Abstract
The objective of this study was to optimize the in vivo activity of proteolipid protein (PLP)-bifunctional peptide inhibitor (BPI) molecule to suppress experimental autoimmune encephalomyelitis (EAE) in SJL/J mice and evaluate pharmacokinetic profiles of PLP-BPI. PLP-BPI is constructed via conjugation of myelin PLP139-151 with CD11a237-246-derived peptide (LABL) via a spacer. The hypothesis is that PLP-BPI binds simultaneously to major histocompatibility complex-II and intercellular adhesion molecule-1 on the antigen-presenting cell (APC) and inhibits the formation of the immunological synapse during T-cell and APC interactions. In this study, the structure of BPI was modified by varying the spacer and was evaluated in the EAE model. Intravenous injections of BPI derivatives inhibited the onset, severity, and incidence of EAE more effectively and induced a lower incidence of anaphylaxis than that produced by unmodified PLP-BPI. As anticipated, production of interleukin-17, a proinflammatory cytokine commonly found in elevated levels among multiple sclerosis (MS) patients, was significantly lower in Ac-PLP-BPI-PEG6- or Ac-PLP-BPI-NH2-2-treated mice than in phosphate-buffered saline-treated mice. These results suggest that BPI-type molecules can be modified to achieve more efficient and better tolerated BPI-based derivatives for the treatment of MS.
Multiple sclerosis (MS), a demyelinating disease affecting the central nervous system, is characterized by inflammatory demyelinating foci in the brain white matter with variable axonal damage (Huizinga et al., 2008; Rueda et al., 2008). Although its etiology is still scarcely understood, the composition of plaques, immunogenetic background, responses to immunomodulation, and data from animal models suggest that MS is mediated by myelin-specific CD4+ T cells (Lu, 2006; Sonobe et al., 2007; Elyaman et al., 2008; Sabatino et al., 2008). It is believed that in autoimmune diseases (e.g., MS, type 1 diabetes, and rheumatoid arthritis), the activation of autoreactive T cells is caused by the recognition of the self-antigen (i.e., fragments of self-protein) before the attack of self-organs. In the case of MS, the activation of a subpopulation of T cells may cause damage to the central nervous system by attacking the myelin sheath proteins on the nerve fibers.
Currently, MS patients are being treated with anti-inflammatory agents (e.g., corticosteroids, interferon, mitoxantrone), Copaxone, and Tysabri. Copaxone (Teva Pharmaceutical Industries Ltd., Petach Tikva, Israel) (Glatiramer) is a synthetic peptide polymer designed as a decoy for the myelin basic protein; it has been suggested to alter the immune response from T helper (Th) type 1 to Th type 2 (Johnson, 1996). Tysabri (natalizumab; Biogen Idec, Cambridge, MA) is a recombinant human monoclonal antibody that inhibits leukocyte adhesion by binding to the α4-subunit of α4β1 and α4β7 integrins on the surface of leukocytes except neutrophils (Langer-Gould et al., 2005; Kappos et al., 2007). Because of fatalities in two patients who were infected with JC virus in the brain, Tysabri was once withdrawn from the market but was later reinstated for clinical use with some cautions. It was speculated that blocking the leukocyte adhesion signal (signal 2) may have compromised the general immune response, including the subpopulation that can fight viral infections. Thus, there is an urgent need to develop therapies that can selectively suppress a subpopulation of T cells that causes the progression of the autoimmune diseases without compromising the general immune response.
The initiating event in the activation of autoreactive CD4+ T cells is triggered by the interaction between T cells and antigen-presenting cells (APCs) through receptor/ligand-like molecular combinations widely referred to as signal 1 and signal 2 (Grakoui et al., 1999; Bromley et al., 2001). Signal 1 includes the interaction between T-cell receptors (TCRs) on the surface of T cells and antigen-loaded major histocompatibility complex (MHC) on the surface of APCs (Frauwirth and Thompson, 2002). Signal 2, also referred to as the costimulatory signal, can be caused by interaction of a variety of molecular pairs such as lymphocyte function-associated antigen-1 (LFA-1)/intercellular adhesion molecule-1 (ICAM-1), CD28/B7, CTLA-4/B7, inducible costimulatory/inducible costimulator ligands, and PD-1/PD-1 ligands (Grakoui et al., 1999; Schaub et al., 1999; Bromley and Dustin, 2002). Initially, the TCR/MHC-peptide complexes (signal 1) are formed in the peripheral region, and the ICAM-1/LFA-1 complexes (signal 2) are formed in the central region. Then, the ICAM-1/LFA-1 clusters translocate to the outer region, and the TCR/MHC-peptide complexes congregate at the center to form the inner region. In the final state, TCR/MHC-peptide complexes congregate at the center to form the central supramolecular activation clusters (SMACs) and the LFA-1/ICAM-1 complexes form a ring around the inner region to form peripheral SMACs. A combination of central SMAC (signal 1) and peripheral SMAC (signal 2) is called the immunological synapse (Monks et al., 1998; Grakoui et al., 1999; Lee et al., 2002).
We discovered a novel and selective way to suppress autoimmune diseases (i.e., MS and type 1 diabetes) by using a bifunctional peptide inhibitor (BPI), which consists of an antigen peptide epitope and an ICAM-1-binding peptide conjugated via a spacer (Kobayashi et al., 2007, 2008; Murray et al., 2007). Although the mechanism of action of BPI is still unknown, we hypothesize that BPI molecules block the formation of the immunological synapse by simultaneously binding to the MHC-II and ICAM-1 on the APC to prevent the translocation and segregation of signal 1 and signal 2 during the formation of the immunological synapse (Kobayashi et al., 2007, 2008; Murray et al., 2007). In this case, the antigenic-peptide epitope 139-151 derived from the proteolipid protein (PLP139-151) was conjugated to LABL peptide derived from αL integrin (CD11a237–246) to make BPI molecules (Kobayashi et al., 2007, 2008). To optimize the efficacy and lower the potential side effects of PLP-BPI molecules, the linker between the PLP and LABL peptides was modified, and the pharmacokinetic properties of the BPI molecule were evaluated. The effect of linker modification of BPI on the prophylactic and therapeutic activities was determined. The data of the pharmacokinetic profiles will be used to assess the safety profile of the BPI molecule and design future formulation and delivery methods for this type of molecule.
Materials and Methods
Peptide Synthesis.
Peptides used in this study are listed in Table 1. The peptides were synthesized with 9-fluorenyl-methoxy-carbonyl-protected amino acid chemistry on appropriate polyethylene glycol (PEG)-PS resin (GenScript Corporation, Piscataway, NJ) by using an automated peptide synthesizer (Pioneer; Applied Biosystems, Foster City, CA). Cleavage of the peptides from the resin and removal of the protecting groups from the side chain were carried out by using trifluoroacetic acid with scavengers. The crude peptides were purified by reversed-phase high-performance liquid chromatography using a preparatory C18 column with a gradient of solvent A [95%/5%, H2O (0.1% trifluoroacetic acid)/acetonitrile] and solvent B (100% acetonitrile). The purity of the peptides was analyzed by high-performance liquid chromatography using an analytical C18 column. The identity of the synthesized peptide was confirmed by matrix-assisted laser desorption ionization/time of flight mass spectrometry.
TABLE 1.
List of peptides used in the present study
BPI is composed of antigen epitope peptide (PLP139-151), varying spacers, and LABL peptide (CD11a237-246), where Acp in the spacer represents ϵ-aminocaproic acid. The peptides are capped at both ends, i.e., N-terminal acetylated (Ac−) and C-terminal amidated (−NH2).
| Peptide | Sequence |
|---|---|
| PLP139-151 | HSLGKWLGHPDKF |
| LABL (CD11a237-246) | ITDGEATDSG |
| Ac-PLP-BPI-NH2-2 | Ac-HSLGKWLGHPDKF-(AcpGAcpGAcp)2-ITDGEATDSG-NH2 |
| Ac-LABL-PLP-NH2 | Ac-ITDEATDSG-AcpGAcpGAcp-HSLGKWLGHPDKF-NH2 |
| Ac-PLP-BPI-PEG3 | Ac-HSLGKWLGHPDKF-Acp(C2H5O)3Acp-ITDGEATDSG-NH2 |
| Ac-PLP-BPI-PEG6 | Ac-HSLGKWLGHPDKF-(C2H5O)3-G-(C2H5O)3-ITDGEATDSG-NH2 |
| Ac-PLP-BPI-PEG6SC | Ac-SLKHGGLWPHKDF-(C2H5O)3-G-(C2H5O)3-TDGITSGEDA-NH2 |
Mice.
SJL/J (H-2S) female mice were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and housed under specific pathogen-free conditions at the Association for Assessment and Accreditation of Laboratory Animal Care-approved facility at the University of Kansas. All protocols involving live mice were approved by the university's Institutional Animal Care and Use Committee.
Induction of EAE and Therapeutic Study.
Five- to 7-week-old SJL/J female mice were immunized subcutaneously with 200 μg of PLP139-151 in a 0.2-ml emulsion composed of equal volumes of phosphate-buffered saline (PBS) and complete Freund's adjuvant (CFA) containing killed Mycobacterium tuberculosis strain H37RA (at a final concentration of 4 mg/ml; Difco, Detroit, MI). The PLP139-151/CFA was administered to regions above the shoulder and the flanks (total of four sites; 50 μl at each injection site). In addition, 200 ng of pertussis toxin (List Biological Laboratories Inc., Campbell, CA) was injected intraperitoneally on the day of immunization (day 0) and 2 days after immunization. The mice received intravenous injections of peptides (100 nmol/mouse) on the indicated days. For the therapeutic study, mice were left untreated until the day of disease onset [determined when the mouse had an experimental autoimmune encephalomyelitis (EAE) clinical score of one or more for the first time]. Upon disease onset, the mice received intravenous injections of peptides (100 nmol/mouse) for 3 consecutive days of the maximum number of injections; peptide injection was discontinued once the disease score returned below one. Disease progression was evaluated by the same observer in a blinded fashion by using a clinical scoring as follows: 0, no clinical signs of the disease; 1, tail weakness or limp tail; 2, paraparesis (weakness or partial paralysis of one or two hind limbs); 3, paraplegia (complete paralysis of two hind limbs); 4, paraplegia with forelimb weakness or paralysis; and 5, moribund (mice were euthanized once they were found to be moribund). Body weight was also measured daily.
Determination of IL-17 Levels in Serum In Vivo.
Blood samples were obtained from peptide-treated and untreated mice on days 12 and 35 (six mice per group). In addition, six unprimed and untreated mice (i.e., normal mice) were also sampled. Blood samples were allowed to clot overnight at 2 to 8°C before centrifuging for 20 min at 2000g. Serum was collected and stored at −20°C until analysis. Enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN) for interleukin-17 (IL-17) in serum were performed according to the manufacturer's instructions. Optical density was measured at 450 nm with correction at 540-nm wavelengths in a spectrophotometric microplate reader (Titertek Multiskan MCC/340, Flow Industries, McLean, VA).
Induction and Monitoring of Anaphylaxis.
Mice received subcutaneous immunization with PLP139-151/CFA on day 0 and intraperitoneal injection of pertussis toxin on the day of immunization and 2 days after immunization. Four to 5 weeks later, the mice were divided into groups and received intravenous injection of described peptides (100 nmol/mouse). To avoid the influence of the variation in their disease severity and history, all of the groups had a very similar set of mice in terms of the average highest disease score, the average cumulative disease score, and the average day of disease onset. Incidence of anaphylactic response was judged by death occurring within 30 min or by the characteristic signs of immediate hypersensitivity, such as piloerection, prostration, erythema of the tail, ears, and footpads, shallow breathing, and lethargy, observed within a few minutes after peptide injection. Any mice that became moribund or did not recover from anaphylactic signs were euthanized.
In Vivo Pharmacokinetic Studies in Rats.
Male Sprague-Dawley rats weighing 250 to 300 g were purchased from Charles River Laboratories, Inc. and housed under specific pathogen-free conditions at the Association for Assessment and Accreditation of Laboratory Animal Care-approved facility at the University of Kansas. All protocols involving live rats were approved by the university's Institutional Animal Care and Use Committee. Before surgery, the animals were anesthetized with intraperitoneally administered ketamine (100 mg/kg) and xylazine (5 mg/kg). The right jugular vein was exposed and cannulated with medical-grade silastic tubing (0.020 inch, i.d. × 0.037 inch, o.d.). The vein was ligated with a 2-0 silk suture and anchored to the surrounding tissue with cyanoacrylate glue. The patency of the jugular catheter was maintained with sterile saline containing heparin (10 U/ml). The animal was maintained unconscious throughout the experiment by subcutaneously administered ketamine (25 mg/kg). Baseline “blank” plasma was drawn from each animal immediately before dosing. An intravenous bolus dose was administered via the jugular vein over 30 s. After dosing, the jugular vein was flushed with sterile saline. Blood samples (∼0.2 ml) were drawn from the jugular vein at scheduled time intervals for 10 h after dosing. The blood samples were immediately centrifuged at 10,000g for 3 min, and plasma was separated and stored at −70°C until analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS).
Plasma Extraction Procedure.
To 95 μl of drug-free rat plasma, 5 μl of working solutions of Ac-PLP-BPI-NH2-2 and Ac-LABL-BPI-NH2 (internal standard, IS) was spiked to yield final concentrations in the range of 15 to 1000 pmol. Quality-control (QC) samples were prepared in a similar manner. All samples, QCs, and standards were diluted with Nanopure water to 1 ml, vortexed for 30 s, and isolated from plasma by solid-phase extraction (Oasis HLB, Waters, Milford, MA). Solid-phase extraction cartridges were pretreated with 1 ml of methanol followed by 1 ml of water before the diluted samples were loaded. After running the samples through the cartridges, the cartridges were washed twice with 1.0 ml of 10% acetonitrile in water. Samples were eluted with 1.0 ml of 60% methanol with 2% trifluoroacetic acid in water, evaporated under nitrogen, and reconstituted with 100 μl of water/acetonitrile (1:1, v/v).
LC-MS/MS Conditions.
The quantity of peptides recovered from plasma was determined by LC/MS/MS using a small, short C18 guard column and monitoring an abundant product ion from activation of a multiple-charged precursor of the peptide. A Waters Acquity UPLC interfaced with a Micromass “triple” quadrupole mass spectrometer (Quattro Ultima Micromass Ltd., Manchester UK) was used. Samples were presented by using a Zorbax C18 (0.32 mm × 25 mm, 5 μm) column (Micro-Tech Scientific, Sunnyvale, CA) heated to 35°C and eluted with a mobile phase gradient at a flow rate of 60 μl/min. The injection volume was 10 μl. Mobile phase consisted of A (H2O with 1% CH3CN) and B (CH3CN with 0.08% formic acid). The gradient was 10% mobile phase B isocratic for 2 min, increased to 60% B in 1 min, then held at 60% B for 3 min. Multiple reaction monitoring mode was used for the quantification. The electrospray source block was 80°C, and the probe desolvation temperature was 200°C. Argon collision gas was set to attenuate the beam to 15% (8e-4 mbar on a gauge near the collision cell). The cone voltage was 25 V. Quadrupoles 1 and 3 were tuned to a resolution of 0.8 U full width at half-maximum. The selected transitions were from the M4H4 precursors, m/z (855.1 > 724) collision energy 28 V for Ac-PLP-BPI-NH2-2 and m/z 988.5 > 1059.8 collision energy 35V for Ac-LABL-BPI-NH2 (IS). Because of the high-charge state of the precursor, the optimum collision gas density and energy occur over a narrower range than commonly observed for singly charged ions.
Pharmacokinetic Data Analysis.
Pharmacokinetic analysis of Ac-PLP-BPI-NH2-2 plasma concentration-time profiles after intravenous bolus injection was conducted by fitting the data to a two-compartment pharmacokinetic model using WinNonlin Professional Version 3.1 (Pharsight, Mountain View, CA) according to eq. 1:
Similarly, the initial volume of distribution (Vinitial) was calculated according to eq. 2:
where C is the plasma concentration of Ac-PLP-BPI-NH2-2, A and B are pre-exponential constants, α is the distribution rate constant, β is the elimination rate constant, t is time, D is the dose, and Cmax is the maximum plasma concentration. The parameters that were calculated for Ac-PLP-BPI-NH2-2 included Cmax, area under curve (AUC)0–∞, systemic clearance, and t1/2. Pharmacokinetic parameters were derived for each animal based on individual observed concentration-time data.
Statistical Analysis.
Statistical comparisons among the groups in clinical disease scores were determined by calculating the average score for each mouse from the day of disease onset to the end of the study and performing a Mann-Whitney U test. Statistical differences among the groups in body weight were analyzed by calculating the average weight for each mouse for 10 days beginning on the day of disease onset and performing a Mann-Whitney U test. Statistical significance in EAE disease morbidity was determined by Cox proportional-hazards regression. Comparison of IL-17 concentration in serum was performed by one-way analysis of variance. All analyses were carried out with StatView (SAS Institute, Cary, NC).
Results
Effect of Linker Modification in BPI on the Suppression of EAE.
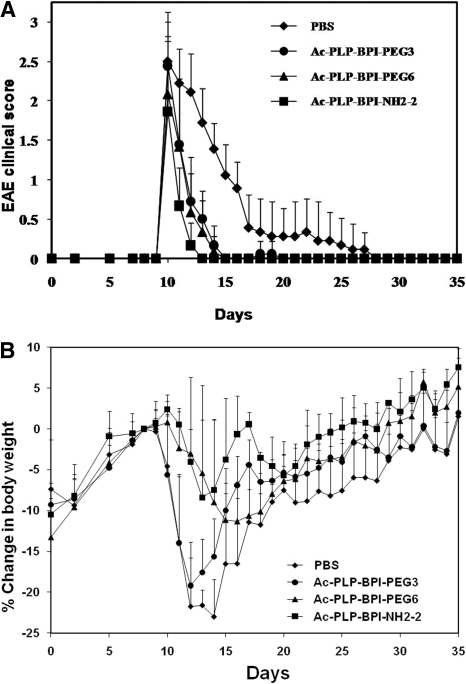
The effects of the nature of the linker and its length were evaluated to optimize the in vivo efficacy of the BPI molecules. The hypothesis is that modification of linker properties will influence the efficacy and side effects of the BPI molecules. In this case, the Acp-G linker in Ac-PLP-BPI-NH2-2 was modified with PEG with different lengths to give Ac-PLP-BPI-PEG3 and Ac-PLP-BPI-PEG6 (Table 1). The in vivo activities of Ac-PLP-BPI-PEG3, Ac-PLP-BPI-PEG6, and Ac-PLP-BPI-NH2-2 were compared with PBS in therapeutic experiments using immunized SJL/J mice. In this case, the animals received intravenous injections of the peptides or PBS after they showed disease signs with a score of 1 or higher. The mice received intravenous injections of the peptides or PBS on consecutive days until the score was less than 1 or a maximum of three injections of the peptides, whichever came first. All three peptides reversed the diseases progression more effectively compared with the control (Fig. 1A) with the following statistical differences in EAE clinical scores on days 10 to 25: Ac-PLP-BPI-NH2-2 versus PBS, p < 0.001; Ac-PLP-BPI-PEG6 versus PBS, p < 0.001; and Ac-PLP-BPI-PEG3 versus PBS, p < 0.001. Ac-PLP-BPI-NH2-2-treated mice recovered from EAE more rapidly compared with those treated with the other two peptides (Ac-PLP-BPI-NH2-2 versus Ac-PLP-BPI-PEG3, p < 0.01; Ac-PLP-BPI-NH2-2 versus Ac-PLP-BPI-PEG6, p < 0.05) as shown by the rapid decline in their clinical scores, particularly from days 10 to 15 (Fig. 1A). In addition, Ac-PLP-BPI-NH2-2-treated mice did not lose as much body weight and/or regained their body weight faster (p < 0.001) than mice treated with Ac-PLP-BPI-PEG3 (Fig. 1B). Although there was no statistical difference in loss of body weight between Ac-PLP-BPI-NH2-2- and Ac-PLP-BPI-PEG6-treated mice (p > 0.05), there was a trend that Ac-PLP-BPI-NH2-2-treated mice regained their body weight faster than Ac-PLP-BPI-PEG6-treated mice (Fig. 1B). No statistical difference (p > 0.05) was observed in EAE clinical scores data between Ac-PLP-BPI-PEG6 and Ac-PLP-BPI-PEG3. However, Ac-PLP-BPI-PEG3-treated mice had significantly (p < 0.001) higher loss in body weight compared with Ac-PLP-BPI-PEG6-treated mice (Fig. 1B), suggesting that Ac-PLP-BPI-PEG3 did not work as well as Ac-PLP-BPI-PEG6. Thus, no further studies were conducted on the Ac-PLP-BPI-PEG3 molecule.
Fig. 1.
The therapeutic efficacy of BPI derivatives in reversing EAE in the mouse model. The mice were immunized with PLP139-151/CFA followed by intravenous treatments up to a maximum of three injections with the BPI molecules starting on the day of disease onset as described in Materials and Methods. A, clinical EAE disease score. B, change in body weight. Results are expressed as the mean ± S.D. (n = 10). There are significant differences between peptide-treated and PBS-treated groups in clinical disease scores: Ac-PLP-BPI-NH2-2, p < 0.001; Ac-PLP-BPI-PEG6, p < 0.001; and Ac-PLP-BPI-PEG3, p < 0.001. Statistical values for the loss of body weight compared with PBS were as follows: Ac-PLP-BPI-NH2-2, p < 0.001; Ac-PLP-BPI-PEG6, p < 0.001; and Ac-PLP-BPI-PEG3, p > 0.05.
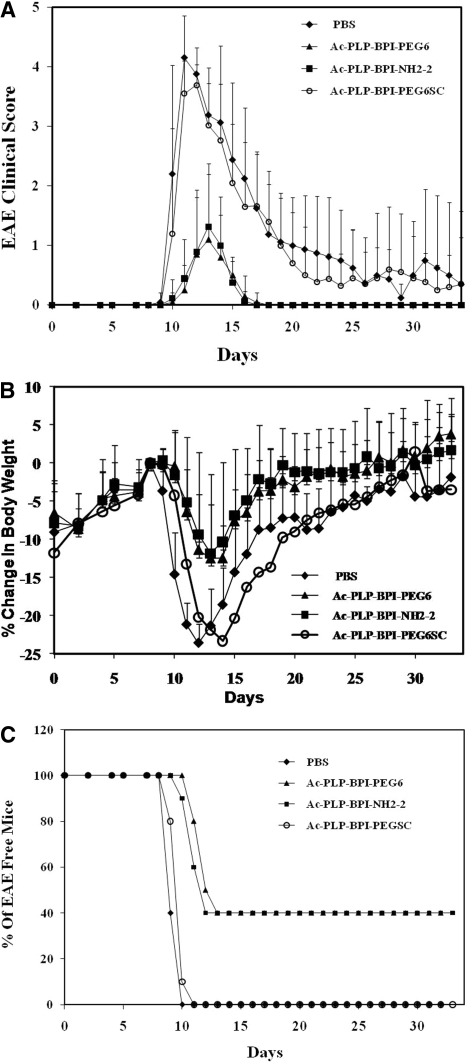
The therapeutic experiments were further corroborated by prophylactic experiments. In this case, the peptides were administered via intravenous route on days 4 and 7 before the onset of EAE. Ac-PLP-BPI-NH2-2-treated mice showed clinical scores of mild EAE compared with the severe EAE found in PBS-treated mice, particularly at the peak of the disease on days 12 to 17 (p < 0.001) (Fig. 2A). Ac-PLP-BPI-NH2-2 treated mice had significant (p < 0.001) minimal loss of body weight compared with the control as shown in Fig. 2B. At the peak of disease severity (days 12–17), PBS-treated mice had as much as 24% loss in body weight compared with a maximum 11% loss in body weight among the Ac-PLP-BPI-NH2-2-treated mice (Fig. 2B). Similarly, intravenous injections of Ac-PLP-BPI-PEG6 inhibited the progression and severity of EAE similar to that of Ac-PLP-BPI-NH2-2 (p > 0.05). The PBS-treated mice recorded EAE clinical scores as high as 4, which included paralysis of their tails and limbs. To examine the necessity of the correct peptide sequence of the BPI molecule, an Ac-PLP-BPI-PEGSC peptide with scrambled sequences at both PLP and LABL fragments was used as a control. This peptide did not suppress the progress of EAE (Fig. 2, A and B); suggesting that the correct sequences of both peptides is necessary for the activity to suppress EAE.
Fig. 2.
In vivo EAE-suppressive activity of BPI derivatives in the mouse EAE model. PLP139-151/CFA-immunized mice received intravenous injections of the indicated peptide (100 nmol/mouse/day) on days 4 and 7. A, clinical EAE disease score. B, change in body weight. C, incidence of disease. Results are expressed as the mean ± S.D. (n = 10). There are significant differences between BPI-treated and PBS-treated groups in clinical disease scores and loss of body weight: Ac-PLP-BPI-NH2-2, p < 0.001 and Ac-PLP-BPI-EG6, p < 0.001. There was no significant difference (p > 0.05) in EAE clinical scores and loss of body weight between scrambled peptide (Ac-PLP-BPI-PEGSC) and PBS groups. In addition, no significant difference (p > 0.05) between Ac-PLP-BPI-NH2-2 and Ac-PLP-BPI-PEG6 was observed.
The effect of linker modification on reducing the anaphylaxis side effect of the peptides was evaluated by multiple injections of the peptides on the treated animals during the late phase of the disease (i.e., 35 days) (Table 2). The animals treated with Ac-PLP-BPI-PEG6 have lower anaphylactic response (22%; Table 2) compared with those of treated with the Ac-PLP-BPI-NH2-2 (45%). As found previously, the animals treated with PLP139-151 had the highest anaphylactic response (87%). This suggests that BPI molecules have lower side effects than the plain PLP139-151; in addition, changing the linker from Acp-G repeat to PEG repeat lowers the side effects of the BPI molecules. As a control, the scrambled peptide (i.e., Ac-PLP-BPI-PEG6SC) did not induce any anaphylactic reaction (0%). Studies by Kuchroo et al., 1994 identified specific residues in the antigenic core that are TCR binding sites and MHC-II binding sites. Their findings indicated that residues Trp-144 and His-147 were the TCR binding sites, whereas residues Leu-145 and Pro-148 were important for MHC-II binding. Thus, any changes in the position of these amino acids will affect the MHC-II and TCR binding properties as well as peptide activity. Because the sequence in Ac-PLP-BPI-PEGSC is scrambled, it is expected that Ac-PLP-BPI-PEGSC has no suppressive activity and anaphylactic reaction in the EAE mouse model.
TABLE 2.
Incidence of anaphylactic response upon peptide challenge
PLP139-151/CFA-immunized mice treated with vehicle or peptide (100 nmol/100 μl) received intravenous injection of the indicated peptide on day 35. Incidence of anaphylactic response was determined as described in Materials and Methods.
| Treatment on Days 4 and 7 | Peptide Challenge on Day 35 | Incidence of Anaphylaxis |
|---|---|---|
| PBS | PLP139-151 | 13/15 (87%) |
| Ac-PLP-BPI-NH2-2 | Ac-PLP-BPI-NH2-2 | 9/20 (45%) |
| Ac-PLP-BPI-PEG6 | Ac-PLP-BPI-PEG6 | 4/18 (22%) |
| Ac-PLP-BPI-PEG6SC | Ac-PLP-BPI-PEG6SC | 0/19 (0%) |
IL-17 Serum Levels in SJL/J Mice In Vivo.
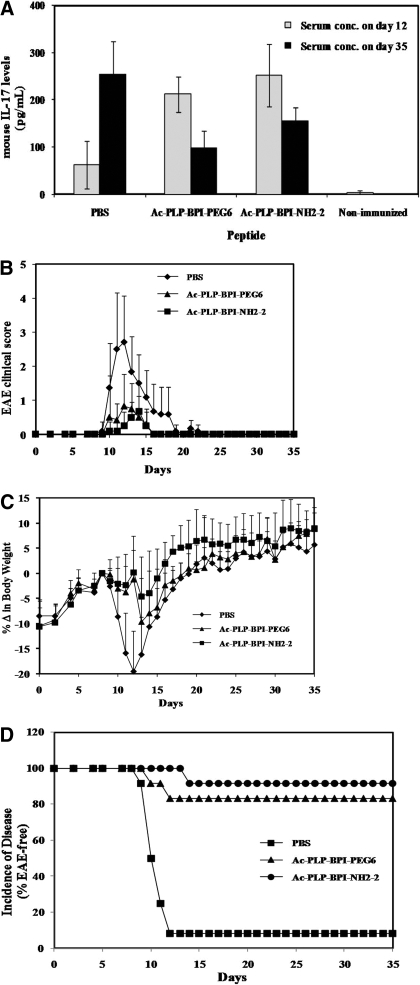
The effect of linker modification of BPI molecules on suppressing the IL-17 levels was evaluated because IL-17 is a marker for the progress of MS. In this study, the animals were treated with Ac-PLP-BPI-NH2-2, Ac-PLP-BPI-PEG6, or PBS on days 4, 7, and 10, and IL-17 levels were measured on days 12 and 35 (Fig. 3A). As expected, the clinical scores, body weight changes, and disease incidence (Fig. 3) indicated that both peptides have better efficacy than PBS. Three injections of the peptides provided lower disease incidence (Fig. 3D) than did two injections on days 4 and 7 (Fig. 2C). In the mice treated with Ac-PLP-BPI-NH2-2 or Ac-PLP-BPI-PEG6, the amount of IL-17 was significantly decreased from days 12 to 35; in contrast, the mice treated with PBS had higher levels of IL-17 on day 35 than on day 12. However, on day 12, mice receiving Ac-PLP-BPI-NH2-2 or Ac-PLP-BPI-PEG6 had unexpected significantly high serum levels of IL-17 compared with the mice receiving PBS alone (p < 0.001) (Fig. 3A). At the end of the study (day 35), mice receiving Ac-PLP-BPI-NH2-2 or Ac-PLP-BPI-PEG6 had significantly lower IL-17 serum concentration (p < 0.01) than did PBS-treated mice. Although there was a trend that the level of IL-17 was lower in the Ac-PLP-BPI-PEG6-treated group than in the Ac-PLP-BPI-NH2-2-treated group on day 35, there was no significant difference (p > 0.05) between these levels of IL-17. Measured serum levels of IL-17 in the unprimed mice were found to be 2.77 ± 4.48 pg/ml (Fig. 3D), representing the background level of IL-17 in mouse. As anticipated, these levels were the lowest IL-17 serum concentration among all of the groups.
Fig. 3.
Production of IL-17 in serum in vivo. SJL/J female mice were immunized subcutaneouslywith PLP139-151/CFA and injected intraperitoneally with pertussis toxin on days 0 and 2. Then, the mice received intravenous injections of the indicated peptide (100 nmol/mouse/day) or PBS on days 4, 7, and 10. A, serum concentration of IL-17 determined on days 12 and 35 in Ac-PLP-BPI-NH2-2-treated, Ac-PLP-BPI-PEG6-treated, PBS-treated, and unprimed mice by using enzyme-linked immunosorbent assays. Data are represented as mean ± S.D. (six mice per group), and experiments were done in duplicate. B to D, disease progression was evaluated by EAE clinical disease score (B), percentage change in body weight (C), and incidence of disease (D). There are significant differences between BPI-treated and PBS-treated groups in clinical disease scores and loss of weight: Ac-PLP-BPI-NH2-2, p < 0.001 and Ac-PLP-BPI-PEG6, p < 0.001. There was no significant difference (p > 0.05) in EAE clinical scores and loss of body weight between Ac-PLP-BPI-NH2-2 and Ac-PLP-BPI-PEG6.
Pharmacokinetic Analysis of Ac-PLP-BPI-NH2-2 in Sprague-Dawley Rats.
For pharmacokinetic studies, plasma concentrations of Ac-PLP-BPI-NH2-2 were determined by LC-MS/MS. We chose Ac-PLP-BPI-NH2-2 as our model BPI compound to study pharmacokinetics because we had done extensive studies on this compound in terms of its efficacy, potential to cause hypersensitivity, and cytokine profiling after treatment in our previous experiments. The initial step in analysis of extracted plasma samples involved the development and validation of the LC-MS/MS analytical method. It was found that a gradient mobile phase composition provided good resolution, symmetric peak shapes of analytes, and short run time. The retention times were 2.44 and 3.48 min for Ac-PLP-BPI-NH2-2 and IS, respectively. No significant interfering peaks caused by endogenous compounds were observed at the retention times of the two analytes of interest. The standard calibration curves of Ac-PLP-BPI-NH2-2 in plasma were linear over the concentration range of 15 to 1000 pmol. Typical mean (n = 3) calibration curves were y = 0.0066x − 0.6754, R2 = 0.999, where y and x are the peak area ratio of analyte to IS and concentration, respectively. The mean plasma extraction recoveries for Ac-PLP-BPI-NH2-2 and Ac-LABL-BPI-NH2 (IS) determined in triplicate in the concentration range of 15 to 1000 pmol were 91.8% (%CV 5.8) and 98.4% (%CV 4.3), respectively. The peak areas of the analytes in the reconstituted QC samples had a CV of less than 10%, indicating that the extracts were “clean” with no coeluting compounds that could influence ionization of the analyte or the IS. The lower limit of quantification defined as the concentration of Ac-PLP-BPI-NH2-2 that can still be determined with acceptable precision (%CV <10) and accuracy (www.fda.gov/cvm) was found to be 75 pmol, and the limit of detection was 35 pmol. Results of the intraday and interday validation assays indicated that the accuracy of the assay was >90% with a CV that did not exceed 10%. For at least 48 h at the autosampler temperature (12°C), no significant degradation was observed in the extracted and reconstituted plasma samples or in working solutions. Ac-PLP-BPI-NH2-2 and the IS in the final extract were considered to be stable when 85 to 115% of the initial concentration was found.
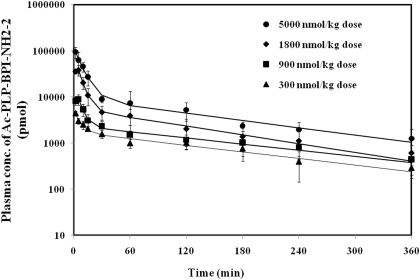
The validated LC-MS/MS method was applied to a pharmacokinetic study of Ac-PLP-BPI-NH2-2 after administration of intravenous injections in Sprague-Dawley rats. Mean plasma concentration-time profiles of Ac-PLP-BPI-NH2-2 after injections of 0.3, 0.9, 1.8, and 5.0 μmol/kg i.v. are shown in Fig. 4, and the related pharmacokinetic parameters are listed in Table 3. Observed plasma concentration-time profile followed a two-compartmental model characterized by a rapid distribution phase and a gradual elimination phase and had a good correlation (R2 = 0.9872) with the predicted plasma concentration. Ac-PLP-BPI-NH2-2 Cmax of 3.84 ± 1.03, 18.17 ± 4.50, 69.59 ± 19.69, and 116.05 ± 24.92 nmol/ml were observed at doses of 0.3, 0.9, 1.8, and 5.0 μmol/kg, respectively.
Fig. 4.
Plasma concentration profiles of Ac-PLP-BPI-NH2-2 after intravenous administration in Spraque-Dawley rats in vivo. Data are represented as mean ± S.D. with (n = 3) and in triplicate. Point marks represent actual plasma levels, and the solid lines represent predicted plasma levels after fitting the raw data into compartmental models using commercial pharmacokinetic software.
TABLE 3.
Summary of pharmacokinetic parameters of Ac-PLP-BPI-NH2-2 after intravenous administration
Data are represented as mean ± S.D. (n = 3).
| Parameter | Units | Ac-PLP-BPI-NH2-2 |
|||
|---|---|---|---|---|---|
| 5 μmol/kg | 1.8 μmol/kg | 0.9 μmol/kg | 0.3 μmol/kg | ||
| AUC | nmol × h/ml | 41.35 ± 3.20 | 22.73 ± 1.84 | 9.97 ± 2.88 | 6.55 ± 0.24 |
| Cmax | nmol/ml | 116.05 ± 24.92 | 69.59 ± 19.69 | 18.17 ± 4.50 | 3.84 ± 1.03 |
| α | h−1 | 7.08 ± 1.70 | 7.88 ± 2.23 | 10.17 ± 2.46 | 4.69 ± 2.83 |
| β | h−1 | 0.36 ± 0.15 | 0.26 ± 0.19 | 0.29 ± 0.06 | 0.23 ± 0.07 |
| A | nmol/ml | 106.71 ± 25.82 | 65.85 ± 19.69 | 15.99 ± 3.71 | 2.51 ± 0.95 |
| B | nmol/ml | 9.34 ± 4.24 | 3.74 ± 2.62 | 2.18 ± 0.81 | 1.34 ± 0.49 |
| Systemic clearance | liter/h/kg | 0.12 ± 0.04 | 0.08 ± 0.01 | 0.01 ± 0.03 | 0.05 ± 0.00 |
| Vd | liter/kg | 0.24 ± 0.07 | 0.25 ± 0.12 | 0.32 ± 0.11 | 0.20 ± 0.05 |
| Vinitial | liter/kg | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.03 |
| t1/2 | h | 2.17 ± 0.86 | 3.55 ± 2.06 | 2.73 ± 0.63 | 3.28 ± 0.91 |
Discussion
In our recent studies, it was found that in vivo activity of PLP-BPI could be improved further by increasing its plasma stability and optimizing the separation distance between PLP and LABL peptides. Because the original PLP-BPI has uncapped N and C termini, it may be susceptible to amino- and carboxyl-peptidase degradation in the blood. The rate of degradation can be decreased tremendously or halted by capping the N and C termini by acetylation and amidation, respectively. In our observations, a single injection of Ac-PLP-BPI-NH2 with capped N and C termini inhibited the onset and progression of EAE more efficiently than uncapped PLP-BPI (Kobayashi et al., 2008), and there was no significant difference between Ac-PLP-BPI-NH2 and PLP-BPI upon four injections of the two different peptides. It is plausible that the improved efficacy of Ac-PLP-BPI-NH2 is caused by increased bioavailability and lower metabolic degradation compared with the parent PLP-BPI. Ac-PLP-BPI-NH2 was further optimized by doubling the linker length to give Ac-PLP-BPI-NH2-2. In vivo studies in SJL/J mice revealed that Ac-PLP-BPI-NH2-2 has activity similar to that of Ac-PLP-BPI-NH2 but lower anaphylaxis reaction, suggesting that the linker properties and length may have a role in the hypersensitivity reaction of the BPI molecules.
The therapeutic effectiveness and the presence of potential side effects of our BPI molecules could also be caused by their pharmacokinetic properties. Therefore, to move closer to clinical use of these peptides, we need to evaluate their pharmacokinetic profiles. For practical reasons such as the need for frequent sampling and sampling volume, male Spraque-Dawley rats were used instead of mice. In the present study, we conducted pharmacokinetic studies of Ac-PLP-BPI-NH2-2 after intravenous administration. Mean plasma concentration-time profiles of Ac-PLP-BPI-NH2-2 after injections of 0.3, 0.9, 1.8, and 5.0 μmol/kg i.v. are shown in Fig. 4, and the related pharmacokinetic parameters are listed in Table 3. Although there was no proportionality, there was an increase in the Cmax with an increase in the dose administered. This trend was also observed in the AUC values, suggesting that systemic exposure (Cmax and AUC) of Ac-PLP-BPI-NH2-2 increased with an increase in dose administered. Except for the smaller clearance values at the dose of 0.3 μmol/kg, there were no significant differences (p > 0.05) in the clearance values and t½ at doses of 0.3, 0.9, 1.8, and 5.0 μmol/kg. This fairly dose-independent disposition (systemic clearance and t1/2) of Ac-PLP-BPI-NH2-2 suggests nonsaturable hepatic extraction. As with any peptide, Ac-PLP-BPI-NH2-2 is expected to have extensive hepatic extraction and, in the case of saturable hepatic extraction, we anticipate that the clearance values should increase with a decrease in the dose administered. On the other hand, elimination t½s are expected to decrease with a decrease in the dose administered. However, the observed pharmacokinetics was inconclusive because of the limited dose range investigated. The results from this study indicate that the BPI molecules are cleared rapidly upon intravenous administration; thus, an alternative route of administration such as subcutaneous injection may be more favorable. Recently, subcutaneous injection of BPI molecules has been shown to be more than or as effective as intravenous injection; thus, the subcutaneous route will be more desirable for delivering the BPI molecules. Pharmacokinetic studies of BPI molecules upon subcutaneous injection with various formulations, including nanoparticle delivery methods, are being investigated.
This study shows that modification of the linker can affect in vivo properties of the BPI molecules, especially in reducing side effects. Modification of Acp-G-linker in Ac-PLP-BPI-NH2-2 to a more hydrophilic PEG6-linker with a similar length in Ac-PLP-BPI-PEG6 lowers the hypersensitivity reaction of the peptides. It was previously shown that PLP-BPI molecules have lower hypersensitivity reactions than PLP139-151 peptide, which has the highest hypersensitivity reactions in this study (Table 2). Ac-PLP-BPI-NH2-2 and Ac-PLP-BPI-PEG6 have similar in vivo activities in suppressing EAE in the animal model, whereas Ac-PLP-BPI-PEG3 with a shorter linker has lower activity, indicating that the distance separation between the two peptides is important for peptide in vivo activity.
Although short linear peptides generally lack the ability to cross-link adjacent IgE molecules on mast cells and basophils (Alexander et al., 2002), repeated injections of peptides have been shown to induce hypersensitivity in MS patients (Smith et al., 2005). Clinical implications of anaphylaxis are illustrated by the termination of a phase II clinical trial using an altered peptide ligand (APL) derived from myelin basic protein 85–99 to regulate MS because some of the patients developed systemic hypersensitivity (Bielekova et al., 2000; Kappos et al., 2000). It has been suggested that the peptide induces the production of anti-IgG1 and anti-IgE antibodies. The administration of anti-IgE antibodies can block myelin peptide-induced anaphylaxis, suggesting that the hypersensitivity reaction is via an IgE-dependent mechanism (Smith et al., 2005). The anaphylaxis reaction in an animal model can be avoided by formation of an APL of myelin oligodendrocyte protein (MOG)35–55 peptide with mutation of the Ala residue at position 37 to Ala to produce MOG-Ala37. The MOG-Ala37 mutant does not bind to IgG1 from the serum of mice immunized with MOG35–55/CFA; this peptide suppressed EAE and, upon repeated injection, did not generate anaphylaxis reaction (Leech et al., 2007). Thus, this method is useful for designing BPI molecules that have no hypersensitivity reaction. In this study, the difference in hypersensitivity of PLP, Ac-PLP-BPI-NH2-2, and Ac-PLP-BPI-PEG6 molecules is possibly also caused by the IgE and IgG1 mechanisms; this hypothesis is still under investigation. We also observed that the administration of lower doses of BPI molecules (<100 nmol/injection) reduces hypersensitivity reactions, suggesting that induction of anaphylaxis by BPI molecules is dose-dependent. A similar observation was found in a clinical trial of APL in which lower doses of peptide administration were shown to reduce side effects of the peptide administration. Therefore, the effect of dose of BPI molecules on the sensitivity reaction is currently being investigated to find the optimal dose that is both low and well tolerated.
The possible mechanisms of action of BPI molecules are elucidated by measuring their effect on IL-17 production during the treatment of EAE. It is widely accepted that T cells that produce proinflammatory cytokines IL-17 (Th17) are the major contributor to the autoimmune pathogenesis of EAE or MS. Elevated levels of IL-17 have been demonstrated in the cerebrospinal fluid and brain lesions of patients with MS (Matusevicius et al., 1999; Lock et al., 2002; Hedegaard et al., 2008) and were shown to correlate with disease activity (Hedegaard et al., 2008). On day 12, the production of IL-17 is significantly higher in mice treated with BPI molecules (Ac-PLP-BPI-NH2-2 or Ac-PLP-BPI-PEG6) than in PBS-treated mice. However, on day 35, the BPI-treated animals have lower IL-17 than the PBS-treated mice, suggesting that the BPI molecules have a long-term suppressive effect. Because both BPI molecules suppressed and ameliorated EAE effectively as indicated by the lower EAE scores, lower EAE incidence, and minimal loss in body weight, the only plausible explanation is that IL-17 up-regulation in the peptide-treated mice is caused by the presence of the antigenic sequence in BPI molecules. In addition, high levels of IL-17 in BPI-treated mice could be attributed to transforming growth factor (TGF)-β production as a result of injection of soluble peptide. It is now known that TGF-β and IL-6 play an important role in the induction of Th17 differentiation (Korn et al., 2009; Morishima et al., 2009). Interestingly, there was a significant decrease in IL-17 production at the end of the study among mice receiving either Ac-PLP-BPI-NH2-2 or Ac-PLP-BPI-PEG6, further corroborating our earlier belief that the initial IL-17 response was caused by the antigen used during priming. On the other hand, there was a significant increase in IL-17 production among PBS-treated mice on day 35, suggesting the possibility of an increased and/or an impending relapse of EAE. A better understanding of the up-regulation/down-regulation of IL-17 would be gained through studies carried out for periods much longer than 35 days and using priming agents other than PLP139-151.
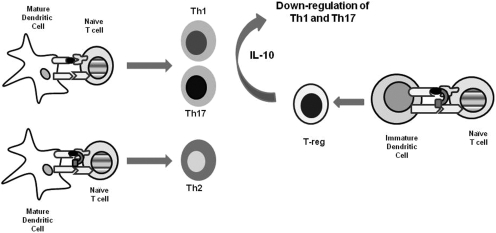
As mentioned previously, the central hypothesis is that the BPI molecules simultaneously bind to MHC-II and ICAM-1 of the immunological synapse at the surface of APCs and selectively alter or suppress the activation of a subpopulation of autoreactive T cells in an antigen-specific manner. Thus, we anticipate that BPI should modulate only those immune responses involved in EAE while minimizing the nonspecific suppression of the entire immune system. Another BPI molecule called GAD-BPI had been shown to colocalize the MHC-II and ICAM-1 on the surface of APCs (B cells) isolated from nonobese diabetic mice (Murray et al., 2007). However, how this colocalization event regulates and alters the commitment of T cells has not been fully elucidated. The mechanisms of action of BPI molecules could also be explained by invoking the mechanism of peptide vaccines as antigen-specific immunotherapy (Fig. 5). When dendritic cells encounter insoluble antigen or complex molecules found on microbes such as bacteria and viruses, they are up-regulated and mature, presenting the processed antigenic peptides using MHC-II molecules on their surface. As they mature, they up-regulate the costimulatory molecules required for full activation of T cells, thus leading to allergic and autoimmunity response upon recognition of the antigenic peptides by the TCRs. This paradigm is based on findings that insoluble peptides can induce local inflammation (Shen et al., 2003), whereas soluble peptides can bind directly to dendritic cells in lymphoid tissues (Santambrogio et al., 1999; Metzler et al., 2000) and may activate regulatory T cells (T-regs) (Dhodapkar et al., 2001; Menges et al., 2002). It has been suggested that the soluble peptide vaccine binds mostly to steady-state (immature) APCs such as dendritic cells. Likewise BPI molecules bind to steady-state APCs, and upon binding of these loaded APCs to naive T cells, the T cells differentiate into T-regs, which produce IL-10 (Larche and Wraith, 2005). The IL-10-producing T-regs suppress the T-cell activation via Th1 and/or Th17 mechanisms (Fig. 5). Our previous data showed that the production of TGF-β- and IL-10-producing CD4+CD25+ T cells were significantly increased in PLP-BPI-treated mice compared with PBS-treated mice, suggesting that the CD4+CD25+ T-regs are in part responsible for the EAE-suppressive activity of BPI (Kobayashi et al., 2007, 2008). However, this does not satisfactorily explain why BPI was much more effective than PLP in suppressing EAE (Kobayashi et al., 2007) or why there were differences in activities of various BPI derivates. Thus, it is also possible that the PLP-BPI molecule binds to mature dendritic cells via simultaneous binding to MHC-II and ICAM-1, which prevents the formation of the immunological synapse formation upon binding of mature dendritic cells to T cells (Fig. 5). This inhibition of the immunological synapse formation generates IL-4-producing Th2 cells as previously observed (Kobayashi et al., 2007). Thus, the BPI molecule acts via two different routes to suppress the T-cell activation whereas PLP peptide alone acts via T-reg activation by binding to immature dendritic cells. These possible mechanisms currently are being investigated in more detail.
Fig. 5.
Potential mechanisms of action of antigenic peptide or BPI molecule in the suppression of EAE. Mature dendritic cells present processed antigenic peptides to naive T cells to generate Th1 and/or Th17 cells. BPI molecule binds simultaneously to MHC-II and ICAM-1 on mature dendritic cells and prevents the formation of the immunological synapse. As a result, BPI molecule inhibits the formation of Th1 and generates Th2 cells. Immature dendritic cells can also present a soluble antigenic and BPI molecule to naive T cells to produce IL-10-producing T-regs. Produced IL-10 suppresses the generation of Th1 and Th17 cells, resulting in the suppression of the disease.
In conclusion, Ac-PLP-BPI-PEG6 effectively inhibited the onset, severity, and incidence of EAE, and its EAE-suppressive activity was comparable with that of Ac-PLP-BPI-NH2-2. The Ac-PLP-BPI-PEG6 treatment has also been shown to lower hypersensitivity reactions compared with Ac-PLP-BPI-NH2-2 and PLP treatment of EAE animals. Further alteration of the structure of BPI molecules may eliminate the hypersensitivity reaction. Currently, the mechanisms of action of BPI molecules and their dosing methods are also being investigated for efficacy optimization and lowering of their side effects. Finally, altering the structure and sequence of BPI molecules will lead to a more efficient and safer BPI-based therapy for MS.
Acknowledgments
We thank Nancy Harmony for proofreading this manuscript.
This work was supported by the National Institutes of Health [Grant R01-AI-0632002] and the National Multiple Sclerosis Society.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161109.
- MS
- multiple sclerosis
- EAE
- experimental autoimmune encephalomyelitis
- PLP
- proteolipid protein
- BPI
- bifunctional peptide inhibitor
- LFA-1
- lymphocyte function-associated antigen-1
- ICAM-1
- intercellular adhesion molecule-1
- MHC
- major histocompatibility complex
- APC
- antigen-presenting cell
- TCR
- T cell receptor
- IL-17
- interleukin-17
- AUC
- area under curve
- Th
- T helper
- SMAC
- supramolecular activation cluster
- PBS
- phosphate-buffered saline
- CFA
- complete Freund's adjuvant
- LC-MS/MS
- liquid chromatography tandem mass spectrometry
- QC
- quality control
- IS
- internal standard
- APL
- altered peptide ligand
- MOG
- myelin oligodendrocyte protein
- TGF
- transforming growth factor
- T-reg
- regulatory T cell.
References
- Alexander C, Kay AB, Larche M. (2002) Peptide-based vaccines in the treatment of specific allergy. Curr Drug Targets Inflamm Allergy 1:353–361 [DOI] [PubMed] [Google Scholar]
- Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, et al. (2000) Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 6:1167–1175 [DOI] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. (2001) The immunological synapse. Annu Rev Immunol 19:375–396 [DOI] [PubMed] [Google Scholar]
- Bromley SK, Dustin ML. (2002) Stimulation of naive T-cell adhesion and immunological synapse formation by chemokine-dependent and -independent mechanisms. Immunology 106:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. (2001) Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 193:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyaman W, Kivisakk P, Reddy J, Chitnis T, Raddassi K, Imitola J, Bradshaw E, Kuchroo VK, Yagita H, Sayegh MH, et al. (2008) Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am J Pathol 173:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth KA, Thompson CB. (2002) Activation and inhibition of lymphocytes by costimulation. J Clin Invest 109:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. (1999) The immunological synapse: a molecular machine controlling T cell activation. Science 285:221–227 [DOI] [PubMed] [Google Scholar]
- Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. (2008) T helper cell type 1 (Th1), Th2, and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology 125:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga R, Gerritsen W, Heijmans N, Amor S. (2008) Axonal loss and gray matter pathology as a direct result of autoimmunity to neurofilaments. Neurobiol Dis 32:461–470 [DOI] [PubMed] [Google Scholar]
- Johnson KP. (1996) Management of relapsing/remitting multiple sclerosis with copolymer 1 (Copaxone). Mult Scler 1:325–326 [DOI] [PubMed] [Google Scholar]
- Kappos L, Bates D, Hartung HP, Havrdova E, Miller D, Polman CH, Ravnborg M, Hauser SL, Rudick RA, Weiner HL, et al. (2007) Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol 6:431–441 [DOI] [PubMed] [Google Scholar]
- Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. (2000) Induction of a nonencephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med 6:1176–1182 [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Kiptoo P, Kobayashi H, Ridwan R, Brocke S, Siahaan TJ. (2008) Prophylactic and therapeutic suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor. Clin Immunol 129:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Kobayashi H, Gu L, Malefyt T, Siahaan TJ. (2007) Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor. J Pharmacol Exp Ther 322:879–886 [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. (2009) IL-17 and Th17 cells. Annu Rev Immunol 27:485–517 [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Greer JM, Kaul D, Ishioka G, Franco A, Sette A, Sobel RA, Lees MB. (1994) A single TCR antagonist peptide inhibits experimental allergic encephalomyelitis mediated by a diverse T cell repertoire. J Immunol 153:3326–3336 [PubMed] [Google Scholar]
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. (2005) Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 353:375–381 [DOI] [PubMed] [Google Scholar]
- Larche M, Wraith DC. (2005) Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med 11:S69–S76 [DOI] [PubMed] [Google Scholar]
- Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. (2002) T cell receptor signaling precedes immunological synapse formation. Science 295:1539–1542 [DOI] [PubMed] [Google Scholar]
- Leech MD, Chung CY, Culshaw A, Anderton SM. (2007) Peptide-based immunotherapy of experimental autoimmune encephalomyelitis without anaphylaxis. Eur J Immunol 37:3576–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8:500–508 [DOI] [PubMed] [Google Scholar]
- Lu B. (2006) The molecular mechanisms that control function and death of effector CD4+ T cells. Immunol Res 36:275–282 [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. (1999) Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 5:101–104 [DOI] [PubMed] [Google Scholar]
- Menges M, Rossner S, Voigtlander C, Schindler H, Kukutsch NA, Bogdan C, Erb K, Schuler G, Lutz MB. (2002) Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med 195:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler B, Anderton SM, Manickasingham SP, Wraith DC. (2000) Kinetics of peptide uptake and tissue distribution following a single intranasal dose of peptide. Immunol Invest 29:61–70 [DOI] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. (1998) Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395:82–86 [DOI] [PubMed] [Google Scholar]
- Morishima N, Mizoguchi I, Takeda K, Mizuguchi J, Yoshimoto T. (2009) TGF-β is necessary for induction of IL-23R and Th17 differentiation by IL-6 and IL-23. Biochem BioPhys Res Commun 386:105–110 [DOI] [PubMed] [Google Scholar]
- Murray JS, Oney S, Page JE, Kratochvil-Stava A, Hu Y, Makagiansar IT, Brown JC, Kobayashi N, Siahaan TJ. (2007) Suppression of type 1 diabetes in NOD mice by bifunctional peptide inhibitor: modulation of the immunological synapse formation. Chem Biol Drug Des 70:227–236 [DOI] [PubMed] [Google Scholar]
- Rueda F, Hygino LC, Jr, Domingues RC, Vasconcelos CC, Papais-Alvarenga RM, Gasparetto EL. (2008) Diffusion tensor MR imaging evaluation of the corpus callosum of patients with multiple sclerosis. Arq Neuropsiquiatr 66:449–453 [DOI] [PubMed] [Google Scholar]
- Sabatino JJ, Jr, Shires J, Altman JD, Ford ML, Evavold BD. (2008) Loss of IFN-gamma enables the expansion of autoreactive CD4+ T cells to induce experimental autoimmune encephalomyelitis by a nonencephalitogenic myelin variant antigen. J Immunol 180:4451–4457 [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Sato AK, Fischer FR, Dorf ME, Stern LJ. (1999) Abundant empty class II MHC molecules on the surface of immature dendritic cells. Proc Natl Acad Sci U S A 96:15050–15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M, Issazadeh S, Stadlbauer TH, Peach R, Sayegh MH, Khoury SJ. (1999) Costimulatory signal blockade in murine relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol 96:158–166 [DOI] [PubMed] [Google Scholar]
- Shen CR, Youssef AR, Devine A, Bowie L, Hall AM, Wraith DC, Elson CJ, Barker RN. (2003) Peptides containing a dominant T-cell epitope from red cell band 3 have in vivo immunomodulatory properties in NZB mice with autoimmune hemolytic anemia. Blood 102:3800–3806 [DOI] [PubMed] [Google Scholar]
- Smith CE, Eagar TN, Strominger JL, Miller SD. (2005) Differential induction of IgE-mediated anaphylaxis after soluble vs. cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 102:9595–9600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonobe Y, Jin S, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. (2007) Chronological changes of CD4+ and CD8+ T cell subsets in the experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. Tohoku J Exp Med 213:329–339 [DOI] [PubMed] [Google Scholar]