Abstract
Because bird species are lost when forests are fragmented into small parcels, trees that depend on fruit-eating birds for seed dispersal may fail to recruit seedlings if dispersal agents disappear. We tested this prediction in rainforest in the East Usambara Mountains of Tanzania, by using the endemic tree Leptonychia usambarensis (Sterculiaceae) and birds that disperse its seeds. We investigated bird abundance and Leptonychia dispersal ecology in fragments isolated for >70 yr, as compared with 3,500 ha of continuous forest. Birds that dispersed Leptonychia seeds in continuous forest were rare or absent in small fragments, where fewer seeds were removed from each tree, far fewer seedlings occurred >10 m from parent trees, and far more seedlings occurred in dense aggregations under parental crowns. Overall, our samples showed that fewer juvenile Leptonychia recruited in fragments than in continuous forest. We provide solid evidence that deficient dispersal due to habitat fragmentation seriously impacts the reproductive cycle of a tropical bird-dispersed tree.
Because large patches of rain forest hold more species of plants and animals than small ones (1), reduction and division of extensive forests into smaller parcels results in local extinctions (2, 3). This loss is reflected by the shift of the mode of log-normal or even more skewed species-abundance distributions to the left, reflecting increasing proportions of species of fragmented communities in smaller and smaller populations (4, 5). In effect, island biogeography theory predicts increasing random extinction with decreasing habitat size because an increasing proportion of species is represented by remnant populations too small to sustain themselves. We argue that even substantial populations of plants or animals may be vulnerable to extinction if key mutualists that they depend on disappear from remnant forest fragments (6). In tropical forests replete with mutualistic relationships, such as those between pollinators and plants or seed dispersers and plants, random loss of one species may have predictable and deleterious effects on others (6, 7).
Disruption of dispersal mutualisms may predispose some tropical trees to local extinction. This is a real possibility in wet tropical forests where as many as 90% of the tree species produce fruits adapted for dispersal by animals (8) and where it is increasingly clear that seed dispersal is critical for seedling recruitment (9). On first principles we expect that forest fragmentation, a virtually universal by-product of agriculture and extensive logging throughout the tropics, will disrupt mutualisms between agents of seed dispersal and their dependent plants, with predictable negative consequences on plant recruitment (10, 11). If small forest fragments lose birds, bats, primates, or other terrestrial or arboreal dispersal agents, we expect that dependent tree species will fail to establish viable cohorts of seedlings and juveniles necessary for the trees to sustain their populations.
Rain forests of the Eastern Arc Mountains of Tanzania are ancient and diverse (12, 13), offering a compelling opportunity to explore the consequences of disruption of mutualisms for diversity in forests that have existed for >30 million years (13). We address seed dispersal and its consequences of Leptonychia usambarensis, a mid-story forest tree endemic to the Eastern Arc and Coastal Forests of Tanzania-Kenya biodiversity hotspot (14), arguably the most endangered of the 25 global biodiversity hotspots (15). Our study area, Amani Nature Reserve and surrounding fragments, holds a large part of the remaining forest of the East Usambara Mountains. These mountains are remarkable in endemism of at least 22% of plant species, contributing to their status as one of the most biologically diverse forests in Africa (16). Deforestation and isolation of remnant forests by agriculture for >70 yr has left <413 km2 of the original 950 km2 of natural forest (16). Because the vast majority of trees of the Usambara forests bear fruits adapted for animal dispersal, the real possibility exists that many species of forest patches left by agriculture persist only as relict nonreproductive populations, evidently healthy as adults but incapable of effective recruitment.
Here, we report fewer dispersal agents, less seed removal, and less recruitment of Leptonychia in fragments than in continuous forest in and near the Amani Nature Reserve. Each of these factors contributes to attrition of populations of this endemic tree from forest remnants.
Methods
Research Site and Tree Species. Our study system involved 3500 ha of submontane forest (800–1,050 m) of the 7500 ha of continuous forest within Amani Nature Reserve and environs (4°S, 38°E). Of the >40 fragments surrounded by tea plantations just outside the reserve, 4 small fragments (2, 9, 13, and 31 ha) separated by 4.9–7.9 km from the continuous forest harbored more than five mature L. usambarensis (Sterculiaceae) trees. Leptonychia is an underto midstory, early to mid successional tree, bearing capsules that dehisce during the day, exposing 1–5 (2.4 ± 0.1, n = 149 fruits, seven trees) black seeds enveloped by a thin, red aril. Arillate seeds may remain exposed in open capsules 1–4 days. Seed length is 90–133 mm (mean ± SE: 111.5 ± 0.8; n = 150 seeds, 15 trees). Individuals in Amani Nature Reserve and environs begin maturity at 6 cm diameter at breast height, but most trees fruit at >8 cm diameter at breast height. Crop sizes vary from 2 to 8,412 seeds (768.4 ± 75.1, 238 trees). Individual trees bear ripe fruit for ≈6 weeks, with peak fruiting over 2–3 weeks. The fruiting season lasts from May to October, with the majority of the population bearing fruit between July and September. Experiments with seeds placed under and away from tree crowns show that seeds germinate within 2–4 weeks, with occasional delay up to 8 weeks in dry weather (unpublished data), with no indication of extended seed dormancy.
Bird Census. To determine whether composition of the disperser assemblage changed with fragment size, we censused birds by using 15-min, unlimited distance point counts (see ref. 11) over a 15-month period: in January and February, May and June, and October 2000 and January and February 2001. Fruit-eating birds were censused in eight (2, 9, 13, 31, 69, 98, 177, and 520 ha) fragments and three sites spaced >300 m apart in continuous forest as part of a larger community-wide study (11). The number of point counts was stratified according to fragment area: 1, 2, 4, and 12 counts for <5-, 5- to 20-, 30- to 200-, and >250-ha fragment size classes, respectively; the number of counts was positively correlated with fragment size (r = 0.74, P < 0.05). We used regressions of species abundance (mean individual birds per count) against log distance to continuous forest and log fragment area to assess which independent variable best explained species responses to the fragmentation process. Regressions of species abundance against the log of distance to continuous forest were not significant for any species, leaving fragment area as the operative variable. Where counts did not meet criteria for regression of tiny greenbul abundance against the log of fragment area, a Spearman rank correlation was used.
Seed Dispersal. To identify the seed dispersers and quantify seed dispersal, focal watches were conducted from 0630 to 1830 hours for each tree in three small fragments (2, 13, and 31 ha) and continuous forest in 2002; fruiting of trees in an additional small (9-ha) fragment failed so watches were not done. Each tree observed as part of the 312 hr of observation was in its period of peak fruiting, which means that it had ≥25% of the capsules open. Although crop sizes were not significantly different in the 16 trees in continuous forest (621 ± 128) as compared with the 10 in fragments (464 ± 97 fruits; t = -1.08, df = 24 for unequal variances, P > 0.14), general comparisons were confirmed with 10 trees in continuous forest similar in size to those in fragments. All vertebrate visitors were identified, enumerated, and classified as dispersers (removed seeds from trees), predators (ate or destroyed seeds), or non-dispersers (did not feed on fruits or seeds, or dropped all seeds under parent crowns). Enumeration of visits combined with notation of the number of seeds removed or dropped under parent trees allowed an estimate of removal effectiveness (17) for each bird species.
Recruitment. To evaluate the consequences of differences in seed dispersal in continuous forest and fragments, we counted Leptonychia seedlings (<15 cm height) and juveniles (15–100 cm) in wedge-transects extending away from the base of parent trees. We evaluated recruitment of Leptonychia seedlings and juveniles by sampling a 20° wedge extending 20 m from the base of the parent tree trunk. The initial direction of the wedge was chosen randomly, with reselection if the wedge approached within 20 m of a conspecific, the forest edge, or large gaps. Seedlings and juveniles were enumerated at 2-m intervals. Eight trees were randomly selected from three widely distributed (>300 m) continuous forest sites, and two each from four small (<35-ha) fragments, resulting in eight trees per fragment size class. Seedlings sampled were from the year 2000 fruiting season; juveniles were >1 yr old but otherwise of unknown age. Following Agresti (18), we used log-linear analyses to identify effects of fragment size (small fragments vs. continuous forest) on Leptonychia life history stages (seedlings and juveniles) and on proximity to tree (near <10 m vs. far 10–20 m), as well as the interaction of proximity to tree on life stage. The main effect model (proximity to tree, fragment size, stage) served as the baseline, and log-likelihood ratios of resulting models with two- and three-way interactions were then subtracted from the main effects model to determine the strength of interacting variables.
We observed no concentrations of adults without young plants or young plants without adults. The small number of Leptonychia in fragments and the patchy distribution of the species within a 150-ha section of continuous forest allow seedlings and juveniles under and near adults to be a representative sample of the landscape occupied by this species.
Results and Discussion
Fewer dispersal agents occurred in fragments than in continuous forest. Six fewer dispersal agents were present in small fragments than in continuous forest; the olive thrush and tiny greenbul all but disappeared in small fragments (Table 1). Abundances dropped with decreasing patch size for Fischer's turaco (R2 = 0.83, slope = -0.16, P < 0.05), Shelley's greenbul (R2 = 0.52, slope = -0.16, P < 0.05), and tiny greenbul (rs = -0.76, P < 0.05). The common bulbul, a bird of second growth, increased in small fragments (R2 = 0.58, slope = +0.18, P < 0.05). Abundances of seven other visitors to Leptonychia (striped-cheek greenbul, green-headed oriole, Waller's starling, dark-backed weaver, green barbet, little greenbul) were unaffected by fragment area. Overall, the mean number of individual visitors from dawn to dusk at a tree was almost three times higher in continuous forest (6.6 ± 1.4) than in small fragments (2.3 ± 0.6; t = 2.86, df = 20.37 for unequal variances, P < 0.01). Sixty-five percent fewer visits occurred per tree in fragments than in continuous forest.
Table 1. Number of visits and number of Leptonychia seeds removed per tree by birds in small fragments (<35 ha) and continuous forest.
| Visits per tree
|
Seeds removed per tree
|
|||
|---|---|---|---|---|
| Species | Cont forest | Fragments | Cont forest | Fragments |
| Striped-cheek greenbul (Andropadus milanjensis) | 2.8 ± 0.5 | 0.7 ± 0.3 | 5.1 ± 1.0 | 1.3 ± 0.6 |
| Shelley's greenbul (A. masukuensis) | 1.6 ± 0.4 | 1.0 ± 0.4 | 2.7 ± 0.8 | 1.0 ± 0.4 |
| Olive thrush (Turdus abyssinicus) | 0.8 ± 0.3 | nr | 1.9 ± 0.8 | nr |
| Green-headed oriole (Oriolus chlorocephalus) | 0.4 ± 0.2 | 0.1 ± 0.1 | 1.3 ± 0.8 | 0.5 ± 0.5 |
| Waller's starling (Onychognathus walleri) | 0.3 ± 0.3 | nr | 1.0 ± 0.8 | nr |
| Tiny greenbul (Phyllastrephus debilis) | 0.3 ± 0.2 | nr | 0.6 ± 0.4 | nr |
| Fischer's turaco (Tauraco fischeri) | 0.1 ± 0.1 | nr | 0.3 ± 0.3 | nr |
| Dark-backed weaver (Ploceus bicolor) | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.1 | x |
| Green barbet (Stactolaema olivacea) | 0.1 ± 0.1 | nr | 0.1 ± 0.1 | nr |
| Little greenbul (A. virens) | 0.1 ± 0.1 | 0.1 ± 0.1 | x | 0.1 ± 0.1 |
| Common bulbul (Pyconotus barbatus) | nr | 0.2 ± 0.1 | nr | 0.3 ± 0.2 |
Results are presented as means ± SE; data are based on 10 and 16 trees in small fragments and continuous (Cont) forest, respectively. Each of the 26 individual trees was watched for a single 12-hr day. Results remain consistent for 10 continuous forest trees with comparable crop sizes to those in fragments, with the exception that two dispersal agents (turaco and barbet) did not visit any of these 10 continuous forest trees during our focal watches. nr, Species not recorded at a site; x, no seeds removed.
More watches in continuous forest could explain the larger number of species, absent of other evidence. However, censuses from this study and a wider study of bird distributions (N.J.C., unpublished data) show that bird species' number quickly reached an asymptote of 24–28 species in small fragments in 5 to 11 sampling visits over 24 months whereas a higher asymptote of 57–62 species required 15–22 sampling visits in continuous forest sites. This potential bias would not in any case influence mean visits or mean seeds taken per tree, both of which were reduced in fragments.
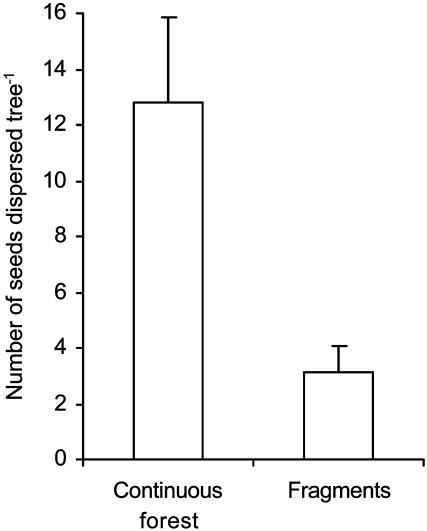
With fewer dispersal agents in fragments, fewer seeds were removed from Leptonychia trees. Focal watches at Leptonychia showed that seed removal per tree in fragments was <25% of that in continuous forest (Fig. 1). Ten bird species in continuous forest and an additional species in small fragments removed seeds from the trees (Table 1). The most effective agents of Leptonychia seed removal in continuous forest, the striped-cheek greenbul, Shelley's greenbul, and green-headed oriole, respectively, removed 75%, 63%, and 62% fewer seeds per tree in small fragments than from trees in continuous forest. A fourth effective disperser in continuous forest, the olive thrush, was absent from fragments. The net result is that seed removal by the “most effective” fruit-eating birds from the plant perspective was greatly reduced in fragments and was not compensated by other birds of secondary forest. When this comparison was done with the 10 trees from continuous forest with crop sizes comparable to the 10 trees in fragments, the striped-cheek greenbul accounted for most of the difference in seed removal by greenbuls between continuous forest and fragments.
Fig. 1.
Seed removal differs between trees in the continuous forest and small fragments (Mann–Whitney U test, U = 31.5, P < 0.01). This difference remains for 10 continuous forest trees clearly comparable in crop size to fragment trees (Mann–Whitney U test, U = 22, P < 0.05).
Reduction of dispersal may affect Leptonychia seedling recruitment in different ways. If birds remove fewer fruits, seed and seedling densities could increase under parents, with lower numbers dispersed further away than in intact forests. With no store of dormant seeds in the soil, and strongly density-dependent seed and seedling mortality under parental crowns (a Janzen–Connell escape effect; see refs. 9, 19, and 20), reduced dispersal might be expected to result in higher seed or seedling mortality near the parent trees in fragments than in continuous forest. Alternatively, if there is weak or no density-dependent mortality under parents, spatial clumping of juvenile trees and ultimately adults should be higher in fragments than in continuous forest (21, 22).
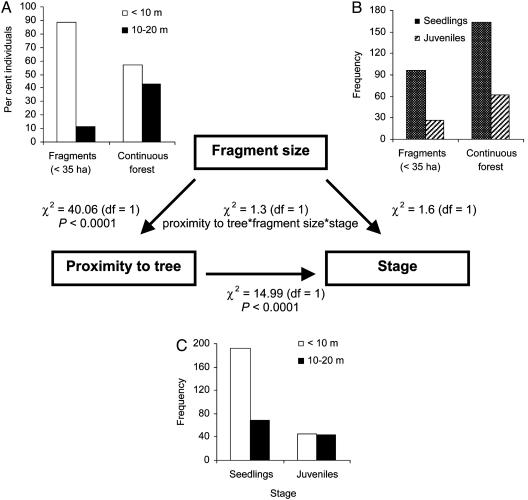
In the Usambara system, log-linear analyses show that fragmentation had multiple effects on seedling and juvenile recruitment (Fig. 2). Fragment size strongly influenced total recruits under and away from parent trees (Fig. 2 A). Overall in our sample, fewer seedlings and juveniles recruited in fragments than in continuous forest (Fig. 2B); there were 44% fewer seedlings and 58% fewer juveniles at trees in fragments than in continuous forest. Consistent with the escape hypothesis, proximity to tree interacted strongly with stage because seedlings were more common under trees than were older juveniles, which had experienced one to several years of attrition (Fig. 2C). In the Usambara system, seedlings were >2.5 times more common near (<10 m) than away (10–20 m) from trees whereas juvenile numbers were similar near and away from parental crowns, indicating a Janzen–Connell effect for Leptonychia recruitment. A similar overall result might occur from an alternative process of adult mortality and long persistence of juveniles in the understory. Longevity and duration of the juvenile stage are unknown in this species, but persistent juveniles are less likely in an early- to mid-successional tree than in a late-successional tree. The best explanation is that it took more seedlings to produce a juvenile near than away from parent trees.
Fig. 2.
Summary of log-linear analyses of recruitment of Leptonychia in relation to fragment size, proximity to tree, and stage. (A) Fragment size interacted with proximity to tree because small fragments had more individuals near than far from trees, a pattern less striking in continuous forest. (B) Absolute numbers for each stage were lower in fragments than continuous forest. (C) Proximity to tree and stage had a significant interaction because disproportionate numbers of seedlings occurred <10 than 10–20 m from parent trees for all sites combined whereas juveniles showed no such differences. Arrows indicate direction of interaction between the operative and dependent variables.
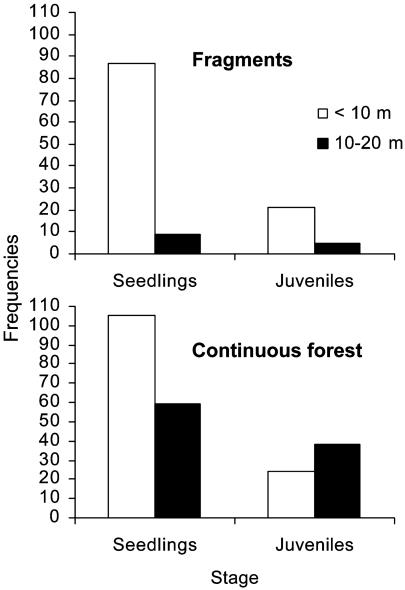
As would be expected with reduced dispersal, more seedlings and juveniles were close to parent trees in small fragments than in continuous forest: the percentage of all seedlings under tree crowns was higher in small fragments (91%) than in continuous forest (64%) whereas four times the proportion of seedlings occurred >10 m away in continuous forest (36%) than in fragments (9%) (Fig. 3). Similarly, more juveniles occurred far from parents in continuous forest (61%) than in fragments (19%). Disproportionately high build-up of seedlings under parents in fragments suggests dispersal limitation whereas the paucity of juveniles suggests stronger density-dependent attrition of seedlings aggregated around parents in the fragments. In contrast, more seedlings and juveniles away from parents in continuous forest suggest better dispersal and less recruitment limitation.
Fig. 3.
Seedling and juvenile abundances under (<10 m) and away (10–20 m) from parent tree crowns in small fragments and continuous forest. Seedlings were equally common under parent trees in small fragments and continuous forest but were much less common 10–20 m away from parent trees in fragments than in continuous forest (χ2 with a Yates correction = 20.83, df = 1, P < 0.00001). Far more juveniles occurred away from parental trees in continuous forest than in fragments (χ2 with a Yates correction = 11.34, df = 1, P < 0.001).
Seed dispersal of tropical forest trees may or may not maintain tropical diversity at regional scales (23–25), but it clearly enhances local forest diversity (9, 20, 21). We provide solid evidence that fragmentation disrupts mutualisms between avian seed dispersers and an endemic tree that depends on them for recruitment (26) in the endemic-rich East Usambara forest patches. A striking result is that most bird species affected by fragmentation in the Usambaras are generalist dispersal agents with low visitation rates. This tree is not visited by birds that diligently seek it and regularly deplete the crops, like the toucans of the well-known Virola dispersal system in Panama (27, 28), or bellbirds of a recently described Ocotea system in the cloud forests of Costa Rica (29). Instead, much lower levels of seed removal in the Leptonychia system occur with decreasing patch area even when the birds are generalist frugivores with low visitation. One might have expected that such species would be replaced by generalist birds of edges, secondary successions, and surrounding open lands, but this substitution did not occur. Even where dispersal agents persisted in fragments in low numbers (e.g., striped-cheek greenbul) (30), they were not as effective from the tree perspective as they were in continuous forest. Dispersal of trees that depend on less common and often more vulnerable specialists might be even more severely depressed by forest fragmentation.
Deficient seed dispersal caused by any human activity, whether harvesting fruits for human consumption (31), excessive hunting (32), or habitat fragmentation (10, 11), may put either common or rare trees at risk in species-rich forests. The danger is that loss of direct and indirect dependencies among pollinators, dispersal agents, and the plants that they serve could drive endemic as well as nonendemic biota to extinction (7, 33, 34). Shifts in plant diversity resulting from disruption of plant-animal mutualisms could cause accelerating local extinctions of interdependent animals and plants in increasingly isolated biodiversity hotspots (15), thereby accelerating regional and global extinctions. We predict that, as the process of forest fragmentation proceeds throughout the tropics, forest integrity will suffer from increasingly less effective mutualisms between plants and animals.
Acknowledgments
We thank the Tanzania Commission for Science and Technology, Amani Nature Reserve, Tanga Regional Forest Office, Amani Parish, East Usambara Tea Company, University of Illinois at Chicago, B. Amritan- and, S. Balcomb, Fr. S. Baruti, C. Challange, T. Challange, H. Gideon, K. M. Howell, M. Joho, H. Karata, S. Lalit, M. Lema, J. Lussenhop, S. Mashauri, C. Mlingwa, D. C. Moyer, B. Mtui, E. Mulungu, B. Munisi, E. Nashanda, W. D. Newmark, H. Nguli, M. W. Pacheco, D. Patrick, V. Pohjonen, and C. Sawe for their assistance on this project. We thank J. Bates, P. Fine, E. Hooper, M. L. Jorge, W. Laurance, D. Levey, C. Martínez-Garza, G. Orians, A. Sullivan, S. J. Wright, and B. Zorn-Arnold for comments on the manuscript. This study was supported by the National Science Foundation, the Wildlife Conservation Society, the Garden Club of America, the Chicago Zoological Society, the Chapman Memorial Fund, the Explorers Club, IdeaWild, Sigma Xi, and the University of Illinois at Chicago.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.MacArthur, R. H. & Wilson, E. O. (1967) The Theory of Island Biogeography (Princeton Univ. Press, Princeton).
- 2.Laurance, W. F. (1997) in Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities, eds. Laurance, W. F. & Bierregaard, R. O., Jr. (Univ. Chicago Press, Chicago), pp. 275–280.
- 3.Sekercioğlu, C. H., Ehrlich, P. R., Daily, G. C., Aygen, D., Goehring, D. & Sandi, R. F. (2002) Proc. Natl. Acad. Sci. USA 99, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preston, F. (1962) Ecology 43, 185–215. [Google Scholar]
- 5.MacArthur, R. H. (1972) Geographical Ecology (Princeton Univ. Press, Princeton).
- 6.Tewksbury, J. J., Levey, D. J., Haddad, N. M., Sargent, S., Orrock, J. L., Welden, A., Danielson, B. J., Brinkerhoff, J., Damschen, E. I. & Townsend, P. (2002) Proc. Natl. Acad. Sci. USA 99, 12923–12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe, H. F. (1977) Ecology 58, 539–550. [Google Scholar]
- 8.Howe, H. F. & Smallwood, J. (1982) Annu. Rev. Ecol. Syst. 13, 201–228. [Google Scholar]
- 9.Harms, K. E, Wright, S. J., Calderón, O, Hernández, A. & Herre, E. A. (2000) Nature 404, 493–495. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso da Silva, J. M. & Tabarelli, M. (2000) Nature 404, 72–74. [DOI] [PubMed] [Google Scholar]
- 11.Cordeiro, N. J. & Howe, H. F. (2001) Conserv. Biol. 15, 1733–1741. [Google Scholar]
- 12.Lovett, J. & Wasser, S. K., eds. (1993) Biogeography and Ecology of the Rain Forests of Eastern Africa (Cambridge Univ. Press, Cambridge, U.K.).
- 13.Griffiths, C. J. (1993) in Biogeography and Ecology of the Rain Forests of Eastern Africa, eds. Lovett, J. & Wasser, S. K. (Cambridge Univ. Press, Cambridge, U.K.), pp. 9–21.
- 14.Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. & Kent, J. (2000) Nature 403, 853–858. [DOI] [PubMed] [Google Scholar]
- 15.Brooks, T. M. Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., Rylands, A. B., Konstant, W. R., Flick, P., Pilgrim, J., Oldfield, S., Magin, G. & Hilton-Taylor, C. (2002) Conserv. Biol. 16, 909–923. [Google Scholar]
- 16.Newmark, W. D. (2002) Conserving Biodiversity in East African Forests: A Study of the Eastern Arc Mountains, Ecological Studies (Springer, Heidelberg), Vol. 155.
- 17.Schupp, E. W. (1993) Vegetatio 107/108, 15–29. [Google Scholar]
- 18.Agresti, A. (1996) An Introduction to Categorical Data Analysis (Wiley, New York).
- 19.Janzen, D. H. (1970) Am. Nat. 104, 501–528. [Google Scholar]
- 20.Connell, J. H. (1971) in Dynamics of Populations, eds. den Boer, P. J. & Gradwell, G. (Center for Agricultural Publication and Documentation, Wageningen, The Netherlands), pp. 298–312.
- 21.Chapman, C. A. & Chapman, L. J. (1995) Conserv. Biol. 9, 675–678. [DOI] [PubMed] [Google Scholar]
- 22.Bleher, B & Böhning-Gaese, K. (2001) Oecologia 129, 385–394. [DOI] [PubMed] [Google Scholar]
- 23.Hubbell, S. P. (2001) The Unified Theory of Biodiversity and Biogeography (Princeton Univ. Press, Princeton).
- 24.Tuomisto, H., Ruokolainen, K. & Yli-Halla, M. (2003) Science 299, 241–244. [DOI] [PubMed] [Google Scholar]
- 25.Nathan, R. & Muller-Landau, H. (2000) Trends Ecol. Evol. 15, 278–285. [DOI] [PubMed] [Google Scholar]
- 26.Bond, W. J. (1994) Phiosl. Trans. R. Soc. London B 344, 83–90. [Google Scholar]
- 27.Howe, H. F. & Vande Kerckhove, G. A. (1980) Science 210, 925–927. [DOI] [PubMed] [Google Scholar]
- 28.Howe, H. F. (1993) Vegetatio 107/108, 149–162. [Google Scholar]
- 29.Wenny, D. (1998) Proc. Natl. Acad. Sci. USA 95, 6204–6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lens, L., Van Dongen, S., Norris, K., Githiru, M. & Matthysen, E. (2002) Science 298, 1236–1238. [DOI] [PubMed] [Google Scholar]
- 31.Moegenburg, S. M. & Levey, D. J. (2002) Ecol. Lett. 5, 320–324. [Google Scholar]
- 32.Wright, S. J. & Duber, H. C. (2001) Biotropica 33, 583–595. [Google Scholar]
- 33.Christian, C. (2001) Nature 413, 635–639. [DOI] [PubMed] [Google Scholar]
- 34.Terborgh, J. (1986) in Frugivory and Seed Dispersal, eds. Estrada, A. & Fleming, T. H. (W. Junk, Dordrecht, The Netherlands), pp. 371–384.