Abstract
Differences in the mechanisms underlying tolerance and μ-opioid receptor desensitization resulting from exposure to opioid agonists of different efficacy have been suggested previously. The objective of this study was to determine the effects of protein kinase C (PKC) and G protein-coupled receptor kinase (GRK) inhibition on antinociceptive tolerance in vivo to opioid agonists of different efficacy. A rapid (8-h) tolerance-induction model was used where each opioid was repeatedly administered to naive mice. Animals were then challenged with the opioid after injection of a kinase inhibitor to determine its effects on the level of tolerance. Tolerance to meperidine, morphine, or fentanyl was fully reversed by the PKC inhibitor 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)carbazole (Gö6976). However, in vivo tolerance to [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO) was not reversed by PKC inhibition. The novel small-molecule GRK inhibitors β-adrenergic receptor kinase 1 inhibitor and 2-(8-[(dimethylamino) methyl]-6,7,8,9-tetrahydropyridol[1,2-a]indol-3-yl)-3-(1-methylindol-3-yl)maleimide (Ro 32-0432) did not reverse the tolerance to meperidine, fentanyl, or morphine but did reverse the tolerance to DAMGO. To correlate GRK-dependent DAMGO-induced tolerance with μ-opioid receptor desensitization, we used in vitro whole-cell patch-clamp recording from mouse locus coeruleus neurons and observed that the GRK inhibitors reduced DAMGO-induced desensitization of μ-opioid receptors, whereas the PKC inhibitor had no effect. These results suggest that tolerance induced by low- and moderate-efficacy μ-opioid receptor agonists is dependent on PKC, whereas tolerance induced by the high-efficacy agonist DAMGO is dependent on GRK.
Opioid analgesics are the most widely used drugs for the management of moderate to severe pain. One of the main drawbacks to this class of drugs is the development of tolerance during chronic use; that is, a decrease in the analgesic effect during prolonged use of the drug. The mechanisms of tolerance to any one opiate are multifaceted and not fully understood. We and others have proposed that μ-opioid receptor desensitization plays an important role in opioid tolerance (Bohn et al., 2000; Zuo, 2005; Bailey et al., 2006). μ-Opioid receptor desensitization can occur in at least two ways, through phosphorylation by G-protein coupled receptor kinase (GRK) and subsequent arrestin binding or by phosphorylation by second messenger kinases such as PKC (for review, see Bailey et al., 2006).
Previous studies have suggested that the intrinsic efficacy of an opioid determines its ability to cause desensitization and that the mechanisms underlying such desensitization may vary according to the agonist (Arden et al., 1995; Yabaluri and Medzihradsky, 1997; Selley et al., 1998; Bailey et al., 2004, 2009). High intrinsic efficacy agonists such as DAMGO produce receptor desensitization through activation of GRKs and subsequent binding of arrestins (Keith et al., 1998; Zhang et al., 1998; Zaki et al., 2000), whereas morphine, a moderate intrinsic efficacy agonist, produces desensitization through second messenger signaling pathways such as the PKC pathway (Selley et al., 1998). For example, in mature brain locus coeruleus neurons μ-opioid receptor desensitization due to morphine exposure is induced primarily through a PKC-dependent mechanism, whereas desensitization due to DAMGO exposure is induced through a GRK-dependent mechanism (Bailey et al., 2004, 2006; Johnson et al., 2006; Kelly et al., 2008).
The main objective of this study was to investigate the effect of PKC and GRK inhibition in vivo on antinociceptive tolerance after repeated administration of opioid agonists of different efficacy to complement the breadth of in vitro experimentation on this topic in the literature. The opioid agonists tested had low (meperidine), moderate (morphine and fentanyl), and high efficacy (DAMGO) at the μ-opioid receptor (Selley et al., 1997, 1998). To do this, we used a rapid (8-h) tolerance induction protocol. We demonstrated previously that tolerance on prolonged exposure to the prototypic opioid morphine in vivo can be reversed by PKC inhibition (Smith et al., 2002, 2006), and others have shown that GRK 3 knockout does not affect morphine tolerance (Terman et al., 2004).
Our in vivo results demonstrate that inhibition of GRK causes reversal of DAMGO-induced tolerance but not meperidine-, morphine- or fentanyl-induced tolerance, whereas the inhibition of PKC results in the reversal of meperidine-, morphine-, and fentanyl-induced tolerance but not DAMGO-induced tolerance. In addition, we found that, at the neuronal level, DAMGO-induced desensitization of μ-opioid receptors was inhibited by small-molecule GRK inhibitors but was not affected by PKC inhibitors. These results indicate that the mechanisms of tolerance to opioid agonists are specific to the efficacy of the agonist.
Materials and Methods
In Vivo Experiments
Animals.
Male Swiss-Webster mice (Harlan, Indianapolis, IN), weighing 25 to 30 g, were housed six to a cage in animal care quarters and maintained at 22 ± 2°C on a 12-h light/dark cycle. Food and water were available ad libitum. The mice were brought to a test room (22 ± 2°C, 12-h light/dark cycle), marked for identification, and allowed 18 h to recover from transport and handling. Protocols and procedures were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University Medical Center and comply with the recommendations of the International Association for the Study of Pain (Seattle, WA).
Tail-Immersion Test.
The warm water tail-immersion test was performed according to Coderre and Rollman (1983) using a water bath with the temperature maintained at 56 ± 0.1°C. Before injecting the mice, a baseline (control) latency was determined. Only mice with a control reaction time from 2 to 4 s were used. The average baseline latency for these experiments was 3.0 ± 0.1 s. The test latency after drug treatment was assessed at 30 min for morphine, fentanyl, and meperidine and at 20 min for DAMGO, with a 10-s maximal cut-off time imposed to prevent tissue damage. Antinociception was quantified according to the method of Harris and Pierson (1964) as the percentage of maximal possible effect (%MPE), which was calculated as [(test latency − control latency)/(10 − control latency)] × 100. The %MPE value was calculated for each mouse using at least six mice per drug.
Intracerebroventricular Injections.
Intracerebroventricular injections were performed as described by Pedigo et al. (1975). Mice were anesthetized with isoflurane, and a horizontal incision was made in the scalp. A free-hand 5-μl injection of drug or vehicle was made in the lateral ventricle (2 mm rostral and 2 mm lateral at a 45° angle from the bregma). The extensive experience of this laboratory has made it possible to inject drugs by this route of administration with greater than 95% accuracy. Immediately after testing, the animals were euthanized to minimize any type of distress, according to Institutional Animal Care and Use Committee Guidelines. Intracerebroventricular injections were used for compounds that are unable to pass the blood-brain barrier such as DAMGO and the PKC, PKA, and GRK inhibitors.
Model of In Vivo Acute Opioid Tolerance.
An 8-h antinociceptive tolerance model to morphine, fentanyl, and meperidine was developed as follows. Mice were injected subcutaneously once every 2 h for 6 h (total of four injections) with the minimal dose of opioid that produces maximal analgesia in naive mice in the tail-immersion test. Two hours after the final dose, mice were administered the kinase inhibitor or vehicle by intracerebroventricular injection and immediately challenged with various subcutaneous challenge doses of the opioid to construct dose-response curves for calculation of ED50 values and potency ratios. In a similar manner, an 8-h antinociceptive tolerance model to DAMGO was developed as follows. Mice were injected intracerebroventricularly once every hour (total of eight injections) with the minimal dose of DAMGO that results in maximal analgesia in naive mice in the tail-immersion test. One hour after the final dose, mice were administered the inhibitor by intracerebroventricular injection and immediately challenged with various intracerebroventricular doses of DAMGO to construct dose-response curves for calculation of ED50 values and potency ratios. The intracerebroventricular morphine tolerance model followed the same dosing scheme as DAMGO, with the exception of a 2-h time period between the final dose of intracerebroventricular morphine and the intracerebroventricular injections of inhibitor and the challenge dose of morphine. The above-mentioned opioid doses producing maximal analgesia were chosen based on the construction of acute dose-response curves in naive mice (data not shown).
Electrophysiological Recordings
Brain Slice Preparation.
C57BL mice (males; approximately 30 g) were killed by cervical dislocation, and horizontal brain slices (250 μm in thickness) containing the locus coeruleus (LC) were prepared as described for rat LC slices (Bailey et al., 2003). All brain slice experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986, the European Communities Council Directive 1986 (86/609/EEC), and the University of Bristol ethical review document.
Whole-Cell Patch-Clamp Recordings.
Slices were submerged in a slice chamber (0.5 ml) mounted on the microscope stage and superfused (2.5–3 ml/min) with artificial cerebrospinal fluid composed of 126 mM NaCl, 2.5 mM KCl, 1.2 mM MgCl2, 2.4 mM CaCl2, 1.2 mM NaH2PO4, 11.1 mM d-glucose, 21.4 mM NaHCO3, and 0.1 mM ascorbic acid; saturated with 95% O2, 5% CO2 at 33–34°C. LC neurons were visualized by Nomarski optics using infrared light, and individual cell somata were cleaned by gentle flow of artificial cerebrospinal fluid from a pipette. Whole-cell voltage-clamp recordings (Vh = −60 mV) were made using electrodes (3–6 MΩ) filled with 115 mM K-gluconate, 10 mM HEPES, 11 mM EGTA, 2 mM MgCl2, 10 mM NaCl, 2 mM Mg ATP, and 0.25 mM Na2GTP (pH 7.3; osmolarity, 270 mOsm). Recordings of whole-cell currents were filtered at 2 kHz using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) and analyzed off-line using pClamp (Molecular Devices). Activation of μ-opioid receptors evoked a transmembrane K+ current, and by performing whole-cell patch-clamp recordings, a real-time index of μ-opioid receptor activation could be continuously recorded. Drugs were applied in the superfusing solution at known concentrations, or for β-adrenergic receptor kinase 1 (β-ARK 1) inhibitor, in the pipette solution.
Statistical Analysis
Opioid dose-response curves were generated for calculation of effective dose-50 (ED50) values using least-squares linear regression analysis followed by calculation of 95% confidence limits (95% CL) by the method of Bliss (1967). Tests for parallelism were conducted before calculating the potency ratio values with 95% CL by the method of Colquhoun (1971) who notes that a potency ratio value of greater than 1, with the lower 95% CL greater than 1, is considered a significant difference in potency between groups.
For the analysis of desensitization, the data obtained in the electrophysiological studies were fitted by nonlinear regression and the curves obtained under different recording conditions were compared using the F test (Prism; GraphPad Software Inc., San Diego, CA) to determine whether the curves were distinguishable (P < 0.05).
Drugs and Chemicals
The PKC inhibitor Gö6976, the PKA inhibitor myristoylated PKI-(14-22)-amide, and the GRK inhibitor β-ARK 1 inhibitor were purchased from Calbiochem (San Diego, CA). The GRK inhibitor Ro 32-0432 HCl was purchased from Sigma-Aldrich (St. Louis, MO). Meperidine hydrochloride, fentanyl hydrochloride, morphine sulfate, and etorphine hydrochloride were obtained from the National Institute on Drug Abuse (Bethesda, MD). DAMGO was purchased from Tocris Bioscience (Ellisville, MO). Morphine sulfate, meperidine, and fentanyl were dissolved in pyrogen-free isotonic saline (Hospira, Lake Forest, IL). DAMGO was dissolved in distilled water. PKI-(14-22)-amide was dissolved in distilled water; the corresponding vehicle-injected mice were injected with distilled water. β-ARK 1 inhibitor, Gö6976, and Ro 32-0432 HCl were dissolved in 10% dimethyl sulfoxide, 20% Cremophor (BASF Wyandotte, Wyandotte, MI), and 70% distilled water; the corresponding vehicle-injected mice were injected with 10% dimethyl sulfoxide, 20% Cremophor, and 70% distilled water. We have published previously on the use of this vehicle for intracerebroventricular injections (Smith et al., 1999, 2002, 2003, 2006). The selected doses of the PKA and PKC inhibitors were shown to reverse morphine tolerance in a 3-day morphine pellet tolerance model (Smith et al., 1999, 2002, 2003, 2006). Drugs and chemicals used in the electrophysiological studies were purchased from Sigma Chemical (Poole, Dorset, UK), except for Gö6976 and β-ARK 1 inhibitor (Calbiochem, Nottingham, UK).
Results
Opioid Antinociceptive Tolerance.
Low-, moderate- and high-efficacy opioid agonists produced similar levels of antinociceptive tolerance, as measured by the tail-immersion test. The repeated administration of the low-efficacy μ-opioid agonist meperidine (40 mg/kg s.c. every 2 h for a total of four injections, with test doses of meperidine administered 2 h after the last injection) resulted in 2.7-fold tolerance in the tail-immersion test. Similarly, the repeated administration of the moderate-efficacy μ-opioid agonists morphine (10 mg/kg s.c. every 2 h for a total of four injections, with test doses of morphine administered 2 h after the last injection) and fentanyl (0.2 mg/kg s.c. every 2 h for a total of four injections, with test doses of fentanyl administered 2 h after the last injection) resulted in a 4.6- and 3.5-fold antinociceptive tolerance, respectively. The repeated administration of DAMGO (25.7 ng/kg i.c.v. every 1 h for a total of eight injections, with test doses of DAMGO administered 1 h after the last injection) resulted in 2.4-fold antinociceptive tolerance (Table 1).
TABLE 1.
Opioid antinociceptive tolerance using an 8-h model
Mice were either repeatedly administered vehicle over 8 h and then challenged with the opioid (vehicle + opioid) or repeatedly administered opioid over 8 h and then challenged with the opioid (opioid + opioid) as well as vehicle intracerebroventricularly. Meperidine (40 mg/kg s.c.), morphine (10 mg/kg s.c.), and fentanyl (0.2 mg/kg s.c.) were administered every 2 h for a total of four injections, with test doses of the opioid administered 2 h after the last injection. DAMGO (25.7 ng/kg i.c.v.) was administered every 1 h for a total of eight injections, with test doses of DAMGO administered 1 h after the last injection. All opioid + opioid groups received vehicle intracerebroventricular injections before the opioid test doses. Thirty minutes (20 min for DAMGO) after test doses were administered, tail-immersion latencies were determined for construction of dose-response curves as well as calculation of ED50 values and potency ratios.
| Treatment | ED50 Value (95% CL) | Potency Ratio (95% CL) | |
|---|---|---|---|
| Vehicle + meperidine | 15.1 mg/kg (11.3, 20.1) | ||
| Meperidine + meperidine | 41.9 mg/kg (37.1, 47.3) | vs. vehicle + meperidine | 2.73 (2.29, 3.23) |
| Vehicle + fentanyl | 0.089 mg/kg (0.08, 0.1) | ||
| Fentanyl + fentanyl | 0.292 mg/kg (0.26, 0.33) | vs. vehicle + fentanyl | 3.50 (3.09, 3.92) |
| Vehicle + morphine | 3.5 mg/kg (2.9, 4.2) | ||
| Morphine + morphine | 16.3 mg/kg (13.4, 19.8) | vs. vehicle + morphine | 4.55 (3.77, 5.69) |
| Vehicle + DAMGO | 11.3 ng/kg (10.3, 12.3) | ||
| DAMGO + DAMGO | 28.3 ng/kg (25.7, 36.0) | vs. vehicle + DAMGO | 2.42 (2.13, 2.73) |
Effects of PKC Inhibition on Opioid Antinociceptive Tolerance.
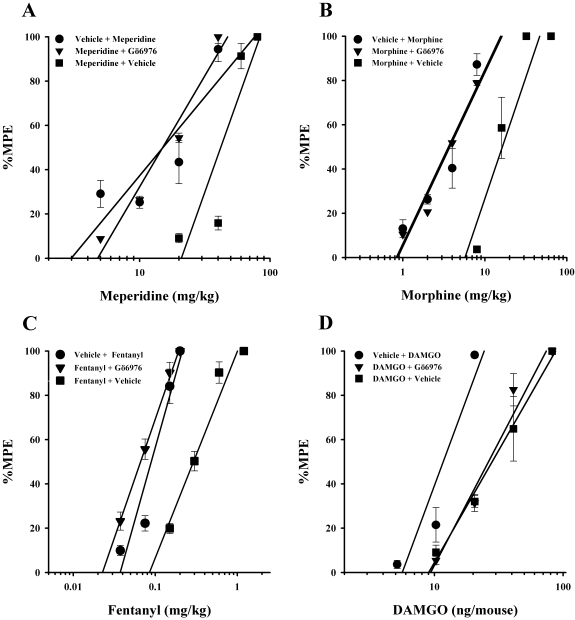
The PKC inhibitor Gö6976 (4 nmol/mouse i.c.v.) administered to tolerant mice immediately before the challenge doses of opioid fully reversed morphine, meperidine, and fentanyl antinociceptive tolerance but did not reverse tolerance induced by DAMGO (Table 2A; Fig. 1). Gö6976 fully reversed the antinociceptive tolerance observed in mice repeatedly administered the low-efficacy μ-opioid agonist meperidine (Fig. 1A).
TABLE 2.
PKC and GRK inhibitors and ED50 and potency ratio values
Mice were either repeatedly administered opioid over 8 h and then administered vehicle intracerebroventricularly immediately before being challenged with the opioid (opioid + vehicle) or repeatedly administered opioid over 8 h and then administered the PKC inhibitor Gö6976 (4 nmol/mouse i.c.v.; A), the GRK inhibitor β-ARK 1 inhibitor (20 nmol/mouse i.c.v.; B), or the GRK inhibitor Ro 32-0432 (2 nmol/mouse i.c.v.; C) immediately before being challenged with the opioid (opioid + inhibitor). Meperidine (40 mg/kg s.c.; A and B), morphine (10 mg/kg s.c.; A–C), and fentanyl (0.2 mg/kg s.c.; A and B) were administered every 2 h for a total of four injections, with inhibitor or vehicle and then test doses of the opioid administered 2 h after the last injection. DAMGO (25.7 ng/kg i.c.v.; A–C) was administered every 1 h for a total of eight injections, with inhibitor or vehicle and then test doses of DAMGO administered 1 h after the last injection. Thirty minutes (20 min for DAMGO) after test doses were administered, tail immersion latencies were determined for construction of dose-response curves as well as calculation of ED50 values and potency ratios.
| Treatment | ED50 Value (95% CL) | Potency Ratio (95% CL) | |
|---|---|---|---|
| A. PKC inhibitor Gö6976 | |||
| Meperidine + vehicle | 41.9 mg/kg (37.1, 47.3) | ||
| Meperidine + Gö6976 | 15.0 mg/kg (12.9, 17.5) | vs. meperidine + vehicle | 2.76 (2.41, 3.16) |
| Fentanyl + vehicle 6 | 0.292 mg/kg (0.26, 0.33) | ||
| Fentanyl + Gö697 | 0.07 mg/kg (0.06, 0.07) | vs. fentanyl + vehicle | 4.65 (4.12, 5.26) |
| Morphine + vehicle | 16.3 mg/kg (13.4, 19.8) | ||
| Morphine + Gö6976 | 3.6 mg/kg (2.6, 4.8) | vs. morphine + vehicle | 4.33 (3.30, 5.20) |
| DAMGO + vehicle | 28.3 ng/kg (25.7, 36.0) | ||
| DAMGO + Gö6976 | 26.2 ng/kg (25.7, 30.8) | vs. DAMGO + vehicle | 1.07 (0.89, 1.30) |
| B. GRK inhibitor β-ARK 1 inhibitor | |||
| Meperidine + vehicle | 51.4 mg/kg (46.4, 56.9) | ||
| Meperidine + β-ARK 1 | 46.3 mg/kg (42.5, 50.5) | vs. meperidine + vehicle | 1.10 (1.00, 1.21) |
| Fentanyl + vehicle | 0.392 mg/kg (0.35, 0.44) | ||
| Fentanyl + β-ARK 1 | 0.395 mg/kg (0.35, 0.45) | vs. fentanyl + vehicle | 1.01 (0.90, 1.13) |
| Morphine + vehicle | 22.4 mg/kg (19.3, 26.1) | ||
| Morphine + β-ARK 1 | 20.3 mg/kg (17.6, 23.3) | vs. morphine + vehicle | 1.10 (0.92, 1.32) |
| DAMGO + vehicle | 30.3 ng/kg (25.7, 36.0) | ||
| DAMGO + β-ARK 1 | 11.3 ng/kg (10.3, 15.4) | vs. DAMGO + vehicle | 2.57 (2.28, 2.88) |
| C. GRK inhibitor Ro 32-0432 | |||
| Morphine + vehicle | 23.2 mg/kg (20.0, 26.8) | ||
| Morphine + Ro 32-0432 | 25.9 mg/kg (22.6, 29.8) | vs. morphine + vehicle | 1.11 (0.93, 1.33) |
| DAMGO + vehicle | 27.7 ng/kg (25.7, 30.8) | ||
| DAMGO + Ro 32-0432 | 11.8 ng/kg (10.3, 15.4) | vs. DAMGO + vehicle | 2.34 (2.04, 2.65) |
Fig. 1.
PKC inhibitor Gö6976-induced reversal of low- and moderate-efficacy but not high-efficacy opioid tolerance. Gö6976 (4 nmol/mouse i.c.v.) completely reversed antinociceptive tolerance in meperidine- (A), morphine- (B), and fentanyl (C)-tolerant mice but not in DAMGO (D)-tolerant mice. Each data point represents six mice. Tolerance was determined by the tail-immersion test 30 min (20 min for DAMGO) after the inhibitor or vehicle and opioid were administered, using various doses of the opioid subcutaneously or intracerebroventricularly for construction of dose-response curves for calculation of ED50 values and potency ratios. ●, animals treated only with vehicle for 8 h before being administered vehicle intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve. ▼, animals treated with the indicated opioid repeatedly over 8 h to build tolerance before being administered inhibitor intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve. ■, animals treated with the indicated opioid repeatedly over 8 h to build tolerance before being administered vehicle intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve.
Similarly, the antinociceptive tolerance observed in mice repeatedly administered either of the moderate-efficacy μ-opioid agonists, morphine or fentanyl, was fully reversed by the administration of the PKC inhibitor Gö6976 (Fig. 1, B and C). Conversely, this same treatment with Gö6976 failed to significantly reverse the antinociceptive tolerance observed in mice repeatedly administered the high-efficacy μ-opioid agonist DAMGO (Fig. 1D).
Effects of GRK Inhibition on Opioid Antinociceptive Tolerance.
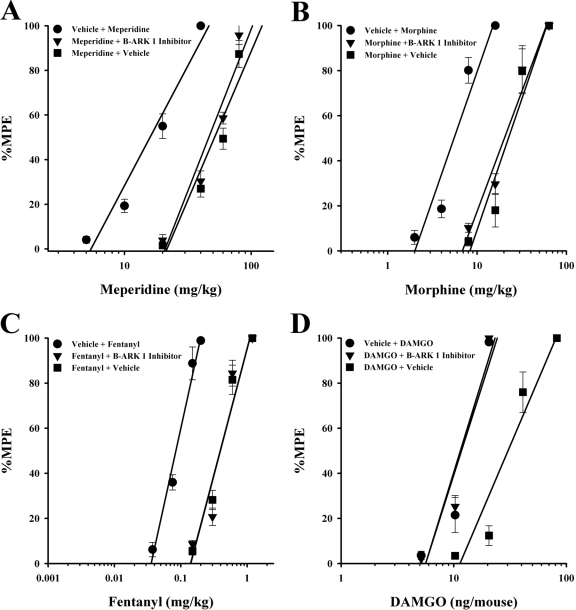
As shown in Table 2B and illustrated in Fig. 2, the GRK inhibitor β-ARK 1 inhibitor (a specific GRK 2 inhibitor; Iino et al., 2002) did not reverse morphine, meperidine, or fentanyl antinociceptive tolerance but did fully reverse DAMGO tolerance. The ED50 values for meperidine, morphine, and fentanyl in the repeatedly opioid-treated mice given β-ARK 1 inhibitor (20 nmol/5 μl i.c.v.), immediately before the challenging doses, were not significantly different from those calculated in the repeatedly opioid-treated mice given vehicle (Table 2B; Fig. 2, A–C).
Fig. 2.
GRK inhibitor β-ARK 1 inhibitor-induced reversal of high-efficacy opioid but not moderate-efficacy opioid tolerance. β-ARK 1 inhibitor (20 nmol/mouse i.c.v.) completely reversed antinociceptive tolerance in DAMGO-tolerant mice (D) but not meperidine- (A), morphine- (B), and fentanyl (C)-tolerant mice. Each data point represents six mice. Tolerance was determined by the tail-immersion test 30 min (20 min for DAMGO) after the inhibitor or vehicle and opioid were administered, using various doses of the opioid subcutaneously or intracerebroventricularly for construction of dose-response curves for calculation of ED50 values and potency ratios. ●, animals treated only with vehicle for 8 h before being administered vehicle intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve. ▼, animals treated with the indicated opioid repeatedly over 8 h to build tolerance before being administered inhibitor intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve. ■, animals treated with the indicated opioid repeatedly over 8 h to build tolerance before being administered vehicle intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve.
It is interesting to note that this same treatment with β-ARK 1 inhibitor fully reversed the antinociceptive tolerance observed in mice repeatedly administered the high-efficacy μ-opioid agonist DAMGO (Fig. 2D). The ED50 value for DAMGO in the repeatedly DAMGO-treated mice, given β-ARK 1 inhibitor immediately before the challenging doses, was significantly different from that of DAMGO-tolerant mice given vehicle inhibitor (Table 2B).
The GRK 5 inhibitor Ro 32-0432 (Ro 32-0432 is less specific than β-ARK 1 inhibitor as it also inhibits GRKs 2 and 3, although to a lesser degree than it does GRK 5; Aiyar et al., 2000) failed to reverse antinociceptive tolerance when administered intracerebroventricularly (2 nmol/mouse) in mice repeatedly administered morphine. The ED50 value for morphine in the repeatedly morphine-treated mice given Ro 32-0432 was not significantly different from that calculated in the morphine-tolerant mice administered vehicle. However, this same inhibitor led to full reversal of antinociceptive tolerance in mice repeatedly administered DAMGO (Table 2C).
Effects of Combined Administration of PKC and PKA Inhibitors on DAMGO Tolerance.
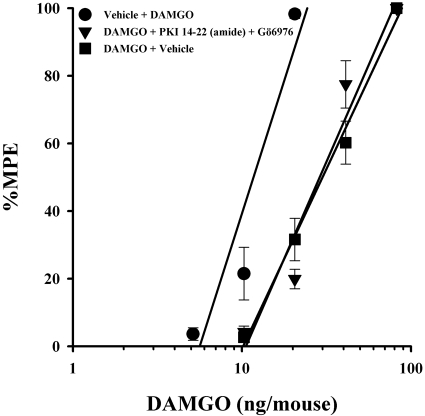
In a previous study, we showed that a higher level of tolerance (45-fold) to morphine was not fully reversed by very high doses of either a PKA inhibitor or a PKC inhibitor alone, but a full reversal was achieved by their combined administration (Smith et al., 2003). To determine whether high-efficacy opioid tolerance, although of a lesser magnitude, was functionally similar to high levels of morphine tolerance, a PKA and a PKC inhibitor were administered simultaneously in DAMGO-tolerant animals. This combined treatment did not significantly reverse tolerance to DAMGO (Fig. 3).
Fig. 3.
Combined inhibition of PKC (Gö6976) and PKA (PKI 14-22) failed to reverse high-efficacy opioid tolerance. Gö6976 (4 nmol/mouse i.c.v.) and PKI 14-22 (3.75 nmol/mouse i.c.v.) administered together failed to reverse antinociceptive tolerance in DAMGO-tolerant mice. Each data point represents six mice. Tolerance was determined by the tail-immersion test 20 min after the inhibitor or vehicle and DAMGO were administered, using various doses of DAMGO intracerebroventricularly for construction of dose-response curves for calculation of ED50 values and potency ratios. ●, animals treated only with vehicle for 8 h before being administered vehicle intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve. ▼, animals treated with the indicated opioid repeatedly over 8 h to build tolerance before being administered inhibitor intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve. ■, animals treated with the indicated opioid repeatedly over 8 h to build tolerance before being administered vehicle intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve.
Effects of PKC and GRK Inhibitors on Morphine Tolerance Developed from Intracerebroventricular Injections.
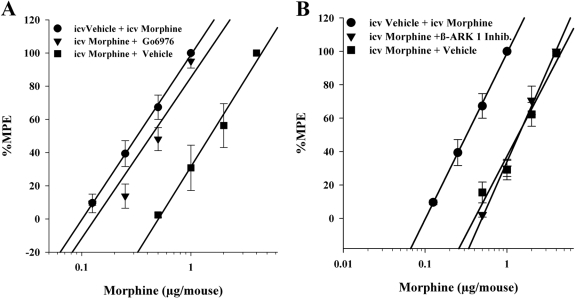
It was important to address whether the differences in the results with PKC and GRK inhibitors between DAMGO and the other opioids was because of the difference in their efficacies or whether it had to do with the dosing scheme and different routes of administration (i.e., DAMGO was administered intracerebroventricularly, whereas other opioids were administered subcutaneously). Therefore, in a new series of experiments morphine was administered in a similar intracerebroventricular dosing scheme as DAMGO and then the animals were administered either Gö6976 (4 nmol/mouse i.c.v.) or β-ARK 1 inhibitor (20 nmol/5 μl i.c.v.) and a challenge dose of morphine intracerebroventricularly to construct a dose-response curve. As can be seen in Fig. 4, morphine tolerance was reversed by the PKC inhibitor (Fig. 4A). However, morphine tolerance was not reversed by the GRK inhibitor (Fig. 4B). These results are the same as those obtained from animals that were administered morphine subcutaneously (Figs. 1B and 2B).
Fig. 4.
Tolerance after the intracerebroventricular administration of morphine was reversed by the PKC inhibitor Gö6976 but not by the GRK inhibitor β-ARK 1 inhibitor. Gö6976 (4 nmol/mouse i.c.v.) reversed intracerebroventricular morphine antinociceptive tolerance (A), whereas β-ARK 1 inhibitor (20 nmol/mouse) failed to reverse intracerebroventricular morphine antinociceptive tolerance (B). Each data point represents six mice. Tolerance was determined by the tail immersion test 30 min after the inhibitor or vehicle and morphine were administered, using various doses of the opioid subcutaneously or intracerebroventricularly for construction of dose-response curves for calculation of ED50 values and potency ratios. ●, animals treated only with vehicle for 8 h before being administered vehicle intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve. ▼, animals treated with the indicated opioid repeatedly over 8 h to build tolerance before being administered inhibitor intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve. ■ indicates animals treated with the indicated opioid repeatedly over 8 h to build tolerance before being administered vehicle intracerebroventricularly and the challenge doses of the indicated opioid to construct a dose-response curve.
Effects of Administration of PKC and GRK Inhibitors on Agonist-Induced Desensitization of the μ-Opioid Receptor in Mature LC Neurons.
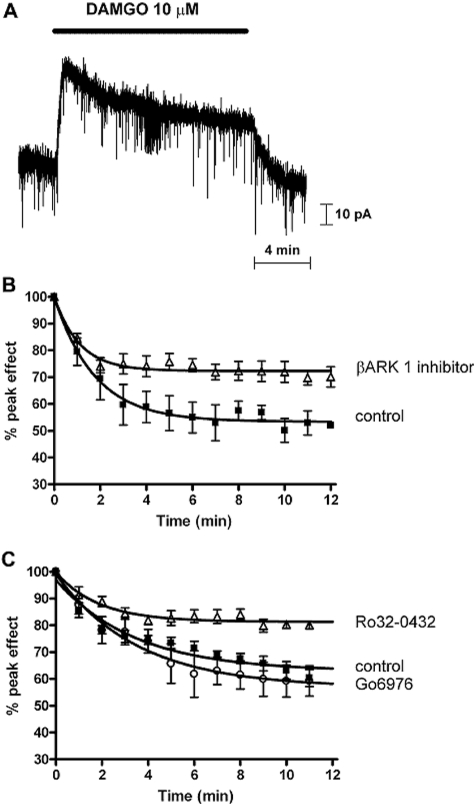
Finally, we wanted to demonstrate that the small-molecule GRK inhibitors we had used in the in vivo experiments also inhibited desensitization of the μ-opioid receptor. We have reported previously that morphine-induced μ-opioid receptor desensitization can be reversed by various PKC inhibitors, including Gö6976 but not by inhibition of GRK (Bailey et al., 2004, 2009). To study the effect of GRK inhibitors on DAMGO-induced desensitization, we performed whole-cell voltage-clamp recordings from LC neurons in vitro in slices taken from mature mice. Application of DAMGO (1 or 10 μM) to the brain slice containing the LC evoked an outward current that peaked within 1 min and then declined (desensitized) in the continued presence of the drug over the 15-min application period (Fig. 5A).
Fig. 5.
Inhibition of DAMGO-induced μ-opioid receptor desensitization by GRK inhibitors. A, current recording from a mouse LC neuron. Application of DAMGO induced an outward current that was not sustained for the period of drug application (indicated by the solid bar) but declined to a steady state. B, decay from the peak of the current induced by DAMGO (10 μM; n = 3) in control (■) and when the βARK 1 inhibitor (100–300 μM; n = 6–7) was present in the recording pipettes solution (△). There was no difference in the degree of inhibition by 100 and 300 μM βARK 1 inhibitor; therefore, the data have been combined. The reduction in DAMGO desensitization in the presence of the βARK 1 inhibitor was statistically significant (p < 0.0001). C, decay from the peak of the current induced by DAMGO (1 μM; n = 6) in control (■) and after slices had been exposed to either Ro 32-0432 inhibitor (0.1–1 μM; n = 6; △) or Gö6976 (1 μM; n = 4; ○) for 20 min before and during the subsequent exposure to DAMGO. There was no difference in the degree of inhibition by 0.1 and 1 μM Ro 32-0432; therefore, the data have been combined. The reduction in DAMGO desensitization in the presence of Ro 32-0432 was statistically significant (p < 0.0001), whereas Go6976 did not significantly alter the DAMGO desensitization.
The β-ARK 1 inhibitor was diffused into the cell from the recording pipette for 15 min before application of DAMGO and during the rest of the experiment. The amount of desensitization induced by 10 μM DAMGO was markedly reduced in the presence of the β-ARK 1 inhibitor at both 100 and 300 μM (Fig. 5B) (there was no difference in the degree of inhibition by 100 and 300 μM β-ARK 1 inhibitor; therefore, the data have been combined). The t1/2 value of desensitization was significantly altered [for DAMGO alone, t1/2 = 73 s (95% CL = 59, 94), whereas for DAMGO in the presence of the β-ARK 1 inhibitor, t1/2 = 46 s (95% CL, = 34, 71)]. Similarly, exposure of LC slices to the GRK inhibitor Ro 32-0432 (0.1–1 μM) for 20 min before and then during the subsequent application of 1 μM DAMGO reduced the amount of DAMGO-induced desensitization (Fig. 5C). In contrast, exposure of the LC slices to Gö6976, a structurally related bisindoylmaleimide that inhibits PKC but not GRK, before and during the application of the opioid failed to alter the desensitization induced by DAMGO (Fig. 5C).
Discussion
We have demonstrated previously that, in isolated neurons, μ-opioid receptor desensitization to the high-efficacy opioid DAMGO differs from that to the moderate-efficacy opioid morphine (Bailey et al., 2003; Johnson et al., 2006), but these results have not yet been demonstrated for tolerance in vivo. To this end, the present study was aimed at investigating whether differences in the mechanism of tolerance to opioids of different agonist efficacy can be found in vivo. To test this hypothesis, a rapid induction in vivo opioid tolerance model was developed using low-, moderate-, and high-efficacy opioids. Specific inhibitors for PKC and GRK were assessed for their ability to reverse opioid tolerance induced by the various opioids.
In these experiments, we demonstrated that antinociceptive tolerance can be developed over 8-h with repeated injections of the low-efficacy μ-opioid receptor agonist meperidine, the moderate-efficacy μ-opioid receptor agonists morphine and fentanyl, and the high-efficacy μ-opioid receptor peptide agonist DAMGO. It is important to note that in various in vitro assays including rat brain tissue, mMOR-CHO cells, and SK-N-SH cells, morphine and fentanyl, although of different potency, are of similar efficacy (Selley et al., 1997, 1998).
We found that the tolerance to meperidine, morphine, or fentanyl was fully reversed by the administration of a PKC inhibitor but not by the administration of GRK inhibitors. However, tolerance to DAMGO was reversed by the GRK inhibitors but not by the PKC inhibitor. We used two different GRK inhibitors; one inhibitor (Ro 32-0432) inhibits GRKs 5, 2, and 3 and the other inhibitor (β-ARK 1 inhibitor) specifically inhibits GRK 2. Because both inhibited tolerance to DAMGO, this suggests that GRK 2 may be the GRK involved in regulating the μ-opioid receptor. These in vivo results studying opioid tolerance are in agreement with our previous results on μ-opioid receptor desensitization in isolated neurons (Bailey et al., 2004, 2009; Johnson et al., 2006) and support the hypothesis that μ-opioid receptor desensitization contributes to tolerance.
Our laboratory has reported on the involvement of PKC in mediating morphine tolerance in vivo (Granados-Soto et al., 2000; Bailey et al., 2006). Most recently, we have used selective inhibitors of different PKC isoforms to demonstrate the involvement of PKCα, PKCγ, and to a lesser extent PKCε in antinociceptive morphine tolerance (Smith et al., 2007). Other workers have demonstrated that morphine tolerance is absent in the PKCγ knockout mouse (Zeitz et al., 2001). In addition, Granados-Soto et al. (2000) demonstrated that rats infused with morphine for 5 days had significantly higher levels of PKCα and PKCγ in the dorsal spinal horn. The higher levels of PKCα and PKCγ, as well as the morphine antinociceptive tolerance, were prevented when the PKC inhibitor chelerythrine was coinfused with morphine during the 5-day treatment.
GRKs have also been suggested to play a key role in μ-opioid receptor desensitization and in tolerance to some opioids. We have reported recently that in both LC neurons and human embryonic kidney 293 cells rapid DAMGO-induced μ-opioid receptor desensitization is GRK-mediated because it is blocked by overexpression of a GRK 2 dominant-negative mutant (Johnson et al., 2006; Kelly et al., 2008; Bailey et al., 2009). In the present study, we found that GRK 2 inhibition reversed DAMGO tolerance but did not reverse tolerance to either morphine or fentanyl in vivo. This is in partial agreement with Terman et al. (2004) who observed that in GRK 3 knockout animals (GRK 2 knockout is lethal) morphine tolerance was unaffected. However, they also reported that tolerance to fentanyl was reduced in the GRK 3 knockout animals, which is different from what we observed with the GRK inhibitors where fentanyl-induced tolerance was unaffected. We do not at present have an explanation for this discrepancy.
One explanation for differences in kinase mechanisms in tolerance between opioid agonists of different efficacies is that opioids induce different conformational changes of the receptor depending upon their efficacy. The ability of a G protein-coupled receptor, such as the μ-opioid receptor, to acquire different conformations when activated by various ligands is referred to as functional selectivity (Mailman, 2007; Urban et al., 2007; Kelly et al., 2008). Opioid agonists would appear to stabilize distinct conformations of the receptor that allow the receptor to couple to the appropriate G proteins to elicit similar downstream responses but that are distinct enough to permit different desensitization mechanisms. Thus, the binding of low- and moderate-efficacy agonists to the μ-opioid receptor induces a conformational change that makes the phosphorylation sites for PKC more readily available, whereas binding of the high-efficacy opioid DAMGO causes a conformational change that makes the phosphorylation sites for GRK more available, thereby differentiating the pathways for tolerance activated by the different opioids.
The ability of some μ-opioid receptor agonists to induce GRK phosphorylation, thereby recruiting arrestin and leading to internalization of the receptor (e.g., DAMGO and etorphine), whereas others are unable to cause such recruitment and internalization (e.g., morphine) has been widely demonstrated in vitro (Keith et al., 1996; Zhang et al., 1998).
One could argue that the difference we found between the low- and moderate-efficacy opioids and the high-efficacy opioid could be due to DAMGO being a peptide rather than due to its high efficacy. However, in vitro experiments have shown that there are only minor differences between DAMGO and other high-efficacy opioids in their stimulation of G proteins despite their structural differences (Saidak et al., 2006). We did attempt to use etorphine in this rapid tolerance induction model; however, possibly due to its very high efficacy, we were unable to develop a rapid induction of tolerance model with this ligand. There is evidence in the literature that under similar conditions, high-efficacy opioids produce significantly less tolerance to analgesic effects than do low-efficacy opioids (Madia et al., 2009). Here, we also saw less tolerance after the 8-h exposure to DAMGO than after the 8-h exposure to the less efficacious opioids. It is possible that, in this tolerance model, animals are unable to produce a measurable amount of tolerance to the analgesic effects of such a high-efficacy opioid as etorphine.
There was also the possibility that the differences we found between the opioids we tested could be a result of the route of administration of the opioid. However, we were able to show, through the experiments where morphine was administered intracerebroventricularly to induce tolerance, that the route of administration of the opioid does not affect which kinase inhibitors are able to reverse the tolerance of that opioid.
In conclusion, these results support our hypothesis that different mechanisms underlie tolerance to opioids of different efficacies. This is the first report of the ability of any PKC inhibitor to reverse in vivo tolerance to meperidine and fentanyl as well as the first in vivo reversal of DAMGO tolerance with a GRK inhibitor. The GRK-dependent mechanism plays a greater role in tolerance to high-efficacy opioids, whereas a PKC-dependent desensitization of the receptor mechanism plays a greater role in tolerance to low- and moderate-efficacy opioid agonists.
This work was supported in part by the National Institutes of Health National Institute of Drug Abuse [Grants DA020836, K05-DA480, DA07027].
These data are a part of the dissertation work: Hull LC (July 2009) Enzymatic Regulation of Opioid Antinociception and Tolerance, Ph.D. dissertation, Virginia Commonwealth University, Richmond, VA.
Parts of this work were presented previously in poster form: Hull LC, Gabra BH, Smith FL, and Dewey WL (2008) PKC and PKA inhibitors reverse acute tolerance to low- and moderate- but not high-efficacy μ-opioid agonists, in Proceedings of the Annual Meeting of the Federation of American Scientists for Experimental Biology; 2008 April 5–9 ; San Diego, CA. Federation of American Scientists for Experimental Biology, Bethesda, MD.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161455.
- PKC
- protein kinase C
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- GRK
- G protein-coupled receptor kinase
- %MPE
- percentage maximal possible effect
- LC
- locus coeruleus
- β-ARK
- β-adrenergic receptor kinase
- β-ARK1 inhibitor
- β-adrenergic receptor kinase 1, methyl-5-[2-(5-nitro-2-furyl)vinyl]-2-furoate)
- PKA
- protein kinase A
- Gö6976
- 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)carbazole
- PKI-(14-22)-amide
- Myr-N-Gly-Arg-Thr-Gly-Arg-Arg-Asn-Ala-Ile-NH2
- Ro 32-0432 HCl
- 2-(8-[(dimethylamino) methyl]-6,7,8,9-tetrahydropyridol[1,2-a]indol-3-yl)-3-(1-methylindol-3-yl)maleimide).
References
- Aiyar N, Disa J, Dang K, Pronin AN, Benovic JL, Nambi P. (2000) Involvement of G protein-coupled receptor kinase-6 in desensitization of CGRP receptors. Eur J Pharmacol 403:1–7 [DOI] [PubMed] [Google Scholar]
- Arden JR, Segredo V, Wang Z, Lameh J, Sadée W. (1995) Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem 65:1636–1645 [DOI] [PubMed] [Google Scholar]
- Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G. (2003) Mu-opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci 23:10515–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Kelly E, Henderson G. (2004) Protein kinase C activation enhances morphine-induced rapid desensitization of μ-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol 66:1592–1598 [DOI] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, et al. (2009) Involvement of PKCalpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. Br J Pharmacol 158:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. (2006) How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci 27:558–565 [DOI] [PubMed] [Google Scholar]
- Bliss CI. (1967) Statistics in Biology, McGraw-Hill, New York: [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723 [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Rollman GB. (1983) Naloxone hyperalgesia and stress-induced analgesia in rats. Life Sci 32:2139–2146 [DOI] [PubMed] [Google Scholar]
- Colquhoun D. (1971) Lectures in Biostatistics: An Introduction to Statistics with Applications in Biology and Medicine, Clarendon Press, Oxford, United Kingdom: [Google Scholar]
- Granados-Soto V, Kalcheva I, Hua X, Newton A, Yaksh TL. (2000) Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain 85:395–404 [DOI] [PubMed] [Google Scholar]
- Harris LS, Pierson AK. (1964) Some narcotic antagonists in the benzomorphan series. J Pharmacol Exp Ther 143:141–148 [PubMed] [Google Scholar]
- Iino M, Furugori T, Mori T, Moriyama S, Fukuzawa A, Shibano T. (2002) Rational design and evaluation of new lead compound structures for selective betaARK1 inhibitors. J Med Chem 45:2150–2159 [DOI] [PubMed] [Google Scholar]
- Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. (2006) Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol 70:676–685 [DOI] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. (1998) mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol 53:377–384 [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. (1996) Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem 271:19021–19024 [DOI] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. (2008) Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol 153 (Suppl 1):S379–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madia PA, Dighe SV, Sirohi S, Walker EA, Yoburn BC. (2009) Dosing protocol and analgesic efficacy determine opioid tolerance in the mouse. Psychopharmacology (Berl) 207:413–422 [DOI] [PubMed] [Google Scholar]
- Mailman RB. (2007) GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedigo NW, Dewey WL, Harris LS. (1975) Determination and characterization of the antinociceptive activity of intraventricularly administered acetylcholine in mice. J Pharmacol Exp Ther 193:845–852 [PubMed] [Google Scholar]
- Saidak Z, Blake-Palmer K, Hay DL, Northup JK, Glass M. (2006) Differential activation of G-proteins by mu-opioid receptor agonists. Br J Pharmacol 147:671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR. (1998) Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamus. J Pharmacol Exp Ther 285:496–505 [PubMed] [Google Scholar]
- Selley DE, Sim LJ, Xiao R, Liu Q, Childers SR. (1997) mu-Opioid receptor-stimulated guanosine-5′-O-(gamma-thio)-triphosphate binding in rat thalamus and cultured cell lines: signal transduction mechanisms underlying agonist efficacy. Mol Pharmacol 51:87–96 [DOI] [PubMed] [Google Scholar]
- Smith FL, Dombrowski DS, Dewey WL. (1999) Involvement of intracellular calcium in morphine tolerance in mice. Pharmacol Biochem Behav 62:381–388 [DOI] [PubMed] [Google Scholar]
- Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. (2007) Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain 127:129–139 [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed R, Elzey MJ, Welch SP, Selley D, Sim-Selley L, Dewey WL. (2002) Prolonged reversal of morphine tolerance with no reversal of dependence by protein kinase C inhibitors. Brain Res 958:28–35 [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Elzey MJ, Dewey WL. (2003) The expression of a high level of morphine antinociceptive tolerance in mice involves both PKC and PKA. Brain Res 985:78–88 [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Smith PA, Dewey WL, Gabra BH. (2006) PKC and PKA inhibitors reinstate morphine-induced behaviors in morphine tolerant mice. Pharmacol Res 54:474–480 [DOI] [PubMed] [Google Scholar]
- Terman GW, Jin W, Cheong YP, Lowe J, Caron MG, Lefkowitz RJ, Chavkin C. (2004) G-protein receptor kinase 3 (GRK3) influences opioid analgesic tolerance but not opioid withdrawal. Br J Pharmacol 141:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Yabaluri N, Medzihradsky F. (1997) Down-regulation of mu-opioid receptor by full but not partial agonists is independent of G protein coupling. Mol Pharmacol 52:896–902 [DOI] [PubMed] [Google Scholar]
- Zaki PA, Keith DE, Jr, Brine GA, Carroll FI, Evans CJ. (2000) Ligand-induced changes in surface μ-opioid receptor number: relationship to G protein activation? J Pharmacol Exp Ther 292:1127–1134 [PubMed] [Google Scholar]
- Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. (2001) Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC gamma mutant mice. Pain 94:245–253 [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A 95:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z. (2005) The role of opioid receptor internalization and beta-arrestins in the development of opioid tolerance. Anesth Analg 101:728–734 [DOI] [PubMed] [Google Scholar]