Abstract
Aggregation of α-synuclein (αsyn) is a hallmark of sporadic and familial Parkinson's disease (PD) and dementia with Lewy bodies. Lewy bodies contain αsyn and several heat shock proteins (Hsp), a family of molecular chaperones up-regulated by the cell under stress. We have previously shown that direct expression of Hsp70 and pharmacological up-regulation of Hsp70 by geldanamycin, an Hsp90 inhibitor, are protective against αsyn-induced toxicity and prevent aggregation in culture. Here, we use a novel protein complementation assay to screen a series of small-molecule Hsp90 inhibitors for their ability to prevent αsyn oligomerization and rescue toxicity. By use of this assay, we found that several compounds prevented αsyn oligomerization as measured by decreased luciferase activity, led to a reduction in high-molecular-mass oligomeric αsyn, and protected against αsyn cytotoxicity. A lead compound, SNX-0723 (2-fluoro-6-[(3S)-tetrahydrofuran-3-ylamino]-4-(3,6,6-trimethyl-4-oxo-4,5,6,7-tetrahydro-1H-indol-1-yl)benzamide) was determined to have an EC50 for inhibition of αsyn oligomerization of approximately 48 nM and was able to rescue αsyn-induced toxicity. In vivo assessment of SNX-0723 showed significant brain concentrations along with induction of brain Hsp70. With a low EC50, brain permeability, and oral availability, these novel inhibitors represent an exciting new therapeutic strategy for PD.
Cytoplasmic depositions of α-synuclein (αsyn) aggregates are a major component of Lewy bodies (Spillantini et al., 1997), the characteristic inclusions observed in both sporadic and familial cases of Parkinson's disease (PD) and related disorders. Point mutations in the αsyn gene (Polymeropoulos et al., 1997; Krüger et al., 1998; Zarranz et al., 2004), as well as gene duplication and multiplication of the αsyn gene locus (Singleton et al., 2003), can lead to αsyn deposits and toxicity, although the mechanism of this process is unclear. However, recent evidence implicates a prefibrillar, oligomeric species of αsyn as a vital toxic intermediate in the process of neurodegeneration (Conway et al., 2000; Uversky et al., 2001; Volles and Lansbury, 2003; Dedmon et al., 2005; El-Agnaf et al., 2006; Danzer et al., 2007; Outeiro et al., 2008). If oligomeric species of αsyn are toxic, an important therapeutic strategy would be to reduce rates of oligomerization or oligomer levels, thereby potentially rescuing αsyn-induced cell death and preventing disease progression.
In general, protein misfolding and aggregation can be prevented by the machinery of the molecular chaperone system. Hsp70, in particular, is up-regulated as part of a stress response to protein misfolding and aggregation and protects against misfolded αsyn-induced toxicity and neurodegeneration by refolding pathogenic species of αsyn and/or directing the misfolded species toward proteasomal or lysosomal degradation (Klucken et al., 2004; McLean et al., 2004; Auluck et al., 2005; Dedmon et al., 2005; Shin et al., 2005; Tetzlaff et al., 2008) We and others have previously shown that Hsp70 interacts with overexpressed αsyn in in vitro H4 neuroglioma cells (Klucken et al., 2004; Shin et al., 2005), in Drosophila (Auluck et al., 2005), and in Masliah line D mouse models (Klucken et al., 2004) in a neuroprotective manner by decreasing higher-molecular-mass αsyn species as well as rescuing αsyn-induced toxicity.
Hsp90 is a molecular chaperone involved in the folding, stabilization, and binding of many client proteins, and is believed to be critical for maintaining the integrity of many signaling cascade pathways in response to cellular stress and perturbations of the pathways by aberrant expression and/or mutation (Schulte and Neckers, 1998; Xiao et al., 1999). Inhibition of Hsp90 chaperone activity results in activation of heat shock factor-1 (HSF-1) and subsequent activation of protective stress-induced HSPs such as Hsp70 (Dickey et al., 2005; Fujikake et al., 2008).
Geldanamycin (GA), a naturally occurring Hsp90 inhibitor, has been found to up-regulate Hsp70 and is cytoprotective in many assays of misfolded protein-related toxicity (McLean et al., 2004; Fujikake et al., 2008). GA itself cannot cross the blood-brain barrier and has considerable toxicity in cancer trials (Waza et al., 2006; Fujikake et al., 2008). 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) and 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17-DMAG) are much less toxic derivatives of GA that are blood-brain barrier permeable (Waza et al., 2006; Fujikake et al., 2008), but they have been difficult to formulate, have limited oral availability, or cause varying degrees of hepatotoxicity in clinical cancer trials, presumably because of the reactivity of the chemical core (Chiosis and Tao, 2006; Cysyk et al., 2006; Okawa et al., 2009).
SNX-2112 represents a class of novel, orally available, nonchemically reactive, and potent Hsp90 inhibitors that exhibit excellent antitumor activity in vitro and in vivo (Chandarlapaty et al., 2008; Okawa et al., 2009). In this study, we screened a group of synthetic, orally active, small-molecule Hsp90 inhibitor compounds in this drug class in an in vitro model of αsyn oligomerization and toxicity and for brain penetration. These compounds are chemically dissimilar to GA and derivatives. We show that novel Hsp90 inhibitors can rescue αsyn-induced toxicity and decrease oligomerization in vitro in a dose-dependent manner at a lower dose than 17-AAG. In vivo pharmacokinetic (PK) and pharmacodynamic studies also indicate that members of this class of Hsp90 inhibitors have good brain absorption and excellent oral bioavailability, thus making them good candidates for further evaluation. Together, these data provide important preclinical information that validates inhibition of Hsp90 as a strong therapeutic strategy in Parkinson's disease and other neurodegenerative disorders linked to protein misfolding.
Materials and Methods
Plasmids
Syn-Luc1 (S1) and Syn-Luc2 (S2) were generated, as described previously (Outeiro et al., 2008), by subcloning αsyn into the Not1/ClaI sites of humanized Gaussia luciferase constructs provided by Dr. Stephen Michnick of the University of Montreal (Remy and Michnick, 2006). The Hsp70 and wild-type αsyn (pSI-WTsyn) plasmids used in this study have been described previously (Klucken et al., 2004). Full-length Gaussia luciferase cDNA was provided by Dr. Bakhos Tannous of the Massachusetts General Hospital (Tannous et al., 2005).
Cell Culture and Transfection
Human H4 neuroglioma cells (HTB-148; American Type Culture Collection, Manassas, VA) were maintained in OPTI-MEM growth media with 10% fetal bovine serum (both from Invitrogen, Carlsbad, CA) and incubated at 37°C in 5% CO2 conditions. H4 cells were plated to 80 to 90% confluence 16 to 24 h before transfection. They were transfected by use of Superfect (QIAGEN, Chatsworth, CA) according to the manufacturer's protocol. Equimolar plasmid ratios for all constructs were used.
Toxicity Assay
Toxicity was measured 24 h after transfection by use of the Toxilight cytotoxicity assay kit (Lonza, Rockland, ME) according to the manufacturer's instructions.
Gaussia Luciferase Protein Complementation Assay
H4 neuroglioma cells were cotransfected with S1 and S2 in 96-well plates as described above. At 24 h after transfection, existing cell media were replaced with serum-free, phenol red-free Opti-MEM (Invitrogen). The cell-permeable substrate, native Coelenterazine (Prolume Ltd, Pinetop, AZ) was resuspended in methanol to 1 mg/ml and dispensed per well by an automated plate reader, the Wallac 1420 Victor2 (PerkinElmer Life and Analytical Sciences, Waltham, MA) to a final concentration of 20 μM. The signal generated from substrate-enzyme interaction was integrated over 2 s before measurement at 480 nm.
Enzyme-Linked Immunosorbent Assay for αsyn
α-Synuclein concentration was quantified using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Invitrogen). In brief, a monoclonal antibody specific for αsyn was coated onto the wells and αsyn binds simultaneously to the immobilized monoclonal (capture) antibody and to the solution phase rabbit polyclonal (detection) antibody specific for αsyn. After washing, a horseradish peroxidase-labeled anti-rabbit IgG (anti-rabbit IgG-horseradish peroxidase) is added which binds to the detection antibody to complete the four-member sandwich. After incubation and washing, substrate solution is added and absorbance is read at 450 nm. The absorbance is directly proportional to the concentration of αsyn present in the original specimen. αSyn concentration was determined by plotting sample absorbances against standards with use of GraphPad Prism fitting software (four parameter algorithm).
Native and Denatured PAGE
H4 cells were plated in 60-mm or 100-mm tissue culture dishes and transfected 18 to 24 h later with use of the transfection procedures described above. Twenty-four hours after transfection, samples were harvested in 4°C lysis buffer. All samples were lysed with detergent-free lysis buffer containing protease inhibitors (50 mM Tris/HCl, pH 7.4, 175 mM NaCl, 5 mM EDTA, pH 8.0).
For denatured SDS-PAGE, samples were sheared with a 27-gauge 1-ml syringe 3 to 4 times and centrifuged for 2 min. The supernatant was then boiled in 2× Tris glycine sample buffer (Invitrogen) containing β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) for 5 min at 100°C, and then run in SDS-containing buffer on 10-well 10 to 20% Tris glycine gels (Invitrogen).
For native blots, samples were harvested 24 h after transfection in the detergent-free lysis buffer detailed above. The supernatant was then mixed with 2× SDS-free native Tris glycine sample buffer and run on 4 to 12% Tris glycine gels. For both denatured and native blots, protein concentrations were determined before electrophoresis by use of the bicinchoninic acid protein assay, and 30 μg of each sample was loaded in per lane.
After electrophoresis, gels were transferred to polyvinylidene difluoride membrane (PerkinElmer Life and Analytical Sciences) and blocked in 5% milk in Tris-buffered saline containing Tween 20 (TBS-T) for 1 h at room temperature or overnight at 4°C. Membranes were immunoblotted with primary antibodies (mouse anti-alpha synuclein, 1:1000; BD Biosciences, San Jose, CA; rabbit anti-Gaussia luciferase, 1:1000, Prolume Ltd.; rabbit anti-Hsp70, 1:10,000, StressGen/Assay Designs, Ann Arbor, MI) for 2 h at room temperature or overnight at 4°C. After three TBS-T washes, membranes were incubated in horseradish peroxidase-conjugated secondary antibodies (1:2000). Immunoblots were analyzed by use of the enhanced chemiluminescence detection system (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Pharmacological Treatments In Vitro
Pretreatment.
H4 cells were plated into 96-well plates and treated 6 to 8 h later with 500 nM 17-AAG or 10 nM to 10 μM synthetic Hsp90 inhibitors, followed by transfection of S1 and S2 16 to 24 h later.
Cotreatment.
H4 cells were plated into 96-well plates, transfected with S1 and S2 16 to 24 h later, and then immediately treated with 500 nM 17-AAG or 10 nM to 10 μM synthetic Hsp90 inhibitors.
pcDNA 3.1+ vector was used as a control for Hsp70 plasmid transfections, and dimethyl sulfoxide was used as a control for drug treatments.
Pharmacokinetics and Pharmacodynamics
The in-life part of the animal studies was conducted at Washington Biotech (Columbia, MD). For each time point, three animals were sacrificed and samples collected. For pharmacokinetic studies, SNX-0723 was administered by oral gavage at 10 mg/kg to female Sprague-Dawley rats weighing 160 to 190 g. Samples were collected at 0, 3, 6, 12, and 24 h. Each animal was immediately necropsied. Brain and lung tissues were collected and flash-frozen. All specimens were stored at −80°C. Extracts from 40 to 100 mg of brain and lung tissue were prepared with a glass Dounce in 100% acetonitrile containing an internal standard. The extracts were centrifuged and a 10-μl aliquot of the supernatant was injected directly into the liquid chromatography-tandem mass spectrometry system [4000 QTRAP (Applied Biosystems, Foster City, CA)] with Shimadzu HPLC (Columbia, MD). Tissue concentrations were calculated from the sample response ratios and tissue masses. Pharmacokinetic parameters were determined by use of the WinNonlin software package (Pharsight Corporation, St. Louis, MO).
For pharmacodynamic studies, SNX-0723 was administered by oral gavage at 10 mg/kg to each of three female Sprague-Dawley rats weighing 160 to 190g. Samples were collected at 0, 1, 3, 6, 12, and 24 h. Lysates were prepared with modification to the Meso Scale Discovery multiarray total Hsp70 assay kit protocol. Brain tissue was homogenized in 1:6 wt/vol of complete lysis buffer using a glass Dounce on ice. Protein concentration was determined by use of the bicinchoninic acid protein assay (Pierce, Rockland, IL). Hsp70 levels were measured according to kit protocol by use of the SI6000 SECTOR imaging instrument (Meso Scale Discovery, Gaithersburg, MD).
Results
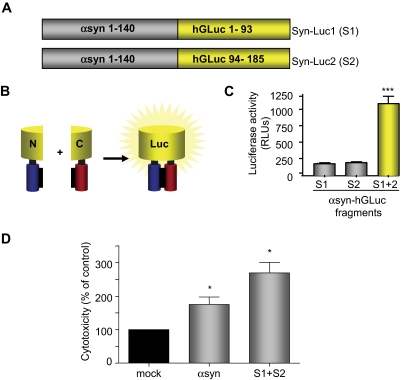
To examine the effect of novel compounds on αsyn oligomerization and toxicity we have developed a bioluminescent protein complementation assay (bPCA). In this strategy, αsyn is fused to nonbioluminescent amino-terminal or carboxyl-terminal fragments of Gaussia princeps luciferase (Remy and Michnick, 2006) that can reconstitute when brought together by αsyn-αsyn interactions (Outeiro et al., 2008), thus providing a readout of αsyn oligomerization (Fig. 1, A and B). With use of this assay, cotransfection of Syn-Luc1 (S1) and Syn-Luc2 (S2) results in reconstituted luciferase activity more than 5-fold above background (Fig. 1C), indicative of αsyn dimer and oligomer formation. Furthermore, like untagged αsyn (Klucken et al., 2004) and αsyn fluorescent protein complementation pairs (Outeiro et al., 2008), cotransfection of S1 and S2 results in significant αsyn-induced cytotoxicity (Fig. 1D). It is noteworthy that, although we have previously shown that cotransfection of αsyn and synphilin-1 results in the formation of intracellular inclusions (McLean et al., 2002; Shin et al., 2005), the oligomeric forms described herein remain soluble and do not lead to macroscopic aggregate formation (Outeiro et al., 2008; Tetzlaff et al., 2008).
Fig. 1.
αSyn bPCA. A and B, schematic representation of the αsyn bPCA constructs. Nonbioluminescent halves of humanized Gaussia luciferase (hGLuc) are fused to αsyn monomers. C, cotransfection of S1 and S2 results in reconstituted luciferase signal at five times greater than background. n = 3; ∗∗∗, p < 0.001. D, like αsyn, overexpression of S1 and S2 results in cytotoxicity. n = 3; ∗ p < 0.05. RLUs, relative light units.
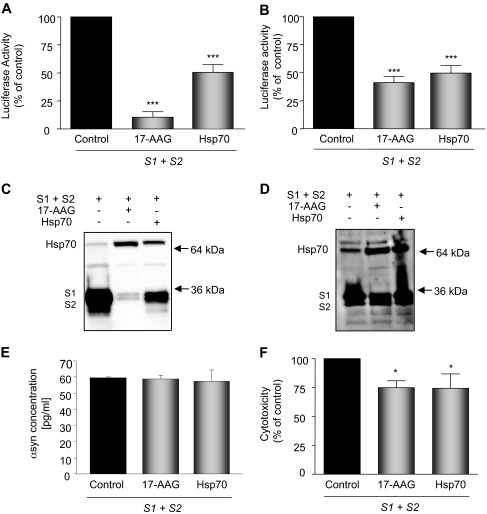
We have previously demonstrated that overexpression of Hsp70 or pharmacological up-regulation of Hsp70 with GA can prevent αsyn aggregation into cell inclusions (McLean et al., 2004). We now examined the effect of Hsp70 on αsyn oligomerization in our αsyn PCA assay by overexpressing Hsp70 or treating with the Hsp90 inhibitor 17-AAG. H4 cells were either pretreated (16–18 h before transfection) or cotreated (at time of transfection) with 17-AAG, or cotransfected with Hsp70. Both pretreatment and cotreatment with 17-AAG prevented αsyn oligomerization (85 ± 8.3 and 59 ± 9.5%, respectively) (Fig. 2, A and B), as did cotransfection with Hsp70 (50 ± 13.7%). Although pretreatment with 17-AAG resulted in a significant decrease in monomeric protein levels (Fig. 2C), which could also account for the decrease in luciferase activity (Fig. 2A), cotreatment with 17-AAG did not significantly reduce protein levels (Fig. 2D) even though it significantly decreased luciferase activity (Fig. 2B). These data suggest that multiple mechanisms, including degradation and refolding, may be at play. To quantify the amount of αsyn in cells after 17-AAG treatment and Hsp70 coexpression we used an αsyn-specific ELISA and found that there was no significant decrease in αsyn levels after cotreatment of 17-AAG and coexpression of Hsp70 (Fig. 2E). Furthermore, in accord with our previous studies (McLean et al., 2004), pretreatment of 17-AAG was found to be effective in rescuing αsyn-induced toxicity (Fig. 2F).
Fig. 2.
Hsp70 and 17-AAG reduce αsyn oligomerization and rescue toxicity. A, pretreatment with 500 nM 17-AAG or overexpression of Hsp70 reduces αsyn oligomerization as measured by the αsyn bPCA. n = 3; ∗∗∗, p < 0.001. B, cotreatment with 500 nM 17-AAG or overexpression of Hsp70 reduces αsyn oligomerization as measured by the αsyn bPCA. n = 3; ∗∗∗, p < 0.001. C, pretreatment with 500 nM 17-AAG or overexpression of Hsp70 reduces αsyn protein levels while up-regulating Hsp70 levels. D, cotreatment with 500 nM 17-AAG or overexpression of Hsp70 does not affect αsyn protein levels while up-regulating Hsp70 levels. E, intracellular αsyn protein levels are not reduced after cotreatment with 500 nM 17-AAG or cotransfection of Hsp70 as measured by αsyn ELISA. F, pretreatment with 17-AAG and coexpression of Hsp70 rescues αsyn-induced toxicity. n = 3; ∗, p < 0.05. C and D are representative of Western blots from n = 3.
Because 17-AAG and GA have limited central nervous system bioavailability and as ansamycins have the potential for problems with chemical reactivity and oral bioavailability, we next screened a novel series of highly selective synthetic Hsp90 inhibitors for their effect on αsyn oligomerization and toxicity. These compounds were discovered de novo from a library designed for targeting purine-binding proteins that, such as Hsp90, have binding sites for purine cofactors such as ATP. They represent a novel and specific chemical platform for Hsp90 inhibition, because they are unrelated to all known Hsp90 inhibitors including 17-AAG, radicicol, and the purine-based analogs (Rodina et al., 2007). This novel series of small-molecule inhibitors of Hsp90 are highly selective and do not bind to other purine-binding proteins (Barta et al., 2008; Chandarlapaty et al., 2008; Rice et al., 2008; Okawa et al., 2009).
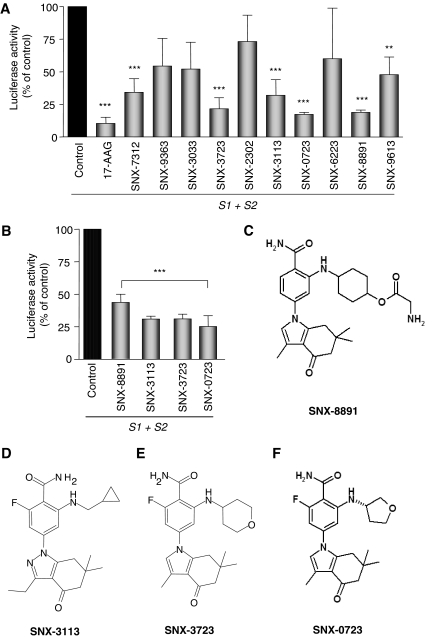
We tested a panel of 10 Hsp90 inhibitors in the αsyn PCA assay that were selected for chemical properties predicted to favor brain penetration. H4 neuroglioma cells were plated in the 96-well plate format, pretreated with 500 nM each inhibitor, and transiently transfected with S1 and S2. Twenty-four hours after transfection, cells were assayed for luciferase activity in an automated plate reader to determine the effect of the inhibitors on αsyn oligomerization as measured by reconstituted luciferase activity. It is noteworthy that a wide range of effectiveness in preventing αsyn oligomerization was observed (Fig. 3A), with several compounds preventing αsyn oligomerization by more than 75% (from 68 ± 20.52 to 82.64 ± 2.53%). Four of the most effective compounds, SNX-8891, SNX-3113, SNX-3723, and SNX-0723, were selected for further cotreatment screening whereby H4 cells were transfected with S1 and S2 and then treated with 500 nM inhibitors (Fig. 3B). All four of the inhibitors (Fig. 3, C–F) also prevented αsyn oligomerization in this treatment paradigm. As a control for all compound treatments, we determined that full-length Gaussia luciferase activity was not affected by Hsp90 inhibitor treatment (not shown).
Fig. 3.
Novel Hsp90 inhibitors prevent αsyn oligomerization and rescue toxicity. A, pretreatment prevents αsyn oligomerization as measured by decreased luciferase complementation. Of the compounds tested, six significantly reduced αsyn oligomerization. n = 3; ∗∗, p < 0.01; ∗∗∗, p < 0.001. B, cotreatment with the four most effective compounds also prevents αsyn oligomerization as measured by decreased luciferase complementation. n = 3; ∗∗∗, p < 0.001. C–F, structures of the four most effective Hsp90 inhibitors used in the study.
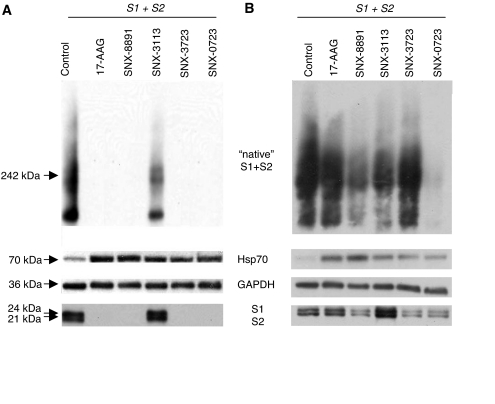
Hsp90 inhibition is thought to be therapeutically effective by specifically degrading misfolded or mutated client proteins that contribute to disease pathology. To determine whether this class of Hsp90 inhibitors was targeting αsyn for degradation, we treated S1/S2 transfected cells (pre- and cotreatment paradigms) with 17-AAG, SNX-8891, SNX-3113, SNX-3723, and SNX-0723 and examined αsyn protein levels via both native and denatured Western blot 24 h after transfection. It is noteworthy that both high-molecular-mass and monomeric forms of S1 and S2 were almost completely degraded by four of the five Hsp90 inhibitors tested after pretreatment (Fig. 4A), whereas levels of the housekeeping gene GAPDH (Fig. 4A) were intact. By contrast, cotreatment was more selective with some decrease in high-molecular-mass αsyn species evident, especially with SNX-0723 (Fig. 4B). It is noteworthy that SNX-3113 seemed to shift the αsyn species from high molecular mass to monomers, suggesting that refolding mechanisms could be at play. As expected, pre- and cotreatment with Hsp90 inhibitors also resulted in concomitant increase of Hsp70 levels by 6- to 8-fold and 2- to 3-fold, respectively (Fig. 4, A and B).
Fig. 4.
Novel Hsp90 inhibitors reduce high-molecular-mass oligomeric αsyn species. Western blots of S1- and S2-transfected H4 cells treated with various Hsp90 inhibitors. A, composite native and denatured PAGE of pretreatment with Hsp90 inhibitors. Blots were probed for αsyn (native and denatured), Hsp70 (denatured only), and GAPDH (denatured only). A reduction in higher-molecular-mass and monomeric αsyn species with a concomitant 6- to 8-fold up-regulation in Hsp70 after pretreatment with Hsp90 inhibitors was observed. B, composite native and denatured PAGE of cotreatment with Hsp90 inhibitors. Blots were probed for αsyn (native and denatured), Hsp70 (denatured only), and GAPDH (denatured only). Cotreatment resulted in some reduction in higher-molecular-mass species (SNX-8891, SNX-3113, and SNX-0723) as well as an apparent redistribution of αsyn species from higher-molecular-mass to monomeric (SNX-3113) with a concomitant 2- to 3-fold up-regulation in Hsp70 after Hsp90 inhibition. Representative blots from n = 3 of each condition.
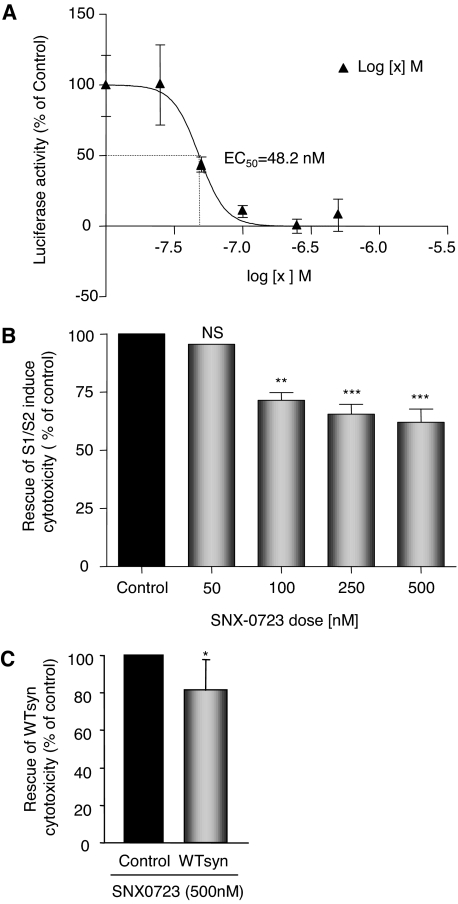
To determine whether the effect of the Hsp90 inhibitors occurred in a dose-dependent manner, we selected a lead compound, SNX-0723, which prevented αsyn oligomerization by 82.6%, and performed a dose-response experiment. H4 cells were pretreated with 10 nM to 1 μM SNX-0723 and assayed for luciferase activity 24 h after transfection. These data yielded a dose-response curve that places the EC50 for inhibition of αsyn oligomerization by SNX-0723 at approximately 48.2 nM (Fig. 5A).
Fig. 5.
SNX-0723 prevents αsyn oligomerization and rescues αsyn-induced toxicity in a dose-dependent manner. A, H4 cells were treated with 10 nM to 10 μM doses of SNX-0723 and assayed for luciferase activity 24 h after transfection. SNX-0723 prevents αsyn oligomerization in a dose-dependent manner with an EC50 of 48.2 nM. n = 4. B, SNX-0723 rescues αsyn-induced toxicity in a dose-dependent manner. n = 3; ∗∗, p < 0.01; ∗∗∗, p < 0.001. C, SNX-0723 also rescues toxicity induced by overexpression of wild-type, untagged αsyn. n = 4; ∗, p = 0.05.
To determine whether SNX-0723 treatment was simultaneously able to rescue αsyn-induced toxicity, we examined cytotoxicity levels after αsyn overexpression and SNX-0723 treatment. In agreement with SNX-0723's ability to prevent oligomerization, it was also effective in preventing αsyn-induced toxicity by up to 40% (Fig. 5B) at doses of 100 to 500 mM. It is noteworthy that SNX-0723 was also effective in preventing αsyn-induced toxicity from overexpression of wild-type, untagged αsyn (Fig. 5C).
The selectivity of SNX-0723 was profiled on over 120 different receptors, enzymes, and transporters in the CEREP BioPrint panel and 36 different kinases by use of the Invitrogen Kinase Selectivity panel. SNX-0723 showed nanomolar potency at inhibiting Hsp90 (IC50 = 14 nM), inducing Hsp70 (IC50 = 31 nM), and decreasing expression of several known Hsp90 client proteins: Human epidermal growth factor receptor 2 (IC50 = 9.4 nM), ribosomal protein s6 (pS6) (IC50 = 13 nM), and protein kinase R-like endoplasmic reticulum kinase (IC50 = 5.5 nM). At 10 μM, no inhibition above 30% was observed for any of the kinases profiled. In addition, broad receptor profiling revealed micromolar potency at the GABAA receptor (IC50 = 23 μM), the melatonin 2 receptor (IC50 = 2.4 μM), the CYP2C19 enzyme (IC50 = 53 μM); and weak binding activity was also observed for phosphodiesterase 11 (47% at 10 μM).
Pharmacokinetic and Pharmacodynamic Assessment of Hsp90 Inhibitors.
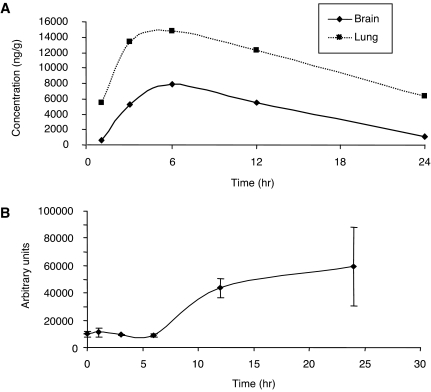
Many currently available Hsp90 inhibitors, including 17-AAG, will not be useful as therapies for neurodegenerative disorders such as Parkinson's disease because of low solubility and limited central nervous system and oral availability (Chiosis and Tao, 2006). Regardless, several studies now support Hsp90 as an important target for PD therapeutics. In vivo pharmacokinetic studies were performed to determine whether SNX-0723 has the potential to be active and brain-permeable. Rats orally dosed with 10 mg/kg SNX-0723 were found to reach maximal brain concentrations 6 h after oral dosing with almost complete clearance by 24 h (Fig. 6A). To validate that the PK properties for SNX-0723 were relevant, a pharmacodynamic evaluation was performed assessing Hsp70 induction in the brain. These studies reveal that SNX-0723 causes a 5-fold induction of Hsp70 in rat brain after a single oral dose of 10 mg/kg (Fig. 6B). These PK and pharmacodynamic data support the potency and brain permeability of this class of Hsp90 inhibitors, making them viable compounds for in vivo validation or further derivation.
Fig. 6.
SNX-0723 is absorbed by the brain and causes Hsp70 induction in vivo. A, SNX-0723 is brain-permeable. Rats orally dosed with 10 mg/kg SNX-0723 reached maximal brain concentration at 6 h after dose with almost complete clearance by 24 h. B, SNX-0723 causes a 5-fold induction of Hsp70 in the rat brain after a single oral dose of 10 mg/kg.
Discussion
Many neurodegenerative diseases such as PD, dementia with Lewy bodies, Alzheimer's disease, amyotrophic lateral sclerosis, and polyglutamine diseases are thought to be caused by misfolding and subsequent accumulation of toxic proteins (Waza et al., 2006; Fujikake et al., 2008). Direct up-regulation of HSF-1 and Hsp70, as well as pharmacological induction by Hsp90 inhibitors in cell culture and animal models, are protective against toxicity induced by pathogenic proteins, ameliorate abnormal transgenic phenotypes in fly and murine models, and suppress protein aggregate formation in several models of neurodegeneration (Dickey et al., 2005; Fujikake et al., 2008).
Hsp90 inhibitors such as 17-AAG and radicicol have been shown to have neuroprotective effects similar to direct HSF-1 and Hsp70 up-regulation (Auluck et al., 2005; Waza et al., 2006), but also have limited oral bioavailability and blood-brain barrier permeability (Chiosis and Tao, 2006; Taldone et al., 2008). 17-AAG is currently in phase II trials as an antitumor compound (Heath et al., 2005; Ramanathan et al., 2005) and is neuroprotective in preclinical models of Huntington's disease and spinocerebellar ataxias (Waza et al., 2006; Fujikake et al., 2008). Furthermore, Hsp90-CHIP complexes can target and selectively degrade phosphorylated tau-protein implicated in Alzheimer's disease (Petrucelli et al., 2004), and Hsp90 inhibitors are also neuroprotective against toxicity caused by Aβ aggregation (Ansar et al., 2007) as well as existing and induced oxidative toxicity (Xiao et al., 1999; Ouyang et al., 2005).
PD is characterized by dopaminergic cell loss in the substantia nigra and the presence of Lewy bodies composed of aggregated αsyn and heat shock proteins among other proteins. We and others have shown that Hsp90 inhibition may play an important role in PD synucleinopathies, where increasing evidence implicates a prefibrillar αsyn species as the toxic moiety, by protecting against αsyn-induced toxicity (McLean et al., 2004; Auluck et al., 2005). In this study we used a bioluminescent protein-fragment complementation assay to study prefibrillar, oligomeric αsyn species. In accord with our previously described fluorescent PCA (Outeiro et al., 2008), no macroscopic aggregates of αsyn were observed under these conditions; instead, prefibrillar, intermediate αsyn species were present. The manipulations measured by the PCA assay were therefore directed toward a range of oligomeric, preaggregate αsyn species.
Like geldanamycin, both 17-AAG and the novel Hsp90 inhibitors decreased αsyn oligomerization and toxicity. At lower doses SNX-0723 was more effective at rescuing αsyn-induced toxicity than 17-AAG (i.e., 100 nM compared with 500 nM), suggesting greater therapeutic potential. However, the data presented herein demonstrate that Hsp70 overexpression, 17-AAG treatment, and the novel Hsp90 inhibitors had a greater effect on αsyn oligomerization than on toxicity rescue. There are several possible explanations for the observed differential effects observed including the fact that other species of αsyn, or other interacting proteins, that are not directly measured by the PCA assay or affected by the inhibitors, may contribute to αsyn-induced toxicity. This suggests that multiple facets contribute to neurodegeneration in PD and raises the possibility that oligomeric αsyn may not represent the only toxic moiety. Indeed, although our own data support oligomeric species as a toxic species, they do not do so conclusively.
We have previously established that pharmacological up-regulation of Hsp70 with GA pretreatment protects against αsyn-induced toxicity and leads to degradation of higher-molecular-mass αsyn (McLean et al., 2004). In line with previous data, we demonstrate here that a novel series of brain-permeable Hsp90 inhibitors can prevent αsyn oligomerization in a dose-dependent manner and concomitantly rescue αsyn-induced toxicity by potentially refolding and/or degrading αsyn. In accord with our previously published studies, significant rescue of αsyn-induced toxicity is only observed by use of a pretreatment paradigm, even though decreased oligomerization occurs with a cotreatment paradigm. The reason for this phenomenon is unclear, but it is possible that specific species of αsyn may have to be ameliorated before significant rescue is achieved and that pretreatment achieves this more effectively than cotreatment.
Current therapies for PD relieve symptoms by restoring levels of dopamine in the brain, but with several side effects, including decreased impulse control, hallucinations, increased risk of cardiovascular side effects, and drug-induced dyskinesia after prolonged treatment (Jankovic and Stacy, 2007; Moller et al., 2008). SNX-0723 exhibits promising PK properties, including robust brain concentration and induction of Hsp70 in rat brains. It is orally available and reaches maximal brain concentrations at 6 h after oral dose, suggesting it could be taken as a therapeutic oral regimen. Although it is uncertain what the side effects of prolonged Hsp90 inhibition will be, the data here support further investigation in vivo to determine whether Hsp90 inhibition can rescue αsyn-induced cell death in animal models.
These novel compounds represent a potential alternative to existing therapeutics, which treat the symptoms of the disease rather than the underlying cause. They specifically induce Hsp70 in vitro as well as in vivo, possess a chemically distinct scaffold with good target specificity, and are orally available. They rescue αsyn-induced toxicity while preventing oligomerization, suggesting a consistent therapeutic effect that is capable of targeting the accumulation and resulting cytotoxicity associated with αsyn in PD. Taken together, these data suggest that these novel compounds may represent an exciting new neuroprotective therapeutic strategy for PD.
Acknowledgments
We thank Dr. Steven Michnick of the University of Montreal for providing the split humanized Gaussia luciferase constructs and Dr. Bakhos Tannous for providing the full-length humanized Gaussia luciferase cDNA.
This work was supported by the National Institutes of Health [Grant NS038372]; and the Michael J. Fox Foundation.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.158436.
- αsyn
- α-synuclein
- PD
- Parkinson's disease
- S1
- αsyn-hGLuc(1)
- S2
- αsyn-hGLuc(2)
- Hsp
- heat shock protein
- HSF-1
- heat shock factor 1
- GA
- geldanamycin
- 17-AAG
- 17-(allylamino)-17-demethoxygeldanamycin
- 17-DMAG
- 17-dimethylaminoethylamino-17-demethoxy-geldanamycin
- PK
- pharmacokinetic
- PAGE
- polyacrylamide gel electrophoresis
- ELISA
- enzyme-linked immunosorbent assay
- bPCA
- bioluminescent protein complementation assay
- SNX-0723
- 2-fluoro-6-[(3S)-tetrahydrofuran-3-ylamino]-4-(3,6,6-trimethyl-4-oxo-4,5,6,7-tetrahydro-1H-indol-1-yl)benzamide
- SNX-2112
- 4-[6,6-dimethyl-4-oxo-3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-indazol-1-yl]-2-[(trans-4-hydroxycyclohexyl)amino]benzamide.
References
- Ansar S, Burlison JA, Hadden MK, Yu XM, Desino KE, Bean J, Neckers L, Audus KL, Michaelis ML, Blagg BS. (2007) A non-toxic Hsp90 inhibitor protects neurons from Abeta-induced toxicity. Bioorg Med Chem Lett 17:1984–1990 [DOI] [PubMed] [Google Scholar]
- Auluck PK, Meulener MC, Bonini NM. (2005) Mechanisms of Suppression of {alpha}-Synuclein neurotoxicity by geldanamycin in Drosophila. J Biol Chem 280:2873–2878 [DOI] [PubMed] [Google Scholar]
- Barta TE, Veal JM, Rice JW, Partridge JM, Fadden RP, Ma W, Jenks M, Geng L, Hanson GJ, Huang KH, et al. (2008) Discovery of benzamide tetrahydro-4H-carbazol-4-ones as novel small molecule inhibitors of Hsp90. Bioorg Med Chem Lett 18:3517–3521 [DOI] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Ye Q, Scott A, Silinski M, Huang K, Fadden P, Partdrige J, Hall S, Steed P, et al. (2008) SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res 14:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiosis G, Tao H. (2006) Purine-scaffold Hsp90 inhibitors. IDrugs 9:778–782 [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr (2000) Acceleration of oligomerization, not fibrilization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A 97:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysyk RL, Parker RJ, Barchi JJ, Jr, Steeg PS, Hartman NR, Strong JM. (2006) Reaction of geldanamycin and C17-substituted analogues with glutathione: product identifications and pharmacological implications. Chem Res Toxicol 19:376–381 [DOI] [PubMed] [Google Scholar]
- Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. (2007) Different species of {alpha}-synuclein oligomers induce calcium influx and seeding. J Neurosci 27:9220–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. (2005) Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem 280:14733–14740 [DOI] [PubMed] [Google Scholar]
- Dickey CA, Eriksen J, Kamal A, Burrows F, Kasibhatla S, Eckman CB, Hutton M, Petrucelli L. (2005) Development of a high throughput drug screening assay for the detection of changes in tau levels—proof of concept with HSP90 inhibitors. Curr Alzheimer Res 2:231–238 [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. (2006) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J 20:419–425 [DOI] [PubMed] [Google Scholar]
- Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. (2008) Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem 283:26188–26197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath EI, Gaskins M, Pitot HC, Pili R, Tan W, Marschke R, Liu G, Hillman D, Sarkar F, Sheng S, et al. (2005) A phase II trial of 17-allylamino-17- demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin Prostate Cancer 4:138–141 [DOI] [PubMed] [Google Scholar]
- Jankovic J, Stacy M. (2007) Medical management of levodopa-associated motor complications in patients with Parkinson's disease. CNS Drugs 21:677–692 [DOI] [PubMed] [Google Scholar]
- Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. (2004) Hsp70 Reduces alpha-Synuclein aggregation and toxicity. J Biol Chem 279:25497–25502 [DOI] [PubMed] [Google Scholar]
- Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. (1998) Ala30Pro mutation in the gene encoding a-synuclein in Parkinson's disease. Nat Genet 18:106–108 [DOI] [PubMed] [Google Scholar]
- McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K, Breakefield XO, Hyman BT. (2002) TorsinA and heat shock proteins act as molecular chaperones: suppression of alpha-synuclein aggregation. J Neurochem 83:846–854 [DOI] [PubMed] [Google Scholar]
- McLean PJ, Klucken J, Shin Y, Hyman BT. (2004) Geldanamycin induces Hsp70 and prevents alpha-synuclein aggregation and toxicity in vitro. Biochem Biophys Res Commun 321:665–669 [DOI] [PubMed] [Google Scholar]
- Moller JC, Eggert KM, Unger M, Odin P, Chaudhuri KR, Oertel WH. (2008) Clinical risk-benefit assessment of dopamine agonists. Eur J Neurol 15 (Suppl 2):15–23 [DOI] [PubMed] [Google Scholar]
- Okawa Y, Hideshima T, Steed P, Vallet S, Hall S, Huang K, Rice J, Barabasz A, Foley B, Ikeda H, et al. (2009) SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood 113:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, Hyman BT, McLean PJ. (2008) Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS ONE 3:e1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Xu L, Giffard RG. (2005) Geldanamycin treatment reduces delayed CA1 damage in mouse hippocampal organotypic cultures subjected to oxygen glucose deprivation. Neurosci Lett 380:229–233 [DOI] [PubMed] [Google Scholar]
- Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, et al. (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13:703–714 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276:2045–2047 [DOI] [PubMed] [Google Scholar]
- Ramanathan RK, Trump DL, Eiseman JL, Belani CP, Agarwala SS, Zuhowski EG, Lan J, Potter DM, Ivy SP, Ramalingam S, et al. (2005) Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res 11:3385–3391 [DOI] [PubMed] [Google Scholar]
- Remy I, Michnick SW. (2006) A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods 3:977–979 [DOI] [PubMed] [Google Scholar]
- Rice JW, Veal JM, Fadden RP, Barabasz AF, Partridge JM, Barta TE, Dubois LG, Huang KH, Mabbett SR, Silinski MA, et al. (2008) Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum 58:3765–3775 [DOI] [PubMed] [Google Scholar]
- Rodina A, Vilenchik M, Moulick K, Aguirre J, Kim J, Chiang A, Litz J, Clement CC, Kang Y, She Y, et al. (2007) Selective compounds define Hsp90 as a major inhibitor of apoptosis in small-cell lung cancer. Nat Chem Biol 3:498–507 [DOI] [PubMed] [Google Scholar]
- Schulte TW, Neckers LM. (1998) The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother Pharmacol 42:273–279 [DOI] [PubMed] [Google Scholar]
- Shin Y, Klucken J, Patterson C, Hyman BT, McLean PJ. (2005) The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysosomal pathways. J Biol Chem 280:23727–23734 [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. (2003) alpha-Synuclein locus triplication causes Parkinson's disease. Science 302:841 [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840 [DOI] [PubMed] [Google Scholar]
- Taldone T, Gozman A, Maharaj R, Chiosis G. (2008) Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol 8:370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. (2005) Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther 11:435–443 [DOI] [PubMed] [Google Scholar]
- Tetzlaff JE, Putcha P, Outeiro TF, Ivanov A, Berezovska O, Hyman BT, McLean PJ. (2008) CHIP targets toxic alpha-synuclein oligomers for degradation. J Biol Chem 283:17962–17968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. (2001) Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem 276:10737–10744 [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT., Jr (2003) Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson's disease. Biochemistry 42:7871–7878 [DOI] [PubMed] [Google Scholar]
- Waza M, Adachi H, Katsuno M, Minamiyama M, Tanaka F, Sobue G. (2006) Alleviating neurodegeneration by an anticancer agent: an Hsp90 inhibitor (17-AAG). Ann N Y Acad Sci 1086:21–34 [DOI] [PubMed] [Google Scholar]
- Xiao N, Callaway CW, Lipinski CA, Hicks SD, DeFranco DB. (1999) Geldanamycin provides posttreatment protection against glutamate-induced oxidative toxicity in a mouse hippocampal cell line. J Neurochem 72:95–101 [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, et al. (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–173 [DOI] [PubMed] [Google Scholar]