Abstract
Muscarinic cholinergic receptors modulate dopaminergic function in brain pathways thought to mediate cocaine's abuse-related effects. Here, we sought to confirm and extend in the mouse species findings that nonselective muscarinic receptor antagonists can enhance cocaine's discriminative stimulus. More importantly, we tested the hypothesis that muscarinic receptor agonists with varied receptor subtype selectivity can blunt cocaine's discriminative stimulus and reinforcing effects; we hypothesized a critical role for the M1 and/or M4 receptor subtypes in this modulation. Mice were trained to discriminate cocaine from saline, or to self-administer intravenous cocaine chronically. The nonselective muscarinic antagonists scopolamine and methylscopolamine, the nonselective muscarinic agonists oxotremorine and pilocarpine, the M1/M4-preferring agonist xanomeline, the putative M1-selective agonist (4-hydroxy-2-butynyl)-1-trimethylammonium-3-chlorocarbanilate chloride (McN-A-343), and the novel M1-selective agonist 1-(1-2-methylbenzyl)-1,4-bipiperidin-4-yl)-1H benzo[d]imidazol-2(3H)-one (TBPB) were tested as substitution and/or pretreatment to cocaine. Both muscarinic antagonists partially substituted for cocaine and enhanced its discriminative stimulus. Conversely, muscarinic agonists blunted cocaine discrimination and abolished cocaine self-administration with varying effects on food-maintained behavior. Specifically, increasing selectivity for the M1 subtype (oxotremorine < xanomeline < TBPB) conferred lesser nonspecific rate-suppressing effects, with no rate suppression for TBPB. In mutant mice lacking M1 and M4 receptors, xanomeline failed to diminish cocaine discrimination while rate-decreasing effects were intact. Our data suggest that central M1 receptor activation attenuates cocaine's abuse-related effects, whereas non-M1/M4 receptors probably contribute to undesirable effects of muscarinic stimulation. These data provide the first demonstration of anticocaine effects of systemically applied, M1 receptor agonists and suggest the possibility of a new approach to pharmacotherapy for cocaine addiction.
Cocaine and other stimulant abuse is a considerable public health problem, for which no established pharmacotherapy is available. Mounting evidence suggests that cholinergic systems are implicated in abuse-related effects of cocaine and other abused drugs. The reinforcing effects of cocaine depend on dopamine systems that arise in the ventral tegmental area (VTA) and project to the nucleus accumbens (NAc; Roberts et al., 1980). Dopamine release in these pathways is regulated by cholinergic input through muscarinic receptors (Oakman et al., 1995; Blaha et al., 1996). In addition, muscarinic receptors within the striatum (including the NAc) colocalize with dopamine receptors and modulate neuronal responses to dopamine receptor activation. Specifically, M4 and D1 receptors exert directly opposing effects on cyclic AMP synthesis, whereas M1 receptors oppose the effects of D2 receptors (Di Chiara et al., 1994; Onali and Olianas, 2002). Systemic administration of muscarinic antagonists induces striatal dopamine release in humans and rats, and was found to potentiate cocaine-induced dopamine increases in rats (Dewey et al., 1993; Chapman et al., 1997; Tanda et al., 2007). Conversely, muscarinic agonists produce functional dopamine antagonism (Bymaster et al., 1998). Based on the above-mentioned interactions with dopamine receptors, the M1 and M4 receptors seem most likely to mediate this functional dopamine antagonism.
Most studies investigating the effects of cholinergic manipulations on rewarded behaviors have focused on the roles of specific brain regions, rather than on systemic pharmacological manipulations. In rats, immunotoxic destruction of cholinergic neurons in the NAc increased the potency of self-administered cocaine, whereas intra-NAc-infused oxotremorine, a muscarinic agonist, reduced cocaine self-administration (Smith et al., 2004; Mark et al., 2006). Those findings indicate that stimulation of NAc muscarinic receptors opposes the reinforcing effects of cocaine. In contrast to the NAc, the nonselective cholinergic agonist carbachol produced conditioned place preference and was self-administered when infused into the VTA (Ikemoto and Wise, 2002). Experimenter-induced elevation of acetylcholine levels in the VTA similarly reinstated lever pressing in rats trained to self-administer cocaine by a mechanism dependent on muscarinic receptors (You et al., 2008). Intra-VTA infusions of muscarinic antagonists opposed cocaine self-administration and blunted its effect on extracellular VTA dopamine (You et al., 2008). Lesions of the pedunculopontine tegmental nucleus were similarly shown to reduce amphetamine reward (Bechara and van der Kooy, 1989; Alderson et al., 2004). Thus, muscarinic receptors in the VTA and pedunculopontine tegmental nucleus seem to facilitate drug reward. Those facilitations most likely depend on M5 receptors (Forster et al., 2002; Thomsen et al., 2005), although recent studies indicate that M5 receptors may modulate effects of different drugs of abuse differentially (Schmidt et al., 2010), and other brain regions and muscarinic receptors may also facilitate abuse-related effects of drugs (Crespo et al., 2006; Carrigan and Dykstra, 2007).
Because of the opposing effects of muscarinic receptors in different brain regions, one cannot easily generalize from these studies what effects systemic administration of muscarinic ligands would have. Although studies targeting specific brain sites are important to help us understand the biology of addiction disorders, it is most likely that pharmacotherapy in humans will be given systemically. Most studies investigating the effect of systemically administered muscarinic ligands on behavioral effects of cocaine have focused on antagonists, in general, showing enhancement of cocaine's effects (Wilson and Schuster, 1973; Acri et al., 1996; Katz et al., 1999; Tanda and Katz, 2007; but see Ranaldi and Woolverton, 2002). Few reports have been published on modulation of cocaine's effects by systemically applied muscarinic agonists or acetylcholinesterase inhibitors. In a single-session tail-vein self-administration procedure in mice, muscarinic agonists decreased rates of cocaine self-administration (Rasmussen et al., 2000). However, because the pretreatments were tested against the peak dose of cocaine, it is difficult to ascertain whether this decrease in response rates reflected a leftward or rightward shift in cocaine's dose-effect function. In addition, nonspecific rate-decreasing effects of the pretreatments could have contributed to those findings. Acetylcholinesterase inhibitor treatment prevented the development of conditioned place preference to both morphine and cocaine and decreased cocaine self-administration (Hikida et al., 2003; Grasing et al., 2009). However, the clinical usefulness of acetylcholinesterase inhibitors and nonselective muscarinic agonists may be limited by opposing effects at different receptor subtypes, and by well recognized adverse effects (e.g., nausea). As a final point, hypercholinergic rats showed a blunted response to cocaine (Fagergren et al., 2005). Here, we tested the hypothesis that systemically administered muscarinic agonists would attenuate the abuse-related effects of cocaine. We further hypothesized that selectivity for M1 or M1/M4 subtypes would confer greater effectiveness and lower risk of adverse effects. We tested various muscarinic ligands, including the novel M1-selective allosteric agonist TBPB (Jones et al., 2008), in a cocaine discrimination procedure and a chronic cocaine self-administration procedure in mice.
Materials and Methods
Animals and Housing.
Male Swiss-Webster mice, male wild-type C57BL/6NTac and male M1−/−M4−/− mice were bred at Taconic Farms (Germantown, NY) and were acquired at 4 to 8 weeks of age. M1−/− and M4−/− mice were generated as described previously by use of 129S6/SvEv embryonic stem cells (Gomeza et al., 1999; Miyakawa et al., 2001) and backcrossed 11 generations to C57BL/6NTac females to produce essentially congenic mice. Double-knockout mice were bred by intercrossing the single-knockout lines and then maintained as a separate line, because of the low yield of double-knockout mice if bred by heterozygous intermating. Age- and sex-matched C57BL/6NTac mice were thus used as wild-type controls. Animals were acclimated to the housing facilities for at least 7 days before experiments were initiated. During this time they were also handled, and they were anesthetized briefly once for subcutaneous implantation of an identification microchip. Animals were kept in a 12-h light/dark cycle at ∼22°C and ∼55% humidity and were group housed up to five per cage. Water was accessible ad libitum and standard rodent chow (rodent diet 5001; PMI Feeds, Inc., St. Louis, MO) was provided once daily after training/testing sessions, 4 g/mouse/day. Rodent “treats,” nesting material, and hiding/nesting devices were provided for enrichment. Running wheels were available, although before catheter implantation, only in the self-administration groups, to avoid potential injuries caused by the protruding catheter base. All testing was conducted during the light phase of the circadian cycle. All procedures were carried out in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Operant-Conditioning Apparatus.
The same type of apparatus was used for drug discrimination and self-administration, but distinct equipment was dedicated to each assay. Operant-conditioning chambers and training and evaluation of food-maintained behavior under a fixed ratio (FR) schedule of reinforcement have previously been described in detail (Thomsen and Caine, 2005). In brief, each operant-conditioning chamber contained two nose-poke holes 10 mm above the grid floor, each equipped with a photocell and a yellow cue light. Centered between the holes was a plate into which liquid food could be delivered. For self-administration, a liquid swivel mounted on a balance arm was used for intravenous drug delivery in the freely moving animals.
Training and Evaluation in Cocaine Discrimination.
Mice were trained to discriminate 10 mg/kg cocaine from saline, administered intraperitoneally 10 min before the session. Liquid food (25 μl of Ensure protein drink, vanilla) was used as the reinforcer, with a maximum of 30 reinforcers available per 20-min session. Mice were trained initially under a FR 1 schedule, with the 10-min pretreatment time spent in the home cage, to ensure that the first nose-pokes were reinforced. The FR was then gradually increased to a final FR 10, and longer portions of the pretreatment time were spent in the operant-conditioning chamber. Final sessions were preceded by a 10-min pretreatment period in the chamber, during which all lights were off and responding had no scheduled consequences. Cocaine and saline were presented in pseudorandom order, and mice were counterbalanced with cocaine trained on the left or right hole. Criteria for stable discrimination were met when at least 7 of 8 consecutive sessions satisfied: 1) ≥10 reinforcers earned per session, 2) ≥80% correct responses for the first reinforcer, and 3) ≥90% correct total responses.
After criteria were met, mice were tested with saline and 0.32 to 18 mg/kg cocaine to generate dose-effect functions. In substitution tests, amphetamine (0.1–1.8 mg/kg i.p.), U-50488 (3.2–18 mg/kg i.p.), scopolamine (0.032–56 mg/kg i.p.), or methylscopolamine (1–56 mg/kg i.p.) was administered immediately before placing the animal in the test chamber. For drug combinations, 0.32 mg/kg scopolamine or 1 mg/kg methylscopolamine was added to cocaine solutions of each dose, and administered as substitutions. In pretreatment tests, oxotremorine (0.032–0.18 mg/kg s.c.) was administered 20 min before cocaine, pilocarpine (1–10 mg/kg s.c.) 30 min before cocaine, xanomeline (0.32–3.2 mg/kg s.c.) 15 min before cocaine, McN-A-343 (3.2–18 mg/kg s.c.) immediately before cocaine, and TBPB (18–100 mg/kg i.p.) 30 min before cocaine. For each drug including cocaine, doses were tested within-subjects according to a Latin square design. At least one training session was interspersed between each test session, and tests were only performed when mice satisfied discrimination criteria.
Training and Evaluation of Cocaine Self-Administration.
Training and evaluation of food-maintained behavior and cocaine self-administration under a FR 1 schedule have been described elsewhere (Caine et al., 2002; Thomsen and Caine, 2005). Responding in the right-sided hole resulted in delivery of a reinforcer and turning on of the cue light for 20 s during which no reinforcer could be earned (i.e., postreinforcer timeout). Cocaine solutions or saline was delivered in 0.56 ml/kg doses, e.g., for a 32-g mouse, 18 μl infused over 3.2 s. Responses in the left-sided hole were counted but had no scheduled consequences. At the start of sessions, a single noncontingent reinforcer was delivered, then the house light was turned on, and remained illuminated until the end of the session. The mice were initially trained with liquid food (Ensure, vanilla): mice were placed in the operant-conditioning chamber daily for 2-h sessions, 5 days/week. The mice were allowed at least five consecutive sessions to acquire responding, and as long as needed until criteria were met (rarely more than five sessions; criteria: ≥20 reinforcers earned per session, with <20% variation over two sessions and ≥70% active responses) After acquisition criteria were met, water was substituted for at least three sessions and until responding was extinguished to <50% of each mouse's food-maintained responding.
An indwelling catheter was then implanted into the right or left external jugular vein under oxygen/sevoflorane vapor anesthesia. The surgical procedure has been described in detail previously (Thomsen and Caine, 2005). In brief, a catheter (Silastic tubing 0.2-mm inner diameter, 0.4-mm outer diameter) was inserted 1.2 cm into the jugular vein and delicately anchored to the vein. The catheter ran subcutaneously to the base located above the midscapular region. The mice were allowed 7 days recovery, during which 0.02 ml of 0.9% saline containing heparin (30 USP units/ml) and antibiotic (cefazoline, 67 mg/ml) were infused daily through the catheter to forestall clotting and infection. After the postoperative recovery period, catheters were flushed with saline containing heparin immediately before and after self-administration sessions, and the free end of the cannula guide was kept closed at all times. Catheter patency was confirmed before initiation of cocaine self-administration and after completion of each dose-effect determination by the infusion of 0.02 to 0.03 ml of 1% brevital in saline. Loss of muscle tone and clear signs of anesthesia within 3 s of infusion indicated catheter patency.
After recovery, mice were again introduced to the operant-conditioning chambers for 3-h sessions, 5 days/week, and allowed to self-administer 1.0 mg/kg/infusion cocaine intravenously under the same FR 1 schedule as above. Criteria for stable cocaine self-administration were the same as for food, followed by saline substitution until extinction criteria were met (<50% of cocaine responding). Cocaine dose-effect functions were determined in each mouse according to a Latin square design, 0.032 to 1.0 mg/kg/infusion. Then dose-effect functions were determined again in the same manner, but with each session preceded by administration of a muscarinic agonist. In some mice, 3.2 mg/kg/infusion cocaine was tested last, without pretreatment and then with pretreatment. Finally, liquid food was again substituted as the reinforcer for two sessions, first with no pretreatment, then with the same muscarinic agonist tested with cocaine, in each mouse.
Drugs.
Cocaine hydrochloride was supplied by the National Institute on Drug Abuse (National Institutes of Health, Bethesda, MD). d-Amphetamine sulfate, scopolamine hydrobromide, (−)-scopolamine methylbromide (methylscopolamine), oxotremorine sesquifumarate, McN-A-343 and S(−)-eticlopride were purchased from Sigma-Aldrich (St. Louis, MO). Pilocarpine hydrochloride and (±)-U-50488 hydrochloride were purchased from Tocris (Ellisville, MO). TBPB was synthesized at the Vanderbilt University. Xanomeline was synthesized at the McLean Hospital according to previously published methods (Kane et al., 2008). TBPB and U-50488 were dissolved in double-deionized water, and eticlopride was dissolved in ethanol followed by dilution in sterile water (final concentration ethanol, 1%). All other drugs were dissolved in sterile 0.9% saline. All drug doses refer to the weights of the respective salts.
Data Analysis.
For the drug discrimination assay, the percentage of drug-appropriate responding (%DAR) for the whole session and total response rates (i.e., responses in both holes combined) are presented. In all cases, comparable effects were observed in %DAR for the first reinforcer (not shown). The common occurrence of missing values for %DAR because of complete suppression of behavior by the test drugs precluded the use of ANOVA on %DAR. Effects were thus analyzed by comparing A50 values in cocaine dose-effect functions with and without the test drug. For A50 calculations, the doses estimated to produce 50% DAR (substitution tests), 50% decrease in DAR (pretreatment tests) and 50% decrease in response rates (all tests), were estimated in each mouse by interpolation of the dose-effect curves, then group means and 95% confidence intervals were calculated. Effects on response rates were analyzed by repeated-measures ANOVA with drug dose as factor. For cocaine self-administration dose-effect functions, data from the first 2 h of the sessions were analyzed and presented. This time frame was chosen based on time course data obtained on the pretreatment drugs by use of a mouse operant rate assay in our laboratory. The numbers of reinforcers earned were compared by use of repeated-measures ANOVA with cocaine dose and pretreatment as factors. Significant effects were followed where appropriate by Bonferroni-corrected two-sided paired-sample t tests. Food reinforcers earned under baseline and pretreatment conditions were compared by two-sided paired-sample t test. Significance level was set at p < 0.05 before the Bonferroni correction.
For isobolographic analysis, the %DAR dose-effect functions for cocaine, scopolamine, and methylscopolamine were fitted by nonlinear regression with use of the equation E = (Emax × Ap) / (Ap + A50 p ), where E denotes the effect, A the dose, Emax the maximal effect achieved by the drug in question, A50 the dose estimated to elicit 50% of this maximal effect, and p a factor related to the slope of the curve (Hill coefficient). The individual data for all mice, rather than means, were used for curve fitting, and 95% confidence limits were also obtained. Curve fitting was executed by use of GraphPad Prism v. 4.0 for Mac. The equations were then rearranged to express dose as a function of effect: A = ((E × A50 p )/(Emax − E))1/p. Thus, the equivalent (equieffective) doses of each drug were calculated, and expected additive DAR values (with 95% confidence limits) were computed by adding the dose of cocaine equivalent to 0.32 mg/kg scopolamine, or 1 mg/kg methylscopolamine, to each dose of cocaine tested, and entering this total dose into the cocaine equation. The 95% confidence limits for each drug (i.e., cocaine and either scopolamine or methylscopolamine) were obtained, but only the largest (most conservative) interval for each data point are reported for brevity.
Results
Baseline Cocaine Discrimination Behavior and Control Tests.
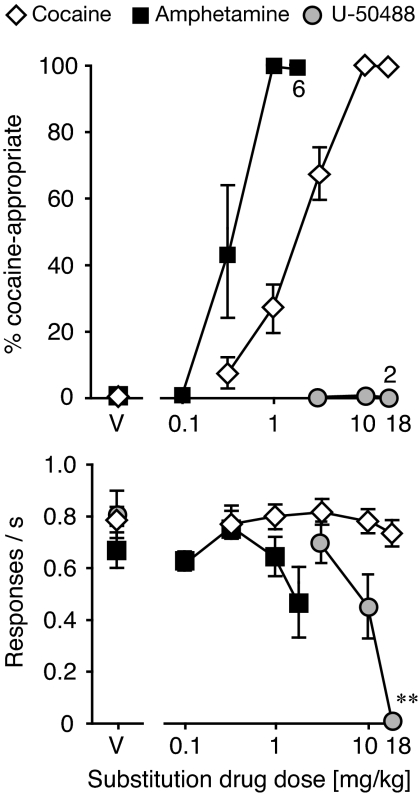
Swiss-Webster mice met criteria for cocaine discrimination after on average 15.1 ± 1.3 weeks (range, 6–31 weeks). Figure 1 shows cocaine dose-effect determinations in all mice from which data are reported in the present investigation. Cocaine produced DAR in a dose-dependent manner, reaching 100% in all mice. Positive and negative controls were established with the psychostimulant amphetamine and the κ-agonist U-50488, respectively, as shown in Fig. 1 and Table 1. Amphetamine reached 100% DAR in all mice, whereas no appreciable DAR was observed after U-50488 treatment up to a dose that almost eliminated responding (peak DAR in any subject, 3%). Table 1 shows the doses estimated to produce 50% DAR and 50% reduction in response rate (relative to saline) for each drug.
Fig. 1.
Effects of cocaine, d-amphetamine, and the κ-agonist U-50488 in mice trained to discriminate cocaine from saline. Abscissae, drug dose in mg/kg; “V” indicates vehicle. Ordinates, percentage cocaine-appropriate responses (top), response rate in responses per second (bottom). Data are groups means ± S.E.M. Groups sizes: cocaine, n = 30; amphetamine, n = 7; U-50488, n = 6. In the top, exceptions to these group sizes are indicated when some mice failed to respond at the highest drug doses. **, p < 0.01 versus vehicle.
TABLE 1.
Substitutions
| Pretreatment (dose) | A50 DAR | n/n | A50 Rate Reduction | n/n |
|---|---|---|---|---|
| Cocaine | 1.96 (1.46–2.64) | 29/29 | Not calculated | 4/29 |
| Amphetamine | 0.33 (0.22–0.54) | 7/7 | Not calculated | 3/7 |
| U-50488 | Not applicable | 0/6 | 9.32 (6.25–13.90) | 6/6 |
| Scopolamine | 0.90 (0.29–2.76) | 4/8 | Not calculated | 3/8 |
| Methylscopolamine | 4.46 (2.95–14.15) | 6/8 | 33.13 (20.34–53.95) | 5/8 |
n/n indicates the number of mice showing ≥50% reduction in %DAR and ≥50% reduction in response rates, respectively, over the number of mice tested.
Effects of Muscarinic Receptor Antagonists in the Cocaine Discrimination Assay.
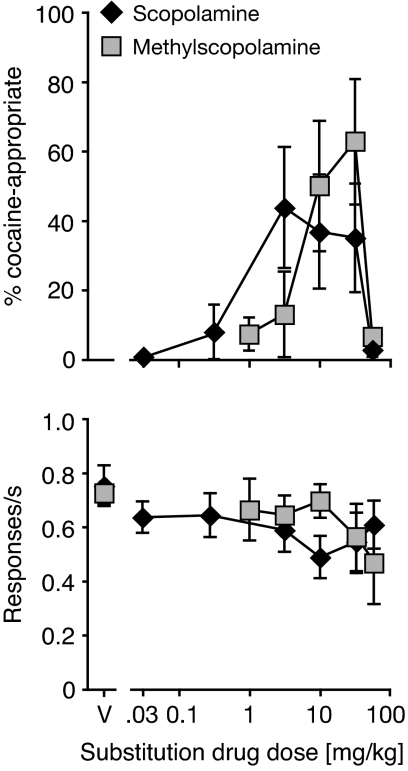
As shown in Fig. 2, scopolamine and methylscopolamine both substituted partially for cocaine (scopolamine peak: 44 ± 17% DAR at 3.2 mg/kg; methylscopolamine peak: 63 ± 18% at 32 mg/kg; mean ± S.E.M.). Methylscopolamine was significantly less potent than scopolamine (see Table 1), consistent with its poorer penetration of the blood-brain barrier. Neither drug significantly affected response rate but 56 mg/kg methylscopolamine resulted in the death of the first mice tested, and evaluation of this dose was therefore discontinued.
Fig. 2.
Substitution of the muscarinic receptor antagonists scopolamine or methylscopolamine. Abscissae, drug dose in mg/kg; “V” indicates vehicle. Ordinates, percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are group means ± S.E.M.; n = 8, with the exception of 56 mg/kg methylscopolamine, n = 5.
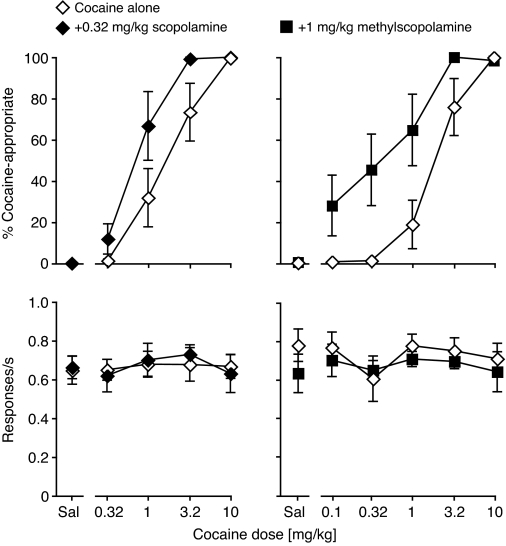
Figure 3 shows that the addition of 0.32 mg/kg scopolamine to cocaine produced a moderate leftward shift in the dose-effect curve, which was significant based on nonoverlapping 95% confidence intervals for cocaine's A50 values: cocaine alone, 1.95 (1.19–3.19) mg/kg; cocaine + scopolamine, 0.64 (0.42–0.97) mg/kg. Scopolamine alone at 0.32 mg/kg did not engender any DAR (0–1% in individual mice). Response rate was not significantly affected by either cocaine or scopolamine dose. The addition of 1.0 mg/kg methylscopolamine produced a more pronounced leftward shift, so that a lower cocaine dose was added to enable A50 calculations (0.1 mg/kg, tested last). The effect of methylscopolamine addition was significant based on nonoverlapping 95% confidence intervals for cocaine's A50 values: cocaine alone, 1.91 (1.12–3.26) mg/kg; cocaine + methylscopolamine, 0.38 (0.18–0.83) mg/kg. Methylscopolamine alone at 1 mg/kg did not engender any appreciable DAR (0–3% in individual mice). Response rate was not significantly affected by either cocaine or methylscopolamine dose.
Fig. 3.
Combinations of cocaine with scopolamine or methylscopolamine. Abscissae, cocaine dose in mg/kg; “Sal” indicates saline. Ordinates, percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are group means ± S.E.M. Group sizes: cocaine/scopolamine, n = 9; cocaine/methylscopolamine, n = 8.
Table 2 shows the results of isobolographic analysis of the cocaine + scopolamine and the cocaine + methylscopolamine combinations. The calculated dose of cocaine equivalent to 0.32 mg/kg scopolamine was 0.409 mg/kg, 95% confidence limits of 0.003 to 1.207 mg/kg. The calculated dose of cocaine equivalent to 1 mg/kg methylscopolamine was 0.431 mg/kg, 95% confidence limits of 0.007 to 0.754 mg/kg. In both cases, the expected dose-effect curve if simple additivity was assumed did not differ significantly from the experimentally determined cocaine-alone curves (data not shown). In contrast, comparison of cocaine alone with the experimentally determined drug combinations indicated that the combination was significantly more potent than expected for additivity, at least for portions of the curve (see Table 2).
TABLE 2.
Predicted additive and experimentally determined dose of cocaine needed to produce a given effect level in combination with 0.32 mg/kg scopolamine or 1 mg/kg methylscopolamine
The doses of cocaine needed to produce 30 to 95%DAR in the mouse discrimination assay were calculated from nonlinear curve fitting of the dose-effect functions, both as predicted if cocaine/scopolamine and cocaine/methylscopolamine combinations were additive, and calculated directly from the experimentally determined drug mixture dose-effect functions. Both antagonists enhanced the discriminative stimulus of cocaine in a more-than-additive manner at some effect levels.
| Effect Level | Cocaine/Scopolamine
Mixture |
Cocaine/Methylscopolamine
Mixture |
||
|---|---|---|---|---|
| Predicted Additive | Actual | Predicted Additive | Actual | |
| 30 | 0.88 (0.5–1.36) | 0.53 (0.35–0.71) | 1.14 (0.78–1.56) | 0.15 (0.06–0.30) a |
| 40 | 1.25 (0.79–1.90) | 0.63 (0.44–0.86) | 1.61 (1.35–2.59) | 0.24 (0.10–0.49) a |
| 50 | 1.65 (1.07–2.50) | 0.75 (0.54–1.03) a | 1.66 (1.39–2.66) | 0.36 (0.17–0.77) a |
| 80 | 3.66 (2.32–6.02) | 1.32 (0.95–1.90) a | 3.52 (2.45–5.11) | 1.54 (0.68–4.15) |
| 95 | 8.90 (4.11–10.0) | 2.50 (1.36–3.98) a | 8.90 (4.11–10.0) | 7.77 (1.55–10.0) |
Nonoverlapping 95% confidence limit relative to predicted additive. All doses calculated in mg/kg; effect levels are in %DAR.
Effects of Muscarinic Receptor Agonists in the Cocaine Discrimination Assay.
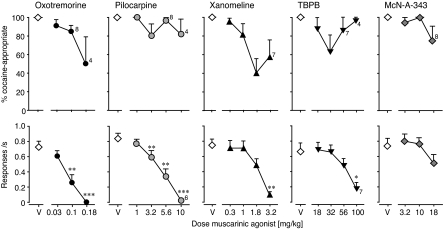
Figure 4 shows the effects on %DAR (top) and response rate (bottom) of pretreatment with the nonselective muscarinic agonists pilocarpine and oxotremorine, the M1/M4-preferring agonist xanomeline, the novel M1-selective agonist TBPB, and the non-brain penetrant M1 agonist McN-A-343.
Fig. 4.
Effects of various muscarinic receptor agonists as pretreatment to 10 mg/kg cocaine. Abscissae, muscarinic agonist dose in mg/kg; “V” indicates vehicle. Ordinates, percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± S.E.M. Group sizes: TBPB, n = 8. all other, n = 9. Exceptions to these group sizes are indicated when some mice failed to respond at a particular drug dose and were not tested on a higher dose, when applicable. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus saline.
Pilocarpine had little effect on %DAR but decreased response rates (F4,32 = 85.9, p < 0.0001). Oxotremorine produced a 50% decrease in DAR, but also suppressed response rates (F3,24 = 30.9, p < 0.0001), so that only four mice emitted responses at the highest dose. Of those, two emitted responses only in the cocaine-appropriate hole, and two emitted responses only in the saline-appropriate hole. Potencies for suppression of DAR and response rates are shown in Table 3, significance of post hoc tests on rate are shown in Fig. 4. Xanomeline decreased DAR by up to 60% (peak effect at 1.8 mg/kg), but also suppressed response rates as a function of dose (F4,32 = 21.5, p < 0.0001). TBPB decreased DAR by 37% at 32 mg/kg, whereas higher doses had little effect on DAR. Response rates decreased with dose of TBPB (F4,28 = 9.7, p < 0.0001). McN-A-343 had little effect on DAR, because only two of nine mice showed decreases at the highest dose. Pretreatment to a range of cocaine doses in those two mice were not indicative of rightward shifts; instead, this pretreatment produced mixed results more consistent with a masking effect or loss of stimulus control (data not shown). Although doses up to 18 mg/kg did not affect response rates significantly, higher doses were not tested because a pilot experiment with 32 mg/kg resulted in lack of responding and/or death in two of four mice tested.
TABLE 3.
Pretreatment-induced decreases in DAR and rate, with 10 mg/kg cocaine
The dose of each pretreatment drug estimated to produce 50% reduction in DAR, and the dose estimated to produce 50% reduction in response rate (relative to vehicle) are shown as group mean (95% confidence interval) of values calculated in each mouse. n/n indicates the number of mice showing ≥50% reduction in DAR and ≥50% reduction in response rates, respectively, over number of mice tested (the first n thus represents the number of mice from which A50 values were calculated). Means were not calculated when an A50 could be calculated in less than half the mice tested.
| Pretreatment Drug | A50 DAR Reduction | n/n (DAR) | A50 Rate Reduction | n/n (Rate) |
|---|---|---|---|---|
| Oxotremorine | Not calculated | 3/9 | 0.07 (0.05–0.10) | 9/9 |
| Pilocarpine | Not calculated | 2/9 | 4.51 (3.34–6.09) | 9/9 |
| Xanomeline | 1.08 (0.78–1.50) | 6/9 | 1.98 (1.59–2.45) | 8/9 |
| TBPB | Not calculated | 3/9 | 67.73 (55.27–83.01) | 7/8 |
| McN-A-343 | Not calculated | 2/9 | Not calculated | 2/8 |
| Eticlopride | 0.05 (0.02–0.10) | 6/9 | 0.11 (0.07–0.18) | 9/9 |
We then assessed whether pretreatment with 0.1 mg/kg oxotremorine, 1.0 or 1.8 mg/kg xanomeline, or 32 mg/kg TBPB could produce a rightward shift in cocaine's %DAR dose-effect function. Figure 5 shows cocaine dose-effect functions with and without pretreatment (within-subjects). Oxotremorine produced a 3-fold rightward shift, which was not statistically significant based on overlapping 95% confidence intervals (see Table 4). However this dose of oxotremorine suppressed response rates (F1,35 = 58.9, p < 0.0001) (no significant effect of cocaine dose, no interaction; see Fig. 5 for significant effects post hoc). Xanomeline produced a significant 5- to 7-fold shift in the cocaine DAR curve. Response rates were also decreased by both 1.0 mg/kg xanomeline (F1,25 = 16.1, p < 0.001) and 1.8 mg/kg xanomeline (F1,35 = 47.5, p < 0.0001), although decreases reached significance post hoc only at the 1.8 mg/kg pretreatment dose. TBPB produced a comparable rightward shift but did not affect response rates.
Fig. 5.
Effects of muscarinic agonist pretreatments on the cocaine dose-effect function. Abscissae, cocaine dose in mg/kg; “Sal” indicates saline. Ordinates, percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± S.E.M. Group sizes: oxotremorine and TBPB, n = 8; xanomeline, n = 9. Exceptions to these group sizes are indicated when some mice failed to respond at a particular drug dose. *, p < 0.05; **, p < 0.01 versus cocaine alone.
TABLE 4.
Pretreatment-induced cocaine dose-effect function shifts
The dose of cocaine estimated to produce 50% DAR, as group mean of values calculated in each mouse (95% confidence interval), was significantly higher after all but the oxotremorine treatment, which produced a smaller, nonsignificant rightward shift. Fold shift indicates the A50 after pretreatment divided by the baseline A50, as group mean and 95% confidence interval of shifts calculated in each mouse.
| Pretreatment (dose) | A50 Cocaine Alone | A50 Pretreatment | Fold Shift | Mean Rate Decrease |
|---|---|---|---|---|
| Oxotremorine (0.032) | 2.06 (1.30–3.24) | 4.92 (2.96–8.16) | 3.4 (1.1–5.8) | −53% (−68 to −39%) |
| Xanomeline (1.0) | 1.22 (0.61–2.44) | 5.76 (3.36–9.88) a | 5.5 (2.8–8.3) | −28% (−45 to −10%) |
| Xanomeline (1.8) | 1.16 (0.69–1.94) | 5.32 (2.86–9.90) a | 7.0 (2.3–11.7) | −51% (−67 to −35%) |
| TBPB (32) | 1.43 (0.86–2.37) | 7.30 (4.04–13.20) a | 6.7 (4.3–9.1) | +6% (−15 to +5%) |
| Eticlopride (0.032) | 1.10 (0.78–1.74) | 4.39 (2.70–7.13) a | 5.4 (0.9–9.8) | −4% (−9 to +17%) |
| Eticlopride (0.1) | 1.10 (0.78–1.74) | 4.45 (2.15–9.21) a | 4.3 (2.6–6.1) | −70% (−87 to −53%) |
Nonoverlapping 95% confidence intervals relative to cocaine alone.
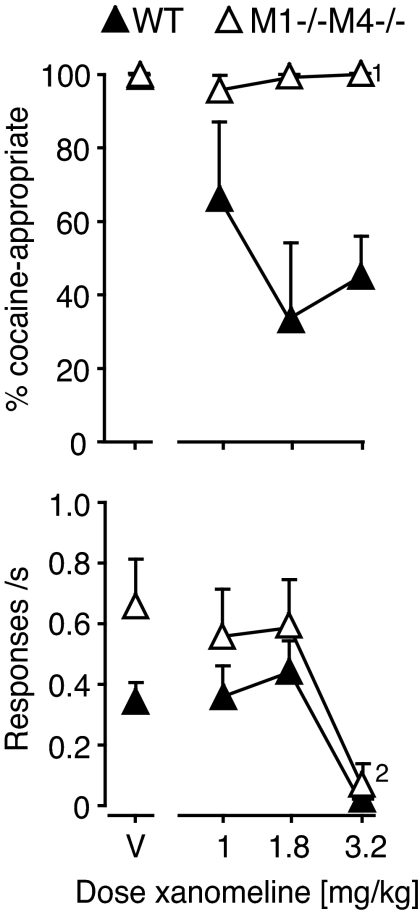
To test the hypothesis that the muscarinic agonists attenuated cocaine's discriminative stimulus by a M1 and/or M4 receptor-dependent mechanism, we tested xanomeline (1.0, 1.8, and 3.2 mg/kg) in knockout mice lacking M1 and M4 receptors. Both wild-type C57BL/6Ntac mice and M1−/−M4−/− mice acquired cocaine discrimination (DAR under saline test, mean ± S.E.M.: wild-type and M1−/−M4−/−, respectively: 1.2 ± 0.6%, 0.5 ± 0.5%; 10 mg/kg cocaine test 99.1 ± 0.3%, 99.4 ± 0.4%; n = 4–9). In the wild-type mice xanomeline decreased DAR similarly to its effects in Swiss-Webster mice, but in the M1−/−M4−/− mice xanomeline had no effect on DAR (Fig. 6). However xanomeline showed similar rate-suppressing effect in wild-type mice [(F3,15 = 10.4, p < 0.001); A50 with 95% confidence limits: 2.36 mg/kg (2.22–2.50)], and in M1−/−M4−/− mice [(F3,6 > 100, p < 0.0001); A50 2.48 mg/kg (2.36–2.61)].
Fig. 6.
Effects of xanomeline pretreatment in M1−/−M4−/− and wild-type mice. Abscissae, xanomeline dose in mg/kg; “V” indicates vehicle. Ordinates, percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are group means ± S.E.M. Group sizes: M1−/−M4−/−, n = 3; wild-type, n = 6. Exceptions to these group sizes are indicated when some mice failed to respond at a particular drug dose and were not tested on a higher dose.
We also tested, in Swiss-Webster mice, whether the cocaine-like stimulus produced by a muscarinic antagonist (methylscopolamine) could be blocked by muscarinic agonist pretreatments (oxotremorine, TBPB; Fig. 7). Oxotremorine produced a rightward shift in DAR, and profoundly decreased response rates (F1,28 > 100, p < 0.001). Post hoc comparisons indicated significant reductions in rate for all methylscopolamine doses (p < 0.05 to p < 0.001 versus methylscopolamine alone), with a trend for saline (p = 0.06). The mean decrease in response rates was −78% (95% confidence limits: −91 to −66%). TBPB decreased DAR across methylscopolamine doses (only one mouse emitted >10% DAR in the pretreatment condition), but did not affect response rates significantly. Meaningful mean A50 values could not be estimated in the pretreatment conditions because of numerous missing values (i.e., failure to respond) in the case of oxotremorine, and because of failure to reach 50% DAR in the case of TBPB.
Fig. 7.
Effects of muscarinic agonist pretreatments in methylscopolamine substitution. Abscissae, methylscopolamine dose in mg/kg; “Sal” indicates saline. Ordinates, percentage of cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± S.E.M. Group sizes (Swiss-Webster mice): oxotremorine, n = 8; TBPB, n = 6. Exceptions to these group sizes are indicated when some mice failed to respond at a particular drug dose. *, p < 0.05; ***, p < 0.001 versus methylscopolamine alone.
Comparison with Dopamine Antagonist Pretreatment in the Cocaine Discrimination Assay.
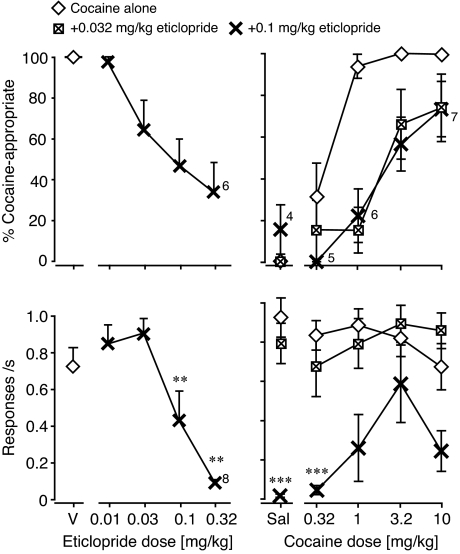
As a positive control for the pretreatment effects, we tested the dopamine D2 antagonist eticlopride as pretreatment to cocaine (Fig. 8). Eticlopride decreased DAR by up to 75%, and decreased response rates profoundly in the same dose range (F5,40 = 26.0, p < 0.0001).
Fig. 8.
Effects of dopamine D2 receptor antagonist pretreatment to cocaine. Abscissae, eticlopride dose in mg/kg (left, “V” indicates pretreatment vehicle; all as pretreatment to 10 mg/kg cocaine), cocaine dose in mg/kg (right, “Sal” indicates saline). Ordinates, percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± S.E.M. Group sizes (Swiss-Webster mice): eticlopride dose-effect and 0.032 mg/kg, n = 9; eticlopride 0.1 mg/kg, n = 8. Exceptions to these group sizes are indicated when some mice failed to respond at a particular drug dose and were not tested on a higher dose, when applicable. **, p < 0.01; ***, p < 0.001 versus cocaine alone.
We then evaluated eticlopride pretreatment, 0.032 and 0.1 mg/kg, over the range of cocaine doses. Both doses of eticlopride produced significant 4- or 5-fold rightward shifts in the DAR curve (see also Table 4). Although the lower dose did not decrease response rate significantly, 0.1 mg/kg did (F1,35 = 68.3, p < 0.0001), with a significant eticlopride by cocaine interaction (F4,35 = 2.8, p < 0.05).
Effects of Muscarinic Receptor Agonists in Cocaine Self-Administration.
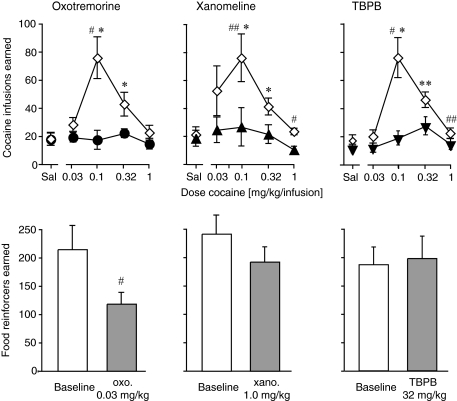
We also tested muscarinic agonists in mice that intravenously self-administered cocaine chronically. Under baseline conditions, cocaine was self-administered in all three groups of mice with inverted U-formed dose-effect functions typical for the FR 1 schedule of reinforcement. This was confirmed by significant effects of cocaine dose [oxotremorine group (F4,20 = 12.0, p < 0.0001), xanomeline group (F4,28 = 6.0, p < 0.01), TBPB group (F4,28 = 15.3, p < 0.0001)]. In each group, 0.1 and 0.32 mg/kg/infusion cocaine were self-administered above saline levels (p < 0.05–0.01 versus saline; Fig. 9, top). Pretreatment with oxotremorine (0.032 mg/kg), xanomeline (1 mg/kg), or TBPB (32 mg/kg) each abolished cocaine self-administration. Repeated-measures ANOVA confirmed a significant effect of treatment for oxotremorine (F1,5 = 15.1, p < 0.05), xanomeline (F1,7 = 35.8, p < 0.001), and TBPB (F1,7 = 41.7, p < 0.001), as well as significant cocaine dose by pretreatment interactions [(F4,20 = 14.7, p < 0.0001), (F4,28 = 5.3, p < 0.01), and (F4,28 = 9.8, p < 0.0001), respectively]. After agonist pretreatment cocaine did not maintain significant self-administration at any dose.
Fig. 9.
Effects of muscarinic agonist pretreatments in cocaine intravenous self-administration and food-maintained operant behavior. Abscissae, cocaine dose in mg/kg/infusion (top, “Sal” indicates saline); treatment (bottom). Ordinates, number of cocaine infusions earned in 2 h (top); number of food reinforcers earned in 2 h (bottom). Data are group means ± S.E.M. Group sizes (Swiss-Webster mice): oxotremorine, n = 6; xanomeline and TBPB, n = 8. *, p < 0.05; **, p < 0.01 versus saline; #, p < 0.05; ##, p < 0.01 versus baseline.
For comparison, the same mice were tested by use of the same operant procedure reinforced with a palatable liquid food instead of cocaine (Fig. 9, bottom). Oxotremorine decreased food-reinforced responding (p < 0.05), although the magnitude of effect was smaller than for cocaine. Xanomeline produced a small, nonsignificant decrease in food-reinforced behavior (p = 0.15), and TBPB did not affect food-reinforced behavior (p > 0.7). Oxotremorine was also tested at doses of 0.032 and 0.1 mg/kg as pretreatment to a full range of liquid food dilutions in water, using the same methods (see Supplemental Fig. 1). Oxotremorine produced parallel downward shifts in the food concentration-effect curves. ANOVA with food concentration and dose of oxotremorine as repeated-measures factors confirmed an effect of food concentration (F4,24 = 36.3, p < 0.0001) and dose of oxotremorine (F2,12 = 18.5, p < 0.001), with no interaction.
Discussion
We examined muscarinic modulation of the discriminative and reinforcing effects of cocaine in mice. Swiss-Webster mice readily acquired and maintained cocaine discrimination in a standard two-manipulandum procedure. Control experiments with amphetamine and a κ-agonist confirmed pharmacological specificity of the training stimulus. We found that muscarinic antagonists enhanced, and muscarinic agonists decreased, the discriminative stimulus of cocaine in a manner consistent with involvement of M1 receptors.
Our findings with scopolamine and methylscopolamine confirm and extend previous studies in rats, in which atropine and scopolamine produced leftward shifts in cocaine's discriminative stimulus (Acri et al., 1996; Katz et al., 1999; Tanda and Katz, 2007). In male Sprague-Dawley rats trained to discriminate 10 mg/kg cocaine from saline, muscarinic antagonists produced little or no cocaine-appropriate responding (Katz et al., 1999; Tanda and Katz, 2007), or partial substitution comparable with the present findings (Acri et al., 1996). Thus, rather than a species difference, it seems that subtle differences using comparable assays (e.g., behavioral or pharmacological history of the subjects) can modify the response to muscarinic antagonists. This is consistent with a weak cocaine-like discriminative stimulus, or a stimulus only partially overlapping with cocaine. Tanda and Katz (2007) showed similar leftward shifts in cocaine discrimination with the M1- or M1/M4-preferring antagonists telenzepine and trihexyphenidyl, and suggested that M1 receptors mediated at least part of those “cocaine-enhancing” effects. Telenzepine and trihexyphenidyl also increased cocaine-induced increases in extracellular NAc dopamine, providing some clues as to the mechanism of this modulation (Tanda et al., 2007). Consistent with this finding, the lower potency of methylscopolamine relative to scopolamine in the present investigation suggests a centrally mediated cocaine-like stimulus of the muscarinic antagonists. Our data also suggested a more-than-additive effect of combining muscarinic antagonists with cocaine. This is consistent with observations of increased effects of cocaine in the absence of appreciable effects of the antagonists alone in some previous studies (Katz et al., 1999; Tanda and Katz, 2007; Tanda et al., 2007).
To the best of our knowledge, modulation of cocaine's discriminative stimulus by muscarinic agonists has not been described previously. In the present study, oxotremorine produced some reduction in DAR, but only at doses that dramatically decreased response rates. Pilocarpine seemed to have little effect on DAR up to doses that eliminated responding. The rate-suppressing effects of both drugs make it difficult to evaluate any effect on DAR, so that the apparent difference in effects between oxotremorine and pilocarpine may not be reproducible or biologically meaningful. Alternatively, the observed results could reflect differences between the two drugs' relative efficacies to activate muscarinic receptor subtypes, as several investigations indicated (e.g., Leiber et al., 1990). The putative non-brain-penetrant M1 agonist McN-A-343 had little effect on DAR, up to doses approaching toxicity. The selectivity of McN-A-343 has been debated, but recent data using muscarinic M1−/− mice supported the notion that McN-A-343 is functionally selective for the M1 receptor in vivo (Hardouin et al., 2002; Kremin et al., 2006). McN-A-343 is a quaternary ammonium structure, thought to penetrate the blood-brain barrier poorly (Walland et al., 1997). Although it cannot be excluded that higher doses may have affected DAR, a pilot experiment indicated that a quarter log increase above the highest dose tested would be lethal in some mice, which is consistent with observations in guinea pigs (Walland et al., 1997). Thus, as with the muscarinic antagonists, the relative lack of effect of McN-A-343 on DAR is consistent with a centrally mediated effect of the muscarinic agonists on cocaine discrimination.
The M1/M4-preferring agonist xanomeline produced clear shifts in the cocaine discrimination curve, at doses that produced low to moderate rate suppression. The novel M1-selective agonist TBPB produced a comparable rightward shift at a dose that did not affect response rate. Thus, M1 receptor stimulation seems sufficient to attenuate cocaine's discriminative stimulus. This finding is also in agreement with the recent finding that TBPB can attenuate amphetamine-induced locomotor stimulation (Jones et al., 2008). It is noteworthy that TBPB lost its effectiveness at higher doses in the present study, presumably because of some antagonist activity at muscarinic non-M1 receptors (unpublished observations), suggesting a possible contribution of other subtypes in “anticocaine” effects. Further supporting the role of M1/M4 receptors in mediating these “anticocaine” effects, xanomeline failed to decrease DAR in the M1−/−M4−/− double-knockout mice up to a dose that nearly eliminated responding. It is noteworthy that xanomeline decreased response rates comparably in both genotypes, indicating that rate-decreasing effects were mediated through non-M1/M4 muscarinic receptors. Xanomeline has moderate functional selectivity for M1/M4 subtypes over M2/M3 subtypes, which are the primary subtypes in peripheral tissues (Shannon et al., 1994; Eglen, 2006). We speculate that M2 and/or M3 receptor stimulation accounts for most of the rate suppression observed with less selective muscarinic agonists, and that selective M1 or M1/M4 agonists may have a low incidence of side effects typically associated with nonselective muscarinic agonists in humans.
For comparison with the muscarinic agonists, we tested the dopamine D2 receptor antagonist eticlopride in the cocaine discrimination assay. Eticlopride dose-dependently reduced DAR and produced rightward shifts in the cocaine dose-effect function, as seen previously in monkeys (Spealman, 1996). The magnitude of shift in cocaine DAR was comparable with that obtained with xanomeline and TBPB, up to a dose of eticlopride that almost eliminated responding when tested alone. Some mutual antagonism was apparent, as might be expected, as increasing doses of cocaine attenuated the rate-suppressing effects of eticlopride, up to a certain point. To our knowledge, the muscarinic agonists therefore produced as large an effect in the cocaine discrimination assay as any other pharmacological manipulation reported. We also verified that muscarinic agonists could attenuate the cocaine-like stimulus induced by a muscarinic antagonist, suggesting a common (reciprocal) mechanism of action for muscarinic manipulations enhancing and antagonizing the discriminative stimulus of cocaine, such as modulation of cocaine-induced increases in extracellular dopamine (see above, and Tanda and Katz, 2007).
In addition to cocaine's discriminative stimulus, we assessed whether muscarinic agonists could blunt the reinforcing effects of cocaine by testing them in mice that intravenously self-administered cocaine chronically. We found that pretreatment with oxotremorine, xanomeline, or TBPB abolished cocaine self-administration. Thus, the muscarinic agonists blocked the reinforcing effects of cocaine more completely than they affected its discriminative stimulus. Although oxotremorine also decreased food-maintained responding, xanomeline had little or no effect on food-maintained behavior and TBPB had no effect on food-maintained behavior under the conditions studied. Although the difference in baseline rates of responding maintained by food somewhat limits the conclusions that can be drawn from this comparison, experiments with lower food reinforcer magnitudes suggested that oxotremorine's effects on operant behavior did not depend on ongoing rates of responding. In a previous report, intra-NAc-infused oxotremorine reduced cocaine self-administration in rats but had no appreciable effect on food-maintained behavior (Mark et al., 2006). This effect was blocked by the M1-preferring antagonist pirenzepine (Mark et al., 2006). Our findings extend those results in that systemically administered oxotremorine similarly reduced cocaine self-administration, but also produced nonspecific rate suppression. Taken together, previous and present findings suggest two things. First, stimulation of M1 receptors in the NAc (with or without participation of M4 receptors) accounts for most or all of the muscarinic agonist-induced suppression of abuse-related effects of cocaine. Second, these effects can be observed in the absence of undesirable nonspecific effects by use of M1-selective ligands.
TBPB has moderate affinity for D2 receptors (Jones et al., 2008), raising the concern that its effects might result from D2 receptor blockade. However, 100 mg/kg TBPB showed no D2 occupancy in a positron emission tomography study in rats (Jones et al., 2008), and oxotremorine and xanomeline have no or low dopamine receptor affinities (Burt et al., 1975; Shannon et al., 1994). Furthermore, results of the knockout experiment are inconsistent with a role of D2 receptors in the observed effects of the muscarinic agonists. It is noteworthy that the modulation of cocaine self-administration by muscarinic agonists is qualitatively different from the effects of dopamine antagonists, which produce reciprocal and surmountable antagonism, manifested by rightward shifts in cocaine dose-effect functions in mice (Caine et al., 2002). Here we saw no increase in self-administration of high cocaine doses, even when 3.2 mg/kg/infusion cocaine was tested in some mice (data not shown). Taken together, those findings indicate that direct dopamine receptor antagonism did not significantly contribute to the muscarinic agonist modulation of cocaine's behavioral effects. Furthermore, our data suggest that muscarinic agonists modulate abuse-related effects of cocaine in a more complex fashion than simply through functional dopamine antagonism.
In summary, we extended previous findings that muscarinic antagonists can produce partial substitution in animals trained to discriminate cocaine from saline and can synergistically enhance the discriminative stimulus of cocaine, and that these effects are most likely mediated through central muscarinic receptors. More importantly, we found that muscarinic agonists can blunt the discriminative stimulus of cocaine and abolish cocaine self-administration behavior, and that these effects are probably mediated in part or completely through striatal M1 receptors. Finally, we found that the M1-selective agonist TBPB produced no suppression of food-maintained operant behaviors, suggesting that novel, highly M1-selective agonists may have a low incidence of adverse effects in humans. These findings collectively raise the possibility of an entirely new approach to pharmacological treatments for cocaine addiction. Further studies are warranted to establish whether those “anticocaine” effects of M1/M4 agonists can be generalized to other strains, species, schedules of reinforcement, and, importantly, chronic treatments. Future studies may also better elucidate the involvement of each receptor subtype in both muscarinic agonist-induced blunting of cocaine's discriminative stimulus and reinforcing effects (e.g., M1, M4), and in undesirable effects of muscarinic agonists (e.g., M2, M3).
Supplementary Material
Acknowledgments
We thank Jennifer Dohrmann and Dana Angood for technical assistance and Dr. Carrie K. Jones for help with coordination between sites.
This work was supported in part by the Intramural Research Program of the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; the National Institutes of Health National Institute on Drug Abuse [Grants DA007252, DA-012142]; the National Institutes of Health National Institute of Mental Health [Grants MH073676, MH-082867]; and by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award and a Eleanor and Miles Shore/Harvard Medical School Fellowship (to M.T.).
Presented in part at the annual meeting of the International Study Group Investigating Drugs as Reinforcers, Reno, Nevada, June 20, 2009.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.162057.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- VTA
- ventral tegmental area
- FR
- fixed ratio
- DAR
- drug-appropriate responding
- NAc
- nucleus accumbens
- McN-A-343
- (4-hydroxy-2-butynyl)-1-trimethylammonium-3-chlorocarbanilate chloride
- (±)-U-50488
- trans-(±)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide
- TBPB
- 1-(1-2-methylbenzyl)-1,4-bipiperidin-4-yl)-1H benzo[d]imidazol-2(3H)-one
- ANOVA
- analysis of variance.
References
- Acri JB, Siedleck BK, Witkin JM. (1996) Effects of benztropine on behavioral and toxic effects of cocaine: comparison with atropine and the selective dopamine uptake inhibitor 1-[2-(diphenylmethoxy)ethyl]-4-(3-phenyl-propyl)-piperazine. J Pharmacol Exp Ther 277:198–206 [PubMed] [Google Scholar]
- Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P. (2004) An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience 125:349–358 [DOI] [PubMed] [Google Scholar]
- Bechara A, van der Kooy D. (1989) The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci 9:3400–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. (1996) Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci 16:714–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DR, Enna SJ, Creese I, Snyder SH. (1975) Dopamine receptor binding in the corpus striatum of mammalian brain. Proc Natl Acad Sci U S A 72:4655–4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Shannon HE, Rasmussen K, Delapp NW, Mitch CH, Ward JS, Calligaro DO, Ludvigsen TS, Sheardown MJ, Olesen PH, et al. (1998) Unexpected antipsychotic-like activity with the muscarinic receptor ligand (5R,6R)6-(3-propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1]octane. Eur J Pharmacol 356:109–119 [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. (2002) Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci 22:2977–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan KA, Dykstra LA. (2007) Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor-deficient mice. Psychopharmacology (Berl) 191:985–993 [DOI] [PubMed] [Google Scholar]
- Chapman CA, Yeomans JS, Blaha CD, Blackburn JR. (1997) Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience 76:177–186 [DOI] [PubMed] [Google Scholar]
- Crespo JA, Sturm K, Saria A, Zernig G. (2006) Activation of muscarinic and nicotinic acetylcholine receptors in the nucleus accumbens core is necessary for the acquisition of drug reinforcement. J Neurosci 26:6004–6010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Simkowitz P, MacGregor RR, Fowler JS, Volkow ND, Wolf AP. (1993) Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography in normal human subjects. Proc Natl Acad Sci U S A 90:11816–11820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. (1994) Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci 17:228–233 [DOI] [PubMed] [Google Scholar]
- Eglen RM. (2006) Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol 26:219–233 [DOI] [PubMed] [Google Scholar]
- Fagergren P, Overstreet DH, Goiny M, Hurd YL. (2005) Blunted response to cocaine in the Flinders hypercholinergic animal model of depression. Neuroscience 132:1159–1171 [DOI] [PubMed] [Google Scholar]
- Forster GL, Yeomans JS, Takeuchi J, Blaha CD. (2002) M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci 22:RC190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. (1999) Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 96:10483–10488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasing K, He S, Yang Y. (2009) Long-lasting decreases in cocaine-reinforced behavior following treatment with the cholinesterase inhibitor tacrine in rats selectively bred for drug self-administration. Pharmacol Biochem Behav 94:169–178 [DOI] [PubMed] [Google Scholar]
- Hardouin SN, Richmond KN, Zimmerman A, Hamilton SE, Feigl EO, Nathanson NM. (2002) Altered cardiovascular responses in mice lacking the M(1) muscarinic acetylcholine receptor. J Pharmacol Exp Ther 301:129–137 [DOI] [PubMed] [Google Scholar]
- Hikida T, Kitabatake Y, Pastan I, Nakanishi S. (2003) Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A 100:6169–6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. (2002) Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci 22:9895–9904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, et al. (2008) Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci 28:10422–10433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane BE, Grant MK, El-Fakahany EE, Ferguson DM. (2008) Synthesis and evaluation of xanomeline analogs—probing the wash-resistant phenomenon at the M1 muscarinic acetylcholine receptor. Bioorg Med Chem 16:1376–1392 [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. (1999) Novel 3alpha-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther 288:302–315 [PubMed] [Google Scholar]
- Kremin T, Gerber D, Giocomo LM, Huang SY, Tonegawa S, Hasselmo ME. (2006) Muscarinic suppression in stratum radiatum of CA1 shows dependence on presynaptic M1 receptors and is not dependent on effects at GABA(B) receptors. Neurobiol Learn Mem 85:153–163 [DOI] [PubMed] [Google Scholar]
- Leiber D, Marc S, Harbon S. (1990) Pharmacological evidence for distinct muscarinic receptor subtypes coupled to the inhibition of adenylate cyclase and to the increased generation of inositol phosphates in the guinea pig myometrium. J Pharmacol Exp Ther 252:800–809 [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader SL, Berger SP, Bechtholt AJ. (2006) Injection of oxotremorine in nucleus accumbens shell reduces cocaine but not food self-administration in rats. Brain Res 1123:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. (2001) Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci 21:5239–5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. (1995) Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci 15:5859–5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onali P, Olianas MC. (2002) Muscarinic M4 receptor inhibition of dopamine D1-like receptor signalling in rat nucleus accumbens. Eur J Pharmacol 448:105–111 [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Woolverton WL. (2002) Self-administration of cocaine: scopolamine combinations by rhesus monkeys. Psychopharmacology (Berl) 161:442–448 [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Sauerberg P, Nielsen EB, Swedberg MD, Thomsen C, Sheardown MJ, Jeppesen L, Calligaro DO, DeLapp NW, Whitesitt C, et al. (2000) Muscarinic receptor agonists decrease cocaine self-administration rates in drug-naive mice. Eur J Pharmacol 402:241–246 [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. (1980) Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 12:781–787 [DOI] [PubMed] [Google Scholar]
- Schmidt LS, Miller AD, Lester DB, Bay-Richter C, Schulein C, Frikke-Schmidt H, Wess J, Blaha CD, Woldbye DP, Fink-Jensen A, et al. (2010) Increased amphetamine-induced locomotor activity, sensitization, and accumbal dopamine release in M(5) muscarinic receptor knockout mice. Psychopharmacology (Berl), 207:547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon HE, Bymaster FP, Calligaro DO, Greenwood B, Mitch CH, Sawyer BD, Ward JS, Wong DT, Olesen PH, Sheardown MJ. (1994) Xanomeline: a novel muscarinic receptor agonist with functional selectivity for M1 receptors. J Pharmacol Exp Ther 269:271–281 [PubMed] [Google Scholar]
- Smith JE, Co C, Yin X, Sizemore GM, Liguori A, Johnson WE, III, Martin TJ. (2004) Involvement of cholinergic neuronal systems in intravenous cocaine self-administration. Neurosci Biobehav Rev 27:841–850 [DOI] [PubMed] [Google Scholar]
- Spealman RD. (1996) Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 278:1128–1137 [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, Katz JL. (2007) Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther 321:334–344 [DOI] [PubMed] [Google Scholar]
- Tanda G, Katz JL. (2007) Muscarinic preferential M(1) receptor antagonists enhance the discriminative-stimulus effects of cocaine in rats. Pharmacol Biochem Behav 87:400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. (2005) Chronic intravenous drug self-administration in rats and mice, in Current Protocols of Neuroscience, Chapter 9: Preclinical Models of Neurologic and Psychiatric Disorders, unit 9.20, Wiley, Hoboken, NJ: [DOI] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wörtwein G, Fink-Jensen A, Wess J, Caine SB. (2005) Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci 25:8141–8149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walland A, Palluk R, Burkard S, Hammer R. (1997) Compensation of muscarinic bronchial effects of talsaclidine by concomitant sympathetic activation in guinea pigs. Eur J Pharmacol 330:213–219 [DOI] [PubMed] [Google Scholar]
- Wilson MC, Schuster CR. (1973) Cholinergic influence on intravenous cocaine self-administration by rhesus monkeys. Pharmacol Biochem Behav 1:643–649 [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Wise RA. (2008) Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci 28:9021–9029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.