Abstract
Alterations in N-methyl-d-aspartate receptor (NMDAR) protein levels or subcellular localization in brain after chronic ethanol exposure may contribute to withdrawal-associated seizures and neurotoxicity. We have investigated synaptic localization of NMDARs in cultured hippocampal pyramidal neurons after prolonged (7 days) exposure to, and acute withdrawal from, 80 mM ethanol using fluorescence immunocytochemistry techniques. After chronic ethanol exposure, there was a significant increase in the clustering of NR1 and NR2B subunits and their colocalization with the synaptic proteins synaptophysin and postsynaptic density protein 95, respectively. There was also increased expression of NR1 variants containing the C2′ cassette after chronic ethanol exposure. The ethanol-induced synaptic clustering and colocalization were rapidly reversed within 4 h after ethanol withdrawal. Surface labeling of NR2B subunits suggested that this rapid reversal involved lateral receptor movement to extrasynaptic sites rather than internalization of receptors. Receptor removal from the synapse during ethanol withdrawal was associated with changes in the phosphorylation state of NR2B Ser1480, controlled by the protein kinase CK2. The redistribution of NMDAR to synapses produced by long-term ethanol exposure, as well as the rapid removal during withdrawal, may not only affect neuronal withdrawal hyperexcitability but also may sensitize the system to subsequent synaptic plasticity.
It is well established that N-methyl-d-aspartate receptor (NMDAR) channel activity can be inhibited by acute ethanol exposure at physiologically relevant concentrations (Hoffman et al., 1989; Lovinger et al., 1989). Conversely, long-term exposure to ethanol in vitro or in vivo has been associated with increased NMDAR binding density and elevated mRNA and/or protein expression in cultured neurons and in many brain regions (Gulya et al., 1991; Follesa and Ticku, 1995; Snell et al., 1996), as well as with increased function in hippocampal slice preparations as measured by enhanced NMDAR currents and induction of long-term potentiation (LTP) (Fujii et al., 2008; Sabeti and Gruol, 2008). The increase in receptor density presumably represents an adaptive response to the prolonged attenuation of channel activity by ethanol. These adaptive changes in NMDARs are of particular concern because the long-term ethanol-induced increases in NMDAR density seen in animals produce central nervous system hyperexcitability once ethanol has been removed, leading to neuronal toxicity and seizures associated with ethanol withdrawal (Grant et al., 1990).
It is not clear whether increased NMDAR density after long-term ethanol exposure involves increased synthesis of receptors, changes in receptor localization, or both. In some cases, increased NMDAR function has been observed in brain tissue without changes in the number of receptors (Cebere et al., 1999). However, other studies of brain (Snell et al., 1996) and cultured neurons (Chen et al., 1999) have found that long-term ethanol treatment results in significant increases in expression of NMDAR subunit proteins.
Recruitment of NMDARs to postsynaptic membranes has been reported to be activity-dependent in multiple neuronal cell types (Rao and Craig, 1997; Rosen et al., 2007), such that long-term blockade of excitatory activity leads to increased synaptic density of NR1 and NR2 subunits. In cultured hippocampal neurons, long-term ethanol exposure was also reported to lead to increased synaptic localization of receptor proteins (Carpenter-Hyland et al., 2004). In vivo studies showed that long-term ethanol-induced changes in hippocampal NMDAR reversed relatively rapidly after withdrawal (Gulya et al., 1991). Although NMDARs become concentrated in the postsynaptic density (PSD) of dendritic spines, populations of extrasynaptic receptors have also been observed (Harris and Pettit, 2008), and it has been suggested that internalization of NMDARs requires that synaptic receptors move to extrasynaptic sites before undergoing endocytosis (Lau and Zukin, 2007). Such lateral movement could account for rapid reversal of activity-dependent increases in synaptic NMDAR density.
In the current report, we have used primary cultures of hippocampal pyramidal neurons, immunocytochemical techniques, and fluorescence microscopy to study the effects of chronic exposure to and withdrawal from ethanol on the synaptic localization of NMDAR subunits. Our results show that long-term ethanol exposure leads to an increase in the synaptic localization of NMDARs, which is quickly reversed on ethanol withdrawal by a mechanism that involves changes in receptor phosphorylation and movement to an extrasynaptic site.
Materials and Methods
Cell Culture.
Primary hippocampal neurons were prepared from neonatal Sprague-Dawley rats (P0-P1) as described in Gomez et al. (2002). In brief, the hippocampus was dissected from brains of neonatal rats and dissociated by papain digestion. For immunocytochemical experiments, neurons were plated at low density (15–30,000 cells/ml) in modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) on glass coverslips coated with poly-d-lysine and laminin (BD Biosciences, San Jose, CA). For immunoblotting and immunoprecipitation experiments, neurons were plated at high density (300–400,000 cells/ml) on 6-cm Petri plates. After 1 day, the medium was replaced with Neurobasal supplemented with B-27 (Invitrogen) and mitotic inhibitors [uridine + fluorodeoxyuridine (Ur+FdUr)] (Sigma-Aldrich, St. Louis, MO) that had previously been conditioned by cultured rat glial cells for 24 h. The neurons were then maintained on glial-conditioned Neurobasal, B-27, and Ur+FdUr and grown for 14 days in vitro.
Ethanol Treatment and Withdrawal.
After 14 days of growth in vitro, hippocampal neurons were treated by addition of a concentrated (50% v/v) ethanol solution in growth medium to yield a final ethanol concentration of 100 mM or by addition of 2-amino-5-phosphonopentanoate (AP5; Sigma-Aldrich) to a final concentration of 100 μM. Ethanol-treated neurons in plates or dishes were placed inside an enclosed 200-ml water bath containing 100 mM ethanol and were maintained for 7 days in a humidified atmosphere of 5% CO2 at 37°C. The bath and cultures were supplemented with ethanol (1.2 ml of 50% ethanol was added to the bath; 6 μl of 50% ethanol/ml of growth medium was added to each dish) once per day. In a mock treatment procedure, gas chromatographic analysis (Tabakoff et al., 1976) of culture media before and after the daily addition of ethanol indicated that the concentration of ethanol was maintained for 7 days between 66 and 100 mM, with an average concentration over the time course of 82 mM. The ethanol concentration in the culture media was routinely measured by gas chromatography at the end of the chronic exposure period to confirm that ethanol was maintained at the proper concentration. The concentration of ethanol (100 mM) was selected to yield a maximal increase in NMDAR clustering to optimize the measurement of any reversal of the effects after acute ethanol withdrawal. Lower concentrations of ethanol (25 mM) were previously shown to be less effective at promoting NMDAR clustering within the same time frame (Carpenter-Hyland et al., 2004).
For ethanol withdrawal, the media were replaced with conditioned Neurobasal, B-27, Ur+FdUr for 24 h, 4 h, 30 min, 15 min, or 5 min before processing for immunocytochemistry or immunoprecipitation. After the replacement of culture media, residual ethanol remaining in the cultures was determined by gas chromatography to be between 0 and 6 mM. In some experiments, 10 μM H89 (Sigma-Aldrich) was added to the neuronal cultures during the final 2 days of chronic ethanol treatment. [This time period was chosen because of the tendency of long-term exposure to protein kinase A (PKA) inhibitors to promote apoptosis in some systems, as noted in Hendricson et al. (2007).] To determine the effects of kinase inhibitors during ethanol withdrawal, 10 μM 4,5,6,7-tetrabromobenzotriazole (TBB; Sigma-Aldrich) or 10 μM H89 was added to the ethanol-free replacement media used during the ethanol withdrawal time course, as noted above.
Immunocytochemistry.
Hippocampal neurons were processed for immunocytochemistry essentially as described in Gomez et al. (2002). Cultured neurons on glass coverslips were washed in phosphate-buffered saline (PBS) and either fixed in ice-cold methanol for 20 min (for NMDAR labeling) or fixed in 3.7% formaldehyde/PBS for 10 min and permeabilized with 0.2% Triton X-100 in PBS for 10 min [for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) labeling]. Cells were then washed with PBS and blocked in PBS + 10% bovine serum albumin (BSA) for 30 to 60 min. The primary antibodies were incubated with the neurons for 1 to 2 h at room temperature in PBS + BSA. Polyclonal antibodies against synaptophysin (syp) (1:500; Sigma-Aldrich), AMPAR GluR1 (1:200; Millipore, Billerica, MA), and NMDAR NR2B subunit (1:200; Zymed Laboratories, South San Francisco, CA), as well as monoclonal antibodies against NMDAR NR1 subunit (1:100; BD Biosciences) and postsynaptic density protein 95 (PSD-95) (1:100, clone 6G6-1C9; Advanced Biomedical Research, Inc., Princeton, NJ), were used. After incubation with primary antibodies, the cells were washed extensively in PBS + BSA and incubated for 1 h with fluorescent secondary antibody conjugates at room temperature. Goat anti-rabbit and goat anti-mouse secondary antibodies conjugated to Alexa-647 (1:500) and Texas Red (1:250), respectively, were obtained from Invitrogen (Carlsbad, CA). Coverslips were washed in PBS and water and mounted on glass slides with Pro-Long mounting medium (Invitrogen). For surface labeling with an NR2B amino-terminal antibody, hippocampal neurons were incubated in cold Neurobasal medium with the primary antibody (1:50; Zymed) for 30 min at 4°C [to minimize endocytosis, as described by Horne and Dell'Acqua (2007)], washed with cold PBS, and fixed in 3.7% formaldehyde/PBS for 10 min. Cells were then permeabilized with 0.2% Triton X-100 in PBS and blocked in PBS + 10% BSA for 60 min before incubating with additional primary or fluorescent secondary antibody conjugates, as above. Total NR2B immunoreactivity was determined using fixed and permeabilized cells processed in parallel with the cell surface labeling samples.
Fluorescence Microscopy.
Cell imaging of neurons was performed on an inverted Carl Zeiss GmbH (Jena, Germany) Axiovert 200M with 100× plan-apo/1.4 numerical aperture objective, 175-W xenon illumination, Coolsnap charge-coupled device camera (Roper Scientific, Trenton, NJ), and Slidebook 4.0 software (Intelligent Imaging Innovations, Denver, CO). Three-dimensional z-stacks of x,y-planes with 0.5-μm steps were collected for the entire cell imaged. Images were deconvolved to the nearest neighbor to generate confocal x,y-sections. In the figures, two-dimensional x,y-projection images of the entire deconvolved z-stack are shown to better represent complete pictures of dendrites and spines. These projection images were used for quantitative mask analysis as described below.
Quantitative Digital Image Analysis.
Image quantitation was performed in Slidebook 4.0 (Intelligent Imaging Innovations) using Masks and Segmentation. For calculation of normalized mean fluorescence intensities for immunostaining, both red-Texas Red and green-Alexa-647 channels were segmented together to generate a total neuron mask including only areas of continuous pixels corresponding to the cell of interest with background removed. A separate mask was drawn for the soma and subtracted from the total neuron mask to generate a dendrite (D) mask. Mean fluorescence intensities were calculated from the D mask for each channel. Segmented dendritic puncta (P) masks were generated showing discrete areas of continuous pixels of fluorescence intensity >2 times the mean dendritic fluorescence for each channel. These discrete P define dendritic “clusters.” A mask showing only areas of dendritic colocalization (C) for red and green channels was generated using the “AND” function. Integrated intensity values were then calculated from the C mask and the individual dendritic P masks for each channel. Fluorescence intensity values from P and C masks were used to calculate the “clustering index” (defined as the ratio of the sum P intensity in a single channel divided by the sum D intensity in the same channel) and percentage C (defined as the sum C intensity in a single channel divided by the sum D intensity in that channel multiplied by 100) scores, respectively. Cluster size and number data were derived from the P mask for each channel using the respective functions of the Slidebook software. The number of clusters was transformed into “cluster density” (clusters/10 μm) by dividing total cluster number by the dendritic area of the D mask. For cell-surface labeling experiments, mean NR2B surface fluorescence intensity in the D mask was normalized to the mean value of total NR2B fluorescence intensities for each condition and expressed as a ratio versus the untreated controls. Colocalization between surface NR2B and PSD-95 was determined using the C mask, as described previously. Fluorescence micrograph images were exported as red-green-blue tagged image file format files and assembled into figures using Adobe Photoshop version 7.0 (Adobe Systems, Mountain View, CA).
Immunoprecipitation and Blotting.
Immunoprecipitations from hippocampal neurons were performed as described in Gomez et al. (2002). In brief, neurons in 6-cm Petri dishes were grown and treated as described above before harvesting in Tris/EDTA buffer (1 mM Tris, pH 8.0, 5 mM EDTA, 10% SDS) with protease and phosphatase inhibitors (2 μg/ml leupeptin/pepstatin, 1 mM benzamidine, 1 mM 4-(2-aminoethyl)benzenesulfonylfluoride, 35 mM sodium fluoride, 1 mM sodium orthovanadate). Five hundred micrograms of total cellular protein was incubated overnight at 4°C with 5 μg of antibody raised against the NR2B subunit (Millipore) or NR1 subunit (BD Biosciences Pharmingen, San Diego, CA) and protein A agarose (Millipore). Bound proteins were precipitated by centrifugation, washed, and separated by SDS-polyacrylamide gel electrophoresis (NuPAGE bis-Tris 4–12% polyacrylamide gel; Invitrogen). Proteins were transferred to polyvinylidene difluoride membranes (25 V, 2 h) and probed with primary antibodies [NMDA-NR2B subunit (Zymed); phospho-Ser1480 NR2B (pSer1480), a gift from Dr. Richard Huganir (Howard Hughes Medical Institute, Johns Hopkins University, Chevy Chase, MD) or obtained from Novus Biologicals, Inc. (Littleton, CO); and NMDA-NR1 C2 and C2′ splice variants (Millipore Bioscience Research Reagents, Temecula, CA)], followed by peroxidase-coupled secondary antibodies (Bio-Rad Laboratories, Hercules, CA), and were processed for visualization using enhanced chemiluminescent reagents (Western Lighting; PerkinElmer Life and Analytical Sciences, Waltham, MA). The blots were developed on X-ray film (Thermo Fisher Scientific, Waltham, MA), and optical densities were analyzed using Quantity One software (Bio-Rad Laboratories) to determine the volume (intensity × mm2) of specific bands. After global background subtraction, pSer1480 band volumes were normalized to immunoprecipitated NR2B protein band volumes (by dividing pSer1480/NR2B) for each lane and expressed as a percentage of the normalized control volume. NR1 C2′ and C2 variant band volumes were expressed as a ratio (by dividing C2′/C2) and averaged across experiments.
Statistics.
Combined data from independent experiments were analyzed using σ-Stat 3.1 (Systat Software, Inc., San Jose, CA) to determine mean values, S.E., and one-way analysis of variance (ANOVA) followed by pairwise comparisons using the Holm-Sidak method to determine statistical significance, except where noted otherwise. Data were found to be distributed normally and exhibited equal variance across groups. When making a single comparison of two groups, data were analyzed by an unpaired t test to determine statistical significance. The n values are presented as the number of coverslips analyzed for each treatment or control condition with an average of 10 cells imaged per coverslip.
Results
Chronic Ethanol Exposure Promotes Synaptic Clustering of NMDARs.
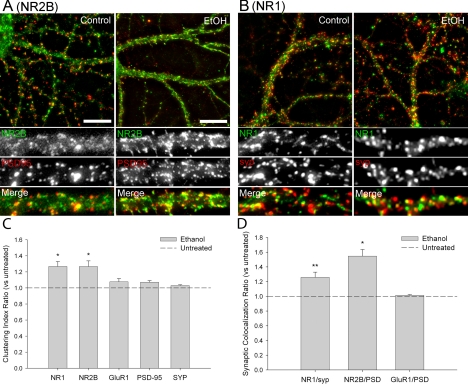
The synaptic localization and clustering of NMDARs were measured by immunocytochemical analyses of primary cultures of rat hippocampal neurons. After 21 days in culture, clusters of NR1 and NR2B immunoreactivity were detected along the dendritic shafts of neurons with pyramidal cell morphology, similar to previous reports (Rao and Craig, 1997; Carpenter-Hyland et al., 2004). In neurons exposed to ∼80 mM ethanol for 7 days (days 14–21 in culture), clusters of NR1 and NR2B immunoreactivity were enhanced relative to untreated controls (Fig. 1, A and B). In particular, the observed -fold increase in the NR1 clustering index in the ethanol-treated cells (defined above, see under Materials and Methods) was 1.27 ± 0.06 (mean ± S.E.M.) above untreated control, and the NR2B clustering index was increased 1.27 ± 0.07-fold above control (Fig. 1C). Changes in the clustering index of any group of puncta could reflect changes in the size, density (e.g., number of puncta per unit area), and/or fluorescence intensity of those clusters of proteins. Significant increases in both the size and density of NR1 and NR2B clusters in response to chronic ethanol treatment were observed (Table 1). However, the increases in NR1 and NR2B clustering were not likely caused by an increase in total protein because the mean dendritic receptor immunoreactivity in ethanol-treated cells was not different from untreated controls (1.01 ± 0.02 above control, NR1; 1.04 ± 0.01 above control, NR2B). Incubation of neuronal cultures with 10 μM H89, an isoquinoline inhibitor of PKA, during the final 48 h of chronic ethanol exposure did not alter the chronic ethanol-induced increases in NR1 and NR2B subunit clustering (data not shown).
Fig. 1.
Chronic ethanol (EtOH) treatment promotes synaptic clustering of NMDAR subunits. Dual immunocytochemistry and confocal imaging of NMDAR subunits (NR2B, NR1) and synaptic marker proteins (PSD-95, syp) were used to examine the effects of chronic ethanol exposure on receptor clustering in cultured hippocampal neurons. A and B, confocal imaging revealed punctate dendritic clusters of NR2B (A; green) and NR1 (B; green) subunits in control cultures that partially colocalized with synaptic proteins PSD-95 (A; red) and syp (B; red). Chronic exposure to ethanol (∼80 mM for 7 days) caused an increase in NR2B and NR1 clustering relative to untreated controls, as well as an increase in colocalization with the respective synaptic proteins (A and B); scale bar: 10 μm (A). C and D, quantitative mask analysis of confocal projection images (see Materials and Methods) revealed a significant chronic ethanol-dependent increase in the clustering (C) and synaptic localization (D) of NR1 and NR2B subunits versus control. The clustering index for each protein after chronic ethanol treatment is reported as a ratio relative to the untreated controls. Asterisk indicates a significant difference from untreated controls (*, p < 0.01; **, p < 0.05; t test comparison within a one-way ANOVA adjusted by Holm-Sidak method; n > 8 coverslips for each condition).
TABLE 1.
Chronic ethanol treatment produces a reversible increase in NMDAR cluster size and density
Cluster size and density expressed as mean ± S.E.M. Student's t test; n > 8 coverslips for each condition.
| Cluster Size (Pixels/Cluster) |
Cluster Density (Clusters/10 μm) |
|||||
|---|---|---|---|---|---|---|
| Control | EtOH | WD 4 h | Control | EtOH | WD 4 h | |
| NR1 | 6.76 ± 0.4 | 12.15 ± 0.5* | 6.86 ± 0.6† | 5.90 ± 0.4 | 9.17 ± 0.4* | 5.56 ± 0.8† |
| syp | 13.70 ± 0.8 | 14.14 ± 1.0 | 14.26 ± 0.7 | 11.06 ± 0.4 | 12.39 ± 0.8 | 12.02 ± 0.7 |
| NR2B | 7.51 ± 0.3 | 17.25 ± 1.1* | 9.28 ± 0.4† | 5.46 ± 0.3 | 8.59 ± 0.6* | 4.82 ± 0.6† |
| PSD-95 | 11.54 ± 0.6 | 11.28 ± 0.5 | 10.45 ± 0.8 | 12.05 ± 1.0 | 11.99 ± 0.9 | 9.92 ± 0.9 |
| GluR1 | 12.6 ± 0.9 | 12.8 ± 0.8 | 12.8 ± 1.0 | 7.44 ± 0.6 | 6.84 ± 0.9 | 6.62 ± 0.9 |
EtOH, chronic ethanol exposure; WD, withdrawal.
p < 0.01 compared with untreated control.
p < 0.01 compared with chronic ethanol treatment.
Comparison of the colocalization of NMDAR subunit immunoreactivity with the immunoreactivity of presynaptic vesicular protein syp or PSD-95 showed the presence of synaptic clusters of NR1 and NR2B, respectively (Fig. 1A). Chronic ethanol exposure produced an increase in the colocalization of clusters of NR1 with syp (1.26 ± 0.07 above control) and clusters of NR2B with PSD-95 (1.55 ± 0.09 above control) (Fig. 1D). However, there was no change observed after chronic ethanol exposure in PSD-95 or syp puncta size or density (Table 1), clustering index (Fig. 1C), or mean dendritic immunoreactive intensity (1.06 ± 0.04 above control, PSD-95; 0.96 ± 0.02 of control, syp). Colocalization of NR1 and NR2B subunits increased 1.18 ± 0.05-fold above control after chronic ethanol treatment (Fig. 3, A and E).
Fig. 3.
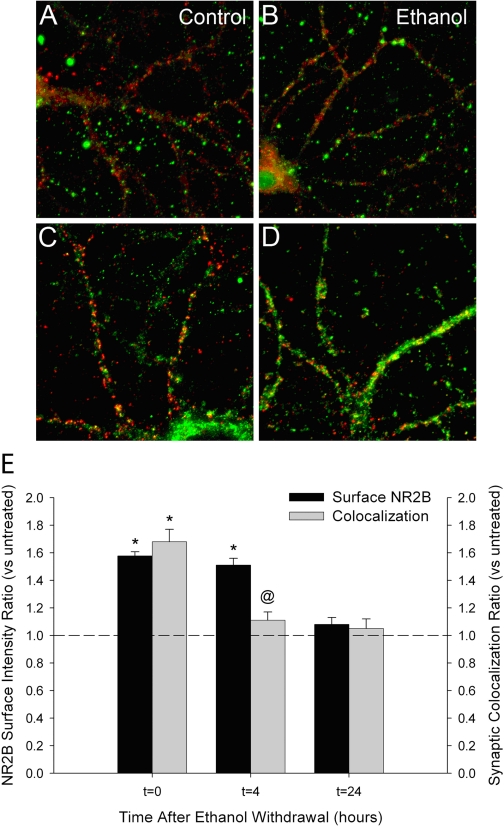
Ethanol withdrawal rapidly reverses the synaptic clustering of NMDAR subunits. A and C, dual immunocytochemistry and confocal imaging of NR2B (red) and NR1 (green) subunits revealed that chronic ethanol-enhanced clustering (t = 0) was reversed shortly after ethanol was removed from the cultures and returned to control levels within 4 h (t = 4) (C). A and E, clusters of NR1 colocalized with NR2B in untreated control cultures, and this colocalization persisted during chronic ethanol treatment and withdrawal. B and D, analysis of clusters of NR2B (green) colocalized with PSD-95 (red) and clusters of NR1 (green) colocalized with syp (red) in chronic ethanol-treated cultures (t = 0) revealed that synaptic colocalization reversed shortly after ethanol was removed from the cultures and returned to control levels within 30 min (t = 0.5) (D). C–E, quantitative mask analysis of confocal projection images. Asterisk indicates a significant difference from untreated controls; @ indicates a significant difference from chronic ethanol-treated cells (*, p < 0.01; @ or **, p < 0.05; pairwise comparison using one-way ANOVA adjusted by Holm-Sidak method, n > 8 coverslips for each condition).
Chronic treatment of cultured neurons with 100 μM d-AP5, a selective NMDAR antagonist, resulted in increased NR1 clustering (1.35 ± 0.02-fold above control) and colocalization with syp (1.26 ± 0.03 above control). Similar to ethanol, chronic AP5 treatment did not alter the clustering indices of PSD-95 or syp (1.01 ± 0.02 and 1.03 ± 0.01 above control, respectively).
The effect of ethanol on the synaptic localization and clustering of AMPAR GluR1 subunits was also measured. Neurons exposed to ethanol for 7 days showed no significant change in GluR1 subunit clustering (1.08 ± 0.04 above control) compared with untreated neurons (Fig. 1C). Chronic ethanol exposure did not alter the size or the density of GluR1 clusters compared with untreated control cells (Table 1). Measurement of the colocalization of GluR1 clusters with PSD-95 revealed no change in the synaptic localization of GluR1 clusters (1.01 ± 0.01 above control) after prolonged ethanol exposure (Fig. 1D). Chronic ethanol exposure did not result in a statistically significant change in NR1 colocalization with GluR1 (1.15 ± 0.05-fold above control, p > 0.05, n = 4). Chronic treatment with 100 μM AP5 did not produce a significant change in GluR1 clusters (size or colocalization with PSD-95 or NR1; data not shown). These findings are consistent with earlier reports in which chronic NMDAR inhibition with AP5 or ethanol failed to promote synaptic clustering of AMPARs (Rao and Craig, 1997; Carpenter-Hyland et al., 2004).
Chronic Ethanol Exposure Increases the C2′/C2 Ratio of NMDAR NR1 Splice Variants.
Variable splicing of exon 22 of NMDAR NR1 mRNA has been shown to affect cell-surface expression of the protein in cultured neurons and heterologous cell types (Okabe et al., 1999; Mu et al., 2003). To determine whether chronic ethanol exposure increases synaptic NR1 subunit density through a change in expression of carboxyl-terminal splice variants, rat hippocampal neurons were cultured at high density and used for Western blot analyses. Exposure to 80 mM ethanol for 7 days did not change the detectable levels of NMDAR NR1 subunit expression in the neurons (data not shown) or the levels of the neuron-specific βIII-tubulin (Fig. 2A). The intensity of a ∼100-kDa band detected with an antibody specific for the C2′ cassette increased, whereas a band detected with an antibody specific for the C2 cassette of NR1 decreased in intensity after chronic ethanol exposure (Fig. 2A). These changes in intensity result in a significant increase in the ratio of C2′ to C2 splice variants (Fig. 2B).
Fig. 2.
Chronic ethanol exposure increases the C2′/C2 ratio of NMDAR NR1 splice variants. Immunoprecipitation and Western blot analyses were used to determine the effects of chronic ethanol exposure and withdrawal on the expression of C2′ and C2 splice variants of NMDAR-NR1 subunits in cultured hippocampal neurons. A, splice variant-selective antibodies directed against NR1-C2′ or -C2 detected ∼120-kDa bands in cultured hippocampal homogenates after immunoprecipitation with monoclonal NR1 antibodies. Band intensity of C2′-containing subunits increased, whereas C2-containing subunits decreased after 7-day exposure to ∼80 mM ethanol. In whole homogenates, bands detected with antibodies against neuronal tubulin did not change with ethanol exposure. B, quantitative analysis of Western blots by densitometry revealed a significant increase in the ratio of C2′ to C2 detected after chronic ethanol exposure. Band intensities were normalized to neuronal tubulin band volume as a loading control. Asterisk indicates a significant difference from untreated controls (*, p < 0.01, comparison using Student's t test; n > 3 blots for each condition).
Withdrawal from Ethanol Reverses Synaptic Clustering of NMDARs.
To determine the time course of reversal of the ethanol-induced increase in the synaptic clustering of NMDA receptors, cells were fixed and processed for immunocytochemistry 30 min, 4 h, and 24 h after the removal of ethanol from the neuronal cultures (Fig. 3). The exchange of ethanol-containing culture medium with untreated medium resulted in a final concentration of ∼4 mM ethanol in the culture as measured by gas chromatography. Removal of ethanol resulted in a rapid return to control clustering levels for NR1 and NR2B (Fig. 3, A and B). The NR1 and NR2B clustering indices were significantly reduced 30 min after withdrawal relative to chronic ethanol-treated cultures (1.09 ± 0.03-fold above control at 30 min versus 1.27 ± 0.06 before withdrawal, NR1; 1.12 ± 0.03-fold above control at 30 min versus 1.27 ± 0.07 before withdrawal, NR2B) (Fig. 3C). Ethanol withdrawal also reversed the increase in NR1 cluster synaptic colocalization with syp (0.77 ± 0.09 of control, 30 min) and NR2B cluster synaptic colocalization with PSD-95 (0.66 ± 0.07 of control, 4 h) (Fig. 3D). In fact, the synaptic colocalization of NR1 with syp and NR2B with PSD-95 at 30 min and 4 h after ethanol withdrawal, respectively, decreased significantly below control levels. Colocalization of NR1 and NR2B subunits was not significantly altered by chronic ethanol treatment or withdrawal (Fig. 3E).
In cultured hippocampal neurons treated for 7 days with 100 μM d-AP5, reversal of synaptic NR1 clustering was also observed soon after the drug was removed by replacement of the culture medium. After chronic treatment with AP5, the clustering index of NR1 subunits (1.35 ± 0.02-fold above untreated control) returned to control levels within 30 min after withdrawal (0.97 ± 0.10 of control, 30 min) and remained at control levels up to 24 h later.
Withdrawal from Ethanol Promotes NMDAR Relocation to Extrasynaptic Membrane Sites.
To determine whether the NMDARs undergo internalization after ethanol withdrawal or translocate to an extrasynaptic localization in the dendritic membrane, cell surface NR2B immunofluorescence intensity was monitored in intact neurons after withdrawal from chronic ethanol exposure (Fig. 4, A–D). Surface NR2B immunoreactivity was observed as punctate staining that overlapped with a fraction of the total PSD-95 immunoreactivity (measured after cells had been fixed and made permeable to the PSD-95 antibody) (Fig. 4, A and B). Exposing intact cells to an antibody directed at the intracellular protein PSD-95 did not result in any detectable immunostaining, as expected (data not shown). Prolonged exposure to ethanol produced an increase in the mean surface NR2B immunoreactivity (1.58 ± 0.02-fold above control) in the absence of any change in mean total NR2B immunoreactivity (1.04 ± 0.01 above control) (Fig. 4E). Surface NR2B colocalization with PSD-95 was also increased by chronic ethanol treatment (1.68 ± 0.06 above control). The increase in surface NR2B fluorescence intensity was not reversed at 4 h after the removal of ethanol from neuronal cultures (1.51 ± 0.04 above control), consistent with NMDAR retention at the plasma membrane. However, colocalization of surface NR2B with PSD-95 was reversed by 4 h after ethanol removal (1.10 ± 0.01 above control), suggesting that the receptors relocated to an extrasynaptic region of the membrane. By 24 h after the removal of ethanol, surface intensity of NR2B clusters and colocalization with PSD-95 had returned to control levels (Fig. 4E).
Fig. 4.
Chronic ethanol treatment increases surface expression of NR2B subunits. Immunocytochemistry and confocal imaging of surface-accessible NR2B subunits (green) and total PSD-95 (red) immunoreactivity were used to examine the effects of chronic ethanol and withdrawal on the surface expression of NMDARs. A and B, antibodies directed against the extracellular amino terminus of NR2B revealed punctate clusters of NR2B immunoreactivity on the surface of cultured hippocampal neurons. Chronic exposure to ethanol resulted in increased surface immunoreactivity of NR2B subunits versus untreated controls (B). Serial labeling with PSD-95 antibodies revealed the synaptic localization of the surface NR2B clusters. No PSD-95 immunoreactivity was detected by simultaneous labeling of intact neurons with NR2B and PSD-95 antibodies (data not shown). C and D, immunocytochemistry of total NR2B subunits and PSD-95 in permeabilized neurons revealed no change in the mean fluorescence intensity of NR2B-immunoreactive dendritic clusters after chronic ethanol exposure. E, quantitative mask analysis of confocal projection images showed a significant chronic ethanol-dependent increase in the mean surface-fluorescence intensity of NR2B subunits and synaptic colocalization with PSD-95 (t = 0). Shortly after removing ethanol from the cultures, synaptic colocalization of surface NR2B with PSD-95 had reverted to control levels (t = 4), whereas mean surface fluorescence remained elevated (E). Mean surface NR2B fluorescence intensity returned to control values 24 h after the removal of ethanol (t = 24) from neuronal cultures. Asterisk indicates a significant difference from untreated controls, and @ indicates a significant difference from chronic ethanol-treated cells (* or @, p < 0.01; t test comparison within a one-way ANOVA adjusted by Holm-Sidak method; n > 4 coverslips for each condition).
Protein Kinase CK2 Regulates the Reversal of NR2B Clustering during Withdrawal from Chronic Ethanol Exposure.
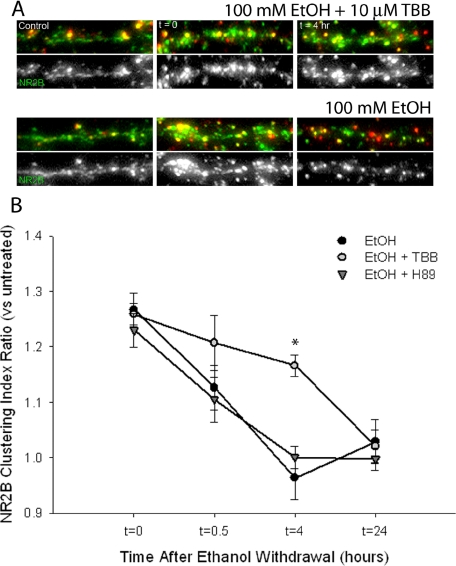
Protein kinase CK2 is a ubiquitous serine/threonine kinase highly enriched in brain that has been previously shown to regulate NMDAR activity (Lieberman and Mody, 1999). Phosphorylation of NR2B subunits by CK2 has been reported to disrupt the interaction between NMDARs and PSD-95 (Chung et al., 2004). Treatment of ethanol-exposed neurons with 10 μM TBB, a selective, cell-permeable ATP site inhibitor of CK2 activity (IC50 = 0.9 μM), during ethanol withdrawal reduced the rate of reversal of NR2B clustering compared with neurons treated with vehicle during withdrawal (Fig. 5). TBB has shown little or no inhibitory efficacy on a panel of 33 other kinases, including the closely related kinase CK1, at this concentration (Sarno et al., 2001). However, CK2 inhibition did not completely attenuate the reversal of NR2B clustering, and receptor clustering in the presence of TBB returned to control levels by 24 h after withdrawal from ethanol. A similar treatment with the PKA inhibitor H89 (10 μM) did not affect the rate of reversal of NR2B clustering (Fig. 5B).
Fig. 5.
Inhibition of protein kinase CK2 stabilizes the ethanol-enhanced clustering of NR2B subunits. Immunocytochemistry and confocal imaging were used to determine the effects of protein kinase CK2 inhibition on the reversal of ethanol-induced clustering of NR2B subunits after withdrawal. A, NR2B-immunoreactive clusters (green) were enhanced by chronic ethanol exposure (t = 0) and reverted to control levels within 4 h after ethanol was removed from the cultures (t = 4 h). The presence of 10 μM TBB, an inhibitor of protein kinase CK2, during withdrawal slowed the reversal of NR2B clustering compared with vehicle (dimethyl sulfoxide). B, quantitative mask analysis of confocal projection images showed the effect of CK2 inhibition on the rate of reversal of NR2B clustering during ethanol withdrawal. Cells treated with TBB, but not 10 μM H89 (an isoquinoline inhibitor of PKA), exhibited a significant increase in NR2B clustering at 4 h after ethanol withdrawal compared with cells treated with vehicle during withdrawal. Asterisk indicates a significant difference from chronic ethanol-treated cells (*, p < 0.01, pairwise comparison using one-way ANOVA adjusted by Holm-Sidak method; n > 6 coverslips for each condition).
Inhibition of CK2 also reduced the effects of withdrawal from chronic ethanol exposure on the size and density of NR2B clusters. Clusters of NR2B-containing receptors increased in size from 7.51 ± 0.3 pixels/cluster to 17.25 ± 1.1 pixels/cluster with chronic ethanol exposure (Table 1). After the removal of ethanol from the cultures, NR2B cluster size decreased to 9.28 ± 0.4 pixels/cluster within 4 h. However, in the presence of 10 μM TBB during ethanol withdrawal, NR2B clusters remained elevated in size 4 h after withdrawal (15.8 ± 0.6 pixels/cluster, p < 0.01, n = 6 coverslips). NR2B cluster density was also increased from 5.46 ± 0.3 clusters/10 μm to 8.59 ± 0.6 clusters/10 μm with chronic ethanol exposure (Table 1). After the removal of ethanol from the cultures, NR2B cluster density decreased to 4.82 ± 0.6 clusters/10 μm within 4 h. Inhibition of CK2 with TBB during ethanol withdrawal lessened the decrease in NR2B cluster density (7.06 ± 0.3 clusters/10 μm after 4 h withdrawal, p < 0.02, n = 6 coverslips).
Chronic Ethanol Exposure Promotes Dephosphorylation of NR2B on Ser1480.
To determine whether chronic ethanol treatment and withdrawal affect the phosphorylation state of NR2B at Ser1480, high-density primary cultures of rat hippocampal neurons were used in Western blot analyses with phosphorylation-specific antibodies. In neurons exposed to ethanol for 7 days in culture, phosphorylation of NR2B at Ser1480 was significantly decreased (48.9 ± 6.8%) relative to untreated controls (Fig. 6C). The amount of detectable NR2B protein in cell lysates (data not shown) or immunoprecipitates was equivalent between untreated controls and chronically ethanol-treated cells, as determined by immunoblot analysis with an NR2B subunit-specific antibody (Fig. 6A). To determine the effect of withdrawal on the phosphorylation state of Ser1480, we prepared samples for immunoprecipitation shortly after withdrawing ethanol from the cultures (withdrawal 5 and 15 min). Ethanol withdrawal for 15 min produced an increase in Ser1480 phosphorylation (pSer1480) that reversed the chronic ethanol-dependent decrease (Fig. 6, A–C). Incubation of neurons with 10 μM TBB during ethanol withdrawal prevented the increased phosphorylation of Ser1480 (Fig. 6, B and C).
Fig. 6.
Changes in NR2B subunit phosphorylation on Ser1480 during chronic ethanol exposure and withdrawal. Immunoprecipitation and Western blot analyses were used to determine the effects of chronic ethanol exposure and withdrawal on the phosphorylation state of Ser1480 on NR2B subunits. A and B, phosphoserine antibodies directed against NR2B Ser1480 detected a ∼170-kDa band in cultured hippocampal homogenates that had been precipitated with NR2B antibodies. This band was decreased in samples from chronic ethanol-treated cells (A) and returned to control levels shortly after ethanol was removed from the cultures (WD 15 min). Incubation with 10 μM TBB during ethanol withdrawal prevented the reversal to control phosphorylation levels (B). C, quantitative analysis of Western blots by densitometry revealed a significant decrease in Ser1480 phosphorylation after chronic ethanol exposure. Inhibition of CK2 with TBB resulted in a decrease in Ser1480 phosphorylation 15 min after withdrawal, relative to cells treated with ethanol alone. Phosphorylation levels were normalized to total NR2B band volume and are expressed as a percentage of untreated control. Asterisk indicates a significant difference from untreated controls, and @ indicates a significant difference between ethanol + TBB and treatment with ethanol alone (* or @, p < 0.01, pairwise comparison using one-way ANOVA adjusted by Holm-Sidak method; n > 4 blots for each condition).
The coordinated activities of PKA and protein kinase C have been shown to regulate surface trafficking of NMDARs by altering phosphorylation of Ser896 and Ser897 of NR1 (Scott et al., 2003). We did not detect substantial immunoreactivity using phospho-specific antibodies against NR1 subunits Ser896 and Ser897, nor was there any change in the intensity of these bands after chronic ethanol treatment (data not shown).
Discussion
There is substantial evidence supporting the role of glutamatergic neurotransmission in the development of alcohol dependence (Gass and Olive, 2008). The NMDAR response to alcohol is characterized by acute inhibition of channel conductance, leading to an adaptive increase in the density and activity of NMDARs after chronic alcohol exposure (Hoffman, 2003). Here we replicate the finding that NMDARs accumulate at the synapses of cultured hippocampal neurons in response to chronic ethanol exposure (Carpenter-Hyland et al., 2004). This accumulation is likely because of the prolonged inhibition of NMDAR activity by ethanol because chronic treatment with AP5 produced similar results as previously reported by Rao and Craig (1997). We also show that the synaptic accumulation of NMDARs is quickly reversed once ethanol or AP5 is removed, suggesting that an activity-dependent process regulates synaptic recruitment and removal of NMDARs. This observation is consistent with another report showing that chronic ethanol-induced changes in paired-pulse facilitation and synaptic NMDAR currents are reversed shortly after ethanol withdrawal (2–8 h) from slice preparations of the central amygdala (Roberto et al., 2006). However, our findings contrast with the report by Carpenter-Hyland et al. (2004), who showed that enhanced NR1 clustering persisted for 4 days after withdrawal from chronic ethanol exposure. It is likely that differences between the studies with respect to the rate of ethanol withdrawal can account for the differing results. Carpenter-Hyland et al. (2004) allowed ethanol to slowly evaporate from the culture, whereas we removed ethanol from the cultures quickly by replacement with ethanol-free media. Neither of these procedures exactly mimics the time course of ethanol metabolism in vivo, where we observed that increased NMDAR ligand binding was reversed sometime between the elimination of ethanol by the animals (8 h after withdrawal) and 24 h after withdrawal (Gulya et al., 1991). However, the abrupt removal of ethanol in the current study allowed us to observe the mechanism and time course of the initial reversal of chronic ethanol-induced changes in NMDAR clustering.
Our results provide evidence for the regulated removal of NR2B-containing receptors from synapses, as well as translocation to extrasynaptic sites, in a CK2 activity-dependent manner. This change may depend on the increased activity of NMDARs after ethanol withdrawal because a similar reversal of clustering was seen on removal of AP5 after chronic exposure. However, it would also be of interest to determine whether changes in other neurotransmitter systems (e.g., GABA) contribute to the activity-dependent reversal of NMDAR clustering during ethanol withdrawal. The rapid reversal of the neuroadaptive response to prolonged ethanol exposure may provide hippocampal neurons with a controlled mechanism for returning to former homeostatic levels of glutamatergic signaling once ethanol has been cleared from the synapse. This mechanism may be protective because aberrant glutamatergic activity has been shown to contribute to central nervous system hyperexcitability and neurotoxicity associated with alcohol withdrawal (Hendricson et al., 2007).
The signaling that underlies the reversible synaptic recruitment of NMDARs in response to chronic ethanol exposure and acute withdrawal has not been fully delineated. Studies using the PKA inhibitor KT-5720 suggested that ethanol-induced increases in NMDAR clustering or surface expression are PKA-dependent (Carpenter-Hyland et al., 2004), whereas others, using the PKA inhibitor H89, reported that these changes are PKA-independent (Hendricson et al., 2007). Note that H89 and KT-5720 have each been reported to produce effects that are independent of PKA (i.e., nonspecific effects), which may confound the interpretation of these data (Murray, 2008). We observed no difference in the chronic ethanol-induced synaptic clustering of NR1 when neurons were incubated with H89 (data not shown).
Although we did not detect a change in serine phosphorylation of NR1 subunits, we did observe an ethanol-dependent increase in the proportion of NR1 containing the C2′ cassette sequence (Fig. 2). Variable splicing of immature mRNA results in four unique carboxyl-terminal sequences of the NR1 subunit that contain C0, C1, C2, or C2′ cassettes (reviewed in Zukin and Bennett, 1995). NR1 splice variants have been reported to display differential surface expression based on the presence of endoplasmic reticulum retention motifs in the C1 sequence (Okabe et al., 1999). Recent reports have shown that chronic inhibition of NMDARs with AP5 promotes the expression of C2′-containing variants, and the presence of the C2′ cassette can abrogate endoplasmic reticulum retention via PDZ-binding and coatamer protein complex interactions (Mu et al., 2003; Horak and Wenthold, 2009). Although our experiments cannot distinguish between total cellular NR1 and membrane-integrated NR1 subunits, our results, in conjunction with previous studies, suggest that chronic ethanol exposure may increase NMDAR cell surface density through selective expression of NR1 variants (Kumari, 2001).
NMDARs are maintained at synapses by association with PDZ-containing scaffold proteins such as PSD-95 (Lin et al., 2004). Whereas chronic ethanol exposure promotes surface expression of NR2B subunits (Fig. 4), we detect no change in the clustering of synaptic marker proteins PSD-95 or syp (Fig. 1C), consistent with an earlier report in which chronic inhibition of NMDARs with AP5 did not alter the distribution of these proteins (Rao and Craig, 1997). Shortly after ethanol withdrawal, NR2B colocalization with PSD-95 is decreased, signifying movement of receptors away from the PSD. However, the elevated surface expression of NR2B does not reverse at the same rate, suggesting that the NMDARs are moving laterally in the plasma membrane. It is unfortunate that antibodies that target the extracellular amino terminus of the NR1 and NR2A subunits are unavailable or of insufficient quality to reliably detect surface NMDARs in intact neurons using direct immunocytochemical approaches. However, because the colocalization of NR1 with NR2B (78 ± 4.0% in control neurons) is not reduced at 4 h after withdrawal (Fig. 3, A and E), it is likely that some proportion of NR1 subunits remain localized at the cell surface and are also moved away from the synapse at this time. Although we were unable to investigate changes in NR2A synaptic density in this study, the observation that NR2B subunits exhibit greater lateral mobility in the plasma membrane than NR2A subunits (Groc et al., 2006) suggests that a change in subunit composition may contribute to NMDAR redistribution after ethanol withdrawal.
Phosphorylation of NMDAR subunits and their association with scaffold and accessory proteins regulates the synaptic stability of these receptors (Wenthold et al., 2003). The decrease in NR2B serine phosphorylation (pSer1480) that we observed in response to prolonged ethanol treatment may be a prerequisite for NMDAR association with PSD-95 and recruitment to the synapse (Chung et al., 2004). Chronic ethanol exposure may decrease NR2B phosphorylation through the inhibition of a kinase (e.g., CK2) or activation of a phosphatase. We have also detected a withdrawal-induced increase in pSer1480 that was attenuated by the CK2 inhibitor TBB. This is the first demonstration of changes in NR2B serine phosphorylation in response to chronic ethanol exposure or withdrawal.
Internalization of NMDARs via clathrin-mediated endocytosis has been reported (Roche et al., 2001), leading to either receptor degradation or recycling to the plasma membrane (Scott et al., 2004). The internalization of NR2B subunit-containing receptors can be regulated by receptor phosphorylation and by interactions between NMDARs and PSD proteins (Lavezzari et al., 2003). Our results show that immediately after ethanol withdrawal, NR2B moves away from the PSD, and clusters of NR2B-containing receptors decrease in size (Table 1). This lateral movement of NMDARs to extrasynaptic sites is likely to precede clathrin-mediated internalization, as has been previously suggested (Lau and Zukin, 2007).
Recent findings support the concept that ethanol and other drugs of abuse use the machinery of normal, experience-dependent plasticity in the brain (i.e., memory and learning) to elicit neuroadaptation that leads to drug dependence. Given the critical role of NMDARs in the formation of long-term synaptic plasticity (MacDonald et al., 2006), the synaptic recruitment or removal of these receptors may regulate the ability of the neuron to undergo subsequent, activity-dependent synaptic modification. Taken together, these observations suggest that the chronic ethanol-induced increase in NMDAR density may initiate temporary metaplastic changes in a neuron's response to subsequent ethanol exposure. The reversible nature of the changes in NMDAR synaptic density that we observed represent the effects of a single, prolonged episode of ethanol exposure followed by withdrawal, whereas the “kindling” effects of serial episodes of exposure and withdrawal may produce changes of a more durable nature. It has been found that chronic intermittent ethanol treatment produces a greater and more persistent increase in NR2B subunit expression, as well as more persistent withdrawal seizure responses, than a single exposure (Becker et al., 1997). Our results show one molecular mechanism that generates a rapid reversal of chronic ethanol-induced changes. One may speculate that this mechanism may be impaired after repeated exposure and withdrawal, leading to the persistent changes in glutamatergic transmission associated with alcohol dependence.
Acknowledgments
We thank Dr. Richard Huganir for the gift of phospho-Ser1480 NR2B antibody.
This work was supported in part by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants AA14101, AA007464]; and the Banbury Fund.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.158741.
- NMDAR
- N-methyl-d-aspartate receptor
- LTP
- long-term potentiation
- PSD
- postsynaptic density
- Ur+FdUr
- uridine + fluorodeoxyuridine
- AP5
- 2-amino-5-phosphonopentanoate
- H89
- N-[2-(4-bromocinnamylamino)ethyl]-5-isoquinoline
- PKA
- protein kinase A
- TBB
- 4,5,6,7-tetrabromobenzotriazole
- PBS
- phosphate-buffered saline
- AMPAR
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- BSA
- bovine serum albumin
- syp
- synaptophysin
- PSD-95
- postsynaptic density protein 95
- D
- dendrite
- P
- puncta
- C
- colocalization
- pSer1480
- phospho-Ser1480
- ANOVA
- analysis of variance
- KT-5720
- (9S,10S,12R)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid hexyl ester.
References
- Becker HC, Diaz-Granados JL, Weathersby RT. (1997) Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol 14:319–326 [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. (2004) Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci 24:7859–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebere A, Cebers G, Liljequist S. (1999) Enhancement of NMDA-induced functional responses without concomitant NMDA receptor changes following chronic ethanol exposure in cerebellar granule cells. Naunyn Schmiedebergs Arch Pharmacol 360:623–632 [DOI] [PubMed] [Google Scholar]
- Chen X, Moore-Nichols D, Nguyen H, Michaelis EK. (1999) Calcium influx through NMDA receptors, chronic receptor inhibition by ethanol and 2-amino-5-phosponopentanoic acid, and receptor protein expression. J Neurochem 72:1969–1980 [DOI] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. (2004) Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci 24:10248–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. (1995) Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Brain Res Mol Brain Res 29:99–106 [DOI] [PubMed] [Google Scholar]
- Fujii S, Yamazaki Y, Sugihara T, Wakabayashi I. (2008) Acute and chronic ethanol exposure differentially affect induction of hippocampal LTP. Brain Res 1211:13–21 [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. (2008) Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75:218–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML. (2002) Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J Neurosci 22:7027–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. (1990) Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol 176:289–296 [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. (2006) NMDA receptor surface mobility depends on NR2A–2B subunits. Proc Natl Acad Sci U S A 103:18769–18774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. (1991) Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res 547:129–134 [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. (2008) Recruiting extrasynaptic NMDA receptors augments synaptic signaling. J Neurophysiol 99:524–533 [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. (2007) Aberrant synaptic activation of N-methyl-d-aspartate receptors underlies ethanol withdrawal hyperexcitability. J Pharmacol Exp Ther 321:60–72 [DOI] [PubMed] [Google Scholar]
- Hoffman PL. (2003) NMDA receptors in alcoholism. Int Rev Neurobiol 56:35–82 [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Moses F, Tabakoff B. (1989) Selective inhibition by ethanol of glutamate-stimulated cyclic GMP production in primary cultures of cerebellar granule cells. Neuropharmacology 28:1239–1243 [DOI] [PubMed] [Google Scholar]
- Horak M, Wenthold RJ. (2009) Different roles of C-terminal cassettes in the trafficking of full-length NR1 subunits to the cell surface. J Biol Chem 284:9683–9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne EA, Dell'Acqua ML. (2007) Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J Neurosci 27:3523–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M. (2001) Differential effects of chronic ethanol treatment on N-methyl-d-aspartate R1 splice variants in fetal cortical neurons. J Biol Chem 276:29764–29771 [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426 [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. (2003) Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology 45:729–737 [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. (1999) Casein kinase-II regulates NMDA channel function in hippocampal neurons. Nat Neurosci 2:125–132 [DOI] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. (2004) Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci 24:10138–10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243:1721–1724 [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Jackson MF, Beazely MA. (2006) Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol 18:71–84 [DOI] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. (2003) Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 40:581–594 [DOI] [PubMed] [Google Scholar]
- Murray AJ. (2008) Pharmacological PKA inhibition: all may not be what it seems. Sci Signal 1:re4 [DOI] [PubMed] [Google Scholar]
- Okabe S, Miwa A, Okado H. (1999) Alternative splicing of the C-terminal domain regulates cell surface expression of the NMDA receptor NR1 subunit. J Neurosci 19:7781–7792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Craig AM. (1997) Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron 19:801–812 [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. (2006) Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology 31:988–996 [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. (2001) Molecular determinants of NMDA receptor internalization. Nat Neurosci 4:794–802 [DOI] [PubMed] [Google Scholar]
- Rosen KM, Moghekar A, O'Brien RJ. (2007) Activity dependent localization of synaptic NMDA receptors in spinal neurons. Mol Cell Neurosci 34:578–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gruol DL. (2008) Emergence of NMDAR-independent long-term potentiation at hippocampal CA1 synapses following early adolescent exposure to chronic intermittent ethanol: role for sigma-receptors. Hippocampus 18:148–168 [DOI] [PubMed] [Google Scholar]
- Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. (2001) Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (“casein kinase-2”). FEBS Lett 496:44–48 [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. (2003) Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology 45:755–767 [DOI] [PubMed] [Google Scholar]
- Scott DB, Michailidis I, Mu Y, Logothetis D, Ehlers MD. (2004) Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J Neurosci 24:7096–7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. (1996) Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res 40:71–78 [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Anderson RA, Ritzmann RF. (1976) Brain acetaldehyde after ethanol administration. Biochem Pharmacol 25:1305–1309 [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. (2003) Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol 43:335–358 [DOI] [PubMed] [Google Scholar]
- Zukin RS, Bennett MV. (1995) Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci 18:306–313 [DOI] [PubMed] [Google Scholar]