Abstract
Treatment with angiotensin II type 1 receptor blockers (ARBs) is the first-line therapy for hypertensive patients with diabetic nephropathy. However, emerging clinical evidence indicates that mineralocorticoid receptor (MR) blockers have blood pressure-independent antiproteinuric effects. We sought to determine whether treatment with an MR blocker, eplerenone, enhances the effects of an ARB, telmisartan, on podocyte injury and proteinuria in type 2 diabetic Otsuka-Long-Evans-Tokushima-Fatty (OLETF) rats. From 20 to 50 weeks old, diabetic OLETF rats showed higher systolic blood pressure (SBP) and urinary protein excretion (UproteinV) than nondiabetic control Long-Evans-Tokushima-Otsuka rats. At 50 weeks old, OLETF rats also showed glomerular sclerosis and podocyte injury, whereas nephrin and podocin mRNA levels in isolated glomeruli were significantly decreased. Treatment with telmisartan (3 mg/kg/day p.o.) decreased SBP and UproteinV, increased nephrin and podocin mRNA levels, and attenuated glomerular sclerosis and podocyte injury. Eplerenone (100 mg/kg/day p.o.) did not alter SBP but elicited similar changes in renal parameters. However, greater reductions in UproteinV and podocyte injury and greater increases in nephrin and podocin mRNA levels were observed in the combination treatment group. Hydralazine (25 mg/kg/day p.o.) decreased SBP but did not alter any renal parameters. These data indicate that MR blockade enhances the SBP-independent antiproteinuric effect of an ARB through inhibiting podocyte injury in type 2 diabetic rats.
The progression of proteinuria increases the risk of renal and cardiovascular diseases in type 2 diabetes. In type 2 diabetic hypertensive patients, treatment with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II (AngII) type 1 receptor blockers (ARBs) is more effective in reducing proteinuria than other traditional antihypertensive therapies (Sasso et al., 2002; Ogawa et al., 2007), suggesting the blood pressure-independent antiproteinuric effects of AngII blockade. Other studies have demonstrated that remission of nephrotic-range proteinuria with ACEIs is associated with substantial reductions in the risk of renal and cardiovascular events, leading to greatly improved survival in type 2 diabetic patients (Rossing et al., 2005). Therefore, most national guideline groups have recommended the use of ACEIs or ARBs in preference to other antihypertensive agents for hypertensive patients with diabetic nephropathy (Buse et al., 2007; Mancia et al., 2007; Ogihara et al., 2009).
There is also increasing clinical evidence indicating that aldosterone blockade with mineralocorticoid receptor (MR) blockers elicits strong antiproteinuric effects (Kiyomoto et al., 2008). In hypertensive patients with type 2 diabetes, monotherapy with a nonselective MR antagonist, spironolactone, elicited blood pressure-lowering effects that are similar to those of the ACEI cilazapril; however, spironolactone is more effective than cilazapril in reducing proteinuria (Rachmani et al., 2004). Furthermore, the addition of spironolactone or a selective MR antagonist, eplerenone, to ACEIs or ARBs has no effect on blood pressure but markedly reduces proteinuria in patients with diabetic nephropathy (Chrysostomou and Becker, 2001; Sato et al., 2005). These observations suggest that targeting aldosterone with MR blockers amplifies the antiproteinuric effects of ACEIs and ARBs. However, the mechanisms by which combination therapy with AngII and MR blockers amalgamate their antiproteinuric effects in diabetes have not been clarified.
Recent studies indicate that glomerular podocyte (glomerular visceral epithelial cells) abnormalities, including functional changes, loss, and injury, are cardinal features of diabetic nephropathy (Wolf et al., 2005; Jefferson et al., 2008) and are closely involved in the progression of proteinuria (Wolf et al., 2005; Shankland, 2006; Jefferson et al., 2008). Therefore, the present study was undertaken to test the hypothesis that in type 2 diabetic rats treated with an ARB, the additive antiproteinuric effect of an MR blocker is associated with the inhibition of podocyte injury. To test this hypothesis, we examined the effects of an ARB, an MR blocker, and their combination on podocyte injury in type 2 diabetic Otsuka-Long-Evans-Tokushima-Fatty (OLETF) rats with overt proteinuria that exhibit pathological features of renal injury similar to those of human type 2 diabetes (Nagai et al., 2005; Nishiyama et al., 2008). We also measured the glomerular expressions of nephrin and podocin, which are functional molecules in the slit diaphragms located between the adjacent foot processes of podocytes (Wolf et al., 2005; Jefferson et al., 2008) and have critical roles in proteinuria in diabetes (Wolf et al., 2005; Jefferson et al., 2008).
Materials and Methods
Animals.
All experimental procedures were performed according to the guidelines for the care and use of animals established by the Osaka City General Hospital, Kagawa University Medical School (Kagawa, Japan) and Tulane University Health Sciences Center (New Orleans, Louisiana). In total, 60 4-week-old male OLETF rats and 10 age-matched male LETO rats (genetic control for OLETF rats) were supplied by Otsuka Pharmaceutical Co. Ltd. (Tokushima, Japan). After obtaining basal measurements at 20 weeks of age, LETO rats were treated with vehicle (0.5% methyl cellulose; Nacalai Tesque, Kyoto, Japan). OLETF rats were randomly divided into groups for treatment with vehicle (n = 12); an ARB, 4′-[(1,4-dimethyl-2′-propyl-[2,6′-bi-1H-benzimidazol]-1′-yl)methyl]-[1,1′-biphenyl]-2-carboxylic acid (telmisartan, 3 mg/kg/day; n = 12); an MR blocker, 9,11α-epoxy-7α-(methoxycarbonyl)-3-oxo-17α-pregn-4-ene-21,17β-carbolactone (eplerenone, 100 mg/kg/day; n = 12); and these in combination (n = 12) or with a nonspecific vasodilator, hydralazine (25 mg/kg/day; n = 12). Previous studies have shown that telmisartan and eplerenone selectively block AngII AT1 receptor and MR, respectively (Wienen et al., 1993; Delyani et al., 2001). Telmisartan, eplerenone, and hydralazine were dissolved in 0.5% methyl cellulose (approximately 0.5 ml) and treated by gavage. All animals continued to receive their treatments and were sacrificed at 50 weeks of age. We reported previously that 20-week-old OLETF rats exhibit higher postprandial blood glucose (PPBG) levels and urinary protein excretion rate (UproteinV; Nagai et al., 2005; Nishiyama et al., 2008). Furthermore, OLETF rats show glomerular sclerosis and overt proteinuria at approximately 50 weeks of age (Nagai et al., 2005). The doses of telmisartan, eplerenone, and hydralazine were determined on the basis of earlier rat studies (Balt et al., 2001; Nagai et al., 2005; Younis et al., 2007). We also have reported that eplerenone at a dose of 100 mg/kg/day prevents aldosterone-induced hypertension and renal injury in rats (Nishiyama et al., 2004). Furthermore, our preliminary experiments showed that eplerenone at 100 mg/kg/day did not change blood pressure or plasma potassium levels in OLETF rats (data not shown).
Systolic blood pressure (SBP) was measured five consecutive times in conscious rats by a tail-cuff plethysmograph (model BP-98A; Softron Co., Tokyo, Japan) after the animals had rested for at least 15 min; the mean value of the lowest three readings was recorded. Twenty-four-hour urine samples were collected using metabolic cages. After pentobarbital anesthesia (50 mg/kg i.p.), blood was collected from the abdominal aorta at 50 weeks of age. Then, retrograde perfusion with isotonic saline was performed after cross-clamping the abdominal aorta above the renal arteries and cutting the inferior caval vein near the renal vein (Nagai et al., 2005; Nishiyama et al., 2008). Thereafter, the right renal artery was clamped, and the right kidney was removed for isolating glomeruli by a sieving technique, as described previously (Kawachi et al., 2000). The left kidney was perfused with 10% buffered paraformaldehyde at a pressure of 95 to 135 mm Hg (depending on individual blood pressure at 50 weeks of age).
Histological Examination.
Kidneys were fixed with 10% formalin, pH 7.4, embedded in paraffin, sectioned into 3-μm-thick slices, and stained with periodic acid-Schiff (PAS) reagent. The extent of glomerular sclerosis was quantitatively evaluated by an automatic image analysis system using PAS-stained sections, as described previously (Kobori et al., 2005). The severity of podocyte injury was evaluated by immunohistochemistry for desmin, as described previously (Nagase et al., 2006; Nishiyama et al., 2008). The ratio of the affected lesions to each glomerulus or microscopic field was calculated using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). In total, 30 glomeruli were examined for each rat, and the average percentages of affected lesions were obtained for each rat. The above-mentioned histological analyses were performed by a robotic system with an image analysis software in a blinded manner to avoid any bias (Kobori et al., 2005; Nishiyama et al., 2008). Kidney samples were also evaluated by electron microscopy, as described previously (Nagase et al., 2006; Nishiyama et al., 2008). The ultrastructure of the podocytes was analyzed using a JEM-1200EX microscope (JEOL, Tokyo, Japan).
Real-Time Reverse Transcription-Polymerase Chain Reaction.
The mRNA levels of glyceraldehyde-3-phosphate dehydrogenase, nephrin, podocin, MR, serum glucocorticoid-dependent kinase Sgk)-1, collagen I and α-smooth muscle actin (SMA) in glomeruli were analyzed by real-time reverse transcription-polymerase chain reaction using a LightCycler FastStart DNA Master SYBR Green I kit (Roche Applied Science, Indianapolis, IN), as described previously (Hoffmann et al., 2004; Nagai et al., 2005). All data were expressed as the relative differences between OLETF and LETO rats after normalization to the corresponding glyceraldehyde-3-phosphate dehydrogenase expression.
Other Analytical Procedures.
Urinary protein and plasma creatinine (Cr) were determined using commercially available assay kits (micro-TP-test and Creatinine-test, Wako Pure Chemicals, Osaka, Japan, respectively). PPBG levels were measured with a glucose analyzer (Sanwa-Kagaku, Co. Ltd., Nagoya, Japan). Plasma aldosterone levels were measured with an enzyme-linked immunosorbent assay kit (Cayman Chemical, Ann Arbor, MI). Plasma sodium and potassium levels were measured using a flame photometer (model 750; Hitachi, Tokyo, Japan).
Statistical Analysis.
Values are presented as means ± S.E. Statistical comparisons of differences were performed using one- or two-way analysis of variance combined with the Newman-Keuls post hoc test. Values of P < 0.05 were considered statistically significant.
Results
Body Weight, Kidney Weight, Blood Glucose, Plasma Sodium, Potassium, Cr, and Aldosterone.
Body weight; right kidney weight; PPBG; and plasma sodium, potassium, Cr, and aldosterone levels are shown in Table 1. At 50 weeks of age, the body weight of vehicle-treated OLETF rats was greater than that of vehicle-treated LETO rats. Furthermore, vehicle-treated OLETF rats showed higher PPBG levels than vehicle-treated LETO rats. None of the treatments affected the body weight and PPBG of OLETF rats. Right kidney weight of vehicle-treated OLETF rats was greater than that of vehicle-treated LETO rats. When treated with telmisartan, eplerenone, and their combination, there was similarly decreased right kidney weight in OLETF rats, whereas hydralazine did not show any significant alteration. Plasma sodium, potassium, and Cr levels were not significantly different among the groups at 50 weeks of age. Plasma aldosterone levels were significantly higher in eplerenone- and telmisartan plus eplerenone-treated OLETF rats than in other groups (Table 1).
TABLE 1.
Animal data at 50 weeks of age
| LETO + Vehicle | OLETF + Vehicle | OLETF + Telmisartan | OLETF + Eplerenone | OLETF + Telmisartan + Eplerenone | OLETF + Hydralazine | |
|---|---|---|---|---|---|---|
| No. | 10 | 12 | 12 | 12 | 12 | 12 |
| BW (g) | 500 ± 15 | 629 ± 25* | 602 ± 28* | 608 ± 23* | 611 ± 21* | 605 ± 29* |
| Right KW (g) | 1.31 ± 0.03 | 2.45 ± 0.13* | 1.92 ± 0.11*† | 1.96 ± 0.15*† | 1.86 ± 0.09*† | 2.28 ± 0.21* |
| Right KW/BW ratio (%) | 0.27 ± 0.02 | 0.38 ± 0.04* | 0.32 ± 0.03* | 0.32 ± 0.04* | 0.29 ± 0.04 | 0.35 ± 0.06* |
| PPBG (mg/dl) | 6 ± 2 | 14 ± 3* | 13 ± 2* | 13 ± 4* | 13 ± 3* | 14 ± 4* |
| Plasma sodium (mEq/l) | 144 ± 5 | 145 ± 8 | 143 ± 6 | 142 ± 5 | 141 ± 6 | 144 ± 8 |
| Plasma potassium (mEq/l) | 4.2 ± 0.3 | 4.4 ± 0.3 | 4.8 ± 0.5 | 4.9 ± 0.4 | 4.9 ± 0.6 | 4.8 ± 0.6 |
| Plasma Cr (mg/dl) | 0.39 ± 0.03 | 0.41 ± 0.05 | 0.41 ± 0.04 | 0.40 ± 0.03 | 0.39 ± 0.05 | 0.43 ± 0.06 |
| Plasma aldosterone (pg/ml) | 281 ± 44 | 368 ± 61 | 216 ± 49† | 873 ± 102*† | 726 ± 74*† | 383 ± 48 |
BW, body weight; KW, kidney weight.
P < 0.05 vs. LETO + vehicle.
P < 0.05; OLETF + vehicle vs. OLETF + telmisartan, eplerenone, or telmisartan + eplerenone.
SBP and UproteinV.
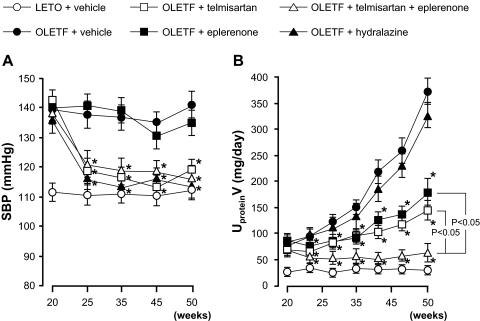
The temporal profile of SBP is shown in Fig. 1A. During the observation period, vehicle-treated OLETF rats showed higher SBP than vehicle-treated LETO rats (141 ± 5 versus 112 ± 3 mm Hg at 50 weeks of age). Treatment with telmisartan and telmisartan plus eplerenone or hydralazine resulted in similar reductions in SBP (119 ± 4, 117 ± 3, and 115 ± 5 mm Hg, respectively, at 50 weeks of age), whereas eplerenone alone did not change SBP.
Fig. 1.
Profiles of SBP (A) and UproteinV (B). Vehicle-treated diabetic OLETF rats develop hypertension and proteinuria. In these animals, treatment with telmisartan, telmisartan + eplerenone or hydralazine decreases SBP to similar levels, whereas SBP is not altered by eplerenone. Treatment with telmisartan or eplerenone similarly attenuates the progression of proteinuria in OLETF rats. In contrast, the combination of telmisartan and eplerenone greatly prevents the progression of proteinuria. Hydralazine does not significantly alter the progression of proteinuria in OLETF rats. ∗, P < 0.05; OLETF + vehicle versus OLETF + telmisartan, eplerenone, telmisartan + eplerenone or hydralazine.
The temporal profiles of UproteinV are shown in Fig. 1B. At 20 weeks of age, OLETF rats exhibited significantly higher UproteinV (79 ± 15 mg/day) compared with LETO rats (25 ± 6 mg/day). After 20 weeks of age, UproteinV of vehicle-treated OLETF rats progressively increased with age (378 ± 21 mg/day at 50 weeks of age). Treatment with telmisartan or eplerenone similarly attenuated the progression of proteinuria in OLETF rats (187 ± 24 and 148 ± 18 mg/day, respectively, at 50 weeks of age). In contrast, the combination of telmisartan and eplerenone greatly prevented the progression of proteinuria (62 ± 17 mg/day at 50 weeks of age). At 50 weeks of age, the value of UproteinV in telmisartan plus eplerenone-treated OLETF rats was significantly lower than that in telmisartan or eplerenone-treated OLETF rats (P < 0.05). Treatment with hydralazine did not significantly alter the progression of proteinuria in OLETF rats (324 ± 22 mg/day at 50 weeks of age).
Histological Findings.
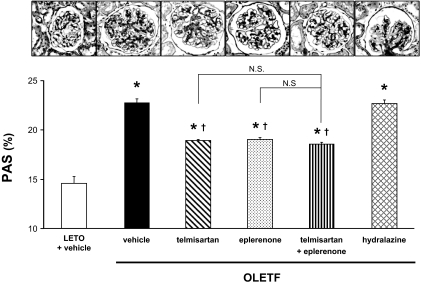
The histological findings after staining with PAS at 50 weeks of age are shown in Fig. 2. In LETO rats, there were no significant findings except for minor alterations in the glomeruli resulting from aging. However, vehicle-treated OLETF showed mesangial expansion accompanied by an accumulation of extracellular matrix. Quantitative analyses confirmed that the glomerular PAS-positive area in vehicle-treated OLETF rats was significantly larger than that in LETO rats at 50 weeks of age (23 ± 1 versus 14 ± 2%). In OLETF rats, treatment with telmisartan, eplerenone, or their combination significantly decreased the PAS-positive area in glomeruli. However, there was no significant difference in glomerular PAS-positive areas among telmisartan-, eplerenone-, or telmisartan- plus eplerenone-treated OLETF rats (18 ± 1, 19 ± 1, and 17 ± 1%, respectively). Hydralazine did not affect the glomerular PAS-positive area (23 ± 1%).
Fig. 2.
Photomicrographs of glomeruli stained with PAS (original magnification, 200×) at 50 weeks of age. Vehicle-treated OLETF rats show mesangial expansion accompanied by an accumulation of extracellular matrix and capillary wall thickening. Quantitative analyses confirm that the glomerular PAS-positive area in vehicle-treated OLETF rats is significantly larger than that in LETO rats. In OLETF rats, treatment with telmisartan, eplerenone, or their combination significantly decreases the PAS-positive area in glomeruli. However, there are no significant differences in glomerular PAS-positive area among telmisartan-, eplerenone- or telmisartan + eplerenone-treated OLETF rats. In contrast, hydralazine does not affect the glomerular PAS-positive area. ∗, P < 0.05 versus LETO rats. †, P < 0.05; OLETF + vehicle versus OLETF + telmisartan, eplerenone, telmisartan + eplerenone or hydralazine.
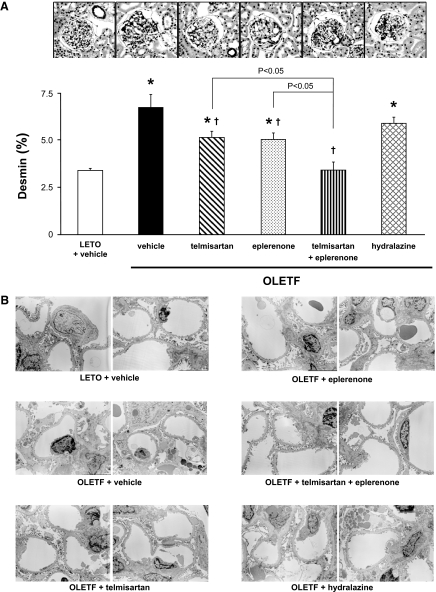
As shown in Fig. 3A, the desmin-positive area in glomeruli was significantly larger in OLETF rats than in LETO rats at 50 weeks of age (6.8 ± 0.7 versus 3.4 ± 0.2%). In OLETF rats, treatment with telmisartan or eplerenone significantly decreased the desmin-positive area in glomeruli (5.1 ± 0.3 and 5.0 ± 0.4%, respectively). Treatment with telmisartan plus eplerenone further decreased the glomerular desmin-positive area to values that did not differ significantly from those of vehicle-treated LETO rats (3.4 ± 0.4%). The value of the desmin-positive area in telmisartan- plus eplerenone-treated OLETF rats was significantly smaller than those in telmisartan- or eplerenone-treated OLETF rats (P < 0.05). Hydralazine did not affect the glomerular desmin positive area (6.0 ± 0.4%).
Fig. 3.
A, glomerular histological findings for desmin immunohistochemistry (original magnification, 200×) at 50 weeks of age. The desmin-positive area is significantly larger in OLETF than in LETO rats. In OLETF rats, treatment with telmisartan or eplerenone significantly decreases the desmin-positive area in glomeruli. Treatment with telmisartan plus eplerenone further decreases the glomerular desmin-positive area to values that do not differ significantly from those of vehicle-treated LETO rats. Alternatively, hydralazine does not affect the glomerular desmin positive area. ∗, P < 0.05 versus LETO rats. †, P < 0.05; OLETF + vehicle versus OLETF + telmisartan, eplerenone, or telmisartan + eplerenone. B, glomerular histological findings for podocyte ultrastructure (original magnification, 3000×). At 50 weeks of age, significant podocyte morphological changes, including foot-process effacement, degeneration of the cell body, and accumulation of cytoplasmic granules, are observed in vehicle-treated OLETF rats. These podocyte morphological changes are barely observed in OLETF rats treated with telmisartan plus eplerenone.
The histological findings for podocyte ultrastructure are shown in Fig. 3B. At 50 weeks of age, significant podocyte morphological changes, including foot-process effacement, degeneration of the cell body, and accumulation of cytoplasmic granules, were observed in vehicle-treated OLETF rats but not in vehicle-treated LETO rats. In OLETF rats, it appears that these podocyte morphological changes were partially decreased by treatment with telmisartan or eplerenone. Furthermore, these podocyte morphological changes were markedly attenuated by their combination treatment. Conversely, these podocyte morphological changes did not differ between vehicle- and hydralazine-treated OLETF rats (Fig. 3B).
Glomerular mRNA Levels of Nephrin, Podocin, MR, Sgk-1, Collagen I, and α-SMA.
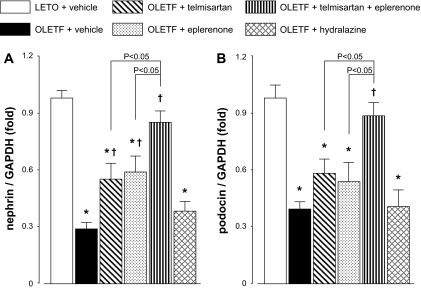
Figure 4, A and B, shows the mRNA levels of functional molecules of nephrin and podocin (Wolf et al., 2005; Jefferson et al., 2008) in isolated glomeruli at 50 weeks of age. The mRNA levels of nephrin and podocin in vehicle-treated OLETF rats were significantly lower than those in vehicle-treated LETO rats. In OLETF rats, treatment with telmisartan or eplerenone partially attenuated the reductions in glomerular nephrin mRNA levels. Reductions in glomerular podocin mRNA levels also tended to be attenuated by telmisartan or eplerenone, but these changes did not reach statistically significant. Alternatively, treatment with telmisartan plus eplerenone prevented the reductions in glomerular nephrin and podocin mRNA levels that did not significantly differ from those in LETO rats. The values of glomerular nephrin and podocin mRNA levels in telmisartan plus eplerenone-treated OLETF rats were significantly higher than those in telmisartan or eplerenone-treated OLETF rats (P < 0.05). Treatment with hydralazine did not alter the levels of nephrin and podocin mRNA in OLETF rats.
Fig. 4.
mRNA levels of the functional molecules of podocyte, nephrin (A) and podocin (B), in isolated glomeruli at 50 weeks of age. The levels of nephrin and podocin mRNA in vehicle-treated OLETF rats are significantly lower than those in vehicle-treated LETO rats. In OLETF rats, treatment with telmisartan or eplerenone partially attenuates the reductions in glomerular nephrin mRNA levels. Reductions in glomerular podocin mRNA levels also tend to be attenuated by telmisartan or eplerenone, but these changes do not reach statistically significant. Treatment with telmisartan + eplerenone prevents the reductions in glomerular nephrin and podocin mRNA levels, which are not significantly different from those in LETO rats. In contrast, treatment with hydralazine does not alter the levels of nephrin and podocin mRNA in OLETF rats. ∗, P < 0.05 versus LETO rats. †, P < 0.05; OLETF + vehicle versus OLETF + telmisartan, eplerenone, or telmisartan + eplerenone.
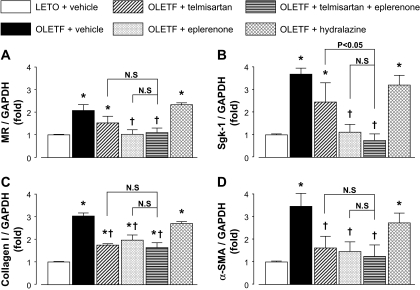
Figure 5, A and B, shows glomerular mRNA levels of MR and its target gene Sgk-1 (Shibata et al., 2007) at 50 weeks of age, respectively. Both MR and Sgk-1 mRNA levels in glomeruli were detectable in all animals. The mRNA levels of MR and Sgk-1 in vehicle-treated OLETF rats were significantly higher than those in vehicle-treated LETO rats. In OLETF rats, treatment with telmisartan did not significantly change MR or Sgk-1 mRNA levels. However, treatment with eplerenone or telmisartan plus eplerenone significantly decreased glomerular expression of MR and Sgk-1. However, treatment with hydralazine did not alter the mRNA levels of MR and Sgk-1 in OLETF rats. Figure 5, C and D, shows glomerular mRNA levels of collagen I and α-SMA at 50 weeks of age, respectively. The mRNA levels of collagen I and α-SMA in vehicle-treated OLETF rats were significantly higher than those in vehicle-treated LETO rats. In OLETF rats, treatment with telmisartan, eplerenone, or telmisartan plus eplerenone decreased glomerular mRNA levels of collagen I and α-SMA; these changes did not significantly differ among the groups. Treatment with hydralazine did not alter glomerular collagen I and α-SMA mRNA levels in OLETF rats.
Fig. 5.
Glomerular mRNA levels of MR (A), Sgk-1 (B), collagen I (C), and α-SMA (D) at 50 weeks of age. The levels of MR, Sgk-1, collagen I, and α-SMA mRNA in vehicle-treated OLETF rats are significantly higher than those in vehicle-treated LETO rats. In OLETF rats, treatment with telmisartan changes neither MR nor Sgk-1 mRNA levels. However, treatment with eplerenone or telmisartan + eplerenone significantly decreases glomerular expression of MR and Sgk-1. Conversely, treatment with telmisartan, eplerenone or telmisartan plus eplerenone decreases glomerular mRNA levels of collagen I and α-SMA, changes that do not significantly differ among the groups. Treatment with hydralazine does not alter MR, Sgk-1, collagen I, and α-SMA mRNA levels in OLETF rats. ∗, P < 0.05 versus LETO rats. †, P < 0.05; OLETF + vehicle versus OLETF + telmisartan, eplerenone, or telmisartan + eplerenone.
Discussion
An emerging body of clinical evidence indicates that adding MR blockers to ACEI or ARBs yields significant decreases in proteinuria in patients with chronic kidney disease (CKD), including diabetic nephropathy (Chrysostomou and Becker, 2001; Rachmani et al., 2004; Sato et al., 2005). Experimental studies have also shown the beneficial effects of combining MR blockers and ACEIs or ARBs on proteinuria in diabetic animals (Kang et al., 2009). However, the mechanisms by which treatment with MR blockers enhances the antiproteinuric effects of ACEIs or ARBs have not been clarified. The present study demonstrates, for the first time, that additive antiproteinuric effect of an MR blocker to that of an ARB is associated with its protective effects on glomerular podocyte injury in type 2 diabetic rats, independently of blood pressure changes. Our data also indicate that these combined effects of an MR blocker and an ARB are accompanied by the maintenance of nephrin and podocin expression during the development of type 2 diabetic nephropathy.
Podocytes constitute interdigitating foot processes that are connected to each other by slit diaphragms composed of nephrin, podocin, and other molecules (Wolf et al., 2005). Podocytes thus serve as the final filtration barrier to prevent the leak of plasma proteins (Wolf et al., 2005; Shankland, 2006; Jefferson et al., 2008). Podocyte injury with decreased nephrin and podocin expression is observed in diabetic patients with nephrotic-range proteinuria (Koop et al., 2003; Benigni et al., 2004). In the present study, we evaluated podocyte injury by desmin staining with a light microscope (Nagase et al., 2006; Nishiyama et al., 2008) and its structural changes with an electron microscope (Nagase et al., 2006; Nishiyama et al., 2008). We observed that the antiproteinuric effect of the ARB was associated with attenuation of podocyte injury and morphological changes (podocyte foot-process effacement, degeneration of the cell body, and accumulation of cytoplasmic granules) in type 2 diabetic rats. Previous studies with immunocytochemical detection of albumin by electron microscopy have revealed that albumin immunosignals are highly concentrated inside the cytoplasmic granules in podocytes of OLETF rats (Nagase et al., 2006; Nishiyama et al., 2008). Furthermore, an antiproteuric effect of an ARB was also associated with maintenance of glomerular nephrin and podocin mRNA levels. These data are in agreement with the observation in Suzuki et al. (2007) that treatment with an ARB ameliorates proteinuria by preventing reduction in glomerular nephrin and podocin expression induced by nephrin antibody in rats. Furthermore, Hoffmann et al. (2004) showed that transgenic rats with overexpression of AngII type 1 receptor in podocytes developed proteinuria without blood pressure changes, suggesting that ARBs have protective effects on podocyte injury through inhibiting local renin-angiotensin system activity in the podocytes. In contrast, a growing body of evidence indicates that podocytes are the target of aldosterone and MR (Nagase et al., 2006; Shibata et al., 2007; Kiyomoto et al., 2008). Nagase et al. (2006) showed that eplerenone attenuates the loss of nephrin and podocin expression and podocyte injury in Dahl salt-sensitive hypertension rats. They also demonstrated that cultured podocytes express substantial amount of MR, which mediates aldosterone-induced Sgk-1 expression (Shibata et al., 2007). In the present study, we found that both MR and Sgk-1 mRNA levels in glomeruli were detectable in all animals. Finally, the progression of proteinuria and of podocyte injury was associated with increases in glomerular MR and Sgk-1 mRNA levels in type 2 diabetic rats. Furthermore, the effects of an MR blocker on proteinuria, podocyte injury, and glomerular nephrin and podocin gene expression were blood pressure-independent but were associated with the attenuation of podocyte injury and Sgk-1 expression. These data are consistent with the hypothesis proposed by Nagase et al. (2006) and Shibata et al. (2007) that the beneficial effect of MR blockade on proteinuria is elicited by inhibiting local MR activity in podocytes. The present study also showed that eplerenone decreased glomerular MR expression in OLETF rats. These data are in agreement with those that show that treatment with spironolactone or eplerenone decreased renal tissues MR expression in uninephrectomized streptozotocin-induced diabetic rats (Guo et al., 2006) and type 2 diabetic mice (Taira et al., 2008).
Throughout the observation period, plasma aldosterone levels were not elevated in type 2 diabetic OLETF rats with overt proteinuria. However, treatment with eplerenone alone showed a strong antiproteinuric effect, independent of blood pressure changes. These data are in agreement with those of clinical studies that MR blockers elicits blood pressure-independent antiproteinuric effects in patients with diabetic nephropathy but without elevated plasma aldosterone levels (Sato et al., 2005). However, we also observed that glomerular Sgk-1 expression was significantly increased, suggesting the activation of MR in glomeruli of diabetic OLETF rats. We have no good explanation as to why glomerular MR is activated under conditions where plasma aldosterone levels are not elevated. Xue and Siragy (2005) showed the expression of an elevated glomerular aldosterone synthase, CYP11B2, in streptozotocin-induced diabetic rats, suggesting that aldosterone is produced locally in glomeruli under hyperglycemic conditions. Other studies using gas chromatography/mass spectrometry have demonstrated that aldosterone is actually produced in cultured human glomerular mesangial cells (Nishikawa et al., 2005). More recently, Shibata et al. (2008) demonstrated that direct Rac1-dependent MR activation in podocytes is an essential contributor to the development of proteinuria and renal injury. This suggests the potential involvement of aldosterone-independent activation of MR in the progression of proteinuria under some pathological conditions. However, the present study clearly cannot extend to addressing these issues.
In some patients, plasma aldosterone levels are initially decreased by treatment with ACEIs or ARBs but are increased in the long term (Sato and Saruta, 2003), a phenomenon termed “aldosterone breakthrough.” Increases in plasma aldosterone levels during long-term treatment with ACEIs or ARBs were observed in 40% of diabetic patients (Sato et al., 2003). However, the present study showed that daily administration of telmisartan at 3 mg/kg from 20 to 50 weeks of age did not cause aldosterone breakthrough in this model. Thus, the beneficial additive effects of an MR antagonist cannot be explained by its effect of inhibiting the activity of increased aldosterone levels in ARB-treated type 2 diabetic rats. Recent in vitro studies report the existence of cross-talk between aldosterone- and AngII-dependent intracellular signaling pathways (Mazak et al., 2004; Montezano et al., 2008). It is hoped that further studies using cultured podocytes will clarify the precise molecular mechanisms of the combined effects of MR blockers and ARBs on podocyte injury.
In agreement with a previous study (Nagai et al., 2005), OLETF rats showed glomerular sclerosis, characterized by mesangial matrix expansion, at approximately 50 weeks of age. Treatment with telmisartan or eplerenone similarly attenuated the progression of glomerular sclerosis. Unlike their effects on podocyte injury, however, their combined effects were not observed in glomerular sclerosis. Although these data are consistent with those of Piecha et al. (2008) who showed that adding spironolactone to an ARB did not improve the regression of glomerular sclerosis in subtotally nephrectomized rats, it is unclear why the combined effects of an ARB and an MR blocker are apparent on podocyte injury but not on glomerular sclerosis. However, these effects may be dependent on complex interactions among several factors that mediate mesangial matrix expansion. For example, in vitro studies have indicated that a high glucose level has a critical role in mediating mesangial cell injury (Suzaki et al., 2004). Furthermore, clinical studies show that moderate glomerular sclerosis in type 1 diabetic nephropathy is regressed 10 years after pancreatic transplantation, suggesting the importance of blood glucose control for the regression of glomerular sclerosis (Fioretto et al., 1998). Thus, strict control of blood glucose levels would be essential for the attenuation of glomerular sclerosis.
Bomback et al. (2008) performed a systematic review of 15 recent clinical studies that examined the effects of adding MR blockers to ACE inhibitors and/or ARBs on proteinuria and the risk of hyperkalemia in patients with CKD. The data showed that adding MR blockers to ACE inhibitors and/or ARBs yields significant decreases in proteinuria with less adverse effects on hyperkalemia in patients with early phase CKD (Bomback et al., 2008). In the present study, eplerenone or eplerenone plus telmisartan did not show hyperkalemia in 50-week-old OLETF rats. However, during the observation period, plasma creatinine levels were not different between nontreated OLETF rats and control nondiabetic LETO rats. Thus, the present study cannot address any issues related to the risk-benefit balance in advanced diabetic nephropathy. It should also be noted that the present study proposes starting drug intervention when proteinuria is well established to resemble the clinical situation where patients usually come under medical supervision. A weak aspect of the present study is that we cannot address issues about “remission” and “regression” of glomerular sclerosis and podocyte injury. This is because we did not evaluate any morphological changes when drug interventions were initiated.
In conclusion, the present study supports the hypothesis that the additive antiproteinuric effects of MR blockers to those of ARBs are associated with their additive inhibiting effects on podocyte injury in type 2 diabetic rats, independently of blood pressure changes. Such data suggest that the dual blockade of AngII and aldosterone could be an effective strategy for preventing the progression of proteinuria through the protection of podocytes in diabetes.
Acknowledgments
We are grateful to Boehringer Ingelheim Co. Ltd., Pfizer Inc., and Otsuka Pharmaceutical Co. Ltd., for supplying telmisartan, eplerenone, and the OLETF and LETO rats, respectively. We acknowledge excellent technical assistance from Shinya Takaiwa (Osaka City General Hospital); Nicolas Perish, Kazi Rafiq, and Gang Liu (Kagawa University); and My-Linh Rauv and Akemi Sato (Tulane University).
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan [Grant-in-Aid for Scientific Research 20590253]; the Kagawa University Project Research Fund 2009 (to A.N.); the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK072408] (to H.K.); and the Medical Research Fund from Osaka City General Hospital (to Y.K. and M.I.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.158113.
- ACEI
- angiotensin-converting enzyme inhibitor
- AngII
- angiotensin II
- ARB
- angiotensin II type 1 receptor blocker
- MR
- mineralocorticoid receptor
- OLETF
- Otsuka-Long-Evans-Tokushima-Fatty
- LETO
- Long-Evans-Tokushima-Otsuka
- PPBG
- postprandial blood glucose
- UproteinV
- urinary protein excretion rate
- SBP
- systolic blood pressure
- PAS
- periodic acid-Schiff
- Sgk
- serum glucocorticoid-dependent kinase
- SMA
- smooth muscle actin
- Cr
- creatinine
- CKD
- chronic kidney disease.
References
- Balt JC, Mathy MJ, Pfaffendorf M, van Zwieten PA. (2001) Inhibition of angiotensin II-induced facilitation of sympathetic neurotransmission in the pithed rat: a comparison between losartan, irbesartan, telmisartan, and captopril. J Hypertens 19:465–473 [DOI] [PubMed] [Google Scholar]
- Benigni A, Gagliardini E, Tomasoni S, Abbate M, Ruggenenti P, Kalluri R, Remuzzi G. (2004) Selective impairment of gene expression and assembly of nephrin in human diabetic nephropathy. Kidney Int 65:2193–2200 [DOI] [PubMed] [Google Scholar]
- Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. (2008) Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis 51:199–211 [DOI] [PubMed] [Google Scholar]
- Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, et al. (2007) Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 115:114–126 [DOI] [PubMed] [Google Scholar]
- Chrysostomou A, Becker G. (2001) Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N Engl J Med 345:925–926 [DOI] [PubMed] [Google Scholar]
- Delyani JA, Rocha R, Cook CS, Tobert DS, Levin S, Roniker B, Workman DL, Sing YL, Whelihan B. (2001) Eplerenone: a selective aldosterone receptor antagonist (SARA). Cardiovasc Drug Rev 19:185–200 [DOI] [PubMed] [Google Scholar]
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. (1998) Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339:69–75 [DOI] [PubMed] [Google Scholar]
- Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, et al. (2006) Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology 147:5363–5373 [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Podlich D, Hähnel B, Kriz W, Gretz N. (2004) Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15:1475–1487 [DOI] [PubMed] [Google Scholar]
- Jefferson JA, Shankland SJ, Pichler RH. (2008) Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int 74:22–36 [DOI] [PubMed] [Google Scholar]
- Kang YS, Ko GJ, Lee MH, Song HK, Han SY, Han KH, Kim HK, Han JY, Cha DR. (2009) Effect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic rats. Nephrol Dial Transplant 24:73–84 [DOI] [PubMed] [Google Scholar]
- Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F. (2000) Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int 57:1949–1961 [DOI] [PubMed] [Google Scholar]
- Kiyomoto H, Rafiq K, Mostofa M, Nishiyama A. (2008) Possible underlying mechanisms responsible for aldosterone and mineralocorticoid receptor-dependent renal injury. J Pharmacol Sci 108:399–405 [DOI] [PubMed] [Google Scholar]
- Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. (2005) Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol 16:2073–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, Bruijn JA. (2003) Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol 14:2063–2071 [DOI] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, et al. (2007) 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 25:1105–1187 [DOI] [PubMed] [Google Scholar]
- Mazak I, Fiebeler A, Muller DN, Park JK, Shagdarsuren E, Lindschau C, Dechend R, Viedt C, Pilz B, Haller H, et al. (2004) Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation 109:2792–2800 [DOI] [PubMed] [Google Scholar]
- Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. (2008) Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol 28:1511–1518 [DOI] [PubMed] [Google Scholar]
- Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, Yukimura T, Shokoji T, Kimura S, Kiyomoto H, et al. (2005) Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol 16:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. (2006) Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 47:1084–1093 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Suematsu S, Saito J, Soyama A, Ito H, Kino T, Chrousos G. (2005) Human renal mesangial cells produce aldosterone in response to low-density lipoprotein (LDL). J Steroid Biochem Mol Biol 96:309–316 [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Nakagawa T, Kobori H, Nagai Y, Okada N, Konishi Y, Morikawa T, Okumura M, Meda I, Kiyomoto H, et al. (2008) Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin II and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. J Hypertens 26:1849–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, et al. (2004) Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 43:841–848 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Takeuchi K, Mori T, Nako K, Tsubono Y, Ito S. (2007) Effects of monotherapy of temocapril or candesartan with dose increments or combination therapy with both drugs on the suppression of diabetic nephropathy. Hypertens Res 30:325–334 [DOI] [PubMed] [Google Scholar]
- Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, et al. (2009) The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res 32:3–107 [PubMed] [Google Scholar]
- Piecha G, Koleganova N, Gross ML, Geldyyev A, Adamczak M, Ritz E. (2008) Regression of glomerulosclerosis in subtotally nephrectomized rats: effects of monotherapy with losartan, spironolactone, and their combination. Am J Physiol Renal Physiol 295:F137–F144 [DOI] [PubMed] [Google Scholar]
- Rachmani R, Slavachevsky I, Amit M, Levi Z, Kedar Y, Berla M, Ravid M. (2004) The effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: a randomized controlled study. Diabet Med 21:471–475 [Retracted]. [DOI] [PubMed] [Google Scholar]
- Rossing K, Christensen PK, Hovind P, Parving HH. (2005) Remission of nephrotic- range albuminuria reduces risk of end-stage renal disease and improves survival in type 2 diabetic patients. Diabetologia 48:2241–2247 [DOI] [PubMed] [Google Scholar]
- Sasso FC, Carbonara O, Persico M, Iafusco D, Salvatore T, D'Ambrosio R, Torella R, Cozzolino D. (2002) Irbesartan reduces the albumin excretion rate in microalbuminuric type 2 diabetic patients independently of hypertension: a randomized double-blind placebo-controlled crossover study. Diabetes Care 25:1909–1913 [DOI] [PubMed] [Google Scholar]
- Sato A, Hayashi K, Naruse M, Saruta T. (2003) Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 41:64–68 [DOI] [PubMed] [Google Scholar]
- Sato A, Saruta T. (2003) Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens 16:781–788 [DOI] [PubMed] [Google Scholar]
- Sato A, Hayashi K, Saruta T. (2005) Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens 18: 44–49 [DOI] [PubMed] [Google Scholar]
- Shankland SJ. (2006) The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69:2131–2147 [DOI] [PubMed] [Google Scholar]
- Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. (2007) Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 49:355–364 [DOI] [PubMed] [Google Scholar]
- Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. (2008) Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14:1370–1376 [DOI] [PubMed] [Google Scholar]
- Suzaki Y, Yoshizumi M, Kagami S, Nishiyama A, Ozawa Y, Kyaw M, Izawa Y, Kanematsu Y, Tsuchiya K, Tamaki T. (2004) BMK1 is activated in glomeruli of diabetic rats and in mesangial cells by high glucose conditions. Kidney Int 65:1749–1760 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, Koike H, Shimizu F, Kawachi H. (2007) Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol 170:1841–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M, Toba H, Murakami M, Iga I, Serizawa R, Murata S, Kobara M, Nakata T. (2008) Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensin-aldosterone system in diabetic rats. Eur J Pharmacol 589:264–271 [DOI] [PubMed] [Google Scholar]
- Wienen W, Hauel N, Van Meel JC, Narr B, Ries U, Entzeroth M. (1993) Pharmacological characterization of the novel nonpeptide angiotensin II receptor antagonist, BIBR 277. Br J Pharmacol 110:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Chen S, Ziyadeh FN. (2005) From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54:1626–1634 [DOI] [PubMed] [Google Scholar]
- Xue C, Siragy HM. (2005) Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension 46:584–590 [DOI] [PubMed] [Google Scholar]
- Younis F, Kariv N, Nachman R, Zangen S, Rosenthal T. (2007) Telmisartan in the treatment of Cohen-Rosenthal Diabetic Hypertensive rats: the benefit of PPAR-gamma agonism. Clin Exp Hypertens 29:419–426 [DOI] [PubMed] [Google Scholar]