heart disease mortality trends: the Year 2000 Age Standard and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Stroke 33:1717–1721 [DOI] [PubMed] [Google Scholar]
- Goldberg MP. (2007) New approaches to clinical trials in neuroprotection: introduction. Stroke 38:789–790 [Google Scholar]
- Green AR, Odergren T, Ashwood T. (2003) Animal models of stroke: do they have value for discovering neuroprotective agents?. Trends Pharmacol Sci 24:402–408 [DOI] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. (2000) Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab 20:631–652 [DOI] [PubMed] [Google Scholar]
- Kaczmarek L, Lapinska-Dzwonek J, Szymczak S. (2002) Matrix metalloproteinases in the adult brain physiology: a link between c-Fos, AP-1 and remodeling of neuronal connections? EMBO J 21:6643–6648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles T, Foerch C, Sitzer M, Schroeter M, Steinmetz H, Rami A, Neumann-Haefelin T. (2005) Tissue plasminogen activator mediated blood-brain barrier damage in transient focal cerebral ischemia in rats: relevance of interactions between thrombotic material and thrombolytic agent. Vascul Pharmacol 43:254–259 [DOI] [PubMed] [Google Scholar]
- Khatri P, Wechsler LR, Broderick JP. (2007) Intracranial hemorrhage associated with revascularization therapies. Stroke 38:431–440 [DOI] [PubMed] [Google Scholar]
- Krause DN, Duckles SP, Pelligrino DA. (2006) Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol 101:1252–1261 [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Chapman DF, Zivin JA. (2000) Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke 31:3034–3040 [DOI] [PubMed] [Google Scholar]
- Lee SR, Tsuji K, Lee SR, Lo EH. (2004) Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J Neurosci 24:671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. (2008) Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol 195:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang X, Liu Q, Yang SH, Simpkins JW. (2007) Dose dependence and therapeutic window for the neuroprotective effects of 17beta-estradiol when administered after cerebral ischemia. Neurosci Lett 415:237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, Yang SH. (2005) 17beta-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res 1060:55–61 [DOI] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. (2003) Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab 14:228–235 [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. (2008) Vascular actions of estrogens: functional implications. Pharmacol Rev 60:210–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A, Ortega-Aznar A, Alvarez-Sabín J, Fernández-Cadenas I, Ribó M, Molina CA, Lo EH, Montaner J. (2006) Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 37:1399–1406 [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. (1998) Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 29:2189–2195 [DOI] [PubMed] [Google Scholar]
- Sare GM, Gray LJ, Bath PM. (2008) Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J 29:2031–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Schlaug G, Caplan L, Selim M. (2005) Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke 36:1447–1451 [DOI] [PubMed] [Google Scholar]
- Sumii T, Lo EH. (2002) Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke 33:831–836 [DOI] [PubMed] [Google Scholar]
- Traystman RJ. (2003) Animal models of focal and global cerebral ischemia. Ilar J 44:85–95 [DOI] [PubMed] [Google Scholar]
- Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH. (2005) Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke 36:1954–1959 [DOI] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. (2001) Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci 21:1809–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lee SR, Guo SZ, Kim WJ, Montaner J, Wang X, Lo EH. (2006) Reduction of tissue plasminogen activator-induced matrix metalloproteinase-9 by simvastatin in astrocytes. Stroke 37:1910–1912 [DOI] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. (2003) Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 9:1313–1317 [DOI] [PubMed] [Google Scholar]
- Wang X, Lo EH. (2003) Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol 28:229–244 [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, Lo EH. (2004) Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke 35:2726–2730 [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wang X, Simpkins JW. (2005) Estrogens as protectants of the neurovascular unit against ischemic stroke. Curr Drug Targets CNS Neurol Disord 4:169–177 [DOI] [PubMed] [Google Scholar]
- Yang SH, Shi J, Day AL, Simpkins JW. (2000) Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke 31:745–749; discussion 749–750 [DOI] [PubMed] [Google Scholar]
- Young AR, Ali C, Duretête A, Vivien D. (2007) Neuroprotection and stroke: time for a compromise. J Neurochem 103:1302–1309 [DOI] [PubMed] [Google Scholar]