Abstract
Chemical coupling to carrier red blood cells (RBCs) converts tissue type plasminogen activator (tPA) from a problematic therapeutic into a safe agent for thromboprophylaxis. The goal of this study was to develop a more clinically relevant recombinant biotherapeutic by fusing a mutant tPA with a single-chain antibody fragment (scFv) with specificity for glycophorin A (GPA) on mouse RBCs. The fusion construct (anti-GPA scFv/PA) bound specifically to mouse but not human RBCs and activated plasminogen; this led to rapid and stable attachment of up to 30,000 copies of anti-GPA scFv/PA per mouse RBC that were thereby endowed with high fibrinolytic activity. Binding of anti-GPA scFv/PA neither caused RBC aggregation, hemolysis, uptake in capillary-rich lungs or in the reticuloendothelial system nor otherwise altered the circulation of RBCs. Over 40% of labeled anti-GPA scFv/PA injected in mice bound to RBC, which markedly prolonged its intravascular circulation and fibrinolytic activity compared with its nontargeted PA counterpart, anti-GPA scFv/PA, but not its nontargeted PA analog, prevented thrombotic occlusion in FeCl3 models of vascular injury. These results provide proof-of-principle for the development of a recombinant PA variant that binds to circulating RBC and provides thromboprophylaxis by use of a clinically relevant approach.
Plasminogen activators (PAs, including tissue-type, tPA), proteases generating plasmin, which cleaves fibrin clots and restores perfusion, are used to achieve urgent thrombolysis within a relatively narrow therapeutic time window after thrombosis (Topol et al., 1987; Holvoet et al., 1993). The safety of this approach is limited by the inability of soluble PAs to discriminate newly formed occluding pathological clots from pre-existing mural hemostatic clots, and their efficacy is limited by delay in initiation of treatment, inactivation by plasma inhibitors, and inadequate delivery into poorly permeable occlusive clots. Paradoxically, endowing tPA derivatives with higher affinity to clot components (Collen, 1996; Runge et al., 1996) further impairs permeation (Sakharov and Rijken, 1995). Increased dosing and potency also increase the risk of bleeding and collateral damage in the brain.
In theory, prophylactic administration of tPA should benefit patients predisposed to a short-term risk of thrombosis (e.g., immobilized patients after surgery, myocardial infarction, or transient ischemic attack). In addition, unfavorable pharmacokinetics (circulation time <20 min) precludes prophylactic use of tPA. However, coupling tPA to carrier red blood cells (RBCs) fundamentally alters tPA pharmacokinetics, converting it from a problematic therapeutic agent into a safe and effective prophylactic agent (Murciano et al., 2003). Studies in animal models have shown that coupling of tPA to RBCs restricts access of the resultant RBC/tPA both to the CNS and to postsurgical hemostatic clots (Zaitsev et al., 2006; Danielyan et al., 2008). RBC/tPA circulate for many hours and incorporate into and rapidly dissolve newly formed, potentially occlusive clots from within (Murciano et al., 2003). Infusion of RBC/tPA in mice, rats, and pigs provides an effective short-term option to prevent thrombotic occlusion in diverse vascular systems, including the cerebral vasculature, without the hemorrhagic and CNS toxicity profile typically seen with free tPA (Murciano et al., 2003; Ganguly et al., 2005; Ganguly et al., 2006; Ganguly et al., 2007; Danielyan et al., 2008; Armstead et al., 2009).
The medical utility of this approach would be enhanced if one could circumvent the need for ex vivo conjugation of tPA to the carrier RBCs before reinfusion. This goal can be achieved by use of tPA derivatives endowed with the ability to bind safely to circulating RBCs. Thus, tPA, chemically conjugated with a monoclonal antibody specific for human complement receptor type I (CR1, an RBC glycoprotein involved in complement regulation and the clearance of immune complexes) (Fearon et al., 1989), can be safely attached onto circulating RBCs, thereby providing thromboprophylaxis in mouse models of thrombosis (Zaitsev et al., 2006). However, CR1 is a low-abundant glycoprotein with significant variation in expression levels among individuals (500–1500 copies per human RBC) (Birmingham and Hebert, 2001). Therefore, dosing of anti-CR1/tPA conjugates is limited and may be insufficient in cases of severe thrombosis. Furthermore, there are technical and regulatory hurdles for industrial development and clinical use of drugs chemically conjugated to antibodies.
The goal of this study was to design a more generally applicable approach to produce RBC-targeted fibrinolytics that would also permit coating RBCs with a wider range of drug doses. To achieve this goal, we produced a recombinant tPA derivative fused to a monovalent scFv fragment derived from the monoclonal antibody Ter-119, specific for mouse glycophorin-A (GPA), an abundant and RBC-specific surface molecule (∼106 copies/RBC) (Kina et al., 2000; Spitzer et al., 2004) similar to its human analog (Furthmayr and Marchesi, 1976). Previous studies showed that the complement regulatory proteins including decay accelerating factor fused with the Ter-119 scFv enhanced the resistance of RBCs to complement-mediated lysis in vitro (Spitzer et al., 2004) and in vivo (Spitzer et al., 2005). In this study, we fused scFv Ter-119 to a truncated form of mouse tPA containing kringle 2 and the protease domain (truncation of auxiliary tPA domains reduces its clearance and side effects) (Martin et al., 1991; Kohnert et al., 1992). Additional mutations homologous to those in Tenectaplase (K296A, H297A, R298A, and R299A) were introduced in the protease domain to confer higher resistance to the plasma inhibitor, PAI-1 (Davydov and Cheng, 2001; Tanswell et al., 2002). Therefore, the PA moiety of the resultant anti-GPA scFv/PA fusion designed and tested in this study combines the mutations found in the human recombinant tPA mutants Retavase and Tenektaplase (Davydov and Cheng, 2001). In the present study, we tested the in vitro activity and pharmacological properties of this anti-GPA scFv/PA fusion protein and demonstrated that this novel agent can be used safely to endow RBCs with high levels of fibrinolytic activity, thus prolonging its bioavailability and providing thromboprophylaxis in vivo.
Materials and Methods
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. The following additional reagents were used: fibrinogen from Enzyme Research Laboratories (South Bend, IN); thrombin from Calbiochem (San Diego, CA); Iodogen from Pierce Chemical (Rockford, IL); QuikChange Site-Directed Mutagenesis kit from Stratagene (La Jolla, CA); Drosophila S2 cells, pMT/Bip/V5-His-A vector, and Schneiders S2 cell medium from Invitrogen (Carlsbad, CA); Drosophila serum-free medium from Lonza (Walkersville, MD); polymerase chain reaction (PCR) core kit and Rapid DNA ligation kit from Roche Diagnostics (Basel, Switzerland); endonucleases from New England Biolabs (Beverly, MA); and Erythrina trypsin inhibitor (ETI) Sepharose from Landing BioTech Inc. (Newton, MA).
Proteins were radiolabeled with Na[125I] (PerkinElmer Life and Analytical Sciences, Boston, MA) with use of the Iodogen method according to the manufacturer's recommendations. The free iodine was removed by use of a Bio-Spin 6 column (Bio-Rad Laboratories, Hercules CA). RBCs were obtained from fresh anticoagulated mouse blood and radiolabeled with [51Cr]Cl2 (PerkinElmer Life and Analytical Sciences), as described previously (Murciano et al., 2003).
Cloning of Anti-GPA scFv/PA and PA.
We followed the template described by us for fusing plasminogen activators with scFv's with the use of a serine-rich linker peptide (Ding et al., 2005, 2008). Ter-119 is a rat monoclonal antibody to mouse GPA and has been characterized previously (Kina et al., 2000). The pNscTDdSeY plasmid served as the source of the scFv Ter-119 cDNA sequence and has been described (Spitzer et al., 2004). In brief, the variable heavy- and light-chain regions of Ter-119 were joined by PCR with a (GGGGS)3 linker to assemble the scFv Ter-119.
Plasmid (pMT/Bip/V5-His-A expression vector) containing mouse tissue type plasminogen activator (tPA) was prepared as follows. Mouse tPA cDNA (Open Biosystems, Huntsville, AL) and cDNA encoding full-length mouse tPA as well as its truncated form containing only the kringle 2 (K2) and protease (P) domains (Retavase analog) was PCR amplified by use of upstream primer 5′-cat ggg agg ttc aga ctc gga gcc cgg tcc tac aga gcg ac-3′ for full-length tPA and 5′-cat ggg agg ttc aga ctc cct aag gga aaa agc gag gac-3′ for the truncated form to introduce BglII restriction site at the 5′ end, and the reverse primer 5′-gag ctg ggc ttc tcg agt cat tgc ttc atg ttg tcg tga atc cag-3′ to introduce an XhoI restriction site at 3′ end. The PCR products were digested with BglII/XhoI restriction enzymes and purified and ligated into pMT/Bip/V5-His-A vector. Point mutations to convert amino acids 296 to 299 (KNKR) to AAAA were introduced into both constructs by use of the QuikChange Site-Directed Mutagenesis Kit (Stratagene) per the manufacturer using the direct primer 5′-cag gct ccc atc ttt gtc gct gcc gca gcg tct cct gga gag aga ttc-3′ and the reverse primer 5′-gaa tct ctc tcc agg aga cgc tgc ggc agc gac aaa gat ggg agc ctg-3′.
cDNA encoding anti-GPA scFv was amplified for cloning in the expression vector pMT/Bip/V5-HisA using the upstream primer 5′-cgt acg act agt cag gtg aag ctg cag gag tca gga gga ggc-3′, which introduces a restriction site for SpeI at the 5′ end, and the downstream primer 5′-ata aga atg cgg ccg cgc cgg aag agc tact ac ccg atg agg aag aag ccc gtt tca gtt cca gct tgg tcc c-3′, which appends the sequence of a short peptide linker (SSSSG)2 and a NotI restriction site at 3′ end. The K2 and P domain fragment of mouse tPA was amplified by using as primers 5′-ata aga atg cgg ccg cac cta agg gaa aaa gcg agg ac-3′, which introduces a NotI restriction site at the 5′ end, and downstream 5′-gag ctg ggc ttc tcg agt cat tgc ttc atg ttg tcg tga atc cag-3′ to introduce an XhoI restriction site at 3′ end. The anti-GPA scFv/PA was assembled as follows. First, the two PCR products were purified and digested with SpeI, NotI, and XhoI, respectively. Second, the two digested fragments were ligated and cloned into the SpeI and XhoI sites of the vector pMT/Bip/V5-HisA. Successful cloning was confirmed by restriction analysis of plasmid and by automated sequencing.
Expression and Purification of Anti-GPA scFv/PA and PA.
Drosophila S2 cells were maintained in Schneiders medium (Invitrogen) supplemented with l-glutamine (Invitrogen), fetal bovine serum (HyClone Laboratories, Logan, UT), and PenStrep (Invitrogen) and cotransfected by use of FuGene6 (Roche Diagnostics) with pMT/Bip/V5-HisA plasmids encoding anti-GPA scFv/PA and PA constructs and pCoBlast (Invitrogen) at a ratio 30:1. Stable transfectants were established by adding blasticidin (Invitrogen) (25 μg/ml). Stable transfectants were then transferred into S2 serum-free medium, SFM (Lonza). Protein production was induced by adding CuSO4 (final concentration, 0.5 mM). Anti-GPA scFv/PA and PA were purified from cell media by affinity chromatography on ETI Sepharose, as described previously (Heussen et al., 1984). The yield was ∼3 and ∼5 mg/l medium for anti-GPA scFv/PA and PA, respectively. Proteins were concentrated to a level not exceeding 2 mg/ml, separated into aliquots, and stored at −80°C until use.
Biochemical Characterization of Anti-GPA scFv/PA and PA.
The size and homogeneity of the fusion protein and its plasminogen activator component were analyzed by use of a 4 to 12% SDS-PAGE gradient. For Western blot analysis, the separated proteins were electrotransferred to a nitrocellulose membrane (NitroBind; GE Osmonics, Minnetonka, MN), and unspecific binding was blocked with Tris-buffered saline containing 10% nonfat milk powder and 0.1% Tween 20. A rabbit polyclonal antibody against mouse tPA (Molecular Innovations, Southfield, MI) served as the primary antibody. The secondary anti-rabbit antibody was conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA), and the antigen-antibody complex was detected with ECL Plus (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
The plasminogen activator activity of anti-GPA scFv/PA and PA was confirmed by use of casein zymography. Aliquots of the SFM from anti-GPA svFv/PA- and PA-expressing cells were mixed with nonreducing Tris-glycine SDS sample buffer for zymography. The samples were resolved under nonreducing SDS-denaturing conditions on a 7.5% gel cast with 1% nonfat dry milk and 20 μg/ml plasminogen incorporated into the gel before polymerization to detect PA activity (Wang et al. 1998). Thereafter, the gels were renatured in Novex Zymogram Renaturating buffer (Invitrogen) and developed in Novex Zymogram Developing buffer (Invitrogen) per the manufacturer's instructions. EDTA (5 mM) was added to both buffers to block potential metalloproteinase activity. Gels were stained with GelCode Blue stain (Pierce Chemical). Gels run in parallel in the absence of added plasminogen served as a control for PA activity (not shown).
The specificity of anti-GPA scFv/PA binding was confirmed by use of an immunocapture assay and Western blot. In brief, mouse and human RBC ghost membranes were prepared (Schwartz et al., 1997), incubated with SFM from the induced S2 cells expressing anti-GPA scFv/PA for 1 h and then washed three times with PBS and lysed in a sample buffer (Invitrogen). The resultant RBC ghost lysates (equalized for total protein) were separated on 4 to 12% SDS-PAGE under nonreducing conditions. Western blot analysis of the samples to detect RBC ghost-captured anti-GPA scFv/PA was performed as described above to detect scFv/PA in SFM medium. An aliquot of the SFM medium from the induced S2 cells expressing anti-GPA svFv/PA served as a positive control for the detection of fusion protein.
Binding of Anti-GPA scFv/PA to RBC.
We measured the binding of 125I-anti-GPA scFv/PA to mouse (target cells) versus human (negative control) RBC as described previously for an anti-CR1 monoclonal antibody/tPA conjugate (Zaitsev et al., 2006). In brief, RBCs were washed by centrifugation (1200g) with PBS/3% BSA, resuspended in the same buffer to a hematocrit of 1 or 10% and incubated with various concentrations of 125I-scFv-PA for 1 h at 37°C (loading) with gentle rotation. Unbound reagent was eliminated by washing the RBC four times with a 20-fold volume of PBS-BSA. The residual radioactivity in the RBC pellets was measured in a γ-counter (PerkinElmer Life and Analytical Sciences). To determine RBC binding, mouse blood was collected in heparin and RBCs were prepared. 125I-anti-GPA scFv/PA was added to whole blood or washed RBCs at a 50% hematocrit to final concentration of 40 μg/ml and binding was measured as described above.
In Vitro Fibrinolysis.
The fibrinolytic activity of anti-GPA scFv/PA bound to RBC was measured by use of 125I-labeled fibrin clots, as described previously (Murciano et al., 2003). RBCs were incubated either with SFM medium from the induced S2 cells expressing anti-GPA svFv/PA or with purified anti-GPA scFv/PA for 1 h, washed three times with PBS, and added to a fibrinogen solution (6 mg/ml in PBS) trace labeled with 125I-fibrinogen. Clotting was induced by adding CaCl2 and thrombin (20 mM and 0.2 units/ml final concentrations, respectively). The clots were then overlaid with 200 μl of PBS and incubated at 37°C; the radioactivity in the supernatants was measured in a γ-counter (PerkinElmer Life and Analytical Sciences).
In Vivo Tracing of RBC and RBC-Anchored Anti-GPA svFv/PA.
Experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Washed RBCs obtained from fresh anticoagulated mouse blood were labeled with 51Cr, as described previously (Zaitsev et al., 2006). 51Cr-RBC or 51Cr-RBC preloaded with 125I-anti-GPA scFv/PA was injected into anesthetized mice via the jugular vein. At designated times, aliquots of blood were drawn in heparin, the animals were sacrificed, and the radioactivity in the blood and major organs was measured.
Pharmacokinetic Analysis of Anti-GPA scFv/PA and PA.
Adult C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were studied. 125I-anti-GPA scFv/PA or 125I-PA (3–5 μg) was injected into anesthetized mice via the jugular vein. At the predesignated times, 100 to 200 μl of blood were withdrawn in heparin and centrifuged at 1200g; the radioactivity in the plasma and pellets was measured. The animals were sacrificed, and the radioactivity in the organs was measured. In a separate set of experiments, RBCs obtained from mice were washed, labeled with 51Cr, loaded with 125I-anti-GPA scFv/PA at a dose of 20,000 molecules/RBC, and injected into anesthetized mice. Blood samples were collected and the major organs were harvested and analyzed for radioactivity as described for 125I.
Fibrinolytic Activity of Anti-GPA scFv/PA Loaded In Vivo on Circulating Carrier RBCs.
We followed the protocol used previously for an anti-CR1 tPA conjugate (Zaitsev et al., 2006). In brief, equimolar doses of anti-GPA scFv/PA or PA, providing an initial blood concentration of 0.85 μM (2 mg/kg PA and 4 mg/kg anti-GPA scFv/PA), were injected in 200 μl of saline vehicle into anesthetized mice via the jugular vein (drug-free saline was injected as a placebo control). Forty-five minutes later, 100-μl aliquots of blood were drawn in the absence of anticoagulant, mixed rapidly with trace amounts of 125I-fibrinogen, and allowed to clot in borosilicate tubes at 20°C. After 20 min of maturation, clots were overlaid with saline and incubated at 37°C and the release of 125I was measured in a gamma-counter (PerkinElmer Life and Analytical Sciences).
Effect of Anti-GPA scFv/PA in a Mouse Model of Carotid Artery Thrombosis.
To test the antithrombotic potential of anti-GPA scFv/PA, we used a mouse model of acute severe carotid thrombotic occlusion in response to vascular injury inflicted by the adventitial application of FeCl3 30 min after administration of anti-GPA scFv/PA, PA, or saline, as described previously (Murciano et al., 2003). An equimolar dose (0.85 μM) of anti-GPA scFv/PA or PA was injected into anesthetized mice as described above. Thrombosis was induced in the exposed contralateral carotid artery by applying a 1 × 2-mm piece of filter paper (Whatman No. 1) saturated with 15% FeCl3 to the adventitia for 2 min. Time to occlusion of the vessel and total blood flow over the ensuing 30 min were measured by Doppler ultrasound by use of a 0.5VB flow probe connected to a recording system (Transonic Systems, Ithaca, NY).
Effect of Anti-GPA scFv/PA in a Mouse Model of Jugular Vein Thrombosis.
To test the antithrombotic potential of anti-GPA scFv/PA, we used a mouse model of acute severe jugular vein thrombotic occlusion in response to vascular injury inflicted by the adventitial application of FeCl3 30 min after administration of anti-GPA scFv/PA, PA, or saline, as described above (Murciano et al., 2003). An equimolar dose (0.85 μM) of anti-GPA scFv/PA or PA was injected into anesthetized mice as described above. Thrombosis was induced in the exposed contralateral jugular vein with use of 15% FeCl3 for 2 min, and the time to 50% occlusion and total blood flow maintained over the 30-min study were measured as described above.
Data Analysis.
All data are presented as the means ± S.E.M. of at least three separate experiments. Differences between groups were tested for statistical significance by use of Student's t test or analysis of variance. Statistical significance was set at P < 0.05.
Results
Design, Synthesis, and Biochemical Properties of the Anti-Gpa scFv/Pa Fusion Protein.
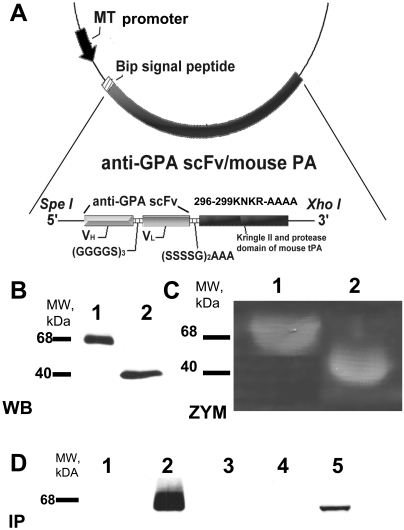
cDNA encoding scFv Ter-119 directed to mouse GPA was ligated to cDNA encoding the kringle II and protease domains of mouse tPA containing an additional Tenectaplase-type mutation by use of a (S4G)2A3 linker within the plasmid pMT-Bip His A (Fig. 1A). Transfection of this plasmid in S2 Drosophila cells lead to the expression of the fusion protein anti-GPA scFv/PA (scFv/PA). The PA part of the fusion protein was also cloned as a BglII/XhoI fragment into pMT-Bip His A and expressed in S2 Drosophila cells. Nonreducing Western blot analysis with an antibody to mouse tPA was then performed to identify the two recombinant proteins. Anti-GPA scFv/PA and PA migrated as single bands with the predicted molecular masses of ∼68 and ∼40 kDa, respectively (Fig. 1B). Electrophoretic zymography demonstrated comparable PA activity for both proteins (Fig. 1C). To test the RBC-binding capacity of anti-GPA scFv/PA, the medium from induced S2 cells transfected with the plasmid encoding anti-GPA scFv/PA was incubated with either mouse or human RBC membranes, washed, and analyzed by immunoprecipitation, size fractionation, and Western blotting as above. This analysis showed that anti-GPA scFv/PA bound to mouse, but not to human RBCs, which served in subsequent studies as the negative control (Fig. 1D).
Fig. 1.
Molecular design, expression, and characterization of anti-GPA scFv/PA fusion protein. A, schematic diagram describing the cloning strategy for the fusion construct anti-GPA scFv/PA. Variable domains of the heavy chain and light chains of the antibody were linked by a (Gly4Ser)3 linker and then fused to the N terminus of the kringle II/protease domain fragment of mouse tPA by a (Ser4Gly)2Ala3 linker. The completed construct was then cloned into the SpeI and XhoI sites in the pMT/BIP/V5/HisA expression vector. The DNA fragment encoding kringle II and protease domain of mouse tPA was PCR amplified and cloned into BglII and XhoI sites in the same vector. B, Western blot (WB) analysis of 40 μl of culture medium from S2 cells expressing either anti-GPA scFv/PA fusion protein or PA after induction by 0.5 mM CuSO4. C, casein zymography (ZYM) analysis of culture medium from S2 cells expressing either the anti-GPA scFv/PA fusion protein or the PA after induction by 0.5 mM CuSO4. D, Western blot analysis of protein immunocaptured with mouse (m) or human (h) RBC ghosts: lane 1, lysate of hRBC ghosts incubated in culture medium from S2 cells expressing anti-GPA scFv/PA fusion protein; lane 2, lysate of mRBC ghosts incubated in culture medium from S2 cells expressing anti-GPA scFv/PA fusion protein; lane 3, lysate of hRBC ghosts; lane 4, lysate of mRBC ghosts; lane 5, 40 μl of culture medium from S2 cells expressing anti-GPA scFv/PA fusion protein (positive control).
RBC-Binding Properties of Anti-GPA scFv/PA Fusion.
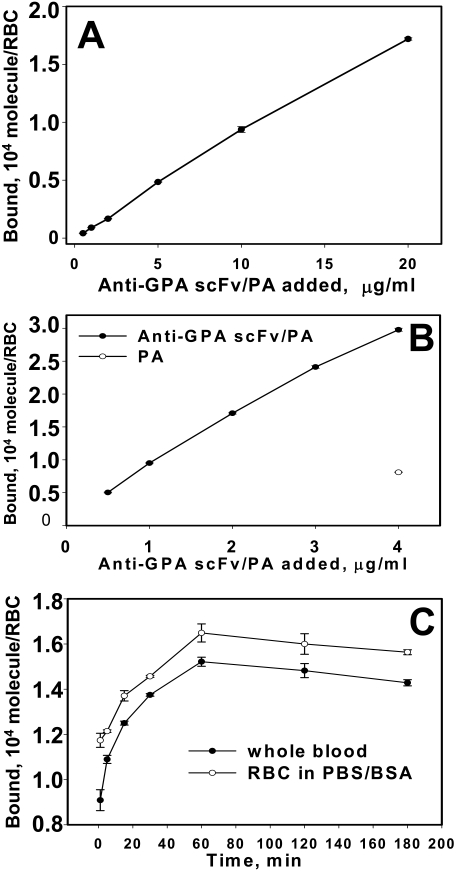
Both anti-GPA scFv/PA and PA were purified from cell media by affinity chromatography with ETI Sepharose to at least 95% purity confirmed by SDS-PAGE (data not shown). Purified 125I-labeled anti-GPA scFv/PA bound to mouse RBC membranes in a dose-dependent manner (Fig. 2A), whereas no significant binding was observed to human RBC at the highest concentration tested (less than 100 copies bound per RBC; data not shown). Even at a 1% hematocrit, binding of anti-GPA scFv/PA (3 × 104 molecules per RBC) did not approach saturation, in agreement with the expression of 1 × 106 copies of GPA per RBC (Kina et al., 2000; Spitzer et al., 2004). The nontargeted PA moiety of the fusion did not bind to mouse RBC (Fig. 2B, ○). Binding of anti-GPA scFv/PA to mouse RBCs was rapid, reaching 50% of maximum within 10 min in whole blood (Fig. 2C).
Fig. 2.
Specific binding and binding kinetics of anti-GPA scFv/PA fusion protein to RBC expressing mouse GPA. A, dose-dependent binding of 125I-anti-GPA scFv/PA fusion protein to washed mouse RBC at 10% hematocrit RBC suspension determined after elimination of unbound material (n = 3). B, dose-dependent binding of 125I-anti-GPA scFv/PA fusion protein (●) versus PA (○) to washed mRBC at 1% hematocrit RBC suspension determined after elimination of unbound material (n = 3). C, binding kinetics of 125I-anti-GPA scFv/PA fusion protein to mRBC in whole heparinized blood versus the same hematocrit washed mouse RBC suspension in PBS/BSA buffer (each time point n = 3). Mean ± 1 S.E.M. are shown.
Fibrinolytic Activity of RBC-Bound Anti-GPA scFv/PA.
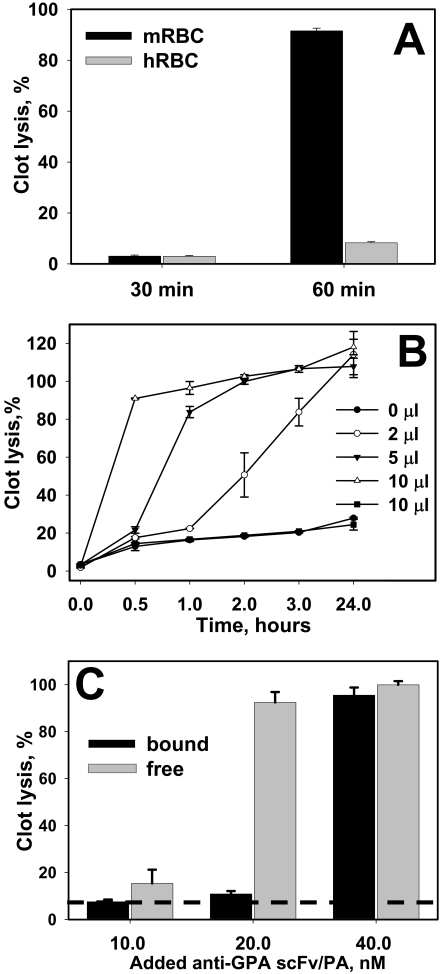
To estimate whether RBC-bound anti-GPA scFv/PA activates plasminogen, mouse and human RBCs were incubated with medium from induced S2 cells transfected with anti-GPA scFv/PA encoding plasmid. Equal amounts of mouse or human RBCs preincubated with anti-GPA scFv/PA were washed and then added to a solution containing 125I-labeled fibrinogen and trace amounts of plasminogen before clotting by adding thrombin. Clot lysis was monitored by release of 125I-labeled fibrin degradation products into the supernatants. Mouse but not human RBCs preincubated with anti-GPA scFv/PA caused nearly complete lysis of the fibrin clot within 1 h (Fig. 3A).
Fig. 3.
Fibrinolytic activity in vitro of RBC-bound and free anti-GPA scFv/PA fusion protein. A, fibrinolytic activity of mouse or human RBC (mRBC versus hRBC) preincubated for 1 h in the medium of the S2 cells expressing anti-GPA scFv/PA fusion protein. RBCs were then washed and 20 μl of 50% RBC suspension was incorporated in a forming 200-μl fibrin clot. After maturation fibrin clots were brought to 37°C and clot lysis was monitored (n = 3). B, dose-dependent lysis of the fibrin clot by mouse RBC loaded with anti-GPA scFv/PA fusion protein. Incorporation in a fibrin clot of 2, 5, and 10 μl of loaded mouse RBCs, 50% hematocrit suspension, corresponds to 1.5, 3.75, and 7.5 nM anti-GPA scFv/PA in a clot, respectively (n = 3). Ten microliters of human RBC suspension incubated with anti-GPA scFv/PA at the same conditions as mouse RBC and 10 μl of intact mouse RBC suspension incorporated in the clot served as controls. C, comparison of fibrinolytic activity of anti-GPA scFv/PA in a free and RBC-bound state. Equal amounts of anti-GPA scFv/PA were incorporated in the formation of fibrin clots in mouse RBC bound or free state (the structure of the clot was kept the same by incorporation of an equal number of human RBCs). Clot lysis was monitored for 30 min (n = 3). Dash line shows the spontaneous lysis of fibrin clots with incorporated intact RBCs. In each experiment the mean ± 1 S.E.M. is shown.
To analyze the fibrinolytic potency of RBC-bound anti-GPA scFv/PA, mouse or human RBCs were incubated with purified anti-GPA scFv/PA at a concentration chosen to attach ∼20,000 molecules per RBC (Fig. 2). After removing unbound anti-GPA scFv/PA by washing, 2-, 5-, or 10-μl aliquots of the RBC preparations (50% hematocrit) were added to a solution containing 125I-labeled fibrinogen and trace amounts of plasminogen before clotting by thrombin, thereby achieving resultant concentrations of 1.5, 3.75, and 7.5 nM anti-GPA scFv/PA in the clots, respectively. 125I-Labeled degradation products were released into the supernatants in a dose- and time-dependent manner as a result of fibrinolysis (Fig. 3B). Release of radioactivity was not detected when mouse RBCs were replaced by human RBCs that do not bind anti-GPA scFv/PA (■). Anti-GPA scFv/PA-RBC retain ∼100% of their initial fibrinolytic activity at 24 h and ∼80% at 48 h (data not shown).
We then used the same approach to compare the lysis of fibrin clots by equal amounts of free or RBC-bound anti-GPA scFv/PA. In samples incubated with free anti-GPA scFv/PA, an equal number of human RBCs was added to account for the effect of cell incorporation on clot structure. Fibrinolysis was assessed after a 30-min incubation, the optimal time to reveal dose-dependent differences (Fig. 3B). At rate-limiting doses, free anti-GPA scFv/PA caused more profound fibrinolysis than RBC-bound anti-GPA scFv/PA in vitro, probably because of diffusional limitations imposed by the RBC carrier (Fig. 3C).
Binding of Anti-GPA scFv/PA Does Not Affect RBC Biocompatibility and Prolongs Anti-GPA scFv/PA Circulation Time in Mice.
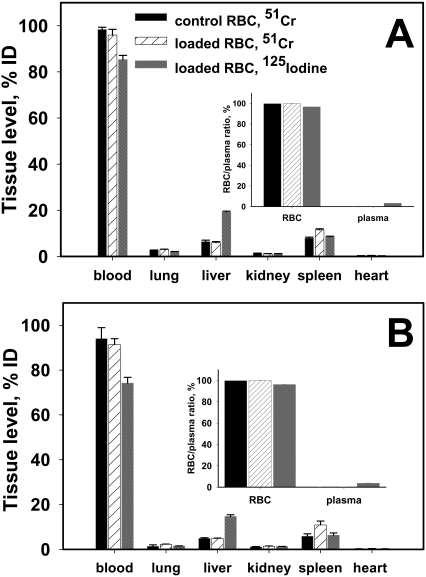
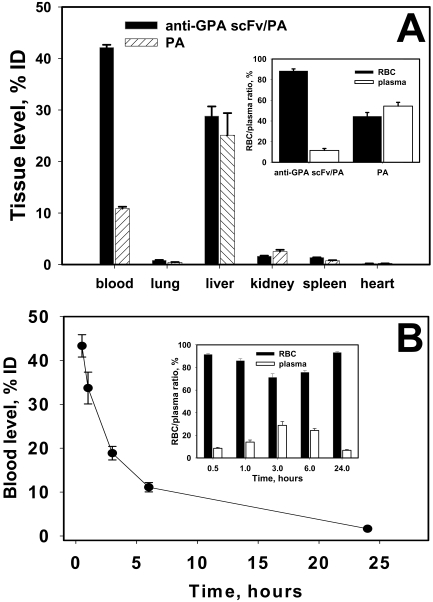
To test the effect of anti-GPA scFv/PA binding on RBC biocompatibility and survival, washed 51Cr-labeled mouse RBCs, either intact or coated with 125I-labeled anti-GPA scFv/PA at a level of ∼30,000 molecules per RBC, were injected into recipient mice. The amount of 51Cr in the blood and major organs was nearly identical in mice injected with either naive RBCs or anti-GPA scFv/PA coated RBCs at 1 and 3 h after injection (Fig. 4, A and B). It is noteworthy that there was no detectable elevation in the uptake of RBCs in the lungs and spleen, indicating that anchoring of anti-GPA scFv/PA did not cause significant damage to the RBCs, nor cause them to aggregate in a manner that would enhance splenic clearance or induce mechanical retention in capillary-rich organs such as the lungs. More than 80% of injected 125I-anti-GPA scFv/PA remained in the circulation, with the rest found primarily in the liver (Fig. 4, A and B). Centrifugation of heparinized blood samples showed that nearly 100% of both isotopes were recovered in the RBC pellet, consistent with stable binding of anti-GPA scFv/PA to RBC circulating in bloodstream as measured in vivo, with minimal elution into the plasma (Fig. 4, A and B, insets).
Fig. 4.
Anti-GPA scFv/PA loading does not damage carrier RBC. 51Cr-labeled mouse RBC, either naive or loaded at the level of 20,000/RBC with 125I-anti-GPA scFv/PA fusion protein, were injected into mice. The animals were killed 1 h (A) and 3 h (B) afterward, and the amount of 51Cr and 125I was measured in blood and the main organs (n = 4). The insets in both A and B indicate 51Cr and 125I distribution in blood components at the indicated times. In each experiment the mean ± 1 S.E.M. are shown.
Next, we compared the pharmacokinetics of 125I-anti-GPA scFv/PA versus 125I-PA injected directly into the bloodstream, simulating their clinical use. This setting differs from the “preloading” strategy described above, because here the binding occurs in the recipient's bloodstream. Therefore, based on the binding kinetics showing complete binding by 1 h after incubation of anti-GPA scFv/PA with RBC in vitro (Fig. 2C), tissue clearance within the first 30 min after injection has the potential to eliminate a significant fraction of free drug. Nevertheless, at this time point, there was a 4-fold higher concentration of the anti-GPA scFv/PA fusion protein than nontargeted PA in the blood (Fig. 5A), and approximately 90% anti-GPA scFv/PA recovered in blood samples was associated with the RBC pellet (Fig. 5A, inset). Nontargeted PA and the unbound fraction of anti-GPA scFv/PA were taken up primarily by the liver; no appreciable accumulation of anti-GPA scFv/PA or PA was observed in the lungs or other major organs (Fig. 5A). It is worth mentioning that, when we measured the pharmacokinetics of free PA, we recovered ∼40% of the injected dose in the major organs 1 h after injection. The half-life of free PA in circulation is several minutes because of rapid elimination by the liver and kidneys. Thus, we infer that the rest of the protein was excreted into urine and bile, which we could not collect and analyze because we did not use metabolic cages. The blood level of anti-GPA scFv/PA decreased gradually over time following a two-phase kinetics with a T1/2 of approximately 2 and 10 h for the rapid and slow phases, respectively (Fig. 5B). The major fraction of anti-GPA scFv/PA recovered in blood circulates bound to the RBCs for at least the first day after intravenous injection, whereas the plasma concentration peaked at 3 to 6 h and was nearly undetectable by 24 h (Fig. 5B, inset).
Fig. 5.
Pharmacokinetics of 125I-anti-GPA scFv/PA fusion protein versus 125I-PA in mice. A, organ distribution of 125I-anti-GPA scFv/PA fusion protein versus 125I-PA after 1 h of circulation in mice. Inset, blood components distribution of 125I-anti-GPA scFv/PA fusion protein versus 125I-PA after 1 h of circulation in mice. B, blood clearance of 125I-anti-GPA scFv/PA fusion protein during 24 h of circulation after intravenous injection in mice. Inset, percentage of 125I-anti-GPA scFv/PA fusion protein recovered in plasma versus RBC pellet in blood obtained at the indicated times after intravenous injection of 125I-anti-GPA scFv/PA fusion protein in mice. (The number of animals in all experiments is 5 per group.) In each experiment the mean ± 1 S.E.M. is shown.
Fibrinolytic Activity of and Prophylactic Thrombolysis by Anti-GPA scFv/PA Injected in Animals.
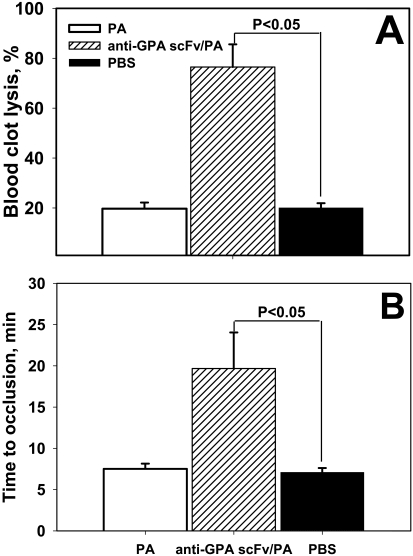
To test whether anti-GPA scFv/PA injected in mice retains its fibrinolytic activity, we analyzed the ex vivo lysis of clots formed from blood collected from mice without anticoagulants 45 min after intravenous injection of vehicle control (PBS), nontargeted PA or anti-GPA scFv/PA at equimolar doses. Clots formed from blood of animals injected with nontargeted PA did not undergo greater lysis than those formed from the blood of PBS-injected mice (Fig. 6A). In agreement with the higher blood level of RBC-targeted anti-GPA scFv/PA observed at this time point, we observed nearly complete lysis of clots formed from blood of mice injected with the fusion protein. These data indicate that the circulating, RBC-bound anti-GPA scFv/PA retains PA activity in vivo.
Fig. 6.
Fibrinolytic activity of circulating anti-GPA scFv/PA/mRBC complex in arterial thrombosis. A, fibrinolytic activity recovered in mouse blood samples obtained 45 min after intravenous injection of 4 mg/kg dose of anti-GPA scFv/PA fusion protein, 2 mg/kg PA or saline. n = 4 per group; p < 0.05. B, occlusive thrombi were formed in the carotid artery of mice by applying FeCl3 to adventitia. Thirty minutes before injury 4 mg/kg dose of anti-GPA scFv/PA fusion protein, 2 mg/kg PA or saline were injected (intravenously jugular vein). Time of complete vessel occlusion was determined with Doppler ultrasound. Data shown as mean ± S.E.M.; n = 8 per group; p < 0.05.
Based on this encouraging outcome, we then injected the same formulations in mice 30 min before inducing a thrombus in the carotid artery. Doppler analysis of perfusion through the carotid artery revealed that prophylactic administration of anti-GPA scFv/PA, but not nontargeted PA, caused a 3-fold delay in the time to vascular occlusion (Fig. 6B). Therefore, in vivo loading of anti-GPA scFv/PA onto circulating RBCs provides thromboprophylaxis not feasible with its soluble nontargeted counterpart in this model, which is characterized by precipitous activation of platelets and coagulation cascade in a high-shear stress vessel.
Last, we tested anti-GPA scFv/PA in a model of jugular venous thrombosis. Venous (“red”) clots, comprising predominantly fibrin and RBCs, might be especially amenable to RBC-tPA, compared with arterial (“white”) thrombi, which are populated predominantly by platelets. Anti-GPA scFv/PA injected 30 min before jugular injury almost completely prevented vascular occlusion for the entirety of the experiment (Fig. 7, C and D), most likely because of expedited lysis of nascent “red” clots as they were forming in response to vascular injury (compare Fig. 7, A and C, showing typical records of blood perfusion in the vessel). In contrast, untargeted PA delayed, but failed to prevent, vascular occlusion (Fig. 7, B and D). Therefore, RBC-targeted anti-GPA scFv/PA, but not untargeted PA, preserved blood flow in the injured vein (Fig. 7E).
Fig. 7.
Fibrinolytic activity of circulating anti-GPA scFv/PA/mRBC complex in venous thrombosis. A–C show the typical records of the blood flow in injured jugular veins in mice monitored by Doppler. Saline (A), untargeted tPA (B), or equimolar dose of anti-GPA scFv/PA fusion protein (C), was injected intravenously in a contralateral jugular vein 30 min before injury inflicted by FeCl3. D, analysis of data collected in these animal groups depicted as the time needed to attain the 50% reduction of the blood flow after the injury. E, analysis of the data collected in these groups presented as percentage of retention of the blood flow in the jugular vein during 30 min after induction of injury in mice treated with prophylactic administration of equimolar doses of PA versus anti-GPA scFv/PA 30 min before injury. In D and E, the mean ± S.E.M. is shown; n = 6 per group; p < 0.05.
Discussion
Thrombosis is the leading cause of mortality and disability in the United States (Jackson and Clagett, 1998). Thrombi are prone to recur within hours to days after a myocardial infarction, stroke, transient ischemic attack, or pulmonary embolism, and when patients are immobilized (Wartenberg et al., 2004). Thromboembolism is also a common and dangerous complication of surgery, a setting that is especially difficult to manage because of the risk of exacerbating bleeding at the operative site. Therefore, situations in which patients are at high and predictable risk for thrombosis to occur (or recur), i.e., postsurgical patients, are known. However, antiplatelet and anticoagulant agents provide only limited prophylaxis and often pose considerable risk of bleeding, especially perioperatively (Zlokovic, 1997; Konstantopoulos and Mousa, 2001). Plasminogen activators (PAs) are used for acute therapy of thrombosis in very circumscribed high-risk settings (Topol et al., 1987; Holvoet et al., 1993). However, inadequate delivery (blood clearance within <15 min (Narita et al., 1995), inactivation by plasma inhibitors such as PAI-1 (Reilly et al., 1991), and impermeability of occlusive clots (Rijken et al., 2004) restrict the effectiveness of therapeutic fibrinolysis by PA. For example, very high doses of PAs (e.g., ∼100 mg of tissue type PA, tPA) are needed to overcome these obstacles. At these high concentrations, drug may diffuse into hemostatic mural clots, predisposing to unwanted bleeding episodes, or into tissues such as the CNS (Wang et al., 1998) where it may cause cerebral hemorrhage, damage the blood-brain-barrier and direct neurotoxicity (Lo et al., 2003). Attempts to improve PA delivery and benefit/risk ratios have not yielded decisively better outcomes because the fundamental limitations with their use have not been overcome (Runge et al., 1996).
We hypothesized that prophylactic delivery of a PA would result in its incorporation into the interior of early nascent thrombi, arresting clot propagation and promoting clot lysis. This strategy would result in a more homogeneous drug delivery into the nascent thrombus rather than “therapeutic” fibrinolysis by external PA only effective on the clot surface. This, in turn, would minimize the incidence of secondary emboli and thrombus re-formation. Short-term prophylactic use of PA in patients at high risk of imminent primary or recurrent thrombosis would also be predicted to reduce formation of occlusive clots impervious to delayed fibrinolysis.
Existing fibrinolytics are not used for prophylaxis because of their rapid clearance and serious side effects. Even newly designed mutant PAs with enhanced potency, including tPA variants with a mutated PAI-1 binding site and deleted accessory domains implicated in tPA clearance and adverse vascular signaling, i.e., Retavase and Tenekteplase (Chapman et al., 2001), are likely to show limited diffusion into occlusive clots. Furthermore, all existing PAs are short-lived (<30 min), which makes them fundamentally inadequate for prophylaxis, and small (proteins with molecular mass of 30–60 kDa, <10 nm diameter), which permits diffusion into hemostatic clots, increasing the risk of bleeding and increasing the propensity for collateral tissue damage, especially in the CNS. As of today, no PA has been designed for use as thromboprophylaxis.
Diverse drug delivery systems including liposomes have been used to improve pharmacokinetics of plasminogen activators (Gupta et al., 2005; Elbayoumi and Torchilin, 2008). Previous studies from our group indicate that RBC provides a good carrier for fibrinolytics. Prior experiments in animal models of thrombosis documented that ex vivo coupling of tPA to carrier RBCs (RBC/tPA) provides effective and safe thromboprophylaxis, with the potential to shift the current paradigm for clot prevention (Murciano et al., 2003; Zaitsev et al., 2006; Danielyan et al., 2008). Such an ex vivo approach to RBC coating by drugs might be suitable in settings where transfusion is common, but would be less practical in other settings. To avoid the need to couple tPA to isolated RBCs ex vivo followed by transfusion, we have targeted PA to circulating RBCs directly by conjugating tPA to a CR1 monoclonal antibody. We showed that the anti-CR1/tPA conjugate binds without harm to circulating RBCs in mice and provides safe and effective thromboprophylaxis (Zaitsev et al., 2006) comparable with that provided by infusion of RBC/tPA (Murciano et al., 2003).
However, antibody conjugates, useful in animal studies, are suboptimal for clinical use and would be challenging to produce in sufficient quantities. Furthermore, there is the potential to form large (molecular mass >270 kDa), heterogeneous, and multimeric conjugates that may activate cellular defense mechanisms and complement via Fc-fragments. To circumvent these problems and to achieve predictable coating levels over a wide range of drug concentrations, we designed recombinant PA variants fused to an antigen-binding vehicle (single-chain variable fragment [scFv]) directed to mouse glycophorin A (Spitzer et al., 2004).
Expression of scFv/PA through recombinant technology enables large-scale production of homogeneous monovalent scFv/PA fusion proteins (Holvoet et al., 1993). Because scFv's lack the Fc portion of an intact antibody, the risk of immune-mediated side effects lessened. Established techniques for humanization and methods to reduce the potential immunogenicity of scFv chimeras further help to minimize the potential for eliciting immune reactions (Almagro and Fransson, 2008). Last, the modular recombinant format used in our studies supports the synthesis of targeted variant PA prodrugs lacking domains that may elicit untoward effects.
The results shown in the present article provide proof-of-principle for the proposed strategy. Anti-GPA scFv/PA (Fig. 1) bound to RBCs and invested them with PA activity (Figs. 2 and 3). When injected at equimolar doses in mice, RBC-targeted anti-GPA scFv/PA exhibited markedly higher fibrinolytic activity in the circulation than a nontargeted PA variant (Figs. 6 and 7). This was probably due to binding of the anti-GPA scFv/PA to circulating RBCs, which enhanced its circulation time and the bioavailability in vivo (Fig. 5). This major change in the pharmacokinetics of anti-GPA scFv/PA compared with nontargeted PA overrode a partial reduction of its resultant specific fibrinolytic activity in vitro compared with free anti-GPA scFv/PA (Fig. 3C). This inequity of fibrinolytic potency in vitro may reflect restricted diffusional freedom within the clot of anti-GPA scFv/PA bound to the large RBC carrier, which is relatively immobile in the clot meshwork. Therefore, it is likely that in vivo comparison of fibrinolytic activity of anti-GPA scFv/PA versus PA at equipotent rather than equimolar doses would reveal an even more profound advantage of the RBC-targeted anti-GPA scFv/PA over its nontargeted counterpart.
Testing of anti-GPA scFv/PA in mouse models of thrombosis provided important new in vivo findings relevant to pharmacological properties of this potential biotherapeutic. Anti-GPA scFv/PA dissolved venous versus arterial thrombi more effectively, as expected. However, alleviation of arterial thrombotic occlusion by anti-GPA scFv/PA implies that sufficient drug-loaded RBCs were incorporated into the clot even at high shear to achieve their intended effect.
The results reported here warrant further systematic evaluation of dosing, timing, and duration of beneficial and potential adverse effects of anti-GPA scFv/PA in laboratory animals. For example, the duration of prophylaxis here was limited and would need to be extended to be of clinical use. However, the stability of the RBC-scFv/PA complex in vivo portends protracted thromboprophylactic activity, although this remains to be proven. Moreover, subtle effects on GPA function with protracted use not evident in this study will have to be excluded. It will also be necessary to replace the anti-murine GPA scFv with an anti-human GPA to invest it with clinical utility. However, this study provides a modular template to design diverse iterations of this novel class of biotherapeutics by use of recombinant and mutagenesis techniques to vary their affinity and binding sites on RBCs and molecular structure of plasminogen activator domains involved in regulation of such important auxiliary features as resistance to plasma inhibitors, interaction with vascular receptors, and regulation of the enzymatic activity by fibrin and other components of clots and clotting cascade. These additional molecular modifications may further enhance the clinical utility, safety, potency, and specificity of RBC-targeted plasminogen activators.
Supplementary Material
This work was supported by the National Institutes of Health [Grant R01 HL090687] (to V.R.M.); the American Heart Association [Grant SDG 0535258N] (to S.Z.); and Fondo de Investigaciones Sanitarias [Grant PI081795] (to J.-C.M.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.159194.
- tPA
- tissue type plasminogen activator
- PA
- plasminogen activator
- PAI-1
- plasminogen activator inhibitor type 1
- scFv
- single chain antibody variable fragment
- GPA
- glycophorin A
- RBC
- red blood cell
- CNS
- central nervous system
- CR1
- human complement receptor type 1
- ETI
- Erythrina trypsin inhibitor
- SFM
- serum-free cell culture medium
- PAGE
- polyacrylamide gel electrophoresis
- PBS
- phosphate-buffered saline
- BSA
- bovine serum albumin
- PCR
- polymerase chain reaction.
References
- Almagro JC, Fransson J. (2008) Humanization of antibodies. Front Biosci 13:1619–1633 [DOI] [PubMed] [Google Scholar]
- Armstead WM, Ganguly K, Kiessling JW, Chen XH, Smith DH, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR. (2009) Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab 29:1463–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham DJ, Hebert LA. (2001) CR1 and CR1-like: the primate immune adherence receptors. Immunol Rev 180:100–111 [DOI] [PubMed] [Google Scholar]
- Chapman DF, Lyden P, Lapchak PA, Nunez S, Thibodeaux H, Zivin J. (2001) Comparison of TNK with wild-type tissue plasminogen activator in a rabbit embolic stroke model. Stroke 32:748–752 [DOI] [PubMed] [Google Scholar]
- Collen D. (1996) Fibrin-selective thrombolytic therapy for acute myocardial infarction. Circulation 93:857–865 [DOI] [PubMed] [Google Scholar]
- Danielyan K, Ganguly K, Ding BS, Atochin D, Zaitsev S, Murciano JC, Huang PL, Kasner SE, Cines DB, Muzykantov VR. (2008) Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation 118:1442–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov and Cheng, 2001.Davydov L, Cheng JW. (2001) Tenecteplase: a review. Clin Ther 23:982–997 [DOI] [PubMed] [Google Scholar]
- Ding et al., 2005.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. (2005) Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood 106:4191–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding et al., 2008.Ding BS, Hong N, Murciano JC, Ganguly K, Gottstein C, Christofidou-Solomidou M, Albelda SM, Fisher AB, Cines DB, Muzykantov VR. (2008) Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood 111:1999–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbayoumi and Torchilin, 2008.Elbayoumi TA, Torchilin VP. (2008) Liposomes for targeted delivery of antithrombotic drugs. Expert Opin Drug Deliv 5:1185–1198 [DOI] [PubMed] [Google Scholar]
- Fearon et al., 1989.Fearon DT, Klickstein LB, Wong WW, Wilson JG, Moore FD, Jr, Weis JJ, Weis JH, Jack RM, Carter RH, Ahearn JA. (1989) Immunoregulatory functions of complement: structural and functional studies of complement receptor type 1 (CR1; CD35) and type 2 (CR2; CD21). Prog Clin Biol Res 297:211–220 [PubMed] [Google Scholar]
- Furthmayr and Marchesi, 1976.Furthmayr H, Marchesi VT. (1976) Subunit structure of human erythrocyte glycophorin A. Biochemistry 15:1137–1144 [DOI] [PubMed] [Google Scholar]
- Ganguly et al., 2006.Ganguly K, Goel MS, Krasik T, Bdeir K, Diamond SL, Cines DB, Muzykantov VR, Murciano JC. (2006) Fibrin affinity of erythrocyte-coupled tissue-type plasminogen activators endures hemodynamic forces and enhances fibrinolysis in vivo. J Pharmacol Exp Ther 316:1130–1136 [DOI] [PubMed] [Google Scholar]
- Ganguly et al., 2005.Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines DB, Muzykantov VR, Murciano JC. (2005) Blood clearance and activity of erythrocyte-coupled fibrinolytics. J Pharmacol Exp Ther 312:1106–1113 [DOI] [PubMed] [Google Scholar]
- Ganguly et al., 2007.Ganguly K, Murciano JC, Westrick R, Leferovich J, Cines DB, Muzykantov VR. (2007) The glycocalyx protects erythrocyte-bound tissue type plasminogen activator from enzymatic inhibition. J Pharmacol Exp Ther 321:158–164 [DOI] [PubMed] [Google Scholar]
- Gupta et al., 2005.Gupta AS, Huang G, Lestini BJ, Sagnella S, Kottke-Marchant K, Marchant RE. (2005) RGD-modified liposomes targeted to activated platelets as a potential vascular drug delivery system. Thromb Haemost 93:106–114 [DOI] [PubMed] [Google Scholar]
- Heussen et al., 1984.Heussen C, Joubert F, Dowdle EB. (1984) Purification of human tissue plasminogen activator with Erythrina trypsin inhibitor. J Biol Chem 259:11635–11638 [PubMed] [Google Scholar]
- Holvoet et al., 1993.Holvoet P, Laroche Y, Stassen JM, Lijnen HR, Van Hoef B, De Cock F, Van Houtven A, Gansemans Y, Matthyssens G, Collen D. (1993) Pharmacokinetic and thrombolytic properties of chimeric plasminogen activators consisting of a single-chain Fv fragment of a fibrin-specific antibody fused to single-chain urokinase. Blood 81:696–703 [PubMed] [Google Scholar]
- Jackson and Clagett, 1998.Jackson MR, Clagett GP. (1998) Antithrombotic therapy in peripheral arterial occlusive disease. Chest 114:666S–682S [DOI] [PubMed] [Google Scholar]
- Kina et al., 2000.Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. (2000) The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol 109:280–287 [DOI] [PubMed] [Google Scholar]
- Kohnert et al., 1992.Kohnert U, Rudolph R, Verheijen JH, Weening-Verhoeff EJ, Stern A, Opitz U, Martin U, Lill H, Prinz H, Lechner M. (1992) Biochemical properties of the kringle 2 and protease domains are maintained in the refolded t-PA deletion variant BM 06.022. Protein Eng 5:93–100 [DOI] [PubMed] [Google Scholar]
- Konstantopoulos and Mousa, 2001.Konstantopoulos K, Mousa SA. (2001) Antiplatelet therapies: platelet GPIIb/IIIa antagonists and beyond. Curr Opin Invest Drugs 2:1086–1092 [PubMed] [Google Scholar]
- Lo et al., 2003.Lo EH, Dalkara T, Moskowitz MA. (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4:399–415 [DOI] [PubMed] [Google Scholar]
- Martin et al., 1991.Martin U, Fischer S, Kohnert U, Rudolph R, Sponer G, Stern A, Strein K. (1991) Pharmacokinetic properties of an Escherichia-coli-produced recombinant plasminogen activator (BM 06.022) in rabbits. Thromb Res 62:137–146 [DOI] [PubMed] [Google Scholar]
- Murciano et al., 2003.Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. (2003) Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol 21:891–896 [DOI] [PubMed] [Google Scholar]
- Narita et al., 1995.Narita M, Bu G, Herz J, Schwartz AL. (1995) Two receptor systems are involved in the plasma clearance of tissue-type plasminogen activator (t-PA) in vivo. J Clin Invest 96:1164–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly et al., 1991.Reilly CF, Fujita T, Mayer EJ, Siegfried ME. (1991) Both circulating and clot-bound plasminogen activator inhibitor-1 inhibit endogenous fibrinolysis in the rat. Arterioscler Thromb 11:1276–1286 [DOI] [PubMed] [Google Scholar]
- Rijken et al., 2004.Rijken DC, Barrett-Bergshoeff MM, Jie AF, Criscuoli M, Sakharov DV. (2004) Clot penetration and fibrin binding of amediplase, a chimeric plasminogen activator (K2 tu-PA). Thromb Haemost 91:52–60 [DOI] [PubMed] [Google Scholar]
- Runge et al., 1996.Runge MS, Harker LA, Bode C, Ruef J, Kelly AB, Marzec UM, Allen E, Caban R, Shaw SY, Haber E, et al. (1996) Enhanced thrombolytic and antithrombotic potency of a fibrin-targeted plasminogen activator in baboons. Circulation 94:1412–1422 [DOI] [PubMed] [Google Scholar]
- Sakharov and Rijken, 1995.Sakharov DV, Rijken DC. (1995) Superficial accumulation of plasminogen during plasma clot lysis. Circulation 92:1883–1890 [DOI] [PubMed] [Google Scholar]
- Schwartz et al., 1997.Schwartz RS, Rybicki AC, Nagel RL. (1997) Molecular cloning and expression of a chloride channel-associated protein pICln in human young red blood cells: association with actin. Biochem J 327:609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer et al., 2004.Spitzer D, Unsinger J, Bessler M, Atkinson JP. (2004) ScFv-mediated in vivo targeting of DAF to erythrocytes inhibits lysis by complement. Mol Immunol 40:911–919 [DOI] [PubMed] [Google Scholar]
- Spitzer et al., 2005.Spitzer D, Unsinger J, Mao D, Wu X, Molina H, Atkinson JP. (2005) In vivo correction of complement regulatory protein deficiency with an inhibitor targeting the red blood cell membrane. J Immunol 175:7763–7770 [DOI] [PubMed] [Google Scholar]
- Tanswell et al., 2002.Tanswell P, Modi N, Combs D, Danays T. (2002) Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet 41:1229–1245 [DOI] [PubMed] [Google Scholar]
- Topol et al., 1987.Topol EJ, Morris DC, Smalling RW, Schumacher RR, Taylor CR, Nishikawa A, Liberman HA, Collen D, Tufte ME, Grossbard EB. (1987) A multicenter, randomized, placebo-controlled trial of a new form of intravenous recombinant tissue-type plasminogen activator (activase) in acute myocardial infarction. J Am Coll Cardiol 9:1205–1213 [DOI] [PubMed] [Google Scholar]
- Wang et al., 1998.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. (1998) Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med 4:228–231 [DOI] [PubMed] [Google Scholar]
- Wartenberg et al., 2004.Wartenberg KE, Patsalides A, Yepes MS. (2004) Is magnetic resonance spectroscopy superior to conventional diagnostic tools in hypoxic-ischemic encephalopathy? J Neuroimaging 14:180–186 [PubMed] [Google Scholar]
- Zaitsev et al., 2006.Zaitsev S, Danielyan K, Murciano JC, Ganguly K, Krasik T, Taylor RP, Pincus S, Jones S, Cines DB, Muzykantov VR. (2006) Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood 108:1895–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic, 1997.Zlokovic BV. (1997) Antithrombotic, procoagulant, and fibrinolytic mechanisms in cerebral circulation: implications for brain injury and protection. Neurosurg Focus 2:e5 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.